Abstract
OBJECTIVE
The objective of the study was to determine the threshold for defining abnormal labor that is associated with adverse maternal and neonatal outcomes.
STUDY DESIGN
This study consisted of a retrospective cohort of all consecutive women admitted at a gestation of 37.0 weeks or longer from 2004 to 2008 who reached the second stage of labor. The 90th, 95th, and 97th percentiles for progress in the first stage of labor were determined specific for parity and labor onset. Women with a first stage above and below each centile were compared. Maternal outcomes were cesarean delivery in the second stage, operative delivery, prolonged second stage, postpartum hemorrhage, and maternal fever. Neonatal outcomes were a composite of the following: admission to level 2 or 3 nursery, 5 minute Apgar less than 3, shoulder dystocia, arterial cord pH of less than 7.0, and a cord base excess of −12 or less.
RESULTS
Of the 5030 women, 4534 experienced first stage of less than the 90th percentile, 251 between the 90th and 94th percentiles, 102 between the 95th and 96th percentiles, and 143 at the 97th percentile or greater. Longer labors were associated with an increased risk of a prolonged second stage, maternal fever, the composite neonatal outcome, shoulder dystocia, and admission to a level 2 or 3 nursery (P < .01). Depending on the cutoff used, 29–30 cesarean deliveries would need to be performed to prevent 1 shoulder dystocia.
CONCLUSION
Although women who experience labor dystocia may ultimately deliver vaginally, a longer first stage of labor is associated with adverse maternal and neonatal outcomes, in particular shoulder dystocia. This risk must be balanced against the risks of cesarean delivery for labor arrest.
Keywords: first stage of labor, labor dystocia
Emanuel Friedman1–3 revolutionized the management of labor with a series of publications describing the patterns of normal labor of nulliparous and multiparous women in the 1950s. These analyses led to the development of labor partograms and definitions of abnormal labor, with specific actions recommended when the labor times exceeded predefined action lines.4,5
Changes in the population combined with rising induction and cesarean rates have led to renewed interest in defining normal labor. Several contemporary labor curves utilizing populations of women who reach 10 cm of dilation have been published using interval censoring and polynomial modeling, techniques that account for repeated cervical measurements and the impact of examination times on individual labor curves.6–13 In these analyses, it has become customary to report the time to achieve 1 cm dilation in terms of the median and 95th percentiles; however, these labor curves have been created in populations in which all women achieved 10 cm of dilation. Thus, even though women exceed the 95th percentile, they may still achieve full dilation and deliver vaginally.
The presentation of the 95th percentile in these publications appears to have been chosen based on statistical customs rather than physiological significance or an association with adverse outcomes. Unfortunately, this custom of reporting the 95th percentile inherently suggests the 95th percentile as a threshold for abnormal labor, despite any documented association of adverse outcomes with exceeding this threshold. Interventions for labors that exceed the 95th percentile may lead to unnecessary cesarean deliveries without improving the maternal and neonatal outcomes.
Therefore, we sought to evaluate the impact of exceeding several percentile thresholds of the first stage of labor on maternal and neonatal outcomes in a contemporary population of women who reached 10 cm of dilation.
Materials and Methods
We conducted a 4 year retrospective cohort study of all consecutive term (gestation of ≥37 weeks) deliveries at Washington University School of Medicine (St. Louis, MO) from July 2004 to June 2008 who reached 10 cm dilation. Institutional board review approval was obtained from Washington University School of Medicine.
Women were included if their gestational age was at least 37 0/7 weeks’ gestation at admission to labor and delivery, carried a singleton pregnancy in vertex presentation, and had an arterial umbilical cord gas obtained at delivery. Women were excluded if they had a prior cesarean, delivered preterm, had fetuses with congenital anomalies, or delivered by cesarean before complete dilation.
Detailed information on maternal sociodemographic, obstetric and gynecological history, medical and surgical history, prenatal history, antepartum history, and labor and delivery course was extracted from the medical charts. The labor and delivery records included medications, labor type, cervical examinations, cervical examination times, length of labor stages, mode of delivery, and postpartum record. All data were extracted using close-ended forms by trained research assistants who underwent regularly scheduled training.
Because the first stage of labor can be defined in many ways, receiver-operator characteristic (ROC) curves were generated to determine the definition of the first stage of labor most closely associated with maternal and neonatal outcomes. The first stage of labor was defined as the time from admission to complete dilation, time from 4 cm of dilation to complete, dilation and time from 6 cm of dilation to complete dilation.
Women presenting with cervical examinations greater than 4 cm or greater than 6 cm were assigned times based on the time of the first cervical examination to complete. The areas under the ROC curves were calculated for each definition of the first stage. ROC curves were created to visually evaluate the relationship between the length of the first stage and maternal and neonatal outcomes, measured as a composite of any of the maternal and neonatal outcomes of interest.
Maternal outcomes considered were cesarean delivery in the second stage, operative vaginal delivery (forceps and vacuum), postpartum hemorrhage (as documented by the delivery physician), prolonged second stage (specific for parity and regional anesthesia use), and maternal fever. Neonatal outcomes considered were analyzed as a composite of the following: 5 minute Apgar less than 3, arterial cord pH less than 7.0, cord base excess −12 or less, admission to a level 2 or 3 nursery, or shoulder dystocia. Shoulder dystocia was documented by the delivery physician and at our institution is typically defined as requiring at least 1 maneuver to deliver the anterior shoulder.
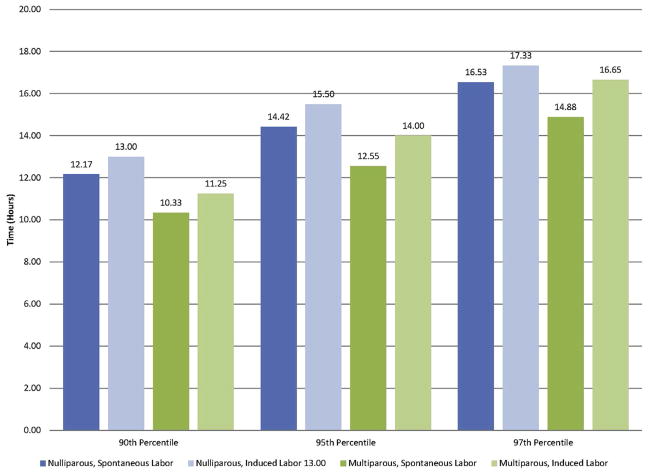
Thereafter the first stage of labor was defined as the time from 4 cm to complete dilation. The exposure group was defined as having a first stage of labor less than the 90th percentile, between the 90th and 94th percentile, between the 95th and 96th percentile, or the 97th percentile or greater. Percentiles were determined for parity (nulliparous vs multiparous) and labor type (induced vs spontaneous) (Figure 1). Maternal and neonatal outcomes were considered as a composite and individually. Because shoulder dystocia is a potentially debilitating complication that can be prevented with cesarean delivery, the number of cesarean deliveries performed to prevent 1 shoulder dystocia was determined for each cutoff of abnormal labor.
FIGURE 1.
Time in hours from 4 cm to 10 cm dilation
Study groups were compared using a Student t test or Mann-Whitney U test for continuous or χ2 for categorical variables as appropriate. Potentially confounding variables of the exposure-outcome association were identified in the stratified analyses. Multivariable logistic regression models were then developed to better estimate the effect of the length of the first stage of labor on maternal and neonatal outcomes while adjusting for potentially confounding effects. Clinically relevant covariates for initial inclusion in the models were selected using the results of the stratified analyses, and factors were removed in a backward stepwise fashion, based on significant changes in the likelihood ratio test. Factors considered included parity, race, body mass index, birthweight, and use of oxytocin. All analyses were completed using Stata SE, version 11 (StataCorp, College Station, TX).
Results
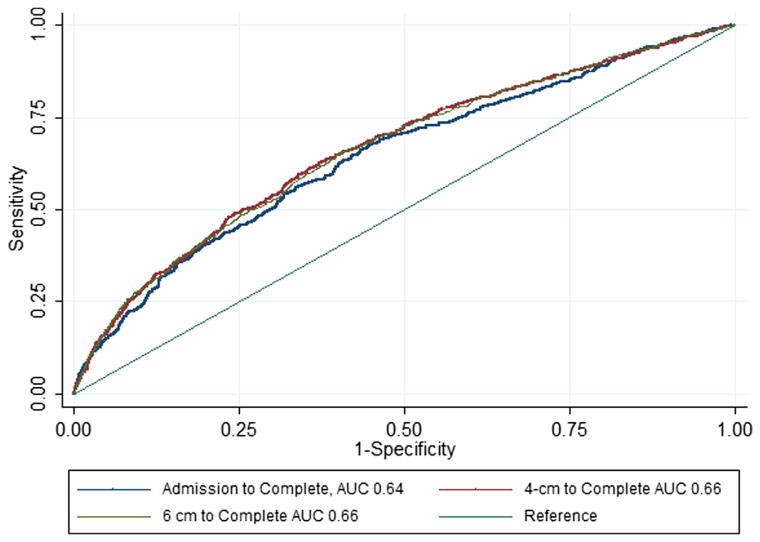
Of 5388 women in the cohort, 5030 were included in the analysis (11 excluded for incomplete time data, 347 for prior cesarean). The ROC curves were created to visually estimate the association between the length of the first stage and adverse outcomes using 3 different definitions of the first stage: time from admission to complete dilation, active phase of labor defined as starting at 4 cm, and the active phase of labor defined as starting at 6 cm (Figure 2). All 3 ROC curves had an area under the curve of 0.64–0.66, demonstrating a moderate association between length of labor and the composite of adverse maternal and neonatal outcomes. No curve demonstrated a clear cut point that could be used as a threshold for determining abnormal labor.
FIGURE 2.
Receiver operator characteristic curve for varying definitions of active labor
Based on this information, we elected to use the time from 4 cm to complete dilation to define the first stage of labor, and this definition was used in the remainder of the analyses. The cutoffs used to define the 90th, 95th, and 97th percentiles from 4 cm to complete dilation by parity and the type of labor can be found in Figure 1; the cutoffs ranged from 10 to 18 hours.
Of the 5030 women, 4534 women experienced a first stage 90th percentile or less for parity and labor type, 251 experienced a first stage between the 90th and 94th percentiles, 102 experienced a first stage between the 95th and 96th percentiles, and 143 experienced a first stage at the 97th percentile or greater. The groups were similar with respect to maternal age, insurance status, and the presence of maternal hypertensive disorders (Table 1). Women with a longer first stage, adjusted for parity and whether labor was induced, were more likely to be white, nulliparous, obese, receive oxytocin, have diabetes, have been induced, and have a macrosomic infant.
TABLE 1.
Maternal characteristics
| Variable | <90th percentile (n = 4534) | 90th–94th percentile (n = 251) | 95th–96th percentile (n = 102) | ≥97th percentile (n = 143) | P value |
|---|---|---|---|---|---|
| Age, y | 24.7 ± 5.9 | 24.5 ± 5.9 | 24.6 ± 5.5 | 25.0 ± 6.0 | .86 |
| Nulliparous | 1790 (39.5%) | 102 (40.6%) | 39 (38.2%) | 59 (41.3%) | .94 |
| Race | .04 | ||||
| Black | 3298 (72.7%) | 185 (73.7%) | 80 (78.4%) | 100 (69.9%) | |
| White | 749 (17.0%) | 45 (17.9%) | 16 (15.7%) | 35 (34.5%) | |
| Hispanic | 292 (6.4%) | 14 (5.6%) | 4 (3.9%) | 3 (2.1%) | |
| Insurance | .10 | ||||
| Private | 644 (14.2%) | 24 (9.6%) | 10 (9.8%) | 23 (16.1%) | |
| Public | 3890 (85.8%) | 227 (90.4%) | 92 (90.2%) | 120 (83.9%) | |
| Obese (BMI ≥ 30 kg/m2) | 2295 (52.5%) | 152 (62.0%) | 62 (61.4%) | 88 (62.4%) | < .01 |
| Hypertension | 112 (2.5%) | 5 (2.0%) | 5 (4.9%) | 7 (4.9%) | .12 |
| Preeclampsia | 298 (6.6%) | 14 (5.6%) | 10 (9.8%) | 8 (5.6%) | .50 |
| Diabetes | 46 (1.0%) | 8 (3.2%) | 0 | 6 (4.2%) | < .01 |
| Induced | 1397 (43.1%) | 77 (78.6%) | 32 (88.9%) | 43 (82.1%) | < .01 |
| Received oxytocin | 2375 (52.4%) | 216 (86.1%) | 91 (89.2%) | 122 (85.3%) | < .01 |
| Birthweight | 3229 ± 522 | 3375 ± 571 | 3349 ± 553 | 3440 ± 719 | < .01 |
| Macrosomia | 221 (4.96%) | 26 (10.4%) | 9 (8.8%) | 21 (14.7%) | < .01 |
BMI, body mass index.
Maternal and neonatal outcomes were examined at each percentile division (Table 2). The risk of a prolonged second stage and maternal fever (P < .01) increased as the first stage length increased, although the risk of cesarean, operative vaginal delivery, and postpartum hemorrhage remained unchanged. The composite adverse neonatal outcome, shoulder dystocia, and admission to a higher-level nursery increased as the length of the first stage increased (P < .01). Apgar score less than 3 at minutes, cord pH less than 7.0, and base excess of −12 or less were not associated with increasing length of the first stage.
TABLE 2.
Maternal and neonatal outcomes by length of the first stage of labor, beginning at 4 cm
| Variable | <90th percentile (n = 4534) | 90th–94th percentile (n = 251) | 95th–96th percentile (n = 102) | ≥97th percentile (n = 143) | P value |
|---|---|---|---|---|---|
| Maternal outcomes | |||||
| Cesarean in second stage | 68 (1.5%) | 3 (1.2%) | 3 (2.9%) | 3 (2.1%) | .60 |
| Operative vaginal delivery | 559 (12.5%) | 39 (15.7%) | 13 (13.1%) | 23 (16.4%) | .27 |
| Prolonged second stage | 194 (4.3%) | 13 (5.2%) | 7 (6.9%) | 21 (14.7%) | < .01 |
| Postpartum hemorrhage | 108 (2.4%) | 9 (3.6%) | 3 (2.9%) | 0 | .16 |
| Maternal fever | 162 (3.6%) | 26 (10.4%) | 11 (10.8%) | 19 (13.3%) | < .01 |
| Neonatal outcomes | |||||
| Composite neonatal outcome | 427 (9.5%) | 45 (18.0%) | 15 (14.7%) | 31 (21.7%) | < .01 |
| Shoulder dystocia | 213 (4.7%) | 20 (8.0%) | 8 (7.8%) | 12 (8.4%) | .01 |
| Admission to level 2–3 nursery | 195 (4.3%) | 25 (10.0%) | 8 (7.8%) | 19 (13.3%) | < .01 |
| Apgar <3 | 3 (0.1%) | 0 | 0 | 1 (0.7%) | .06 |
| pH <7.00 | 5 (0.11%) | 0 | 1 (1.0%) | 0 | .08 |
| Base excess −12 or less | 48 (1.1%) | 3 (1.2%) | 1 (1.0%) | 2 (1.4%) | .98 |
Table 3 displays the relative risks and adjusted odds of adverse outcomes at each percentile: less than 90th vs 90th percentile or greater, less than 95th vs 95th percentile or greater, and less than 97th vs 97th percentile or greater. At each percentile examined, a significant increased risk existed for a prolonged second stage, maternal fever, the composite neonatal outcome, and admission to a level 2–3 nursery. The risk of shoulder dystocia was significantly increased for exceeding the 90th and 95th percentiles, although this did not reach significance at the 97th percentile. The relative risk and adjusted odds ratios do not appear to be significantly different between each percentile, as signified by the overlapping confidence intervals.
TABLE 3.
Maternal and neonatal outcomes associated with labor duration of the ≥90th percentile, ≥95th percentile, and ≥97th percentile from 4–10 cm
| Variable | ≥90th percentile compared with <90th percentile
|
≥95th percentile compared with <95th percentile
|
≥97th percentile compared with <97th percentile
|
|||
|---|---|---|---|---|---|---|
| RR (95% CI) | AOR (95% CI) | RR (95% CI) | AOR (95% CI) | RR (95% CI) | AOR (95% CI) | |
| Maternal outcomes | ||||||
|
| ||||||
| Cesarean in second stage | 1.21 (0.61–2.41) | — | 1.65 (0.72–3.76) | — | 1.39 (0.44–4.34) | — |
|
| ||||||
| Operative vaginal delivery | 1.23 (0.99–1.54) | 1.22 (0.93–1.61)a | 1.19 (0.87–1.62) | 1.19 (0.82–1.73)a | 1.29 (0.88–1.89) | 1.30 (0.81–2.08)a |
|
| ||||||
| Prolonged second stage | 1.93 (1.40–2.67) | 1.78 (1.23–2.56)b | 2.64 (1.82–3.84) | 2.53 (1.64–3.90)b | 3.35 (2.21–5.08) | 3.19 (1.94–5.26)b |
|
| ||||||
| PPH | 1.01 (0.56–1.83) | 0.97 (0.53–1.78)c | 0.50 (0.16–1.56) | 0.47 (0.15–1.48)c | — | — |
|
| ||||||
| Maternal fever | 3.16 (2.37–4.22) | 3.26 (2.34–4.54)d | 3.12 (2.17–4.48) | 3.08 (2.00–4.74)d | 3.26 (2.10–5.07) | 3.01 (1.76–5.15)d |
|
| ||||||
| Neonatal outcomes | ||||||
|
| ||||||
| Composite neonatal outcome | 1.94 (1.58–2.38) | 1.88 (1.45–2.44)e | 1.89 (1.44–2.49) | 1.70 (1.20–2.41)e | 2.16 (1.56–2.98) | 1.90 (1.23–2.93)e |
|
| ||||||
| Shoulder dystocia | 1.72 (1.24–2.38) | — | 1.67 (1.08–2.60) | — | 1.70 (0.98–2.97) | — |
|
| ||||||
| Admission to level 2–3 nursery | 2.42 (1.81–3.24) | — | 2.38 (1.63–3.47) | — | 2.83 (1.82–4.38) | — |
|
| ||||||
| Apgar <3 | 3.05 (0.32–29.2) | — | 6.51 (0.68–62.4) | — | 11.4 (1.19–108.8) | — |
|
| ||||||
| pH <7.0 | 1.83 (0.21–15.6) | — | 3.92 (0.46–33.44) | — | –– | — |
|
| ||||||
| Base excess −12 or less | 1.14 (0.49–2.66) | — | 1.15 (0.36–3.67) | — | 1.32 (0.33–5.38) | — |
AOR, adjusted odds ratio; PPH, postpartum hemorrhage; RR, risk ratio.
Adjusted for black race, parity, and oxytocin use;
Adjusted for black race, macrosomia, and oxytocin use;
Adjusted for parity and macrosomia;
Adjusted for parity, prolonged second stage, and macrosomia;
Adjusted for parity, black race, macrosomia, and obesity.
We calculated the number of cesarean deliveries needed to prevent 1 shoulder dystocia and 1 event of the composite neonatal outcome when abnormal was defined at the 90th, 95th, and 97th percentiles of the first stage of labor (Table 4). Using a cutoff of the 90th percentile, 30 cesarean deliveries (95% confidence interval [CI], 18–58) would be required to prevent 1 shoulder dystocia. At the 95th percentile, 30 cesarean deliveries (95% CI, 15–128) would be required, and 29 cesarean deliveries (95% CI, 12–1426) would be required at the 97th percentile.
TABLE 4.
Number of cesarean deliveries needed to prevent 1 adverse event
| Percentile | Number of cesarean deliveries needed to prevent 1 shoulder dystocia |
|---|---|
| ≥90th | 30 (18–58)a |
| ≥95th | 30 (15–128)a |
| ≥97th | 29 (12–1426)a |
95% confidence interval.
Comment
In this analysis, a longer first stage of labor was associated with adverse maternal and neonatal outcomes. Although these women ultimately reached 10 cm dilation and the vast majority ultimately delivered vaginally, exceeding the 90th, 95th, and 97th percentiles was associated with an increased risk of maternal fever, shoulder dystocia, and neonatal admission to a level 2 or 3 nursery.
Given the potential devastating nature of shoulder dystocia, we specifically examined the risk of shoulder dystocia and the number of cesarean deliveries needed to prevent 1 shoulder dystocia at each percentile cutoff. Prior studies have been conflicting regarding the association of a prolonged first stage of labor and the risk of shoulder dystocia, possibly because of varying definitions of prolonged labor and shoulder dystocia.14–17 In one prospective study of labor management, a prolonged first stage of labor in nulliparas (defined as >2 hours with no cervical change despite adequate contractions) was associated with a 13-fold increase in the risk of shoulder dystocia.16
We noted that the incidence of shoulder dystocia increased as the first stage of labor increased beyond the 90th percentile. Depending on the percentile cutoff used, 29–30 cesarean deliveries for dystocia (95% CI, 12–1426) would need to be performed to prevent 1 shoulder dystocia. The number of cesarean deliveries needed to prevent brachial plexus injury will be significantly higher because only a small percentage of shoulder dystocia results in brachial plexus injury.17 This finding deserves further investigation in prospective studies.
Several prior studies have demonstrated that prolonged latent and active phases of labor are associated with adverse maternal and neonatal outcomes.18–21 However, these studies utilize the definitions for abnormal published by Friedman.1–3 Although his work was revolutionary for the time, a mounting body of literature suggests that these definitions need to be reevaluated using a contemporary patient population and more advanced analytical methods that can account for both repeated measures and differences in the timing between exams.
These more recent analyses have created the custom of presenting time to progress in labor as the median and 95th percentiles. Implicit in this custom is the suggestion that surpassing the 95th percentile is abnormal despite the fact that in these analyses all women reached 10 cm of dilation.
A more recent analysis by Cheng et al22 examined the length of the first stage of labor in nulliparous patients in spontaneous labor by percentile. The investigators also noted that the vast majority of women with a labor longer than the 95th percentile (defined as longer than 30 hours in their analysis) would ultimately delivery vaginally. In this study, longer labor was associated with an increased risk of chorioamnionitis but not with any adverse neonatal outcomes. One major difference between this study and our study is that Cheng et al included women who did not reach 10 cm of dilation; inclusion of women who did not reach the second stage may have falsely shortened the first stage, resulting in a misclassification bias.
Using ROC curves, we sought the most appropriate definition of the first stage of labor: time from admission, time from 4 cm, and time from 6 cm to complete dilation. Each was associated with the composite adverse outcomes with areas under the curve of 0.64–0.66. We then sought a threshold for defining normal vs abnormal labor based on adverse outcomes; however, no clear cutoff in which the risk of adverse outcomes was substantially increased was identified. Therefore, we then examined several cutoffs for defining abnormal: 90th percentile or greater, 95th percentile or greater, and 97th percentile or greater. As the length of labor increased, the risks of a prolonged second stage, maternal fever, shoulder dystocia, and admission to a level 2–3 nursery increased. However, lowering the definition of an abnormal, or protracted, first stage of labor from the 95th to the 90th percentile will likely result in an increased incidence of cesarean for arrest of dilation, which carries its own costs and maternal risks. However, because these percentile definitions have not been prospectively applied in comparison with traditional Friedman cutoffs of labor dystocia, it is difficult to determine the real-world impact on costs and outcomes.
The strength of this study lies in the detailed information available on labor progress as well as maternal and neonatal outcomes. This enabled us to examine a well-defined exposure (length of the first stage) in relationship to clinically relevant outcomes. Additionally, our large sample size enabled us to define the exposure specific to parity and whether labor was induced, both of which are very important factors influencing the length of the first stage.
Our study is not without limitations, the first of which was the rarity of some of the neonatal outcomes of interest, such as acidemia and base excess in term infants. Consequently, we had limited power to examine these outcomes individually as a function of length of the first stage. However, given that our institution routinely performs fetal heart rate monitoring on all patients in labor, we would not expect to find differences in these outcomes based solely on length of labor because physicians will typically intervene for nonreassuring fetal heart rate tracings. In addition, we may have had limited power to detect a difference in outcomes at the level of the 97th percentile because this is a rare exposure by definition.
Another limitation is that because of the rarity of the exposure, we are unable to analyze multiparous and nulliparous women separately or spontaneous vs induced labor separately. However, the exposures were defined specific to parity and labor type. Additionally, some of the outcomes we examined are surrogate markers. For example, prolonged second stage was evaluated because it is associated with adverse neonatal outcomes, such as shoulder dystocia and acidemia, but may not necessarily be harmful in and of itself.
Most importantly, as a retrospective study, we are inherently limited by residual confounding variables, such as differences in patient management. Only patients who reached the second stage were included in this study, and outcomes may have differed in subjects undergoing cesarean prior to a dilation of 10 cm. Therefore, although our retrospective study is hypothesis generating, it would be difficult to apply these findings prospectively to a patient population.
In summary, a clear cutoff was not obvious from the ROC curves; adverse maternal and neonatal outcomes appear to be related in a continuous fashion to length of labor without an obvious threshold in which adverse outcomes dramatically increase. A first stage of labor 90th percentile or greater, 95th percentile, and 97th percentile is associated with increasing risks of maternal fever, prolonged second stage, shoulder dystocia, and adverse neonatal outcomes; however, between these groups, the risks did not change dramatically, and the number of cesarean deliveries needed to prevent 1 event of shoulder dystocia is similar at each cutoff. In light of these findings, we recommend a prospective evaluation of labor management using contemporary definitions of the first stage of labor, examining in particular cutoffs of the 95th percentile or greater.
Acknowledgments
L.M.H. is supported by grant K12HD001258-13, Eunice Kennedy Shriver National Institute of Child Health and Human Development (principal investigator, William W. Andrews). A.G.C. is a Robert Wood Johnson Foundation Physician Faculty Scholar, which partially supports this work.
Footnotes
The authors report no conflict of interest.
Presented at the 33rd annual meeting of the Society for Maternal-Fetal Medicine, San Francisco, CA, Feb. 11–16, 2013.
Reprints not available from the authors.
References
- 1.Friedman E. The graphic analysis of labor. Am J Obstet Gynecol. 1954;68:1568–75. doi: 10.1016/0002-9378(54)90311-7. [DOI] [PubMed] [Google Scholar]
- 2.Friedman EA. Primigravid labor: a graphicostatistical analysis. Obstet Gynecol. 1955;6:567–89. doi: 10.1016/s0029-7844(02)02398-0. [DOI] [PubMed] [Google Scholar]
- 3.Friedman EA. Labor in multiparas; a graphicostatistical analysis. Obstet Gynecol. 1956;8:691–703. [PubMed] [Google Scholar]
- 4.American College of Obstetrics and Gynecology. Committee on Practice B-O. Dystocia and augmentation of labor. ACOG practice bulletin no. 49, December 2003. Obstet Gynecol. 2003;102:1445–54. doi: 10.1016/j.obstetgynecol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization partograph in management of labour. World Health Organization Maternal Health and Safe Motherhood Programme. Lancet. 1994;343:1399–404. [PubMed] [Google Scholar]
- 6.Graseck AS, Odibo AO, Tuuli M, Roehl KA, Macones GA, Cahill AG. Normal first stage of labor in women undergoing trial of labor after cesarean delivery. Obstet Gynecol. 2012;119:732–6. doi: 10.1097/AOG.0b013e31824c096c. [DOI] [PubMed] [Google Scholar]
- 7.Harper LM, Caughey AB, Odibo AO, Roehl KA, Zhao Q, Cahill AG. Normal progress of induced labor. Obstet Gynecol. 2012;119:1113–8. doi: 10.1097/AOG.0b013e318253d7aa. [DOI] [PubMed] [Google Scholar]
- 8.Norman SM, Tuuli MG, Odibo AO, Caughey AB, Roehl KA, Cahill AG. The effects of obesity on the first stage of labor. Obstet Gynecol. 2012;120:130–5. doi: 10.1097/AOG.0b013e318259589c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vahratian A, Zhang J, Troendle JF, Savitz DA, Siega-Riz AM. Maternal prepregnancy over-weight and obesity and the pattern of labor progression in term nulliparous women. Obstet Gynecol. 2004;104:943–51. doi: 10.1097/01.AOG.0000142713.53197.91. [DOI] [PubMed] [Google Scholar]
- 10.Vahratian A, Zhang J, Troendle JF, Sciscione AC, Hoffman MK. Labor progression and risk of cesarean delivery in electively induced nulliparas. Obstet Gynecol. 2005;105:698–704. doi: 10.1097/01.AOG.0000157436.68847.3b. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281–7. doi: 10.1097/AOG.0b013e3181fdef6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Troendle J, Mikolajczyk R, Sundaram R, Beaver J, Fraser W. The natural history of the normal first stage of labor. Obstet Gynecol. 2010;115:705–10. doi: 10.1097/AOG.0b013e3181d55925. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Troendle JF, Yancey MK. Reassessing the labor curve in nulliparous women. Am J Obstet Gynecol. 2002;187:824–8. doi: 10.1067/mob.2002.127142. [DOI] [PubMed] [Google Scholar]
- 14.Mehta SH, Bujold E, Blackwell SC, Sorokin Y, Sokol RJ. Is abnormal labor associated with shoulder dystocia in nulliparous women? Am J Obstet Gynecol. 2004;190:1604–7. doi: 10.1016/j.ajog.2004.03.067. discussion 1607–9. [DOI] [PubMed] [Google Scholar]
- 15.Ouzounian JG, Korst LM, Miller DA, Lee RH. Brachial plexus palsy and shoulder dystocia: obstetric risk factors remain elusive. Am J Perinatol. 2013;30:303–7. doi: 10.1055/s-0032-1324698. [DOI] [PubMed] [Google Scholar]
- 16.Rouse DJ, Owen J, Savage KG, Hauth JC. Active phase labor arrest: revisiting the 2-hour minimum. Obstet Gynecol. 2001;98:550–4. doi: 10.1016/s0029-7844(01)01516-2. [DOI] [PubMed] [Google Scholar]
- 17.Sokol RJ, Blackwell SC American College of Obstetricians and Gynecologists, Committee on Practice B-G. Should dystocia. ACOG practice bulletin no. 40, November 2002 (replaces practice pattern no. 7, October 1997) Int J Gynaecol Obstet. 2003;80:87–92. doi: 10.1016/s0020-7292(02)90001-9. [DOI] [PubMed] [Google Scholar]
- 18.Henry DE, Cheng YW, Shaffer BL, Kaimal AJ, Bianco K, Caughey AB. Perinatal outcomes in the setting of active phase arrest of labor. Obstet Gynecol. 2008;112:1109–15. doi: 10.1097/AOG.0b013e31818b46a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjaergaard H, Olsen J, Ottesen B, Dykes AK. Incidence and outcomes of dystocia in the active phase of labor in term nulliparous women with spontaneous labor onset. Acta Obstet Gynecol Scand. 2009;88:402–7. doi: 10.1080/00016340902811001. [DOI] [PubMed] [Google Scholar]
- 20.Selin L, Wallin G, Berg M. Dystocia in labour— risk factors, management and outcome: a retrospective observational study in a Swedish setting. Acta Obstet Gynecol Scand. 2008;87:216–21. doi: 10.1080/00016340701837744. [DOI] [PubMed] [Google Scholar]
- 21.Chelmow D, Kilpatrick SJ, Laros RK., Jr Maternal and neonatal outcomes after prolonged latent phase. Obstet Gynecol. 1993;81:486–91. [PubMed] [Google Scholar]
- 22.Cheng YW, Shaffer BL, Bryant AS, Caughey AB. Length of the first stage of labor and associated perinatal outcomes in nulliparous women. Obstet Gynecol. 2010;116 doi: 10.1097/AOG.0b013e3181f5eaf0. [DOI] [PubMed] [Google Scholar]