Abstract
Adult Lytechinus variegatus were fed eight formulated diets with different protein (ranging from 12 to 36%) and carbohydrate (ranging from 21 to 39 %) levels. Each sea urchin (n = 8 per treatment) was fed a daily sub-satiation ration of 1.5% of average body weight for 9 weeks. Akaike information criterion analysis was used to compare six different hypothesized dietary composition models across eight growth measurements. Dietary protein level and protein: energy ratio were the best models for prediction of total weight gain. Diets with the highest (> 68.6 mg P kcal−-1) protein: energy ratios produced the most wet weight gain after 9 weeks. Dietary carbohydrate level was a poor predictor for most growth parameters examined in this study. However, the model containing a protein × carbohydrate interaction effect was the best model for protein efficiency ratio (PER). PER decreased with increasing dietary protein level, more so at higher carbohydrate levels. Food conversion ratio (FCR) was best modeled by total dietary energy levels: Higher energy diets produced lower FCRs. Dietary protein level was the best model of gonad wet weight gain. These data suggest that variations in dietary nutrients and energy differentially affect organismal growth and growth of body components.
Keywords: Sea Urchin, nutrition, protein, carbohydrate, production, growth
Introduction
Protein is one of the most necessary and costly nutrients of most aquatic animal diets. Adequate provision of dietary protein decreases feed intake (Frantzis and Gremare, 1992; Fernandez and Bourdouresque, 1998; McBride et al., 1998; Meidel and Scheibling, 1999; Agatsuma, 2000; Fernandez and Bourdouresque, 2000; Hammer et al., 2004; Daggett et al., 2005; Hammer et al., 2006) and increases growth (Fernandez, 1997; Cook et al. 1998; Fernandez and Bourdouresque, 1998; Fernandez and Pergent ,1998; Meidel and Scheibling, 1999; Agatsuma 2000; Akiyama, 2001; Hammer et al., 2004; Hammer et al., 2006a; Taylor, 2006) and roe production (de Jong-Westman et al., 1995a; Fernandez, 1997; Barker et al., 1998; Cook et al., 1998; Meidel and Scheibling, 1999; Pearce et al., 2002b; Hammer et al., 2004; Chang et al., 2005; Schlosser et al., 2005; Hammer et al., 2006a; Marsh and Watts, 2007; Woods et al., 2008) in a number of sea urchin species. However, several studies have hypothesized there is a level of protein level at which growth is maximized (McBride et al., 1998; Kennedy et al., 2005; Senaratna et al., 2005; Hammer et al., 2006a; Marsh and Watts, 2007).
Despite its value as a nitrogen source, metabolism of protein as an energy source is energetically inefficient (Marsh and Watts, 2007) and nitrogenous waste is a water pollutant (Basuyaux and Mathieu, 1999). Furthermore, high protein levels (Pearce et al., 2002b; Woods et al., 2008) and possibly protein sources or the presence of specific amino acids (Komata et al., 1962; Hirano et al., 1978; Hoshikawa et al., 1998; Murata et al., 2001, 2002; Pearce et al. 2002a; Robinson et al., 2002; Osako et al., 2007; Woods et al. 2008) have been suggested to have an adverse effect on the quality of sea urchin roe. Therefore, a formulated diet should provide individuals with adequate protein for maximal growth and production, but excess protein should be avoided. Exact dietary protein requirements (amino acid requirements) for sea urchins have not been established but, as with other animals, requirements may vary among species and age classes.
In addition to a source of amino nitrogen, urchins require energy for production. Soluble carbohydrates are easily digested by sea urchins, and numerous carbohydrases have been identified in the sea urchin gut (Lawrence et al., 2007), indicating that sea urchins can most likely utilize carbohydrates from a wide array of sources. Carbohydrates are also a much more efficient energy source than protein (Marsh and Watts, 2007).
Recent studies indicate that sea urchins may adjust feed intake to satisfy energy requirements regardless of other nutrient levels (Otero-Villanueva et al., 2004; Hammer, 2006; Taylor, 2006; Lawrence et al., 2009). In some cases, decreased protein intake resulting from energy satiation led to decreased somatic growth and organ production in adult sea urchins (Fernandez and Pergent, 1998; Hammer 2006) and decreased growth in juvenile sea urchins (Taylor, 2006). Consequently, feed intake must be measured to accurately determine dietary requirements for these nutrients. Other studies reported compensation for an imbalance in calorie: protein ratio by selective nutrient absorption (reviewed in Lawrence and Lane, 1982). In cases where dietary carbohydrate levels are limiting, sea urchins may use dietary protein as an additional energy source, thus, decreasing growth and production (Schlosser et al., 2005; Hammer et al., 2006a).
Few studies have examined the relationship between dietary protein and dietary energy requirements in sea urchins. Understanding this relationship may be an important step in the formulation of a feed suitable for sustainable sea urchin aquaculture. The purpose of this study is to examine the effect of combinatorial variations in dietary protein and carbohydrate level, presented in a defined daily ration, on organismal growth and roe production in the sea urchin Lytechinus variegatus.
Materials and methods
Collection and Initial Measurements
Adult Lytechinus variegatus (ca. 19.5 ± 2.01g wet weight) were collected from St. Joseph Bay (30°N, 85.5°W), FL and transported to Texas AgriLIFE Mariculture Research Laboratory in Port Aransas, Texas. Nineteen individuals were randomly selected for initial evaluation. Individuals were weighed (to the nearest mg) and dissected by a circular incision around the peristomial membrane. The gut (esophagus, stomach, and intestine combined), and gonads were removed. The gut was cleaned in seawater to remove remaining food pellets. The organs were blotted on a clean paper towel to remove excess water and weighed. Organs were dried at 60°C for 48 hours to constant weight, and dry weights were recorded. Mean dry organ and total dry weights (the sum of the organ dry weights) were calculated for the initial sub-sample and used as estimated initial dry organ and total dry weights for the remaining 64 urchins. The remaining urchins, were weighed and assigned randomly to one of eight dietary treatments (n = 8 per diet). Initial wet weights did not vary significantly among dietary treatments (P<0.05).
Culture Conditions
Sea urchins were held in a semi-recirculating system with both mechanical and biological filtration and UV sterilization. The culture system (2400 L) was comprised of 16 interconnected 20 L fiberglass tanks containing water distributed from a central sump. Each tank held four cylindrical plastic mesh cages (12 cm dia., 30 cm height, constructed of 4 mm open mesh). Each plastic cage was inserted into a PVC coupling (11.5 cm I.D.) and elevated with PVC spacers to allow unimpeded water circulation throughout the cage. Each cage housed one individual. A 12:12 light: dark photoperiod was maintained.
Water volume in each tank was maintained by a central standpipe, and natural seawater was supplied to each mesh enclosure at a ca. rate of 25 L hr−1 (water exchange rate of 3000% per day). Fresh seawater was passed through a sand filter and a stratified Diamond water filter (5 μ, Diamond Water Conditioning, Horton, WI). Water in the entire culture system was exchanged in the system at a rate of 10% per day. Water quality parameters were determined by color metric analysis.
Feed and Feed Preparation
Eight semi-purified diets were formulated and produced using both purified and practical ingredients. Levels of dietary protein and carbohydrate (Table 1, Table 2) ranged from 12 to 36 % protein (using purified plant and animal protein sources) and 21 to 39% carbohydrate (using a purified starch source). Total levels of protein and carbohydrate were adjusted with acid washed diatomaceous earth which has no effect on sea urchins at the levels used (unpublished data). All other nutrients were constant among treatments. The proximate components are shown in Table 2. Dry ingredients were mixed with a PK twin shell® blender (Patterson-Kelley Co., East Stroudsburg, PA) for 10 minutes. Dry ingredients were then transferred to a Hobart stand mixer (Model A-200, Hobart Corporation, Troy, OH) and blended for 40 minutes. Liquid ingredients were added, and the mixture was blended for an additional 10 minutes to a mash-like consistency. The diets were extruded using a meat chopper attachment (Model A-200, Hobart Corporation, Troy, OH) fitted with a 4.8 mm die. Feed strands were separated and dried on wire trays in a forced air oven (35°C) for 48 hours. Final moisture content of all feed treatments was 8– 10%. Feed was stored in air-tight storage bags at 4°C until used.
Table 1.
Calculated protein and carbohydrate levels (as fed), total energy, protein: energy, and protein: carbohydrate ratios in each of the eight diets tested.
| Protein (%) | Carbohydrate (%) | Total Energy (cal g-1) | Protein:Energy Ratio (mg P kcal-1) | Protein:Carbohydrate Caloric Ratio |
|---|---|---|---|---|
| 36 | 21 | 3749 | 95 | 1.7 |
| 28 | 30 | 3299 | 76 | 0.93 |
| 19 | 21 | 2783 | 68 | 0.90 |
| 19 | 30 | 3130 | 60 | 0.63 |
| 19 | 39 | 3478 | 54 | 0.49 |
| 12 | 21 | 2380 | 50 | 0.57 |
| 12 | 30 | 2727 | 44 | 0.40 |
| 12 | 39 | 3075 | 39 | 0.31 |
Table 2.
Proximate composition of the formulationa used to produce diets varying in protein and carbohydrate levels.
| 12% P: 21% C | 12% P: 30% C | 12% P: 39% C | 19% P: 21% C | 19% P: 30% C | 19%P: 39% C | 28% P: 30% C | 36% P: 21% C | |
|---|---|---|---|---|---|---|---|---|
| Crude protein (%) | 12 | 12 | 12 | 19 | 19 | 19 | 28 | 36 |
| Carbohydrate (%) | 21 | 30 | 39 | 21 | 30 | 39 | 30 | 21 |
| Fiber (%) | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.6 | 4.6 |
| Diatomaceous Earth (DE, %) | 27 | 18 | 9 | 19 | 10 | 1 | 0 | 0 |
| Non-DE Ash (%) | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 25 |
| Crude fat (%) | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
All values are approximate, calculated, and on an “as fed” basis unless otherwise indicated.
All diets contain up to 28% marine ingredients, 28.7% plant ingredients, 1.1% carotenoids, 0.7% vitamin premix, 24 % mineral mix, 7.2% binder and antifungal-antioxidant.
Feeding Rate
Each sea urchin was proffered a limiting daily ration equal to 1.5% (sub-satiation) of the initial average wet body weight. Feeding at sub-satiation ensured that urchins consumed all their food in a 24 hour period and allowed for direct measure of feed intake. A sub-satiation feeding regime also prevented individuals from compensating for a dietary deficiency by increasing consumption. Individuals were weighed every three weeks and feed rations were adjusted to be equivalent to 1.5% of the average body weight (Table 3). Feed intake of the presented diet was confirmed by direct observation. Feces were removed by siphoning immediately prior to feeding each day.
Table 3.
AIC scores for each growth model. Rows delineate the response variable while columns delineate the variables in the model. Scores are only comparable for models with the same response variable. All models within one information unit of the best model are in bold, while the best model is in bold and underlined. P + C + (PxC) = Protein + Carbohydrate + (Protein x Carbohydrate). TE= Total Dietary Energy. P:E = Protein:Energy Ratio. PC = Protein:Carbohydrate Ratio.
| Response Variable | P + C + (PxC) | Protein + Carbohydrate | % Protein | TE (cal/g) | P:E mg P/kcal) | P:C(mg P/mgC) |
|---|---|---|---|---|---|---|
| Wet Weight (g) | 345.65 | 343.67 | 342.60 | 359.03 | 339.41 | 344.21 |
| Gonad Wet Weight (g) | 208.20 | 208.81 | 207.44 | 213.02 | 208.74 | 215.30 |
| Gut Wet Weight (g) | −40.40 | − 41.03 | −37.53 | −31.52 | − 41.29 | − 41.57 |
| Dry Matter Production (g) | 177.07 | 175.10 | 173.52 | 182.67 | 176.88 | 181.28 |
| Gonad Dry Matter Production (g) | 62.64 | 61.46 | 62.45 | 60.72 | 65.48 | 69.55 |
| Gut Dry Matter Production (g) | − 211.45 | −209.14 | −201.11 | −192.86 | −206.49 | −208.46 |
| FCR | −34.51 | −36.15 | −36.81 | − 39.74 | −29.19 | −36.59 |
| PER | 13.79 | 18.45 | 16.98 | 46.57 | 16.06 | 31.12 |
Daily feeding rate was calculated as:
(1)Average wet weight of individuals (g) x 0.015
Protein: energy ratio of each feed was calculated as:
(2) Protein (mg) / energy content (kcal)
Total energy content of each feed (per g) was calculated based on the methods of Phillips (1972):
(3) % protein / 100 x 5650 (cal g−1) + % carbohydrate / 100 x 4000 (cal g−1) + % lipid / 100 x 9450 (cal g−1)
Weight Gain and Production
Individuals were weighed every three weeks. Wet weight gain over the 9-week period was calculated as:
(4) Final wet weight (g) – initial wet weight (g)
Estimated total dry matter production was calculated as:
(5) Final dry weight (g) - average initial dry weight (g)
Dry weight of protein consumed for each individual was calculated as:
(6) Feed consumed (g) x % protein in feed
Estimated protein efficiency ratio (PER) for each individual was calculated as:
(7) Dry matter produced (g) / dry weight protein consumed (g)
Production efficiency for each individual was calculated as:
(8) [Final dry weight (g) – initial dry weight (g)/dry feed intake (g)] x 100
Individuals were dissected as previously described, and estimated organ (gut and gonad) dry matter production for each individual was calculated as:
(9) Final dry weight of organ (g) – initial average dry weight of organ (g)
Final dry organ, gut and gonad, index was calculated for each individual as:
(10) Final dry weight of organ (g) / final dry weight of individual (g) x 100
Feed Conversion Ratio (FCR) for each individual was calculated as:
(11) Total feed consumed (g, as fed) / wet weight gain (g)
Statistics
To determine the relationship between carbohydrate and protein level on various urchin growth measurements, multiple linear regressions were conducted in R 2.11.1 (www.r-project.org). For each physical growth response, the fit of different models was compared using the Akaike Information Criterion (AIC) score. The models were; 1) protein level, carbohydrate level, and their interaction, 2) total energy, 3) protein: energy ratio, and 4) protein: carbohydrate ratio (descriptions of equations used to derive values given above). Because initial analyses showed that, at the levels used in this study, the interaction between protein and carbohydrate levels as well as carbohydrate levels themselves were often statistically unimportant, two parameter-reduced models were considered. These included models with protein and carbohydrate level and only the protein level. The assumptions for all models were checked by examining the residuals for normality and homoscedasticity visually.
Results
Water Quality
Water conditions were maintained as follows: 32 ± 0.5 ppt salinity, 22 ± 2°C, D.O. 7 ± 2 ppm., ammonia 0 ppm, nitrite 0 ppm, nitrate 0 ppm, and pH 8.2.
Weight Gain and Production
Urchins in all dietary treatments increased in weight during the 9-week study. Across the various growth and production measurements after 9 weeks, no model was consistently best fitting (Table 3 and 4).
Table 4.
Parameter estimates and tests of significance for various measures of Lytechinus variegatus growth models. Only statistically significant terms (P < 0.05) are included (if an interaction was found to be significant, main effects were included regardless of associated p-values).
| Separate effects | Combined effects | |||||
|---|---|---|---|---|---|---|
| Response Variable | % Protein | % Carbohydrate | P X C | TE (cal/g) | (mg P/kcal) | P:C (mg P/mg C) |
| Wet Weight (g) | 0.50*** | - | - | 0.0067*** | 0.236*** | 11.909*** |
| Gonad Wet Weight (g) | 0.134*** | - | - | 0.0022*** | 0.059*** | 2.806*** |
| Gut Wet Weight (g) | 0.007* | - | - | 0.0041** | 0.220** | |
| Dry Matter Production (g) | 0.116*** | - | - | 0.0018*** | 0.051*** | 2.541*** |
| Gonad Dry Matter Production (g) | 0.029*** | - | - | 0.0006*** | 0.012*** | 0.575** |
| Gut Dry Matter Production (g) | 0.007* | 0.001 | −0.0002* | 0.0011*** | 0.064*** | |
| FCR | −0.01*** | −0.0052*** | −1.23* | −0.196*** | ||
| PER | 0.0078 | 0.038* | −0.002* | −0.0006*** | −0.02*** | −0.77*** |
Associated p-values for parameter estimates being significantly different than 0 are included as
p < 0.05
p < 0.01
p < 0.001.
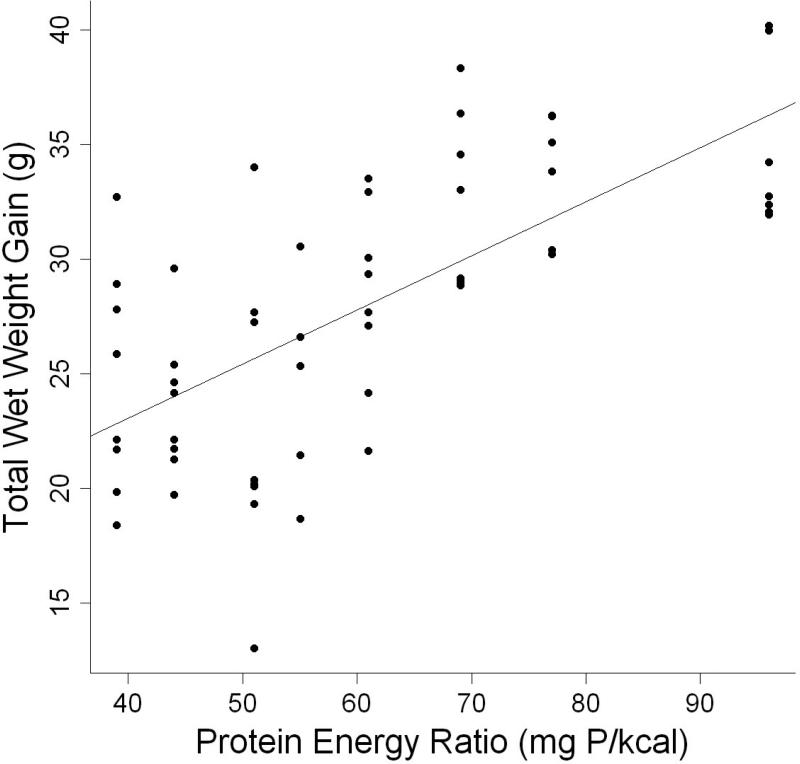
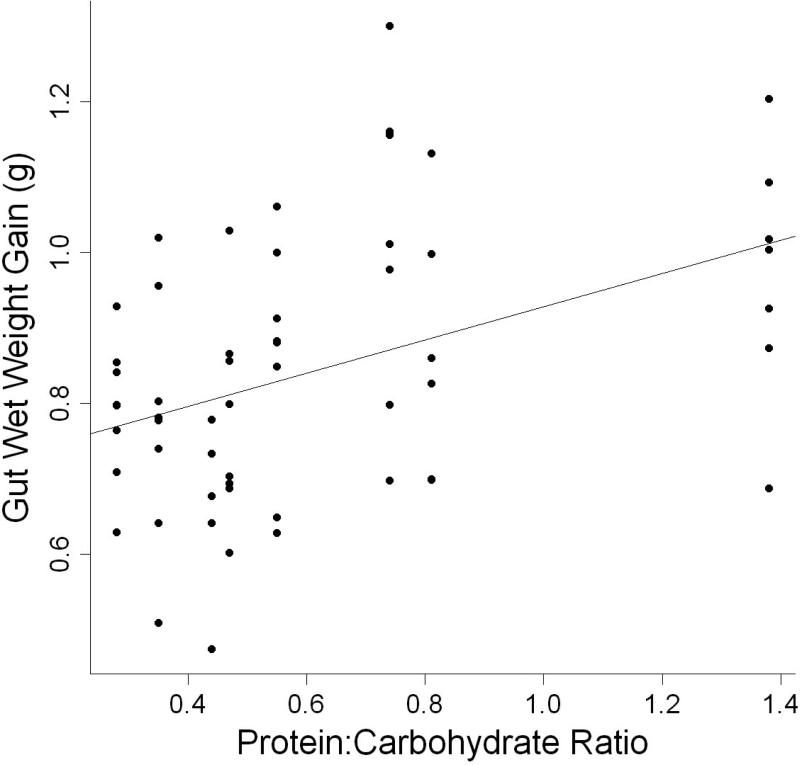
AIC scores indicate that, within the levels used in this study, the best models included dietary protein level. Dietary carbohydrate level provided little information within the range tested. Parameter estimates for wet weight gain in terms of dietary protein showed that individuals gained 0.5g of wet weight (Table 4) for every one percent increase in protein. However, protein: energy ratio was a slightly more parsimonious model of wet weight gain within the ranges of nutrients used (Table 3, Fig. 1). Parameter estimates for wet weight gain in terms of protein: energy ratio indicated that wet weight of individuals increased by 0.236 g for every one mg P kcal−1 increase in protein: energy ratio (Table 4). Consequently, diets with the highest (≥ 68.6 mg P kcal−1) protein: energy ratios had the highest increase in wet weight at the end of 9 weeks. Individuals fed diets with low (≤ 54.9 mg P kcal−1) protein: energy ratios had the lowest increase in wet weight (Table 5).
Figure 1.
Relationship between total wet weight gain (g) and protein energy ratio (mg P kcal−1) of individual L. variegatus fed one of eight semipurified diets for 9 weeks.
Table 5.
Mean total wet weight gain and dry matter production of Lytechinus variegatus feddiets with varying protein and carbohydrate levels, protein:energy ratios (P:E), total energy (TE), and protein:carbohydrate ratios (P:C). P:E represents mg of protein per kilocalorie. TE represents total dietary energy in calories per gram. P:C represents protein:carbohydrate ratio (mg mg-1).
| % Protein | % Carbohydrate | P:E (mg P kcal-1) | TE (cal g-1) | P:C | Final Wet Weight (g) | Wet Weight Gain (g) | Final Dry Weight (g) | Dry Matter Production (g) |
|---|---|---|---|---|---|---|---|---|
| 12 | 21 | 50.79 | 2380 | 0.57 | 42.02 +/- 2.37 | 22.74 +/- 2.30 | 9.18 +/- 0.35 | 4.98 +/- 0.35 |
| 12 | 30 | 44.32 | 2728 | 0.40 | 42.69 +/- 1.54 | 23.59 +/- 1.09 | 9.79 +/- 0.37 | 5.59 +/- 0.37 |
| 12 | 39 | 39.31 | 3075 | 0.31 | 44.51 +/- 2.02 | 24.68 +/- 1.75 | 10.04 +/- 0.39 | 5.84 +/- 0.39 |
| 19 | 21 | 68.56 | 2783 | 0.90 | 51.53 +/- 1.53 | 32.43 +/- 1.31 | 11.04 +/- 0.36 | 6.84 +/- 0.36 |
| 19 | 30 | 60.95 | 3131 | 0.63 | 47.74 +/- 1.77 | 28.30 +/- 1.44 | 10.92 +/- 0.49 | 6.72 +/- 0.49 |
| 19 | 39 | 54.85 | 3478 | 0.49 | 43.86 +/- 2.47 | 24.53 +/- 2.06 | 10.10 +/- 0.44 | 5.89 +/- 0.44 |
| 28 | 30 | 76.88 | 3647 | 0.93 | 53.11 +/- 1.65 | 33.68 +/- 1.13 | 12.09 +/- 0.29 | 7.89 +/- 0.29 |
| 36 | 21 | 95.57 | 3749 | 1.71 | 55.04 +/- 1.39 | 34.79 +/- 1.39 | 12.20 +/- 0.30 | 8.00 +/- 0.30 |
Initial average wet weight was 19.28+/-2.37 g. Initial average dry weight was 4.20+/-0.08 g.
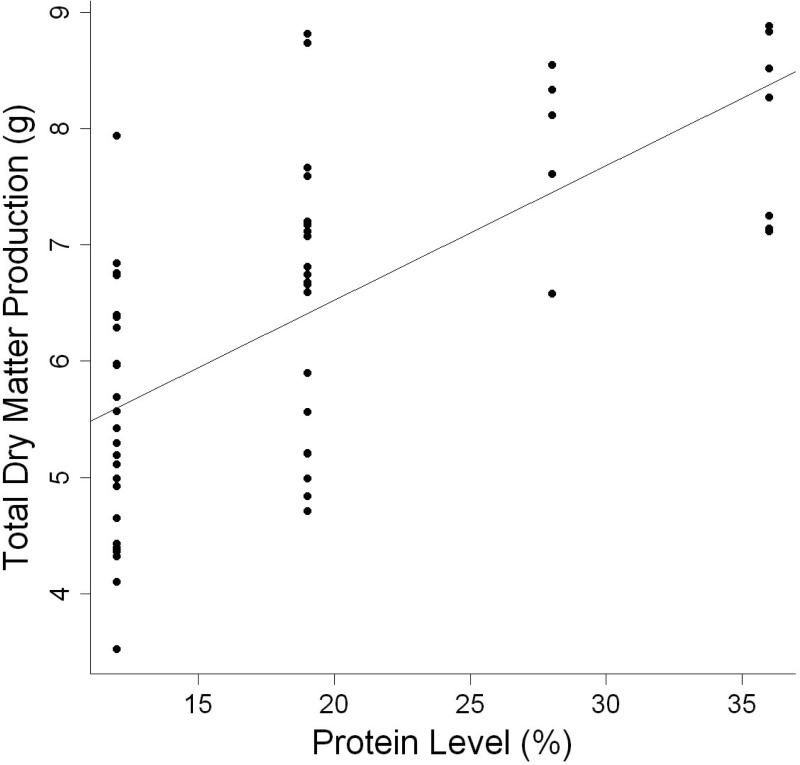
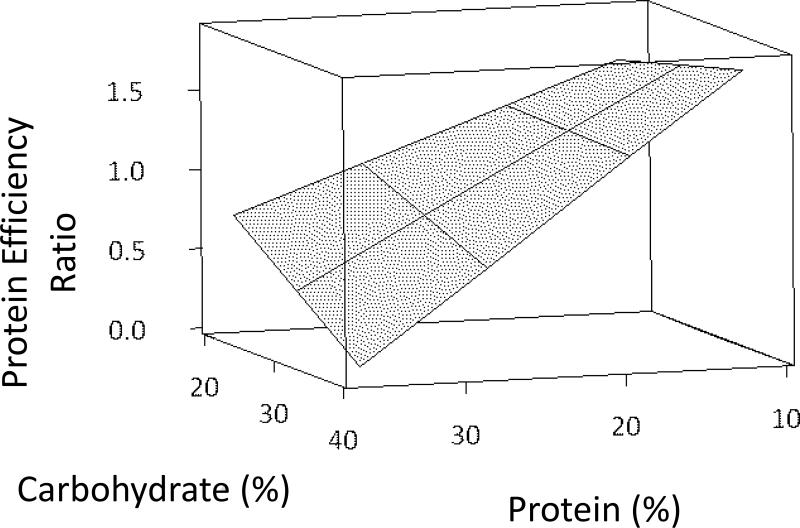
The model using dietary protein level was the best indicator of dry matter production (Table 3, Fig. 2). Parameter estimates indicated that dry matter production increased by 0.116 g for every one percent increase in dry protein consumed, (Table 4). Despite the significance of dietary protein level on both wet weight gain and dry matter production, protein efficiency ratio (PER) of individuals was best modeled by the interaction effect between dietary protein level and dietary carbohydrate level (Table 3, Fig.3).
Figure 2.
Relationship between total dry matter production (g) and dietary protein level (%) of individual L. variegatus fed one of eight semipurified diets for 9 weeks.
Figure 3.
Relationship between protein efficiency ratio and protein × carbohydrate interaction effect of individual L. variegatus fed one of eight semipurified diets for 9 weeks.
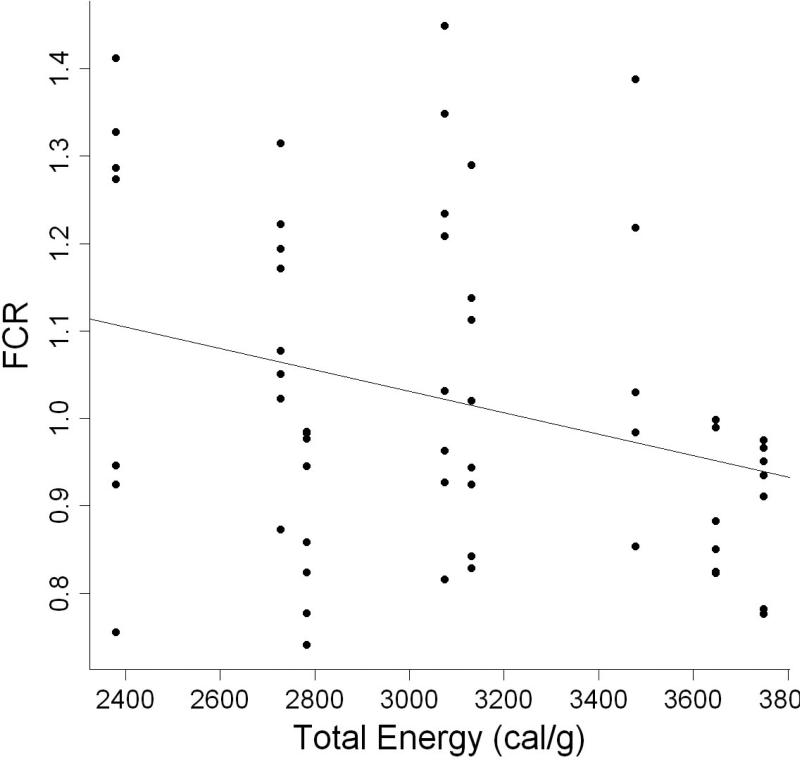
AIC analysis indicated that food conversion ratio (FCR) was best modeled by total dietary energy level (Table 3). Parameter estimates show that FCR of individuals decreased by 0.0052 for every one calorie gram−1 increase in total dietary energy level (Table 4, Fig. 4).
Figure 4.
Relationship between food conversion ratio and total dietary energy level cal g−1 of individual L. variegatus fed one of eight semipurified diets for 9 weeks.
Gut Analysis
AIC analysis indicated that the models containing dietary protein or dietary carbohydrate levels alone were relatively poor indicators of wet gut weight gain (Table 3). However, the model containing protein: carbohydrate ratio was the best indicator of gut wet weight gain (Table 3, Fig. 5). Parameter estimates indicate that gut wet weight increased by 0.220 g for every 1 unit increase in protein: carbohydrate ratio. Consequently, individuals fed a higher protein: carbohydrate ratio (≥0.63) gained the most gut wet weight (Table 6). When gut wet weight gain was examined in terms of the model containing the combined effect of protein and carbohydrate or in terms of the model containing protein: energy ratio, both were relatively equal predictors (Table 3). However, neither provided as parsimonious an explanation as the model containing protein: carbohydrate ratio (Table 3).
Figure 5.
Relationship between gut wet weight gain (g) and protein:carbohydrate ratio of individual L. variegatus fed one of eight semipurified diets for 9 weeks.
Table 6.
Mean final gut wet weight gain, dry gut index, and gut dry matter production of Lytechinus variegatus fed diets with varying protein and carbohydrate levels, protein:energy ratios (P:E), total energy (TE), and protein:carbohydrate ratios (P:C). P:E is mg of protein per kilocalorie. TE is total dietary energy in calories per gram. P:C is protein:carbohydrate ratio (mg mg-1).
| % Protein | % Carbohydrate | P:E (mg P kcal-1) | TE (calg-1) | P:C | Final Wet Gut Weight (g) | Wet Gut Weight Gain (g) | Dry Gut Index (%) | Final Dry Gut Weight (g) | Dry Gut Production (g) |
|---|---|---|---|---|---|---|---|---|---|
| 12 | 21 | 50.8 | 2380 | 0.57 | 1.08 +/- 0.05 | 0.78 +/- 0.05 | 2.99 +/- 0.00 | 0.27 +/- 0.01 | 0.23 +/- 0.01 |
| 12 | 30 | 44.3 | 2728 | 0.40 | 1.08 +/- 0.06 | 0.78 +/- 0.06 | 2.74 +/- 0.00 | 0.27 +/- 0.01 | 0.23 +/- 0.01 |
| 12 | 39 | 39.3 | 3075 | 0.31 | 1.09 +/- 0.03 | 0.79 +/- 0.03 | 2.73 +/- 0.00 | 0.27 +/- 0.01 | 0.23 +/- 0.01 |
| 19 | 21 | 68.6 | 2783 | 0.90 | 1.26 +/- 0.09 | 0.96 +/- 0.09 | 2.90 +/- 0.00 | 0.32 +/- 0.02 | 0.28 +/- 0.02 |
| 19 | 30 | 60.9 | 3131 | 0.63 | 1.16 +/- 0.05 | 0.86 +/- 0.05 | 2.61 +/- 0.00 | 0.28 +/- 0.01 | 0.24 +/- 0.01 |
| 19 | 39 | 54.9 | 3478 | 0.49 | 0.96 +/- 0.05 | 0.66 +/- 0.05 | 2.33 +/- 0.00 | 0.23 +/- 0.01 | 0.19 +/- 0.01 |
| 28 | 30 | 76.9 | 3647 | 0.93 | 1.17 +/- 0.07 | 0.87 +/- 0.07 | 2.35 +/- 0.00 | 0.28 +/- 0.02 | 0.24 +/- 0.02 |
| 36 | 21 | 95.6 | 3749 | 1.71 | 1.27 +/- 0.06 | 0.97 +/- 0.06 | 2.70 +/- 0.00 | 0.33 +/- 0.01 | 0.29 +/- 0.01 |
Initial average wet gut weight was 0.30+/-0.01 Initial average dry gut weight was 0.04+/-0.01
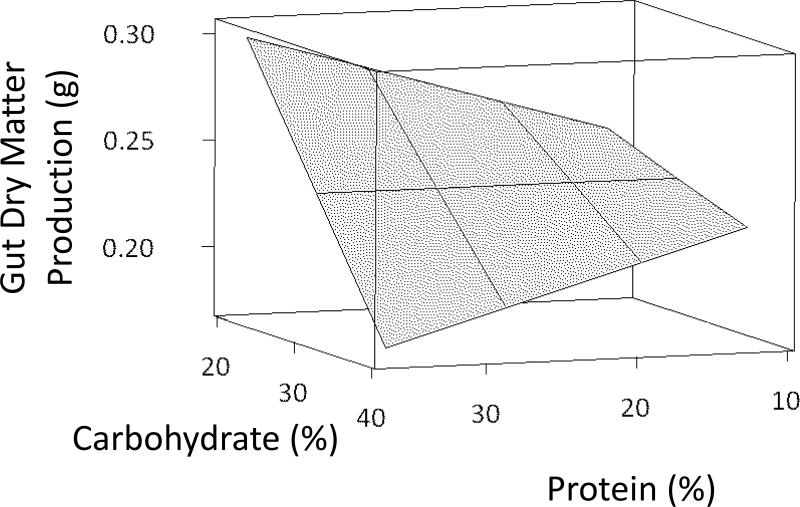
AIC analysis showed that the models containing dietary protein or dietary carbohydrate levels alone were relatively poor indicators of gut dry matter production (Table 3). However, the model containing a protein × carbohydrate interaction effect was the best model of gut dry matter production (Table 3, Fig. 6).
Figure 6.
Relationship between gut dry matter production (g) and protein + carbohydrate + (protein × carbohydrate) of individual L. variegatus fed one of eight semipurified diets for 9 weeks.
Gonad Analysis
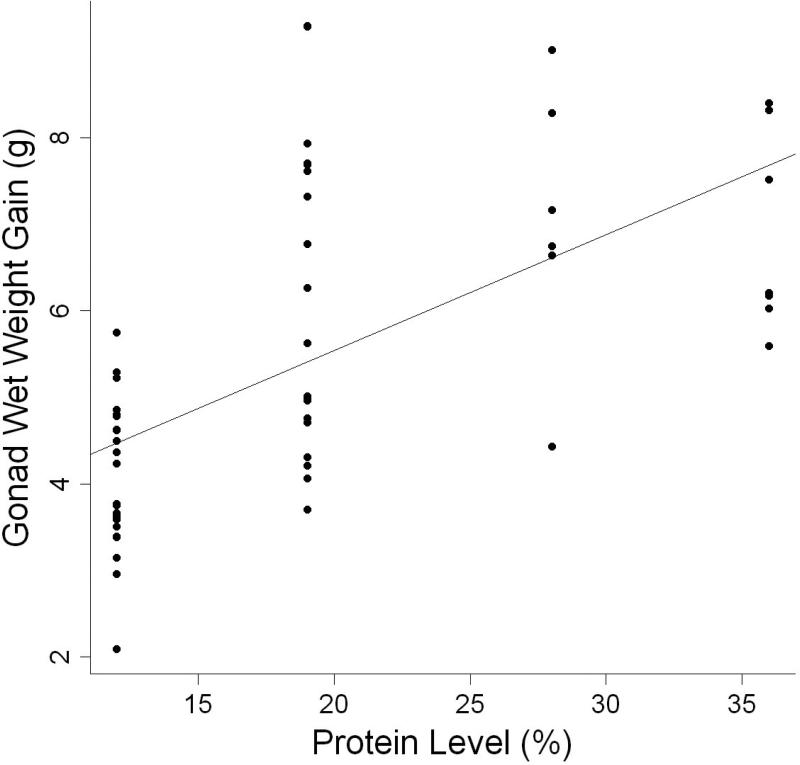
AIC analysis showed that the best indicator of gonad wet weight gain was the model using dietary protein level (Table 3, Fig. 7). Parameter estimates indicated that gonad wet weight of individuals increased by 0.134 g for every one percent increase in protein. Gonad wet weight gain was lowest in individuals fed diets containing less than 19% protein (Table 7). When a protein × carbohydrate interaction effect was considered, additional information was added to the model, but not enough to indicate that this was a better model of gonad wet weight gain (Table 3). The model containing dietary carbohydrate level was a relatively poor indicator of gonad wet weight gain.
Figure 7.
Relationship between gonad wet weight gain (g) and dietary protein level ratio of individual L. variegatus fed one of eight semipurified diets for 9 weeks.
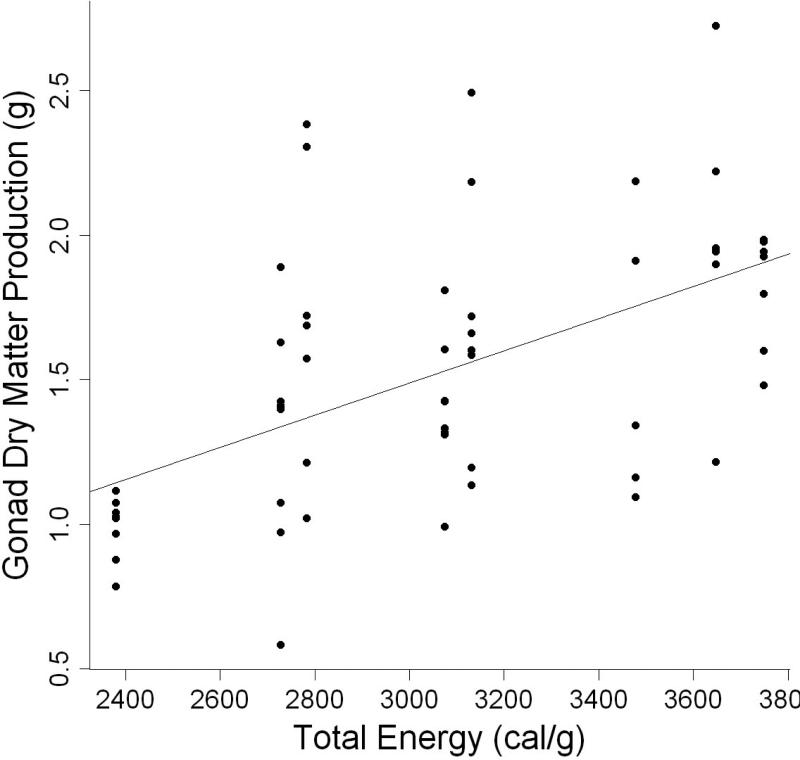
The model containing total dietary energy level was the best indicator of gonad dry matter production (Table 3, Fig 8). Parameter estimates indicated that gonad dry matter production increased by 0.0006 g for every one calorie gram−1 increase in dietary energy level (Table 4). Consequently, the highest gonad dry matter production occurred in individuals fed diets containing ≥ 2783 calories gram−1 of total dietary energy.
Figure 8.
Relationship between gonad dry matter production (g) and total dietary energy of individual L. variegatus fed one of eight semipurified diets for 9 weeks.
Discussion
Water Quality
Water quality parameters maintained in this study were within the ranges suitable for sea urchins (Basuyaux and Mathieu, 1999). This is further supported by the high survivorship and high growth rates exhibited by all treatments, despite sub-satiation rations.
Organismal Growth
Individuals in all treatments grew throughout the study, indicating that all diets were adequate for maintenance and growth. Direct observation indicated that feed rations were below satiation level for L. variegatus in this study. Since previous studies have shown that sea urchins will adjust feed intake to satisfy nutritional requirements (McBride et al., 1998; Fernandez and Boudouresque, 2000; Wallace, 2001; Taylor, 2006), feeding at sub-satiation ensured that all individuals consumed equal amounts of feed and that urchins were not able to compensate for nutritional deficiencies in the diets by increasing consumption.
Determination of dietary protein requirements for optimal growth and production in sea urchins is a complex challenge. In this study, observed limitations in weight gain and dry matter production based on suggested limitations in protein content may, in fact, be the result of limitations in essential amino acids, as indispensable amino acid requirements have not been identified in sea urchins. The total dietary protein and carbohydrate levels were adjusted by varying the level of diatomaceous earth (DE), which has been shown to have no effect on sea urchins in the range of DE used in this study (unpublished data). Thus, any differences in parameters among dietary treatments at the end of nine weeks can be attributed to variations in the amount of protein (amino acids) or carbohydrate consumed, or any nutrient/energy combination thereof.
Among all factors examined, growth of individuals also varied within dietary treatments. This variation can be attributed to intrinsic, most likely genetic, differences in growth rates among individuals (unpublished data; Pawson and Miller, 1982; Grosjean, 2001; Vadas et al., 2002). The results suggest that variations in dietary nutrients and energy differentially affect organismal growth and growth of body components. That is, no one model (protein level, carbohydrate level, protein combined with carbohydrate, protein carbohydrate interaction, protein: carbohydrate ratio, protein: energy ratio, or total energy content) was best for all the growth parameters examined. These data indicate that nutrient allocation and storage in different body components are dependent on specific nutrients, nutrient combinations, and/or energy levels in the diet. Thus, in the future, it may be possible to customize a commercial feed that will maximize gonadal growth and production.
Studies have shown that dietary protein levels affect somatic growth of sea urchins (Fernandez, 1997; Cook et al. 1998; Fernandez and Bourdouresque, 1998; Fernandez and Pergent, 1998; Meidel and Scheibling, 1999; Agatsuma 2000; Akiyama et al., 2001; Hammer et al., 2004; Hammer et al., 2006a; Taylor, 2006). Similarly, AIC analysis and parameter estimates indicate that the model containing dietary protein levels had a proportional effect on total growth and production in sea urchins and was generally an important model in consideration of organismal growth.
Hammer et al. (2006a) reported that growth of adult L. variegatus fed a 20:23% protein: carbohydrate diet was comparable to that of urchins fed a 31:12% diet. Somatic growth of S. droebachiensis was maximized at dietary protein levels of 19-20% (Pearce et al., 2002b; Kennedy and Robinson, 2005). Akiyama (2001) concluded that 20% protein was optimal in a purified diet for P. depressus. However, Hammer et al. (2004) reported reduced growth and survivorship in small L .variegatus fed a formulated diet with 19% protein (as compared to 27%). These studies suggest that dietary protein levels around 20% are adequate for organismal growth of these sea urchin species at this life stage.
Sea urchin growth can be described relatively well by the Tanaka growth model (Ebert, 1997; McShane and Anderson, 1997), which consists of slow initial growth followed by a period of exponential growth and then a period of slow but constant growth. It is reasonable to assume that sea urchins may require different levels of dietary protein and/or carbohydrate at different life stages. Hammer et al. (2004) reported juvenile sea urchins should be in the exponential growth phase and, thus, may have a higher requirement for dietary protein.
Protein efficiency ratio (PER) in sea urchins has been reported to vary with both season and with dietary energy levels (Schlosser et al. 2005). Hammer (2006) observed no difference in PER of adult L. variegatus among diets with high carbohydrate levels. However, when dietary energy from carbohydrates was limiting, PER decreased with increasing dietary protein levels, suggesting that protein was metabolized as an energy source (Hammer et al., 2006b). In the current study, protein efficiency ratio decreased with increasing protein level, more so at increasing carbohydrate levels, suggesting that individuals processed protein more efficiently when dietary protein and carbohydrate levels were low. In this study, dietary carbohydrate was in excess. These data indicate that high dietary carbohydrate levels may have, in some manner, limited the ability of individuals to process dietary protein efficiently. Protein is an expensive feed ingredient and nitrogen release from protein catabolism can contribute to water fouling, thus, it may be beneficial to consider protein efficiency ratio when formulating a commercial feed for use in aquaculture. Although the model containing dietary protein level was a good indicator of urchin growth in this study, there is a cost-benefit consideration, as individuals were less efficient at processing protein when dietary levels were high. Additionally, it appears that excessively high dietary carbohydrate levels should be avoided.
Carbohydrates are the preferential energy source for many animals and sea urchins are most likely no exception (Marsh and Watts, 2007). As such, formulated diets should supply enough energy from dietary carbohydrates to fulfill the energetic requirements of sea urchins so that more expensive nutrients like protein will be spared. In this study, carbohydrate levels varied from 21-39% among diets. Carbohydrate consumption was highest in urchins fed the 12:39 and 19:39% protein: carbohydrate diets, but at the levels used in this study, dietary carbohydrate level was not associated with wet weight gain or dry matter production. We can assume that carbohydrate energy was not limiting in any of the diets, indicating that L. variegatus at this life stage and under these conditions are unlikely to require dietary carbohydrate levels in excess of 21%. Since dietary carbohydrate was not limiting in this study, dietary protein was most likely spared as an energy source.
Studies with L. variegatus ( Hammer, 2006; Taylor, 2006; Gibbs et al., 2009), Psammechinus miliaris (Otero-Villanueva et al., 2004) and Paracentrotus lividus (Fernandez and Pergent, 1998) have reported that sea urchins fed a high energy diet may become satiated and may not consume adequate quantities of other nutrients that might be necessary for optimal growth and development. Other marine and fresh water organisms also adjust feed intake levels according to the level of dietary carbohydrates. Feed intake by channel catfish (reviewed by Gatlin et al., 1986) was limited by increased dietary energy, as was that of rainbow trout (Boujard and Medale, 1994), tilapia (Bowen et al., 1995), and shrimp (Siccardi, 2006; Davis and Arnold, 1995). Sea urchins in this study did not adjust feed intake, consequently, carbohydrate or energy intake could not be adjusted to compensate for the dietary level proffered.
Under the conditions of this study, the model containing total dietary energy (energy from protein, carbohydrates, and lipids) was a relatively poor indicator of urchin growth in terms of wet weight gain and dry matter production. As lipid levels did not vary among diets, energy from lipids was the same among diets. Total energy content of diets did not vary directly with dietary protein levels, and thus total energy was limited in its ability to model growth.
Overall, the model containing protein: energy ratio was a good predictor of urchin growth. The effect of protein: energy ratio on sea urchin growth has not been evaluated, but it is known that protein: energy ratio affects both shrimp and fish. Bautista (1986) studied protein: energy ratios in penaeid shrimp and found that the most weight gain and lowest mortality rate occurred at protein: energy ratios between 120-174 mg p kcal−1. When dietary protein levels were raised from 40 to 50%, individuals did not grow as well unless energy levels were also raised (Bautista, 1986), indicating that not just level of dietary protein, but the ratio of dietary protein to dietary energy is an important consideration when evaluating the nutritional requirements of penaeid shrimp. Recent studies suggest that dietary protein: energy ratio may influence growth and production in sea urchins in a similar manner (Hammer, 2006; Taylor, 2006). Due to their sedentary lifestyle and low respiration rate, energy requirements of sea urchins are low (Lawrence and Lane, 1982; Marsh and Watts, 2007). As such, diets with high protein: energy ratios would be expected to provide the greatest growth and production. In this study, protein: energy ratio was the best model of total wet weight gain. However, while a quadrative effect of protein: energy ratio was not statistically supported, it is likely that at higher protein: energy ratio values (>70), the gain in total wet weight is diminished. High dietary protein: energy levels, while not detrimental from a nutritional standpoint, may only minimally enhance growth and production and actually be disadvantageous in terms of cost and pollution.
Food conversion ratio (FCR) is typically low in sea urchins (Hammer et al. 2004, Hammer, 2006). This is partially attributed to the fact that FCR calculations include the weight associated with the large volume of coelomic fluid which fills the body cavity of sea urchins (Hammer et al., 2004). Regardless, FCR remains an important metric for practical determination of feed utilization by organisms in culture. Lytechinus variegatus fed diets with high protein levels typically have a comparatively low FCR. Hammer (2006) reported FCRs as low as 0.56 in adult L. variegatus fed a high protein: high carbohydrate diet. FCRs in the current study are generally higher that those reported by Hammer (2006), and most likely represent a decrease in energy available for growth relative to maintenance energy when diets are sub-satiating. In the current study, individuals fed diets high in total energy were most efficient at converting feed consumed to body mass. Diets with the highest total energy were also highest in dietary protein. This suggests individuals were able to convert dietary protein consumed (g) to body mass (g wet weight), provided the diet contained sufficient energy to process the protein.
Organ Growth
Typically, gut size varies in response to food availability (Hammer et al., 2006b; Bishop and Watts, 1992). Consequently, most models examined in this study (using protein level, carbohydrate level, total energy, or protein: energy ratio) were poorly associated with gut production or growth. Protein: carbohydrate interaction effect was the best model for gut dry matter production, but the biological significance of this relationship is not understood at this time. Studies with adult S. franciscanus (McBride et al., 1998) and adult L. variegatus (Hammer et al., 2006b), have shown that variations in dietary nutrients affected the biochemical composition of the gut but not gut wet mass or gut dry matter production. Biochemical analysis was not performed on individuals in the current study, so it is unknown whether or not variations in nutrients affected the biochemical composition of the gut tissue.
Dietary carbohydrates are stored primarily in the gonads (Marsh and Watts, 2007); However, under the conditions of this study, variations in dietary carbohydrate levels were poorly associated with wet weight gain or dry matter production of the gonads. Schlosser et al. (2005) found decreased gonad production in P. lividus fed low (presumably inadequate) carbohydrate algal diets as compared to urchins fed a prepared diet with adequate carbohydrate energy. This also suggests that the range of dietary carbohydrate levels tested in the current study was adequate in all diets.
Dietary protein levels are often directly correlated with gonad production (de Jong-Westman et al., 1995; Fernandez, 1997; Barker et al., 1998; Cook et al., 1998; Meidel and Scheibling, 1999; Schlosser et al., 2005; Pearce et al., 2002b; Hammer et al., 2004; Chang et al., 2005; Hammer et al., 2006a; Marsh and Watts, 2007; Woods et al., 2008) and can possibly influence fecundity of individuals (Hammer et al., 2006b). Under the conditions of this study, dietary protein level was the best model of wet gonad weight. In a previous study, Lytechinus variegatus fed diets with 20% protein had significantly larger gonads at 32 days than urchins fed a diet with a 9% protein level (Hammer et al., 2006a). Olave et al. (2001) found that gonad production in Loxechinus albus was higher at dietary protein levels of 20% than at levels of 11 and 17%, but no diets with protein levels higher than 20% were examined for comparison. Gonadal growth of Strongylocentrotus droebachiensis was maximized at 19-20% protein (Pearce et al., 2002b, McBride et al., 1998; de Jong-Westman, 1995). Adult Paracentrotus lividus fed a 29% protein diet had significantly higher gonad index than those fed a 13% protein diet but the gonad index of individuals fed the 29% protein diet was not different than that of individuals fed a 47% protein diet (Fernandez et al., 1997), suggesting that dietary protein levels of 47% are excessive forParacentrotus lividus. Akiyama et al. (2001) found no statistical difference in gonad index among Paracentrotus depressus fed diets with protein levels of 10, 20, 30 and 40%, but comparison of the somatic growth data shows that individuals fed the 10% diet were significantly smaller than individuals fed the higher protein diets.
Both high protein levels and protein source have been suggested to adversely affect roe quality (Pearce et al., 2002a 2002b; Woods et al., 2008; Hoshikawa, 1998; Lawrence et al., 2001; Murata et al., 2001, 2002; Robinson et al., 2002; Senaratna et al., 2005; Woods et al. 2008). As such, culturists must find the balance between optimal roe yield and quality. Further studies are needed to establish protein and amino acid requirements for maximal gonad production in L. variegatus.
In summary, dietary protein was highly associated with most parameters of growth under the conditions of this study. In addition to levels of dietary protein and carbohydrate, sea urchin growth and gonad production can vary in response to changing season and temperature (Hill and Lawrence, 2006; Gibbs et al., 2007; Lawrence et al., 2011), water quality (Basuyaux and Mathieu, 1999), life stage (Pearce et al., 2004; Watts et al., 2010), and other essential nutrients (Jones, 2007; Gibbs et al., 2009; Trawick, 2009; Watts et al., 2010). The development of large-scale sea urchin aquaculture techniques will depend upon our ability to answer questions surrounding these and many other nutritional issues.
Highlights.
Adult Lytechinus variegatus were fed eight formulated diets with different protein (ranging from 12 to 36%) and carbohydrate (ranging from 21 to 39 %) levels.
Dietary protein level and protein: energy ratio were the best models for prediction of total weight gain.
Dietary carbohydrate level was a poor predictor for most growth parameters examined in this study.
Higher energy diets produced lower food conversion ratios.
These data suggest that variations in dietary nutrients and energy differentially affect organismal growth and growth of body components.
Acknowledgments
The authors thank Jeff Barry, Patty Waits Beasley, and the rest of the staff at the Texas AgriLIFE Research Mariculture Laboratory for providing technical support and facilities for this study. We thank Dorothy Moseley and Warren Jones for technical assistance. We also thank Dr. Renee Desmond (Department of Biostatistics) and Dr. Robert Angus for statistical consultation. This report was prepared by S.A.W. under award NA07OAR4170449 from the University of Alabama at Birmingham, U.S. Department of Commerce. The statements, findings, conclusions, and recommendations are those of the authors’ and do not necessarily reflect the views of NOAA or the U.S. Department of Commerce.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Agatsuma Y. Food consumption and growth of the juvenile sea urchin Strongylocentrotus intermedius. Fisheries Science. 2000;66:467–472. [Google Scholar]
- Akiyama T, Unuma T, Yamamoto T. Optimum protein level in a purified diet for young red sea urchin Pseudocentrotus depressus. Fisheries Science. 2001;67:361–363. [Google Scholar]
- Barker MF, Keogh JA, Lawrence JM, Lawrence AL. Feeding rate, absorption efficiencies, growth, and enhancement of gonad production in the New Zealand sea urchin Evechinus chloroticus Valenciennes (Echinoidea: Echinometridae) fed prepared and natural diets. Journal of Shellfish Research. 1998;17:1583–1590. [Google Scholar]
- Basuyaux O, Mathieu M. Inorganic nitrogen and its effect on growth of the abalone Halitotis tuberculata Linnaeus and the sea urchin Paracentrotus lividus Lamarck. Aquaculture. 1999;174:95–107. [Google Scholar]
- Bautista MN. The response of Penaeus monodon juveniles to varying protein/energy ratios in test diets. Aquaculture. 1986;53:229–242. [Google Scholar]
- Bishop CD, Watts SA. Biochemical and morphometric study of growth in the stomach and intestine of the echinoid Lytechinus variegatus (Echinodermata). Marine Biology. 1992;114:459–467. [Google Scholar]
- Boujard T, Medale F. Regulation of voluntary feed intake in juvenile rainbow trout fed by hand or by self-feeders with diets containing two different protein/energy ratios. Aquatic Living Resource. 1994;7:211–215. [Google Scholar]
- Bowen SH, Lutz EV, Ahlgren MO. Dietary protein and energy as determinants of food quality: trophic strategies compared. Ecology. 1995;76(3):899–907. [Google Scholar]
- Chang Y-Q, Lawrence JM, Cao X-B, Lawrence AL. Food consumption absorption, assimilation and growth of the sea urchin Strongylocentrotus intermedius fed a prepared feed and the alga Laminaria japonica. Journal of the World Aquaculture Society. 2005;36:68–75. [Google Scholar]
- Cook EJ, Kelly MS, McKenzie JD. Somatic and gonadal growth of the sea urchin Psammechinus miliaris (Gmelin) fed artificial salmon feed compared with a macroalgal diet. Journal of Shellfish Research. 1998;17:1549–1555. [Google Scholar]
- Daggett TL, Pearce CM, Tingley M, Robinson SMC, Chopin T. Effect of prepared and macroalgal diets and seed stock source on somatic growth of juvenile green sea urchins (Strongylocentrotus droebachiensis). Aquaculture. 2005;244:263–281. [Google Scholar]
- Davis DA, Arnold CR. Effects of two extrusion processing conditions on the digestibility of four cereal grains for Penaeus vannamei. Aquaculture. 1995;133:287–294. [Google Scholar]
- de Jong-Westman M, March BE, Carefoot TH. The effect of different nutrient formulations in artificial diets on gonad growth in the sea urchin Strongylocentrotus droebachiensis. Canadian Journal of Zoology. 1995;73:1495–1502. [Google Scholar]
- Ebert TA. Growth and survival of postsettlement sea urchins. In: Lawrence JM, editor. Edible Sea Urchins: Biology and Ecology. Second Edition Elsevier Science B.V.; Amsterdam: 1997. pp. 95–134. [Google Scholar]
- Fernandez C. Effect of diet on the biochemical composition of Paracentrotus lividus (Echinodermata; Echinoidea) under natural and rearing conditions (effect of diet on biochemical composition of urchins). Comparative Biochemistry and Physiology. 1997;118A:1377–1384. [Google Scholar]
- Fernandez C, Pergent G. Effect of different formulated diets and rearing conditions on growth parameters in the sea urchin Paracentrotus lividus. Journal of Shellfish Research. 1998;17:1571–1581. [Google Scholar]
- Fernandez C, Boudouresque C-F. Evaluating artificial diets for small Paracentrotus lividus (Echinodermata: Echinoidea). In: Mooi R, Telford M, editors. Echinoderms: San Francisco. Balkema; Rotterdam: 1998. pp. 651–656. [Google Scholar]
- Fernandez C, Boudouresque C-F. Nutrition of the sea urchin Paracentrotus lividus (Echinodermata: Echinoidea) fed different artificial food. Marine Ecology Progress Series. 2000;204:131–141. [Google Scholar]
- Frantzis A, Grémare A. Ingestion, absorption, and growth rates of Paracentrotus lividus (Echinodermata: Echinoidea) fed different macrophytes. Marine Ecology Progress Series. 1992;95:169–183. [Google Scholar]
- Gatlin DM, Poe WE, Wilson RP. Protein and energy requirements of fingerling channel catfish for maintenance and maximum growth. Journal of Nutrition. 1986;116:2121–2131. doi: 10.1093/jn/116.11.2121. [DOI] [PubMed] [Google Scholar]
- Gibbs VK, Watts SA, Lawrence AL. Effect of temperature on gamete production and biochemical composition of gonads in the sea urchin Lytechinus variegatus. Gulf of Mexico Science. 2007;(2):119–130. [Google Scholar]
- Gibbs VK, Watts SA, Lawrence AL, Lawrence JM. Dietary phospholipids affect growth and production of juvenile Lytechinus variegatus. Aquaculture. 2009;292:95–103. [Google Scholar]
- Grosjean P. These Doctoral Dissertation. Universite Libre de Bruxelles; 2001. Growth model of the reared sea urchin Paracentrotus lividus (Lamarck, 1816) [Google Scholar]
- Hammer BW, Hammer HS, Watts SA, Desmond RA, Lawrence JM, Lawrence AL. The effects of dietary protein concentration on feeding and growth of small Lytechinus variegatus (Echinodermata: Echinoidea). Marine Biology. 2004;145:1143–1157. [Google Scholar]
- Hammer HS. Ph.D. Dissertation. University of Alabama at Birmingham; Birmingham, Alabama, USA: 2006. Determination of dietary protein, carbohydrate, and lipid requirements for the sea urchin, Lytechinus variegatus, fed semi-purified feeds. [Google Scholar]
- Hammer HS, Watts SA, Lawrence AL, Lawrence JM, Desmond RA. The effect of dietary protein on consumption, survival, growth, and production of the sea urchin Lytechinus variegatus. Aquaculture. 2006a;254:483–495. [Google Scholar]
- Hammer HS, Hammer BW, Watts SA, Lawrence AL, Lawrence JM. The effect of dietary protein and carbohydrate concentration on the biochemical composition and gametogenic condition of the sea urchin Lytechinus variegatus. Journal of Experimental and Marine Biology and Ecology. 2006b;334:109–121. [Google Scholar]
- Hill SK, Lawrence JM. Interactive effects of temperature and nutritional condition on the energy budgets of the sea urchins Arbacia punctulata and Lytechinus variegatus (Echinodermata: Echinoidea). Journal of Marine Biology. 2006;86:783–790. [Google Scholar]
- Hirano T, Yamazawa S, Suyama M. Chemical composition of gonad extract of sea-urchin Strongylocentrotus nudus. Bulletin of Japanese Society Scientific Fisheries. 1978;44:1037–1040. (in Japanese with English abstract) [Google Scholar]
- Hoshikawa H, Takahashi K, Sugimoto T, Tuji K, Nobuta S. The effects of fish meal feeding on the gonad quality of cultivated sea urchins, Strongylocentrotus nudus (A. Agassiz). Sci. Rep. Hokkaido Fish. Exp. Stn. 1998;52:17–24. (in Japanese with English abstract) [Google Scholar]
- Jones WT. Masters Thesis. University of Alabama at Birmingham; Birmingham, AL, USA: 2007. The effect of dietary selenium on weight gain and gonad production in the sea urchin Lytechinus variegatus. [Google Scholar]
- Kennedy EJ, Robinson SMC, Parsons GJ, Castell JD. Effect of protein source and concentration on somatic growth of juvenile green sea urchins Strongylocentrotus droebachiensis. Journal of the World Aquaculture Society. 2005;36:320–336. [Google Scholar]
- Komata Y, Kosugi N, Ito T. Studies on the extractives of “uni” . I. Free amino acid composition. Bulletin of Japanese Society Scientific Fisheries. 1962;28:623–629. (in Japanese with English abstract) [Google Scholar]
- Lawrence JM, Lane JM. The utilization of nutrients by post-metamorphic echinoderms. In: Jangoux M, Lawrence JM, editors. Echinoderm Nutrition. AA Balkema; Rotterdam: 1982. pp. 331–371. [Google Scholar]
- Lawrence JM, Lawrence AL, McBride SC, George SG, Watts SA, Plank LR. Developments in the use of prepared feeds in sea-urchin aquaculture. Journal of the World Aquaculture Society. 2001;32:34–39. [Google Scholar]
- Lawrence JM, Lawrence AL, Watts SA. Feeding, digestion, and digestibility. In: Lawrence JM, editor. Edible Sea Urchins: Biology and Ecology. Second Edition Elsevier Science B.V.; Amsterdam: 2007. pp. 135–158. [Google Scholar]
- Lawrence JM, Cao X, Chang Y, Wang P, Yu Y, Lawrence AL, Watts SA. Temperature effect on feed consumption, absorption and assimilation efficiencies and production of the sea urchin Strongylocentrotus intermedius. Journal of Shellfish Research. 2009;28(2):389–395. [Google Scholar]
- Marsh AG, Watts SA. Energy metabolism and gonad development. In: Lawrence JM, editor. Edible Sea Urchins: Biology and Ecology. Second Edition Elsevier Science B.V.; Amsterdam: 2007. pp. 35–50. [Google Scholar]
- McBride SC, Lawrence JM, Lawrence AL, Mulligan TJ. The effect of protein concentration in prepared feeds on growth, feeding rate, total organic absorption, and gross assimilation efficiency of the sea urchin Strongylocentrotus franciscanus. Journal of Shellfish Research. 1998;17:1563–1570. [Google Scholar]
- McShane PE, Anderson OF. Resource allocation and growth rates in the sea urchin Evechinus chloroticus (Echinoidea: Echinometridae). Marine Biology. 1997;128:657–663. [Google Scholar]
- Meidel SK, Scheibling RE. Effects of food type and ration on reproductive maturation and growth of the sea urchin Strongylocentrotus droebachiensis. Marine Biology. 1999;134:155–166. [Google Scholar]
- Murata Y, Sata NU, Yokoyama M, Kuwahara R, Kaneniwa M, Oohara I. Determination of a novel bitter amino acid pulcherrimine, in the gonad of the green sea urchin Hemicentrotus pulcherrimus. Fisheries Science. 2001;67:341–345. [Google Scholar]
- Murata Y, Yokoyama M, Unuma T, Sata NU, Kuwahara R, Kaneniwa M. Seasonal changes of bitterness and pulcherrimine content in gonads of green sea urchin Hemicentrotus pulcherrimus at Iwaki in Fukushima Prefecture. Fisheries Science. 2002;68:184–189. [Google Scholar]
- Olave S, Bustos E, Lawrence JM, Cárcamo P. The effect of size and diet on gonad production by the Chilean sea urchin Loxechinus albus. Journal of the World Aquaculture Society. 2001;32:210–214. [Google Scholar]
- Osako K, Fujii A, Ruttanapornvareesakul Y, Nagano N, Kuwahara K, Okamoto A. Differences in free amino acid composition between testis and ovary of sea urchin Anthocidaris crassispina during gonadal development. Fisheries Science. 2007;73:660–667. [Google Scholar]
- Otero-Villanueva MM, Kelly MS, Burnell G. How diet influences energy partitioning in the regular echinoid Psammechinus miliaris; constructing an energy budget. Journal of Experimental Marine Biology and Ecology. 2004;304:159–181. [Google Scholar]
- Pawson DL, Miller JE. Studies of genetically controlled phenotypic characters in laboratory-reared Lytechinus variegatus (Lamarck) (Echinodermata: Echinoidea) from Bermuda and Florida. In: Jangoux M, Lawrence JM, editors. Echinoderm Nutrition. AA Balkema; Rotterdam: 1982. pp. 165–171. [Google Scholar]
- Pearce CM, Daggett TL, Robinson SMC. Optimizing prepared feed ration for gonad production of the green sea urchin Strongylocentrotus droebachiensis. Journal of the World Aquaculture Society. 2002a;33:268–277. [Google Scholar]
- Pearce CM, Daggett TL, Robinson SMC. Effect of protein source ratio and protein concentration in prepared diets on gonad yield and quality of the green sea urchin, Strongylocentrotus droebachiensis. Aquaculture. 2002b;214:307–322. [Google Scholar]
- Pearce CM, Daggett TL, Robinson SMC. Effect of urchin size and diet on gonad yield and quality in the green sea urchin (Strongylocentrotus droebachiensis) Aquaculture. 2004;233:337–367. [Google Scholar]
- Phillips AM. Calorie and Energy Requirement. pp1-28. In: Halver JE, editor. Fish Nutrition. First Edition Academic Press; New York: London: 1972. [Google Scholar]
- Robinson SMC, Castell JD, Kennedy EJ. Developing suitable colour in the gonads of cultured green sea urchins (Strongylocentrotus droebachiensis). Aquaculture. 2002;206:289–303. [Google Scholar]
- Senaratna M, Evans LH, Southam L, Tsvetnenko E. Effect of different feed formulations on feed efficiency, gonad yield and gonad quality in the purple sea urchin Heliocidaris erythrogramma. Aquaculture Nutrition. 2005;11:199–207. [Google Scholar]
- Siccardi AJ. PhD Dissertation. Texas A&M University; College Station, TX, USA: 2006. Daily digestible protein and energy requirements for growth and maintenance of sub-adult pacific white shrimp (Litopenaeus vannamei). [Google Scholar]
- Schlosser SC, Lupatsch I, Lawrence JM, Lawrence AL, Shpigel M. Protein and energy digestibility and gonad development of the European sea urchin Paracentrotus lividus (Lamarck) fed algal and prepared diets during spring and fall. Aquaculture Research. 2005;36:972–982. [Google Scholar]
- Taylor AM. Masters Thesis. University of Alabama at Birmingham; Birmingham, Alabama, USA: 2006. Effects of dietary carbohydrates on weight gain and gonad production in small sea urchins, Lytechinus variegatus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawick KN. Masters Thesis. University of Alabama at Birmingham; Alabama, USA: 2009. The effects of dietary zinc on growth and reproduction of the sea urchin Lytechinus variegatus. [Google Scholar]
- Vadas RL, Sr., Smith BD, Beal B, Dowling T. Sympatric growth morphs and size bimodality in the green sea urchin (Strongylocentrotus droebachiensis). Ecological Monographs. 2002;72(1):113–132. [Google Scholar]
- Wallace BD. Masters Thesis. University of Alabama at Birmingham; Birmingham, Alabama, USA: 2001. The effects of dietary protein concentration on feeding and growth of small Lytechinus variegatus (Echinodermata: Echinoidea). [Google Scholar]
- Watts SA, Lawrence JM, Lawrence AL. Approaches to the study of sea urchin nutrition. In: Harris LG, Boettger SA, Walker CW, Lesser MP, editors. 12th International Echinoderm Conference. AA Balkema; Durham: 2010. [Google Scholar]
- Woods CMC, James PJ, Moss GA, Wright J, Siikavuopio S. A comparison of the effect of urchin size and diet on gonad yield and quality in the sea urchin Evechinus chloroticus Valenciennes. Aquaculture International. 2008;16:49–68. [Google Scholar]