Abstract
Accumulating evidence has shown that immunoglobulin (Ig) is ‘unexpectedly' expressed by epithelial cancer cells and that it can promote tumor growth. The main purpose of this study was to explore the components of the cancerous Ig and its possible function. The presence of cancerous Ig in the Golgi apparatus was confirmed by immunofluorescence, indirectly suggesting that the cancerous Ig was processed and packaged in cancer cells. Western blot analysis and ELISA results indicated that cancer cells produced membrane Ig and secreted Ig into the supernatant fraction. The cancerous Ig consists of an α heavy chain and a κ light chain. Finally, by analyzing the Ig components pulled down by protein A beads, the cancerous Ig was found to be structurally distinct from normal Ig. The cancerous Ig was truncated or aberrant. Although the underlying mechanism that causes the abnormalities has not been determined, our current discoveries strengthen our previous findings and promise fruitful future explorations.
Keywords: immunoglobulin, truncated immunoglobulin, tumor immunology
Introduction
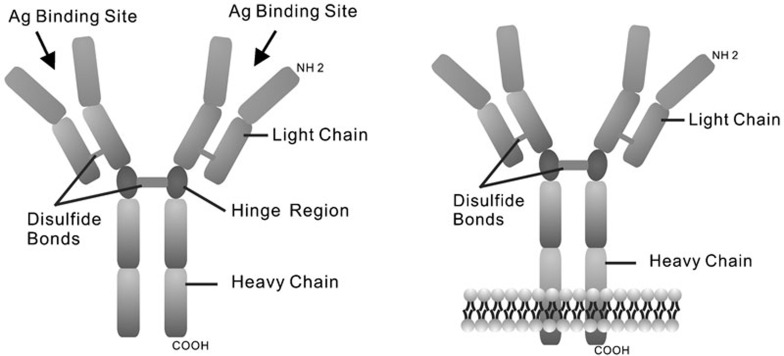
An immunoglobulin (Ig) monomer produced by B cells is known to be a tetramer composed of two identical light chains and two identical heavy chains linked by disulfide bonds (Figure 1). Two types of light chains (λ and κ) and five classes of heavy chains (α, γ, δ, ε and µ) have been identified. After translation and processing, Ig is anchored on the B-cell membrane or secreted into the serum. The membrane Ig (mIg) forms a B-cell receptor (BCR) complex, which transfers transmembrane signals that interact with a specific antigen. Secreted Ig (sIg) circulates as a free antibody or localizes on the mucosal surface.
Figure 1.
Schematic illustration of Ig structures and membrane Ig. The heavy chains and light chains are held together by disulfide bonds. The membrane Ig (mIg) is anchored on the membrane by a transmembrane domain.
Accumulating evidence has indicated that Ig is ‘unexpectedly' expressed in epithelial cancer cells1, 2, 3 or epithelial tissues.4 Cancerous Ig shares some features with normal Ig produced by B lymphocytes. Like normal mIg, cancerous Ig anchors on the cell membrane through its hydrophobic transmembrane domain. It is also secreted into the supernatant fraction, as does normal sIg. Our previous studies provided evidence of sIg secretion by cancer cells.5 Conversely, cancerous Ig and normal Ig are quite different with respect to genetic processing,1, 5 transcription,6 expression level,5 protein structure,7 post-translational modification7 and biological function1, 8, 9, 10, 11 (Table 1). Most of the cancerous Ig is aberrant or abnormal. For example, cancerous Ig lacks a variable (V) region.1, 12 Interestingly, some researchers found that under certain pathological conditions the B cell itself could produce aberrant or truncated Ig.13, 14, 15, 16, 17, 18 For example, in Waldenstrom's macroglobulinemia patients, a 64-kDa truncated µ heavy chain fused with the Ig κ sequence at its NH2 terminal was identified by sequence analysis.13 Additionally, even though the underlying cause is not clear, in one case of heavy-chain deposition disease, a γ heavy chain lacking the CH1 region was identified by immunoblot analysis.18
Table 1. The common features and differences between Ig produced by cancer cells and Ig produced by B cells.
| Ig produced by cancer cells | Ig produced by B cells | ||
|---|---|---|---|
| Heavy chain classes | α, γ, µ, ε and δ | ||
| Light chain types | Only κ-chain was found | κ- or λ-chain | |
| Genetic processing | V(D)J is rearranged | ||
| Germline configuration of C region Lacking V regionSpecial rearrangement pattern | Normal V(D)J rearrangement and class switching under control | ||
| Transcription | Regulated by cis-acting elements and transacting factors | ||
| J1-5 all transcribed | One of J1-5 is chosen to be transcribed | ||
| Protein expression | Expression level is low | Expressing level is high | |
| Protein structures | Unknown | Free antibody tetramer, IgA dimer, IgM pentamer | |
| Post translational modification | N-acetyl and N-glycoyl neuraminic acid↑ N-acetylglucosamine↓ | N-glycans attached to Fc region modulates Ig's biological activity | |
| Function | Favoring tumor growth | Specific antigen receptor; combine Fc receptor; activate complement system | |
Abbreviation: Ig, immunoglobulin.
The function of the cancerous Ig is not well understood. Our previous studies demonstrated that cancerous Ig promoted tumor growth. Our recent studies showed that the level of the cancerous Ig κ light chain could be upregulated by the exogenous oncoprotein, latent membrane protein 1 (LMP1), which is encoded by the Epstein–Barr virus in nasopharyngeal carcinoma (NPC) and triggers several signal pathways mediated through nuclear factor-kappa B (NF-KB) and activator protein-1 (AP-1).19, 20 Our findings indicated that cancerous Ig plays a potential role in the process of cell transformation. Overall, cancerous Ig is different from normal Ig. Based on this finding, the molecular structures of cancerous Ig were explored in the present study.
Materials and methods
Cell lines and cell culture
Five epithelial cell lines, including HeLa (cervical cancer), SW480 (colon cancer), MGC (gastric cancer), MCF-7 (breast cancer) and HNE2 (nasopharyngeal carcinoma), were used for consistency with previous studies.21 Three malignant lymphocyte lines were used as controls. XG6, a multiple myeloma cell line22 that selectively expresses the Ig λ light chain, was used as a negative control for the Ig κ light chain. XG7,22 a multiple myeloma cell line that selectively expresses the Ig κ light chain, was used as a positive control for the Ig κ light chain. Raji (ATCC CCL-86), a Burkitt lymphoma cell line, was also used as a positive control for the Ig κ light chain. A negative control of the α heavy chain is difficult to identify because B lymphocytes are capable of undergoing class switching and thus may express the α chain. Based on our results, XG6, XG7 and Raji all express the α heavy chain. All cell lines were maintained in RPMI1640 (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco), 1% glutamine and 1% antibiotics at 37 °C in humidified atmosphere with 5% CO2.
ELISA analysis
The levels of the κ light chain and α heavy chain secreted by cancer cells were determined using the Human kappa ELISA Quantitation Set (Cat. No. E80–115; Bethyl, Montgomery, TX, USA) and the Human IgA ELISA Quantitation Set (Cat. No. E80–102; Bethyl), respectively, following the instructions provided. The cell culture media were centrifuged at 1500 g, and the supernatant fractions were sealed in dialysis bags and immersed into fresh flowing double distilled water at 4 °C overnight. Finally, samples were concentrated via PGE20000.
Western blot analysis
Western blot analysis was performed according to the method previously described.19 Native membrane proteins were extracted using the ProteoExtract Naive Membrane Protein Extraction Kit (Cat. No. 444810; Calbiochem, Darmstadt, Germany) according to the instructions provided. β-mercaptoethanol (0.8 mM) was applied to destroy disulfide bonds as required. Protein concentration was determined using the BCA Assay Reagent (Cat. No. 23228; Pierce, Rockford, IL, USA). The following primary antibodies at appropriate dilutions were used for immunodetection: rabbit anti-human IgA (Cat. No. A0262; DAKO, Glostrup, Denmark); mouse anti-human IgA (Cat. No. I0636; Sigma, St Louis, MO, USA); rabbit anti-human kappa light chains (Cat. No. A0192; DAKO); mouse anti-human kappa light chains (Cat. No. K4377; Sigma); mouse anti-β−actin (Cat. No. sc-8432; Santa Cruz Biotechnology, Santa Cruz, USA); goat anti-EGFR (Cat. No. 03-G; Santa Cruz); donkey anti-rabbit IgG-HPR (Cat. No. sc2004, Santa Cruz); donkey anti-mouse IgG-HPR (Cat. No. sc2005, Santa Cruz Biotechnology); and donkey anti-goat IgG-HPR (Cat. No. 2033, Santa Cruz Biotechnology).
Immunofluorescence
Cells were fixed and stained using a standard immunofluorescence protocol (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA). The following antibodies were used: Alexa Fluor 488-labeled anti-mouse IgG (Cat. No. A11001; Invitrogen, Carlsbad, CA, USA); goat anti- rabbit IgG-FITC (Cat. No. F6005; Sigma); mouse anti-human IgA (Cat. No. I0636; Sigma); and mouse anti-human kappa light chains (Cat. No. K4377; Sigma). Following the instructions provided, BODIPY TR C5-ceramide complexed to BSA (Cat. No. B-34400; Invitrogen) was applied to stain the Golgi apparatus.
Results
Cancerous Ig chains are found in the Golgi apparatus of cancer cells
The Golgi apparatus is crucially important for the membrane orientation and secretion of proteins. Before arriving at their final destination, newly synthesized proteins must be processed and packaged by the Golgi apparatus. In cancer cells, the Ig protein universally enters the Golgi apparatus for processing. The observation that the cancerous Ig protein appears in the Golgi apparatus of the cancer cell further supports the finding that cancer cells can produce the Ig protein. This suggests that the cancerous Ig protein is processed and packaged for biological function.
Among the five classes of heavy chains and two types of light chains of the Ig molecule, the Ig α heavy chain and Ig κ light chain are the forms most commonly detected in cancer cells.2 Therefore, the α heavy chain and κ light chain were chosen for study in this research project.
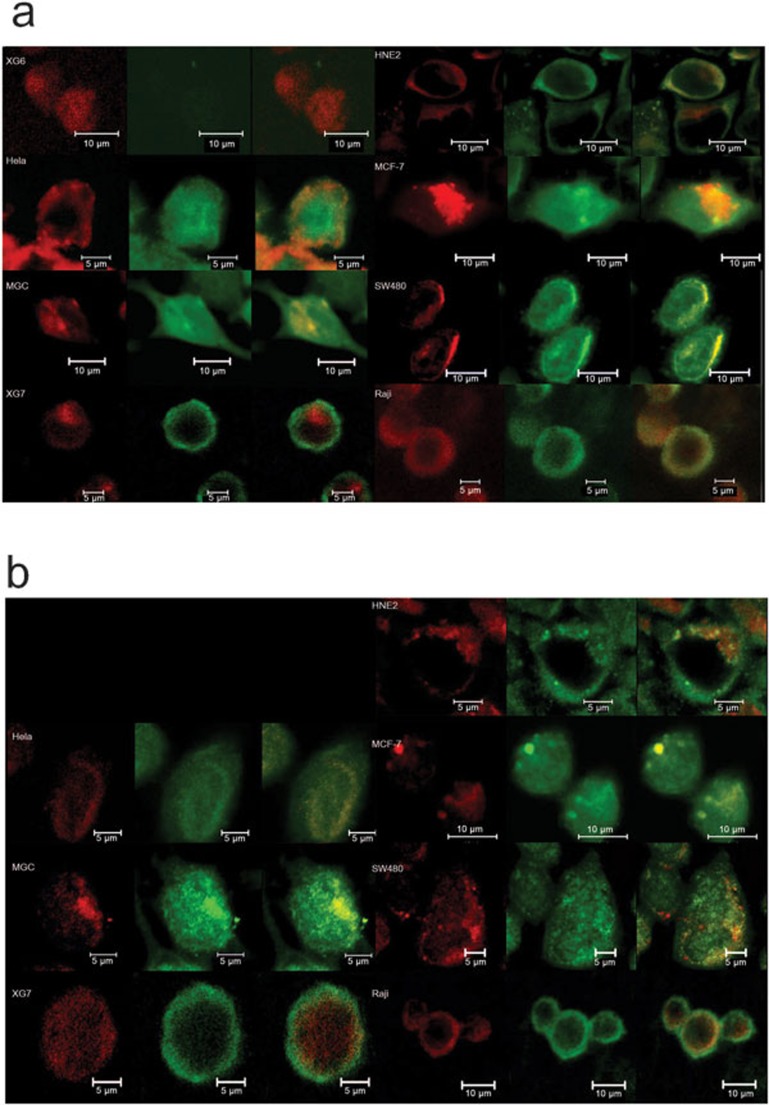
An immunofluorescence assay indicated that the Golgi apparatus staining signal overlapped with the staining of either the Ig κ light chain (Figure 2a) or the Ig α heavy chain (Figure 2b) in all epithelial cancer cell lines studied. This demonstrated the presence of the Ig protein in the Golgi apparatus of cancer cells.
Figure 2.
Cytoimmunofluorescence study of Ig chains merged with the Golgi apparatus in cancer cell lines. The Golgi apparatus is stained by TR C5-ceramide (red) and the Ig chains are stained by Alexa Fluor 488 (green). (a) Ig κ and Golgi apparatus. (b) Ig α and Golgi apparatus. XG6 is used as a negative control for Ig κ, as described in the section on ‘Materials and methods'. Ig, immunoglobulin.
Cancerous mIg and sIg produced by cancer cell lines
The mIg is produced by cancer cell lines
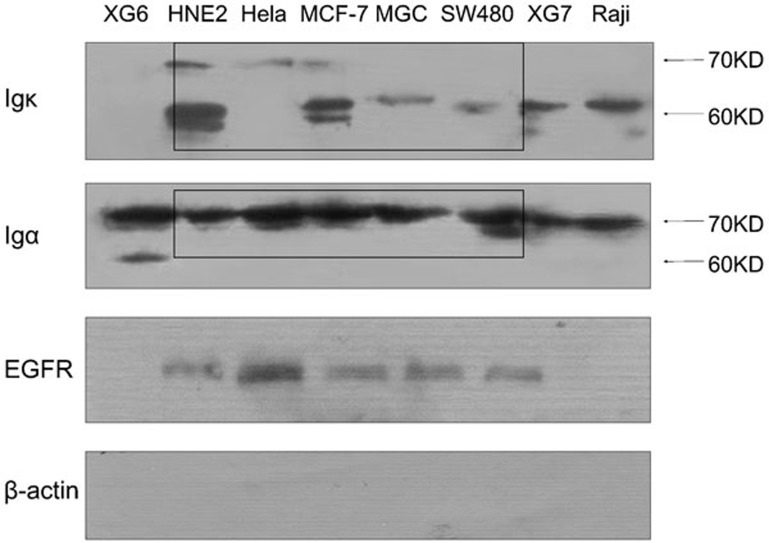
Membrane proteins were extracted from several cancer cell lines. To protect the disulfide bond, β-mercaptoethanol was omitted from the extraction buffer. After extraction, the proteins were denatured and the Ig κ light-chain and α heavy-chain monomers were detected in the membrane extracts by western blot analysis. The epidermal growth factor receptor, a known receptor located on the cell membrane, was used as a membrane marker in the present studies. β-actin, which is a cytoskeleton protein that is not membrane-bound, was used as a marker for cytoplasm proteins. Results demonstrated that both cancerous Ig κ light chain and α heavy chain were located in the membrane extracts (Figure 3). The transmembrane domain of the normal mIg molecule is the C-terminal of the heavy chain, and the light chain is linked to the N-terminal of the heavy chain by a disulfide bond (Figure 1). Thus, detection of the cancerous Ig κ light chain in the membrane protein extract strongly suggested that the light chains are combined with the heavy chains in cancerous mIg.
Figure 3.
mIg is detected in the naive membrane protein extract of several cancer cell lines. The EGFR serves as a membrane protein marker. Detection of β-actin was used to verify the exclusion of cytoplasmic protein contamination. EGFR, epidermal growth factor receptor; Ig, immunoglobulin; mIg, membrane Ig.
The molecular weight of the mIg detected was 60 kDa and/or 70 kDa (Figure 3) and varied among the different cancer cell lines. This molecular weight was smaller than the molecular weight of the ‘normal' tetramer mIg, which suggested that these Ig chains might be truncated and aberrant or abnormal in cancer cells.
The diversity of the protein expression levels and molecular weights further provides evidence showing the heterogeneity and complexity of cancerous Ig. The molecular weight of the κ light chain was observed at both 60 and 70 kDa in the HNE2 and MCF-7 cell lines. In the HeLa cell line, the molecular weight of the Ig κ was 70 kDa, whereas the molecular weight of the Ig κ in the other cancer cell lines studied was approximately 60 kDa. Meanwhile, the molecular weight of the α heavy chain was consistently observed as 70 kDa with one exception: the XG6 cells exhibited molecular weights of both 60 and 70 kDa (Figure 3).
The sIg is secreted by cancer cell lines
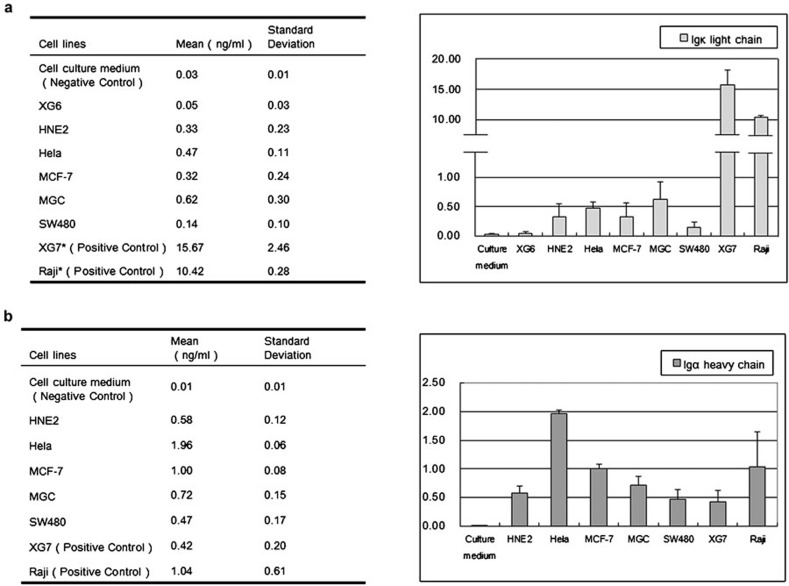
ELISA was used to assess the level of cancerous sIg in concentrated cancer cell culture medium. A highly specific antibody was utilized to prevent false-positive reaction with concentrated fresh cell culture medium (Figure 4). The results demonstrate that all the cancer cell lines were able to secrete Ig κ (Figure 4a) and Ig α (Figure 4b). Compared with the positive control, the protein level of Ig κ was lower in the epithelial cancer cell lines. In contrast, HeLa cells exhibited a moderately higher expression level of Ig α compared with any of the other epithelial cancer cell lines.
Figure 4.
The level of sIg was determined in cell culture medium by ELISA. (a) Ig κ-chain. (b) Ig α-chain. Fresh cell culture medium was tested for both Ig κ and Ig α as a negative control. Ig, immunoglobulin; sIg, secreted Ig.
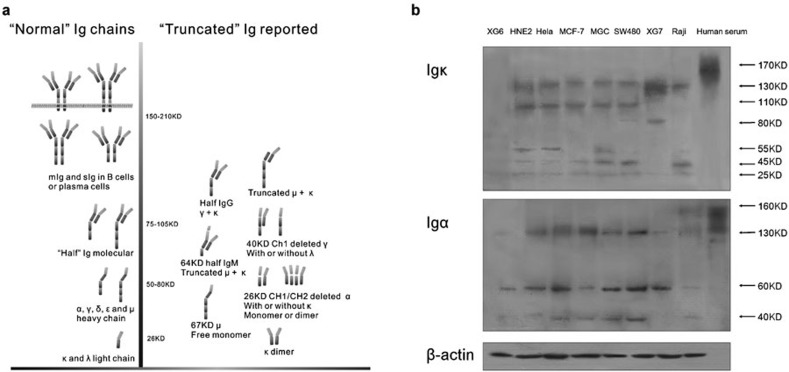
Heterogeneity of Ig-truncated Ig is produced by cancer cells
A previous research study identified an aberrant Ig κ without the V region.1 The results above demonstrated diversity in the molecular weights of the mIg in several cancer cell lines, which suggested the existence of truncated Ig chains. Meanwhile, the production of truncated Ig by B cells has been reported by others13, 14, 15, 16, 17, 18 (Figure 5a). These results strongly imply that the protein structure of cancerous Ig differs from that of the normal antibody tetramer.
Figure 5.
(a) Schematic illustration of truncated Ig in B cells under pathological conditions is shown. (b) Ig κ and Ig α found in naive protein extracts of various cancer cell lines show multiple molecular weights. A healthy human serum sample was used to illustrate normal Ig tetramers. Ig, immunoglobulin.
Identification of the components of naive cancerous Ig
To identify the components of cancerous Ig, total naive cell proteins were extracted without destroying the disulfide bonds. The Ig κ light and Ig α heavy chains were visualized by western blot analysis, and free Ig in human serum was used as a positive control (Figure 5b).
The results revealed that the cancerous Igs from different cancer cells are comprised of multiple molecular weights. The molecular weight of the whole Ig tetramer is larger than 150 Da, primarily comprising circulating IgG (160 kDa), IgM (210 kDa) and IgA (160 kDa). In contrast, Ig κ ranging from 25 to 130 kDa was detected in lymphocyte and epithelial tumor cell lines (Figure 5b). The weak 25-kDa band appeared to be a ‘normal' Ig κ monomer, whereas all the others, including Igs with molecular weights estimated at 130, 110, 80, 55 and 40 kDa, might be truncated Ig chains sticking to each other. Interestingly, in the malignant lymphocyte control cells, similar phenomena were observed, although the molecular weight patterns differed from those of epithelial cells.
Similarly, the molecular weights for Ig α in the epithelial cancer cell lines were approximately 130, 60 and 40 kDa, as compared to 160 kDa observed in the human serum sample (Figure 5b).
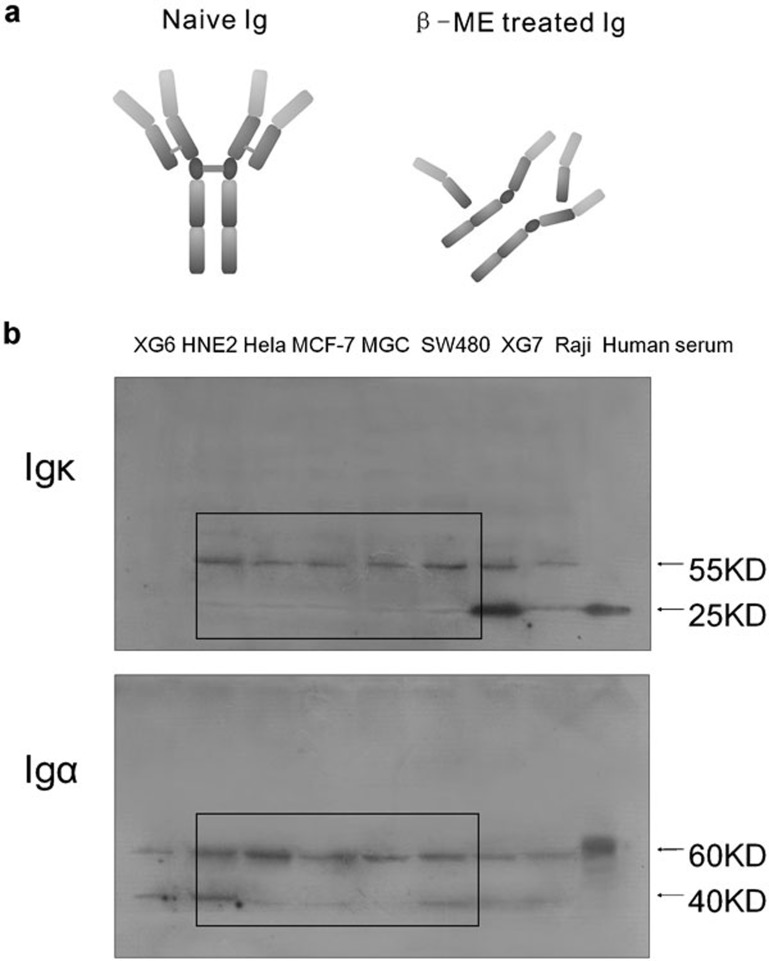
Identification of the monomer components of cancerous Ig
Extracted protein was denatured and treated with β-mercaptoethanol (β-ME) to destroy potential disulfide bonds (Figure 6a). The expression of Ig κ light chain and Ig α heavy chain was again examined. Human serum was used as a positive control, and the molecular weights of Ig κ light chain and Ig α heavy chain (Figure 6b) were approximately 25 and 60 kDa respectively, which are the normal components of the human antibody. All the normal Ig chains detected were monomers owing to the destruction of the disulfide bond. In the cancer cell lines, the molecular weights of the κ light chain were observed as 55 and 25 kDa, whereas the molecular weights of the α heavy chain were 60 and 40 kDa (Figure 6b), again suggesting that the cancerous Ig chains were aberrant or truncated.
Figure 6.
(a) Illustration of naive untreated Ig and β-ME-treated Ig (to destroy disulfide bonds). (b) After treatment with β-ME, Ig in a human serum sample was degraded into light-chain and heavy-chain monomers. The degraded Ig chains in cancer cell lines showed abnormal molecular weights. β-ME, β-mercaptoethanol; Ig, immunoglobulin.
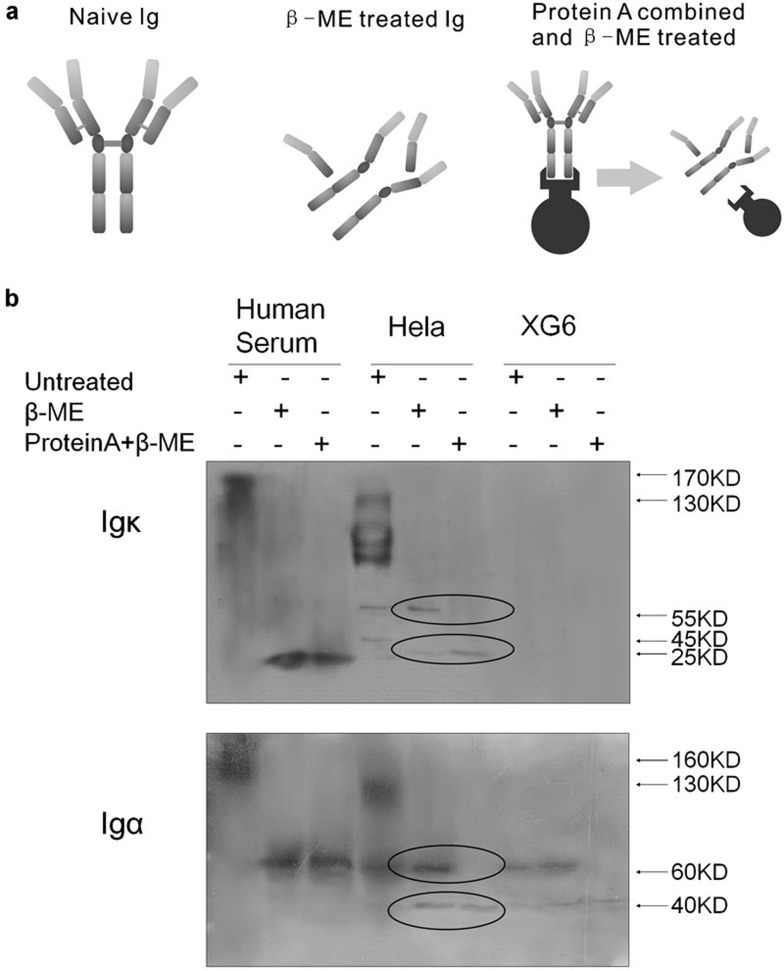
Further analysis of Ig components by protein A pull-down analysis
Protein A beads were used to pull down Ig components in human serum (positive control), HeLa cells and XG6 (negative control) cells. Protein A specifically and sensitively binds to the Fc region of Igs. Untreated, β-ME-treated and β-ME–protein A-treated protein extracts were used to pull down all the Ig molecules with an Fc region (Figure 7a). Results (Figure 7b) showed that both the Ig κ light chain and Ig α heavy chain in human serum could be pulled down by the protein A beads. For Ig κ light chains that do not contain an Fc region, the Ig κ is inferred to be pulled down while binding with the heavy chains. In addition, only the 25-kDa κ chain in HeLa cells was pulled down, suggesting that the 55-kDa κ chain was aberrant or at least incapable of binding the heavy chains. Furthermore, only the 40-kDa α heavy chain could be pulled down. Although the 60-kDa α heavy chain appeared to have a “normal” molecular weight, it did not have a valid Fc region. Therefore, we concluded that both the 40- and 60-kDa α chains produced by cancer cell lines were aberrant and truncated. We had similar results from MGC and MCF-7 cell lines (Supplementary Figure 1).
Figure 7.
(a) Untreated naive Ig, Ig treated with β-ME and Ig treated with β-ME and protein A to enrich the Ig chains containing the Fc region. (b) Human serum, HeLa cells and XG6 cells were untreated, treated with β-ME, or with protein A plus β-ME. Protein A pulled down a 25-kDa κ-chain and a 40-kDa α-chain. β-ME, β-mercaptoethanol; Ig, immunoglobulin.
Discussion
Studies have shown that the expression of Igs is widespread in epithelial cancers originating from different organs. Igs produced by cancer include all types of the heavy-chain classes. In this study, we demonstrated that Ig molecules produced by cancer cells were truncated, aberrant and structurally different from normal Ig tetramers produced by B cells. In normal B cells, the expression of Ig is precisely regulated and tightly controlled, accomplished in multiple stages under successive microenvironments; thus, the phenomenon of aberrant Ig expression by cancer cells is reasonable. Disturbed and unbalanced control over these processes is likely to result in abnormal Ig production. This is supported by the finding that malignancy of B cells leads to the production of truncated Ig chains.13, 14, 15, 16, 17, 18 According to these studies, cancerous Igs comprise components that appear to include normal Igs and truncated Igs. The heterogeneity of Igs produced by cancer cells is important, suggesting that the Ig produced by cancer cells and B cells in vivo could be distinguished. This strongly indicates that aberrant or truncated Igs might be an important cancer biomarker in clinical prediction and diagnosis.
Recently, a series of studies carried out by Lee et al. showed that CA215, a pan-cancer diagnostic marker, was in fact cancer cell-expressed Ig with unique carbohydrate modifications.7, 23, 24 The research by Lee and colleagues demonstrated that Ig produced by cancer cells had unique epitope(s) associated with carbohydrate modification, which could be specifically recognized by PR215, the tumor marker CA215 detecting antibody.7 The question regarding the clinical relevance of this phenomenon remains open. In the present study, we clearly demonstrated the existence of mIg and sIg in various cancer cell lines. The present findings, especially the discovery of cancerous sIg, further substantiate the observation of that Ig produced by tumors could be a pan-cancer marker.
Serum antibody levels are elevated in malignant cancer patients.25, 26, 27, 28, 29 Additionally, high-output proteomics studies revealed that Ig chain fragments were elevated in cancer patients compared with normal healthy controls.30, 31, 32 The elevated serum antibody levels were thought to be produced from autoimmune reactions that were stimulated by the disordered immune system.25, 29 Based on our findings, some of these Ig chains could be inferred to arise from the cancer cell itself.
The existence of mIg in cancer cells is another significant finding. In B cells, mIg together with CD79A and CD79B forms the B-cell receptor complex, which is responsible for conducting transmembrane signaling for B-cell activation.33 CD79 was reportedly not found in epithelial cancer cells; thus, the function of mIg in cancer cells is unknown. Without CD79, the mIg anchored on the cell membrane appears to be a receptor with no cytoplasmic functioning domain. If mIg has a role in tumor development, its function might be in relation to its specialized ‘binding and blocking' ability.
Supplementary information accompanies the paper on Cellular & Molecular Immunology's website(http://www.nature.com/cmi)
Acknowledgments
This work was supported by the National High Technology Research and Development Program (863) of China (no. 2006AA02A404), the National Nature Science Foundation of China (nos. 30471968 and 30772465) and the CMB Educational Thrust Project (04-799).
The authors declare no conflict of interest.
Supplementary Information
References
- Cao Y, Sun Y, Poirier S, Winterstein D, Hegamyer G, Seed J, et al. Isolation and partial characterization of a transformation-associated sequence from human nasopharyngeal carcinoma. Mol Carcinog. 1991;4:297–307. doi: 10.1002/mc.2940040408. [DOI] [PubMed] [Google Scholar]
- Hu D, Zheng H, Liu H, Li M, Ren W, Liao W, et al. Immunoglobulin expression and its biological significance in cancer cells. Cell Mol Immunol. 2008;5:319–324. doi: 10.1038/cmi.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wu L, Zhang L, Hao P, Zhang S, Huang J, et al. Distinct regulatory mechanism of immunoglobulin gene transcription in epithelial cancer cells. Cell Mol Immunol. 2010;7:279–286. doi: 10.1038/cmi.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Mao Y, Huang J, Ma T, Zhang L, Zhu X, et al. Immunoglobulin gene locus events in epithelial cells of lactating mouse mammary glands. Cell Mol Life Sci. 2010;67:985–994. doi: 10.1007/s00018-009-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Li M, Ren W, Zeng L, Liu HD, Hu D, et al. Expression and secretion of immunoglobulin alpha heavy chain with diverse VDJ recombinations by human epithelial cancer cells. Mol Immunol. 2007;44:2221–2227. doi: 10.1016/j.molimm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Li M, Tang M, Deng X. Positive immunoglobulin A expression in human epithelial carcinoma cell lines. Zhonghua Zhong Liu Za Zhi. 2001;23:451–453. [PubMed] [Google Scholar]
- Lee G, Laflamme E, Chien CH, Ting HH. Molecular identity of a pan cancer marker, CA215. Cancer Biol Ther. 2008;7:2007–2014. doi: 10.4161/cbt.7.12.6984. [DOI] [PubMed] [Google Scholar]
- Li M, Feng DY, Ren W, Zheng L, Zheng H, Tang M, et al. Expression of immunoglobulin kappa light chain constant region in abnormal human cervical epithelial cells. Int J Biochem Cell Biol. 2004;36:2250–2257. doi: 10.1016/j.biocel.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Liu HD, Zheng H, Li M, Hu DS, Tang M, Cao Y. Upregulated expression of kappa light chain by Epstein–Barr virus encoded latent membrane protein 1 in nasopharyngeal carcinoma cells via NF-kappaB and AP-1 pathways. Cell Signal. 2007;19:419–427. doi: 10.1016/j.cellsig.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Hu D, Tong S, Wei R, Hui Z, Haidan L, Zhi D, et al. The polymorphisms on Igkappa gene are related to susceptibility of breast cancer and gastric cancer. Genet Test. 2008;12:575–580. doi: 10.1089/gte.2008.0062. [DOI] [PubMed] [Google Scholar]
- Ren W, Zheng H, Li M, Deng L, Li XL, Pan KF, et al. A functional single nucleotide polymorphism site detected in nasopharyngeal carcinoma-associated transforming gene Tx. Cancer Genet Cytogenet. 2005;157:49–52. doi: 10.1016/j.cancergencyto.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Li M, Ren W, Weng XX, Liao W, Xia LQ, Deng X, et al. Nucleotide sequence analysis of a transforming gene isolated from nasopharyngeal carcinoma cell line CNE2: an aberrant human immunoglobulin kappa light chain which lacks variable region. DNA Seq. 2001;12:331–335. doi: 10.3109/10425170109084456. [DOI] [PubMed] [Google Scholar]
- Imoto M, Ishikawa K, Yamamoto K, Sinohara H, Irimajiri K, Kubo N, et al. Occurrence of heavy chain of 7S IgM half-molecule whose NH2-terminal sequence is identical with that of kappa light chain sequence in patients with Waldenstrom macroglobulinemia. Clin Chim Acta. 1999;282:77–88. doi: 10.1016/s0009-8981(99)00020-0. [DOI] [PubMed] [Google Scholar]
- Tamura A, Yamashiro A, Mizutani F, Oita T, Maeda A, Takahashi T.Immunochemical properties of free mu-chain protein in a patient with µ-heavy chain disease Rinsho Byori 200351847–851.Japanese [PubMed] [Google Scholar]
- Cogne M, Aucouturier P, Brizard A, Dreyfus B, Duarte F, Preud'homme JL. Complete variable region deletion in a µ heavy chain disease protein (ROUL). Correlation with light chain secretion. Leuk Res. 1993;17:527–532. doi: 10.1016/0145-2126(93)90129-9. [DOI] [PubMed] [Google Scholar]
- Gallango M L, Suinaga R, Ramirez M. An unusual case of Waldenstrom macroglobulinemia with half molecules of IgG in serum and urine. Blut. 1984;48:91–97. doi: 10.1007/BF00320035. [DOI] [PubMed] [Google Scholar]
- Cheng IK, Ho SK, Chan DT, Ng WK, Chan KW. Crescentic nodular glomerulosclerosis secondary to truncated immunoglobulin alpha heavy chain deposition. Am J Kidney Dis. 1996;28:283–288. doi: 10.1016/s0272-6386(96)90315-7. [DOI] [PubMed] [Google Scholar]
- Moulin B, Deret S, Mariette X, Kourilsky O, Imai H, Dupouet L, et al. Nodular glomerulosclerosis with deposition of monoclonal immunoglobulin heavy chains lacking CH1. J Am Soc Nephrol. 1999;10:519–528. doi: 10.1681/ASN.V103519. [DOI] [PubMed] [Google Scholar]
- Zheng H, Li M, Liu H, Ren W, Hu DS, Shi Y, et al. Immunoglobulin alpha heavy chain derived from human epithelial cancer cells promotes the access of S phase and growth of cancer cells. Cell Biol Int. 2007;31:82–87. doi: 10.1016/j.cellbi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Liu H, Zheng H, Duan Z, Hu D, Li M, Liu S, et al. LMP1-augmented kappa intron enhancer activity contributes to upregulation expression of Ig kappa light chain via NF-kappaB and AP-1 pathways in nasopharyngeal carcinoma cells. Mol Cancer. 2009;8:92. doi: 10.1186/1476-4598-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Huang J, Mao Y, Liu S, Sun X, Zhu X, et al. Immunoglobulin gene transcripts have distinct VHDJH recombination characteristics in human epithelial cancer cells. J Biol Chem. 2009;284:13610–9. doi: 10.1074/jbc.M809524200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XG, Gaillard JP, Robillard N, Lu ZY, Gu ZJ, Jourdan M, et al. Reproducible obtaining of human myeloma cell lines as a model for tumor stem cell study in human multiple myeloma. Blood. 1994;83:3654–3663. [PubMed] [Google Scholar]
- Lee G. Cancer cell-expressed immunoglobulins: CA215 as a pan cancer marker and its diagnostic applications. Cancer Biomark. 2009;5:137–142. doi: 10.3233/CBM-2009-0610. [DOI] [PubMed] [Google Scholar]
- Lee G, Ge B. Cancer cell expressions of immunoglobulin heavy chains with unique carbohydrate-associated biomarker. Cancer Biomark. 2009;5:177–188. doi: 10.3233/CBM-2009-0102. [DOI] [PubMed] [Google Scholar]
- Gajewski T F, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Yu J, Sreekumar A, Varambally S, Shen R, Giacherio D, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353:1224–1235. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- Olubuyide IO, Salimonu LS, Adeniran S O. Soluble immune complexes and immunoglobulin (IgG, IgA and IgM) levels in Nigerians with primary liver cell carcinoma. Afr J Med Med Sci. 1993;22:57–62. [PubMed] [Google Scholar]
- Jager E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jager D, et al. Humoral immune responses of cancer patients against ‘Cancer–Testis' antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84:506–510. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gercel-Taylor C, Bazzett LB, Taylor DD. Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol Oncol. 2001;81:71–76. doi: 10.1006/gyno.2000.6102. [DOI] [PubMed] [Google Scholar]
- Okabe H, Satoh S, Kato T, Kitahara O, Yanagawa R, Yamaoka Y, et al. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 2001;61:2129–2137. [PubMed] [Google Scholar]
- Li J, Tan C, Xiang Q, Zhang X, Ma J, Wang JR, et al. Proteomic detection of changes in protein synthesis induced by NGX6 transfected in human nasopharyngeal carcinoma cells. J Protein Chem. 2001;20:265–271. doi: 10.1023/a:1010912311564. [DOI] [PubMed] [Google Scholar]
- Xiao T, Ying W, Li L, Hu Z, Ma Y, Jiao L, et al. An approach to studying lung cancer-related proteins in human blood. Mol Cell Proteomics. 2005;4:1480–1486. doi: 10.1074/mcp.M500055-MCP200. [DOI] [PubMed] [Google Scholar]
- Pleiman CM, D'Ambrosio D, Cambier JC. The B-cell antigen receptor complex: structure and signal transduction. Immunol Today. 1994;15:393–399. doi: 10.1016/0167-5699(94)90267-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.