Abstract
BACKGROUND
Benign prostatic hyperplasia (BPH) is an age-related disease frequently associated with lower urinary tract symptoms (LUTS) that involves hyperplasia of both epithelial and stromal cells. Stromal fibrosis is a distinctive feature of BPH, but the exact mechanisms underlying this phenomenon are poorly understood.
METHODS
In the current study, proteomics analyses were utilized to identify proteins altered in the BPH stromal compartment from patients with symptomatic BPH. Stromal cells were isolated from histological nodules of BPH by laser capture microdissection (LCM) and subjected to liquid chromatography/mass spectrometry.
RESULTS
Proteins identified included several stromal-specific proteins involved in extracellular matrix remodeling, focal adhesion and cellular junctions. Additionally, the proteomics array identified the presence of luminal epithelial secretory protein PSA. Immunostaining, ELISA, and in situ hybridization analyses of BPH tissues verified the presence of PSA protein but absence of PSA mRNA in the stromal compartment. E-cadherin was down-regulated in BPH epithelial cells compared to normal adjacent tissues, suggesting that alteration of cellular junctions could contribute to the presence of luminal epithelial secreted proteins PSA and KLK2 in the stromal compartment.
CONCLUSIONS
The above findings suggest that the presence of secreted proteins PSA and KLK2 from prostate luminal epithelial cells in BPH stroma is a hallmark of BPH nodules which could in part be due to alterations in cellular junction proteins and/or increased epithelial barrier permeability. Elucidating the cause and consequence of these secreted proteins in the stromal compartment of BPH may lead to new understanding of BPH pathogenesis as well as approaches to prevent and/or treat this common disease.
INTRODUCTION
Benign prostatic hyperplasia (BPH) affects aging men and is often associated with lower urinary tract symptoms (LUTS) including frequent urination, weak stream and nocturia [1–3]. By the age of 60, the incidence of BPH in men is 50% and increases by 10% with each subsequent decade of life [4]. Although BPH is not life threatening, it constitutes a significant burden on the healthcare system with an estimated annual cost of more than $4 billion [5]. As the average life expectancy increases, it is expected that both the incidence and costs of BPH will increase even further. BPH is frequently treated with alpha blockers and/or 5α-reductase inhibitors and surgery is necessary when this treatment fails [3, 6–11]. New and more effective approaches to prevent and treat BPH are needed to reduce the suffering of patients as well as the cost to society.
BPH develops in the transitional zone of the prostate, and consists of hyperplastic nodules comprised primarily of stromal cells and to a lesser degree, epithelial cells. The mechanisms underlying BPH pathogenesis are poorly understood and it is likely that multiple factors are involved. Inflammation is one major factor associated with the development of BPH [12, 13]. Acute and chronic inflammation are implicated in BPH pathogenesis by the increased presence of inflammatory infiltrates and elevated cytokines and chemokines [12, 14, 15]. Chronic inflammation has been associated with the subsequent development of tissue fibrosis. Prostate stromal fibrosis is characterized by an increase in myofibroblasts, collagen deposition and extracellular matrix remodeling (reviewed in [16]). Additionally, altered androgen metabolism in aging men results in the accumulation of DHT and prostate enlargement [17]. The importance of androgens in BPH pathogenesis is illustrated by the observation that men with mutations that impair the activation of the androgen receptor (AR) or who were castrated before puberty do not develop BPH [3]. Inflammation, estradiol, non-androgenic testicular factors, and other elements could also play important roles in BPH pathogenesis [18–21].
We previously identified several androgen-responsive genes that were up-regulated in BPH, including prostate specific antigen (PSA) [22]. While abnormal up-regulation of androgen signaling in prostatic epithelial cells is likely to play an important role in BPH development and progression, BPH is also characterized by stromal growth. Thus, we examined the differential expression of proteins in BPH stromal cells compared to normal adjacent cells isolated from patient specimens. In addition to identifying a group of BPH-specific stromal proteins involved in extracellular matrix (ECM) remodeling, we also identified the presence of several luminal epithelial secreted proteins. PSA, which is regulated by androgens and secreted by prostatic luminal epithelial cells [23–25], was identified in the stromal cells of nodular BPH. These findings suggest that BPH is characterized by an altered microenvironment that could allow the leakage of epithelial secreted proteins into the stromal compartment.
MATERIALS AND METHODS
Human BPH Tissue Acquisition
Human BPH specimens for proteomics were obtained from prostate tissues resected for lower urinary tract symptoms (LUTS). Sections of typical BPH nodules, which consisted of both epithelial and stromal cells, along with normal adjacent tissues were derived from either simple prostatectomy for enlarged prostate with lower urinary tract symptoms. For PSA immunostaining, some specimens were from men undergoing radical prostatectomy for cancer with prostates weighing over 40 g. All tissues were acquired from the Health Sciences Tissue Bank at the University of Pittsburgh Medical Center under approval by the University of Pittsburgh Institutional Review Board and verified by a board certified genitourinary pathologist (AVP, M.D., Ph.D.).
Histologic criteria for a BPH nodule and normal adjacent stroma
BPH foci were present predominantly in the transitional zone of the prostate, and consisted of hyperplastic nodules comprising of both glandular and stromal proliferations. The benign hyperplasia foci were readily identifiable because they had a lobulated and “nodular” appearance. They were most often centered on prostatic urethra in the transition zone of the prostate. The hyperplasia was either epithelial predominant, stromal predominant or displayed mixed epithelial and stromal hyperplasia. Histological features of BPH included the presence of columnar epithelium that was lining the acini which were organized into papillary projections. Less frequently a cuboidal lining was present in the acinar units. Cytoplasm was pale to clear and nuclei had open type chromatin. Glands were medium to large-sized with some of them showing cystic dilation. Two cell layers (basal and columnar cells) were present. There was prominent hyperplasia of glandular and stromal tissue with papillary buds, infoldings and cystic spaces. Occasional foci displayed squamous metaplasia and infarction. The basal cell layer was continuous. In the foci with a stromal predominance, prominent stromal changes included the presence of increased smooth muscle. The stromal cells were bland with very little cytoplasm and contained plump ovoid nuclei with open chromatin. Some stromal rich foci were hypercellular with an abundance of small capillaries. Occasional foci demonstrated the presence of lymphocytes around prostatic ducts. The transition from a hyperplastic nodule to the adjacent stroma was distinct and easily identifiable at low microscopic power. In contrast to the hyperplastic nodules, the adjacent normal stroma was hypocellular with occasional spindle cells with bland cytological appearance.
Liquid Chromatography–Tandem Mass Spectrometry
Histological nodules of BPH stroma as well as normal adjacent stroma were laser capture microdissected (LCM) from paraffin embedded sections as described previously [22], with the exception of incubation in xylenes to deparaffinize before staining. The total microdissected area consisted of 6 × 106 µm2, which was determined by the Leica LMD 6000. LCM tissues were brought up to 100 mM NH4HCO3, pH 8.4, 20% acetonitrile and heated to 100 °C for 1 h, followed by 65 °C for 2 h. Protein was quantitated using the BCA assay (Pierce, Rockford, IL). Samples were digested with trypsin and incubated for 16 h at 37 °C. Peptide digests were de-salted using PepClean spin columns (ThermoFisher Scientific, Inc, San Jose, CA) according to manufacturer’s protocol, dried by vacuum centrifugation and reconstituted in 0.1% trifluoroacetic acid.
Peptide digests were analyzed in triplicate by nanoflow reversed-phase liquid chromatography (nRPLC) coupled online to a hybrid linear Iontrap-Orbitrap mass spectrometer (LTQ-Orbitrap, ThermoFisher Scientific, Inc., San Jose, CA), which was based on procedures described by Hood et al [26]. Separation of the peptide digests was performed using an integrated electrospray ionization (ESI)-fused silica capillary column (100 µm ID × 360 µm OD × 20 cm length) packed in-house with 5 µm 300 Å pore size C18-reversed-phase stationary phase (Jupiter, Phenomenex, Torrance, CA). Mobile phase flow was supplied by a nanoflow HPLC system (Ultimate 3000, Dionex, Corp., Sunnyvale, CA). After injection, the sample was loaded onto a pre-column cartridge (C18, 5 µm, 100 Å, 300 µm ID × 5 mm, LC Packings, Dionex Inc., CA) with 98% mobile phase A (0.1% formic acid in water) at a flow rate of 30 µL/min for 3 min using the loading pump and valve set to waste. After which, the nano pump was switched in line with the pre-column and peptides were eluted using a linear gradient of 2% to 40 % mobile phase B (0.1% formic acid in acetonitrile) over 115 min at a constant flow rate of 200 nL/min followed by a column wash consisting of 95% mobile phase B for 30 min at a constant flow rate of 400 nL/min. Full scan MS spectra were collected in the Orbitrap using full ion scan mode over the m/z range 375–1800 with a resolution of 60,000 at m/z 400. Thirteen most abundant molecular ions dynamically determined from the full MS scan were selected for sequencing by collision-induced dissociation (CID) in the ion trap using a normalized CID energy of 35%. Dynamic exclusion of 60 s was used for ions already selected for fragmentation to minimize redundancy. MS data was recorded for 120 min.
Tandem mass spectra were searched against the UniProt human proteome database (http://www.expasy.org) using SEQUEST (ThermoFisher Scientific, Inc.). Peptides were considered legitimately identified if they achieved specific charge state and proteolytic cleavage-dependent cross-correlation (Xcor) scores of 1.9 for [M + H]1+, 2.2 for [M + 2H]2+, and 3.5 for [M + 3H]3+, and a minimum delta correlation score (ΔCn) of 0.08. Differences in protein abundance between the samples were derived by spectral counting (SC) using only uniquely identified (e.g. “proteotypic”) peptide sequences.
Enzyme-linked immunosorbent assay (ELISA)
Histological nodules of BPH stroma as well as normal adjacent stroma were microdissected and collected into RIPA buffer as described previously [22]. Frozen tissues were utilized in order to avoid cross-linking of proteins during fixation. The area of tissue dissected for the ELISA was 6 × 106 µm2. Samples were assayed for PSA expression using the Human Prostate Specific Antigen (PSA) ELISA kit (Alpha Diagnostic International, San Antonio, TX) according to the manufacturer’s instructions. The reaction was run in duplicate and read on a SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA) at 450 nm and the mean plotted according to provided kit standards.
In situ hybridization
Visualization of RNA was performed using the QuantiGene® View RNA In Situ Hybridization kit according to manufacture instructions (Affymetrix, Santa Clara, CA). Briefly, FFPE prostate sections from the same patient specimens used for immunohistochemical analysis were deparaffinized using xylenes and rehydrated using graded alcohol washes. Specimens were incubated with probes designed and manufactured by Affymetrix to PSA and counterstained with DAP to view nuclei of cells. Incubation times were specific for human prostate tissue as specified by manufacturer’s protocol (QuantiGene 2.0 Assay, Affymetrix).
Rat model of prostatic inflammation
Prostatic inflammation in male Sprague-Dawley rats was induced by intraprostatic injection of 50 µl of 5% formalin or saline vehicle control as described [27]. A total of 14 rats were treated with formalin, and 9 rats were treated with saline. After 28 days, animals were euthanized and the prostates were frozen immediately in OCT for immunohistochemical analyses.
Immunohistochemistry
Immunohistochemical staining of patient tissues was performed on 5-µm sections of paraffin blocks. Briefly, sections were deparaffinized and rehydrated through a graded series of ethanol. Heat induced epitope retrieval was performed using 10 mmol/L of citrate buffer (pH 6), followed by rinsing in TBS buffer. Primary antibodies used were specific for PSA (1:20,000, N1517: Dako), PSA (1:500, ab49395, Abcam), KLK2 (1:250, SAB1406062, Sigma), αSMA (1:300, Cat. # CME305B: Biocare), E-cadherin (1:250, sc-7870, Santa Cruz), and AR (1:50, sc-816: Santa Cruz). Slides were then counterstained in hematoxylin and cover-slipped. Extent of immunostaining was determined according to the presence or absence of specific staining when compared to positive. PSA staining intensity in the stromal compartment was evaluated semi-quantitatively. The percentage of prostate stromal cells of a specific histological phenotype (normal and BPH) that expressed the antigen was estimated in three randomly selected fields at a final magnification of 40X. Staining intensity for PSA was evaluated by two parameters (staining intensity and percentage of cells exhibiting each level of intensity). Intensity of reaction product was based on a 4-point scale – none, faint/equivocal, moderate and intense. A staining score was calculated for each immunostain by cell type using the following formula: Score = 0(% no stain) + 1(% faint/equivocal) + 2(% moderate) + 3(% intense). All tissues were examined by a board certified genitourinary pathologist in a blinded fashion (AVP, M.D., Ph.D.).
Immunostaining of rat prostate tissues was performed on 8 µm frozen sections, fixed in 4% cold acetone and processed for immunohistochemistry. Primary antibody used was E-cadherin (1:250, sc-7870, Santa Cruz) followed by counterstaining with hematoxylin.
Sections were imaged using a Leica DM LB microscope (Leica Microsystems, Wetzlar, Germany) and imaging done with QCapture Suite (QImaging, Surrey, BC, Canada). Composite images were constructed with Photoshop CS (adobe Systems, San Jose, CA).
Statistics and Calculations
Comparison between normal and BPH spectral protein counts of LC/MS was performed using the Mann-Whitney rank-sum followed by Fisher’s exact test. The Student’s t-test was used to determine significance of the PSA ELISA and histological scores between BPH and normal tissue. GraphPad Prism version 5 was used in statistical calculations where mentioned as well as in the rendering of graphs (La Jolla, CA). Values are expressed as means +/− SEM. A p-value < 0.05 was considered significant.
RESULTS
Identification of altered proteins in nodular BPH stroma
To determine molecular changes associated with BPH stroma, LCM of stromal cells followed by mass spectrometry (LC-MS/MS) was performed on BPH and matched normal adjacent tissues from a cohort of 3 men with symptomatic BPH. All BPH and normal adjacent tissues were resected from the prostate transitional zone of patients naïve to medical or hormonal treatment. Differential expression of several proteins was identified in BPH vs. normal adjacent stroma, including 42 up-regulated proteins and 20 down-regulated proteins (Supplemental Table 1). As several proteins known to be preferentially expressed by luminal epithelial cells were identified (i.e., PSA, KRT8 and KRT18), differentially expressed proteins were screened against the previously determined cell-specific transcriptome profiles of normal human prostate cell types stromal, luminal epithelial, basal epithelial and endothelial [28]. The stromal-specific proteins identified as down-regulated in the BPH stroma included several proteins that play a role in cellular junctions: vinculin (VCL), filamin A, (FLNA) and filamin C (FLNC). Additionally, several proteins involved in ECM remodeling were identified, including up-regulated collagen type XV, alpha 1 (COL15A1), elastin microfibril interface 3 (EMILIN3); and down-regulated collagen type XII, alpha 1 (COL12A1), fibulin 5 (FBLN5), myosin, heavy chain 11 (MYH11), and tensin 1 (TNS1).
PSA protein in the stroma of nodular BPH
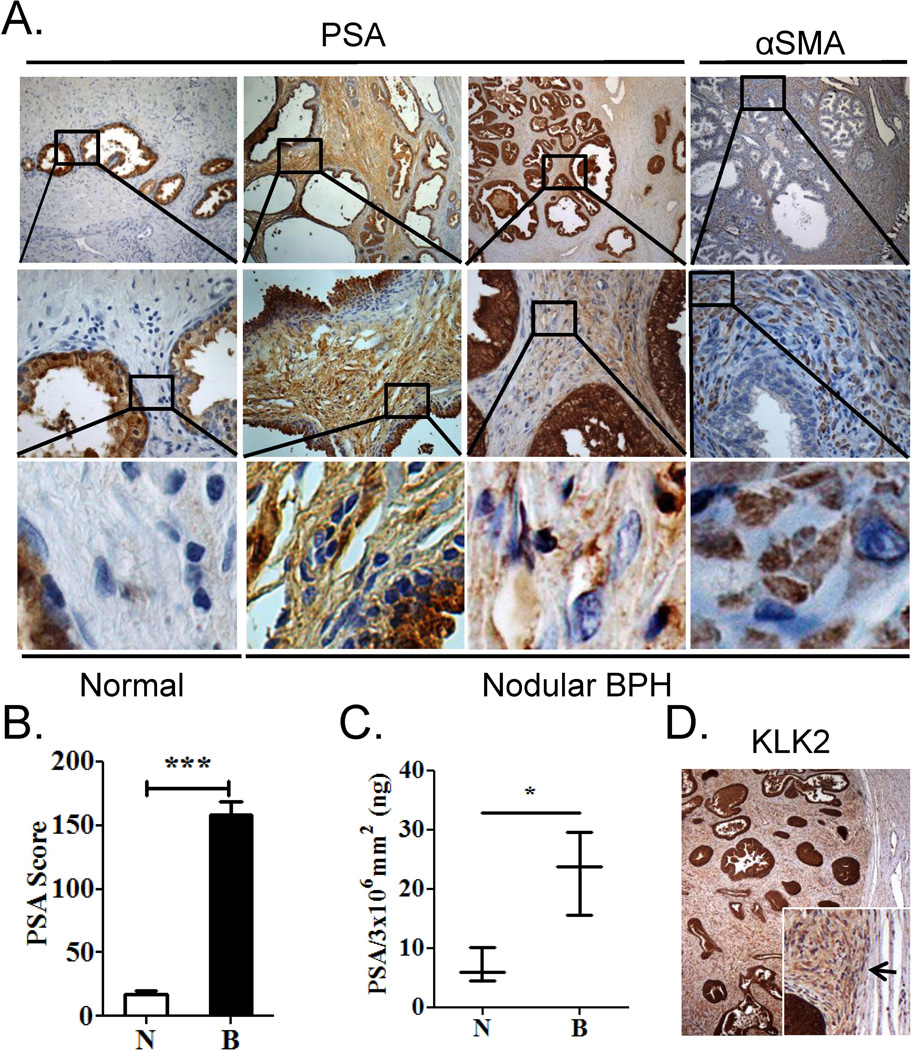
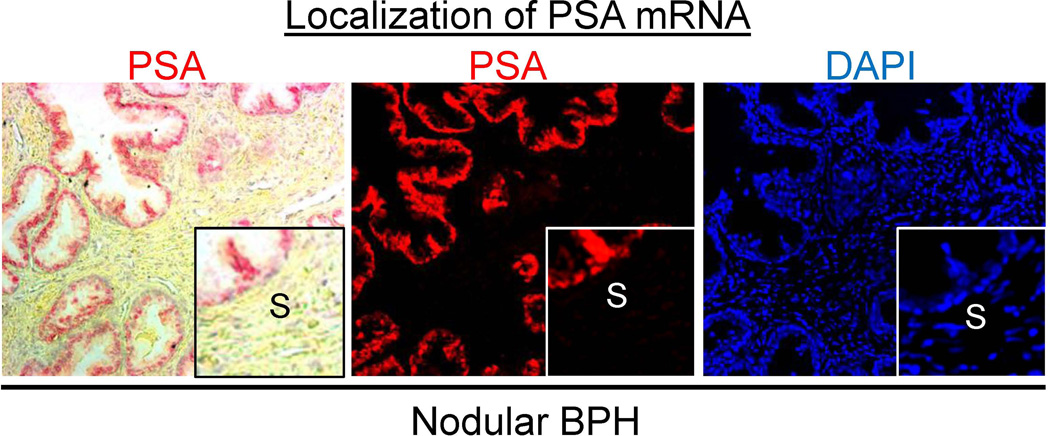
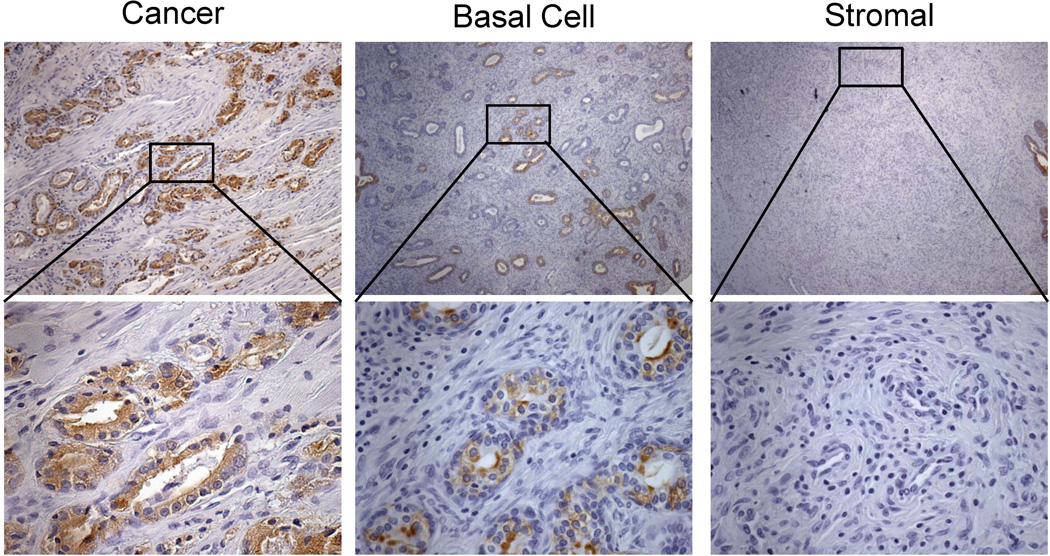
The proteomics array identified several proteins known to be secreted by luminal epithelial cells, including PSA (see Supplemental Table 1). PSA is a secreted protein expressed by luminal epithelial cells in the prostate and has never been reported to be expressed or secreted by prostate stromal cells. In immunohistochemical analyses, PSA protein was identified in the stromal compartment of BPH nodules, but not in normal adjacent stroma in 11 out of 11 patients examined (Fig. 2A). The IHC score for PSA was significantly higher (9.0-fold, p < 0.0001) in areas of BPH stroma compared to that of normal adjacent stroma (Fig. 2B). PSA protein was also detected by ELISA in BPH stroma isolated by LCM in 3 additional patients (p = 0.02) (Fig. 2C) suggesting that PSA protein identified by immunostaining was not due to antibody non-specificity. This finding of PSA protein in the stromal compartment of BPH was confirmed by Dr. William Ricke (University of Wisconsin, personal communication). Furthermore, luminal epithelial secreted protein kalikrein 2 (KLK2) was also identified in the BPH stroma and not in the adjacent normal stroma (Fig. 2D). As expected, PSA mRNA expression was confined to the luminal epithelial cells and was not detected in the stromal compartment of BPH by in situ hybridization (Fig. 3), confirming that the BPH stromal compartment was not expressing PSA mRNA. In order to verify that the presence of PSA protein in the stromal compartment was specific to BPH, PSA immunostaining was performed on several cancer patients. PSA protein expression in prostate cancer was confined to the cancer cells, in agreement with previous findings [29, 30] (Fig. 4).
Figure 2. Detection of PSA protein in the stroma of nodular BPH.
A. Immunostaining of serial sections of normal human prostate and treatment naïve BPH with PSA and stromal marker alpha smooth muscle actin (αSMA). Data are representative of 11 out of 11 (100%) patient specimens examined. B. Stromal PSA immunostaining score from representative BPH patient specimens (n=20). Pathology and scores were determined by a board certified genitourinary pathologist (AVP). C. ELISA for stromal PSA protein performed on LCM dissected stroma of BPH and normal adjacent tissue (n=3). Significance was determined by Student’s t-test (*** p, 0.0001, * p, 0.05). D. Stromal KLK2 immunostaining in BPH node. Inset – Arrow denotes edge of BPH node coincides with KLK2 staining in BPH stroma but not normal adjacent stroma.
Figure 3. PSA mRNA expression in nodular BPH.
In situ hybridization of PSA mRNA expression (in red) was confined to the luminal secretory epithelial cells in the prostate and was not apparent in the stromal (S) compartment (see DAPI nuclear staining in blue). Left panel is brightfield image depicting PSA in red; center panel is fluorescent image depicting PSA in red; right panel is DAPI nuclear staining in blue.
Figure 4. PSA protein expression in prostate cancer.
Immunostaining of serial sections of human prostate cancer, stromal hyperplasia, (negative for PSA n = 0/8) and basal cell hyperplasia (no PSA expression seen in the stromal areas within n= 0/5).
PSA is not expressed in stromal cells of stromal or basal cell hyperplasia nodules
The same human prostate sections used for stromal PSA staining in figure 2 were analyzed in regions of stromal hyperplasia and basal cell hyperplasia. Stromal nodular hyperplasia consists of large regions of new stromal cell growth within the transitional zone of the prostate. They are less common than mixed stromal-epithelial nodules but still occur in many men with enlarged prostates. Stromal nodules found on the same tissue sections with mixed nodules were analyzed for stromal PSA expression and PSA protein was absent all (0/8) nodules analyzed by IHC (Fig. 4). As well, basal cell hyperplastic nodules were found in 5 of the patients in our study. There was no PSA expression in the stromal areas.
Interestingly, we did detect spotted epithelial PSA expression in the glands within the basal cell hyperplasia but not all epithelial cells were PSA positive (Fig. 4). This observation is consistent with a recent finding that prostatic basal-to-luminal differentiation can occur and is induced by inflammation [31]
E-cadherin protein expression was down-regulated in nodules of BPH and in the rat model of prostatic inflammation
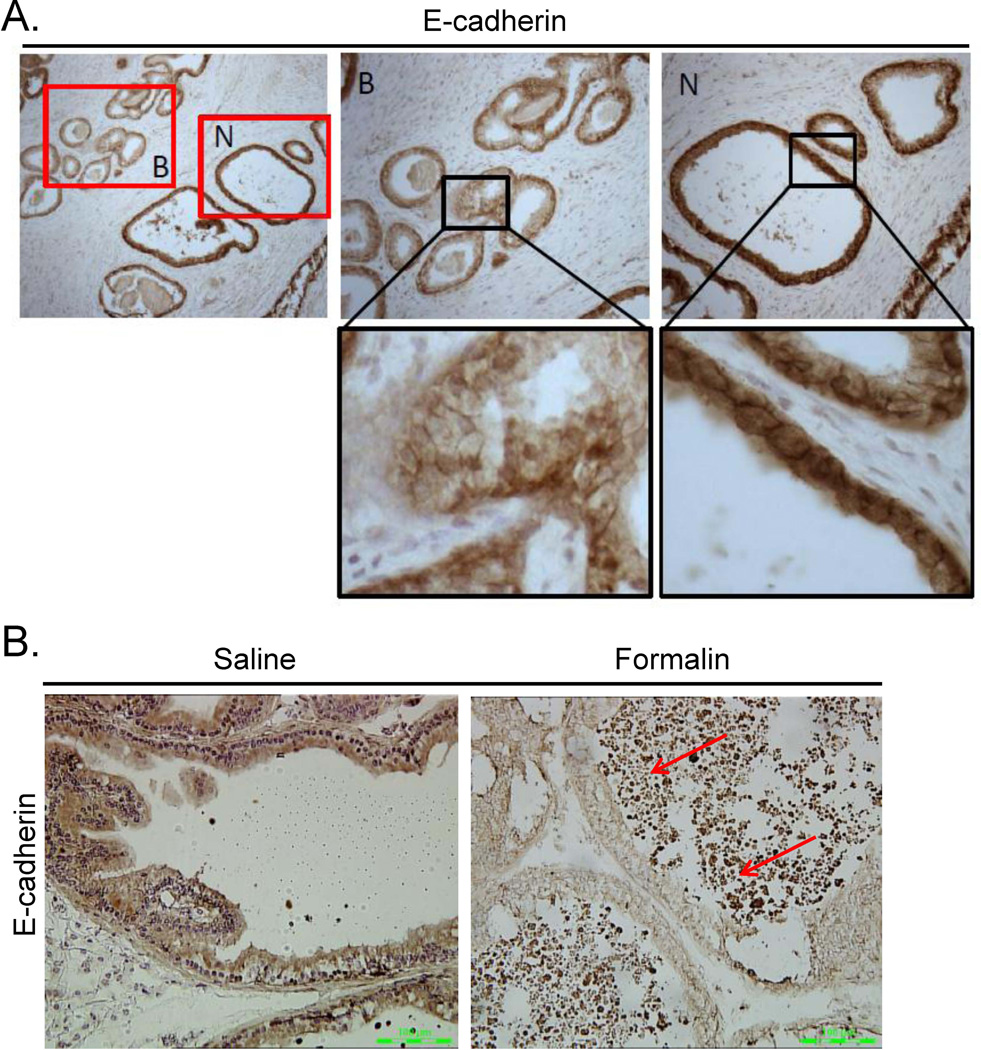
The finding of proteins known to be secreted by luminal epithelial cells in the stromal compartment of BPH nodules and the altered expression of several collagens and extracellular matrix proteins in BPH stromal cells suggested that disruption of the basement membrane or altered cellular junctions in BPH might allow PSA leakage into the stromal compartment. The expression of E-cadherin was decreased in epithelial cells within BPH nodules (Fig. 5A). Down-regulation of E-cadherin has been reported in BPH tissues previously [32]. Inflammation is often associated with BPH. Thus, we tested if inflammation could down-regulate E-cadherin in the rat prostatitis model. Decreased expression of E-cadherin was identified in formalin-induced rat prostate but not in saline treated animals (Fig. 5B).
Figure 5. Expression of E-cadherin in BPH and the rat model of prostatic inflammation.
A. Immunostaining of normal human prostate (N) and treatment naïve BPH (B) with E-cadherin. Data are representative of 11 out of 11 (100%) patient specimens examined. B. E-cadherin immunostaining in rat ventral prostate stimulated with saline (top panel) or formalin (bottom panel). Red arrows indicate areas of inflammation. Data are representative of at least 6 animals per group.
DISCUSSION
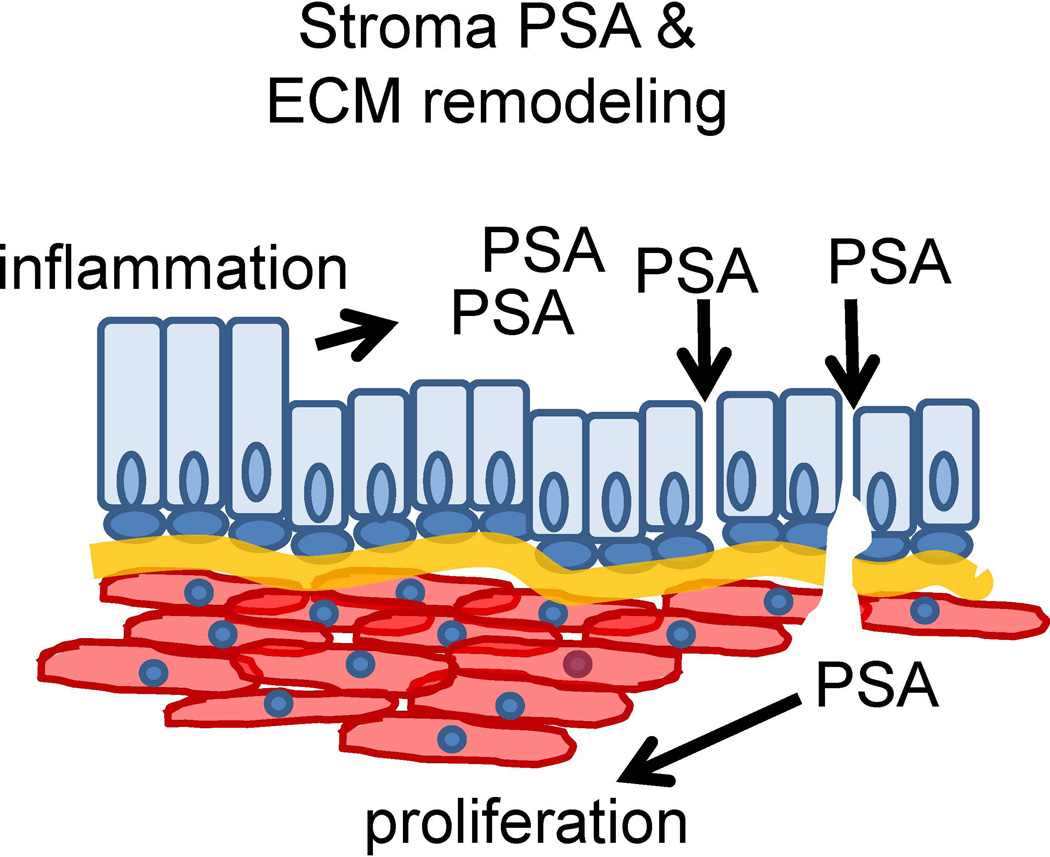
The current study identified a number of proteins exhibiting altered levels in BPH stromal cells compared to normal adjacent stromal cells. These proteins included several cellular junction, extracellular matrix, collagens and focal adhesion proteins: elastin microfibril interfacer 3, fibulin 5, filamin A, filamin C, and vinculin (see Supplemental Table 1). Furthermore, two luminal epithelial proteins PSA and KLK2 were identified within the stromal compartment of all BPH specimens analyzed (14/14). PSA, the most abundant protein in prostatic secretions, functions to enhance sperm motility through degradation of ECM proteins including fibronectin and laminin [33, 34]. PSA has also previously been shown to induce proliferation in prostate stromal fibromuscular cells [35], and its expression is increased in BPH epithelial cells [22]. Increased PSA levels could contribute to a decrease in epithelial barrier integrity and degradation of the basement membrane, ultimately resulting in the leakage of PSA into the stromal compartment and a subsequent stromal response. E-cadherin was also down-regulated in luminal epithelial cells of BPH patient tissues as well as in areas of inflammation in the rat prostatitis model, suggesting that inflammation could contribute to alteration of cellular junctions in BPH nodules (Figure 4). Altered epithelial cellular junctions and/or defects in basement membrane could permit the infiltration of PSA, KLK2 and other luminal epithelial secretory proteins into the BPH stroma (Fig. 6).
Figure 6. Schematic of stromal PSA and ECM remodeling in BPH.
Increased epithelial barrier permeability due to loss of cellular junctions in BPH could allow the infiltration of PSA into the stromal compartment.
Alterations in collagen deposition and extracellular matrix remodeling are indicative of tissue fibrosis in response to tissue inflammation. Collagens play a critical role in basement membrane regulation. EMILIN3 is an extracellular matrix protein expressed in the basement membrane and stroma that can function as an extracellular regulator of the activity of TGF-β ligands [36]. The TGF-β pathway is known to play a major role in fibrosis, and is frequently up-regulated in BPH [37]. Fibulins are extracellular matrix proteins that influence cellular adhesion and migration and the formation and function of elastic fibers; FBLN5 mutations cause cutis laxa, a connective tissue disorder characterized by a loss of elasticity in skin [38]. In the prostate, FBLN5 expression has been reported in the extracellular matrix [39]. FLNA and FLNC are actin filaments. FLNA is expressed by both epithelial and stromal cells in the prostate and loss of nuclear FLNA expression is associated with resistance to androgen deprivation therapy (ADT) in prostate cancer [40]. Overexpression of cytoplasmic FLNA has a tumor promoting effect, whereas nuclear FLNA acts to suppress tumor growth and inhibit metastasis [41]. FLNC hypermethylation has been associated with prediction of biochemical, local, and systemic recurrence of prostate cancer [42]. Vinculin (VCL) is a membrane-cytoskeletal protein associated with focal adhesion and adherens junctions. The head domain of vinculin associates to E-cadherin via α-, β-, and γ-catenins. The tail domain of vinculin binds to membrane lipids and to actin filaments, including FLNA and FLNC. The alterations in extracellular matrix, collagens and focal adhesion proteins compared to normal tissues in the same patient suggested significant changes in the stromal microenvironment of BPH.
The presence of epithelial secreted proteins PSA and KLK2 in the stromal compartment suggests that the integrity of the epithelial barrier separating the epithelial and stromal compartments in BPH might be compromised, allowing secreted proteins from the luminal epithelial cells to leak into the surrounding stroma. These secreted proteins could potentially induce a stromal reaction, such as the increased proliferation, inflammation and fibrosis characteristic of BPH nodules. PSA, the most abundant protein in prostatic secretion, has previously been shown to induce proliferation in prostate stromal fibromuscular cells [35] and is specifically expressed by prostate luminal epithelial cells [22, 24, 25, 43, 44]. Previously, we showed that androgen-responsive gene expression was upregulated in BPH epithelial cells when compared to normal adjacent glandular epithelial cells also in all BPH specimens analyzed [22]. The contribution of the increased luminal epithelial cell expression of androgen-responsive genes such as PSA and KLK2 to the presence of these proteins in the BPH stromal compartment remains to be elucidated. Additionally, PSA protein was identified in the stroma of hyperplastic epithelial nodules, but not in purely stromal BPH nodules or basal cell hyperplasia, suggesting that alternative mechanisms could be contributing the formation of BPH stromal nodules or basal cell hyperplastic nodules. Future studies will be required to determine if defects in the epithelial barrier contribute to proliferation and inflammation or are a consequence thereof. Elucidating the mechanism and effects of PSA protein in the stromal compartment of BPH could provide significant insight into BPH pathogenesis and potential targets for prevention and treatment.
Supplementary Material
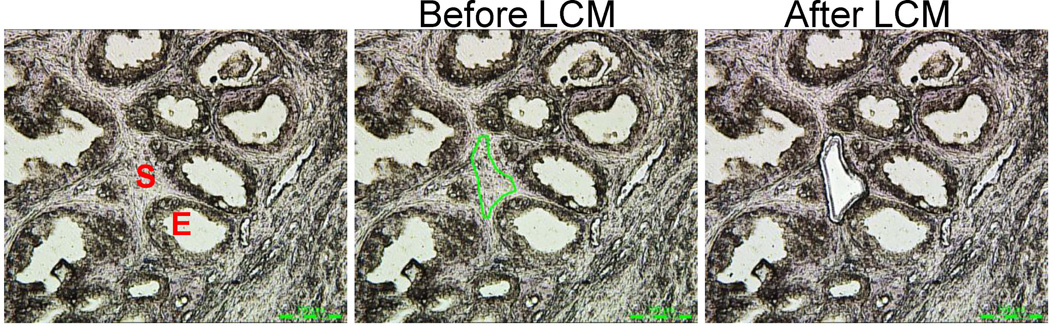
Figure 1. Laser capture microdissection of BPH and normal adjacent stroma.
Areas of prostate stroma (S) were isolated away from epithelial (E) areas in human prostate BPH and normal adjacent paraffin embedded tissues. Areas captured are outlined in green (center panel, Before LCM) and shown after microdissection (right panel, After LCM).
ACKNOWLEDGEMENTS
We would like to thank the members of the Wang lab for their insightful discussions throughout this study. We thank Dr. William Ricke for helpful discussion and confirmation of stromal PSA immunostaining. This research was supported in part by NIH grants R01 DK088793, P20 DK090919, R37 DK51193, and T32 DK007774. This project used the UPCI Animal Facility and Tissue and Research Pathology Services (TARPS) and Cancer Biomarkers Facility/Mass Spectrometry Platform Laboratory and was supported in part by award P30 CA047904.
REFERENCES
- 1.Grayhack JT. Benign prostatic hyperplasia. The scope of the problem. Cancer. 1992;70(1 Suppl):275–279. doi: 10.1002/1097-0142(19920701)70:1+<275::aid-cncr2820701314>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P. New insights into the epidemiology and natural history of benign prostatic hyperplasia. Prog Clin Biol Res. 1994;386:3–18. [PubMed] [Google Scholar]
- 3.Kirby RS. The natural history of benign prostatic hyperplasia: what have we learned in the last decade? Urology. 2000;56(5 Suppl 1):3–6. doi: 10.1016/s0090-4295(00)00747-0. [DOI] [PubMed] [Google Scholar]
- 4.Berry S, et al. The development of human benign prostatic hyperplasia with age. Journal of Urology. 1984;132(3):474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 5.Kortt MA, Bootman JL. The economics of benign prostatic hyperplasia treatment: a literature review. Clin Ther. 1996;18(6):1227–1241. doi: 10.1016/s0149-2918(96)80078-6. [DOI] [PubMed] [Google Scholar]
- 6.Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6 Suppl 9:S31–S39. [PMC free article] [PubMed] [Google Scholar]
- 7.Tempany CM, et al. The influence of finasteride on the volume of the peripheral and periurethral zones of the prostate in men with benign prostatic hyperplasia. Prostate. 1993;22(1):39–42. doi: 10.1002/pros.2990220106. [DOI] [PubMed] [Google Scholar]
- 8.Roehrborn CG. The Clinical Benefits of Dutasteride Treatment for LUTS and BPH. Rev Urol. 2004;6 Suppl 9:S22–S30. [PMC free article] [PubMed] [Google Scholar]
- 9.O'Leary MP, Roehrborn CG, Black L. Dutasteride significantly improves quality of life measures in patients with enlarged prostate. Prostate Cancer Prostatic Dis. 2007 doi: 10.1038/sj.pcan.4500990. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano F. Impact of medical treatments for benign prostatic hyperplasia on sexual function. BJU Int. 2006;97 Suppl 2:34–38. doi: 10.1111/j.1464-410X.2006.06104.x. discussion 44-5. [DOI] [PubMed] [Google Scholar]
- 11.Jonler M, et al. Benign prostatic hyperplasia. Endocrinol Metab Clin North Am. 1994;23(4):795–807. [PubMed] [Google Scholar]
- 12.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51(5):1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Sciarra A, et al. Inflammation and chronic prostatic diseases: evidence for a link? Eur Urol. 2007;52(4):964–972. doi: 10.1016/j.eururo.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Fibbi B, et al. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010;33(3):475–488. doi: 10.1111/j.1365-2605.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 15.Penna G, et al. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol. 2009;182(7):4056–4064. doi: 10.4049/jimmunol.0801875. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol. 2013;10(9):546–550. doi: 10.1038/nrurol.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths K, et al. Steroid hormones and the pathogenesis of benign prostatic hyperplasia. European Urology. 1991;20 Suppl 1:68–77. doi: 10.1159/000471750. [Review]. [DOI] [PubMed] [Google Scholar]
- 18.Nickel JC, et al. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84(9):976–981. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 19.Juniewicz PE, et al. The requirement of the testis in establishing the sensitivity of the canine prostate to develop benign prostatic hyperplasia. J Urol. 1994;152(3):996–1001. doi: 10.1016/s0022-5347(17)32641-1. [DOI] [PubMed] [Google Scholar]
- 20.Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73(3):233–244. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grayhack JT, Kozlowski JM, Lee C. The pathogenesis of benign prostatic hyperplasia: a proposed hypothesis and critical evaluation. J Urol. 1998;160(6 Pt 2):2375–2380. doi: 10.1097/00005392-199812020-00003. [DOI] [PubMed] [Google Scholar]
- 22.O'Malley KJ, et al. The expression of androgen-responsive genes is up-regulated in the epithelia of benign prostatic hyperplasia. Prostate. 2009;69(16):1716–1723. doi: 10.1002/pros.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gleave ME, et al. Serum prostate specific antigen levels in mice bearing human prostate LNCaP tumors are determined by tumor volume and endocrine and growth factors. Cancer Research. 1992;52(6):1598–1605. [PubMed] [Google Scholar]
- 24.Lee C, et al. Regulation of proliferation and production of prostate-specific antigen in androgen-sensitive prostatic cancer cells, LNCaP, by dihydrotestosterone. Endocrinology. 1995;136(2):796–803. doi: 10.1210/endo.136.2.7530653. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery B, et al. Hormonal regulation of prostate-specific antigen (PSA) glycoprotein in the human prostatic adenocarcinoma cell line, LNCaP. Prostate. 1992;21(1):63–73. doi: 10.1002/pros.2990210107. [DOI] [PubMed] [Google Scholar]
- 26.Hood BL, et al. Proteomic analysis of formalin-fixed prostate cancer tissue. Mol Cell Proteomics. 2005;4(11):1741–1753. doi: 10.1074/mcp.M500102-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Funahashi Y, et al. Upregulation of androgen-responsive genes and transforming growth factor-beta1 cascade genes in a rat model of non-bacterial prostatic inflammation. Prostate. 2014;74(4):337–345. doi: 10.1002/pros.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oudes AJ, et al. Transcriptomes of human prostate cells. BMC Genomics. 2006;7:92. doi: 10.1186/1471-2164-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsurusaki T, et al. Expression profile of prostate-specific antigen messenger RNA assessed by in situ hybridization is a novel prognostic marker for patients with untreated prostate cancer. Clin Cancer Res. 1998;4(9):2187–2194. [PubMed] [Google Scholar]
- 30.Yin M, Dhir R, Parwani AV. Diagnostic utility of p501s (prostein) in comparison to prostate specific antigen (PSA) for the detection of metastatic prostatic adenocarcinoma. Diagn Pathol. 2007;2:41. doi: 10.1186/1746-1596-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon OJ, et al. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc Natl Acad Sci U S A. 2014;111(5):E592–E600. doi: 10.1073/pnas.1318157111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alonso-Magdalena P, et al. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc Natl Acad Sci U S A. 2009;106(8):2859–2863. doi: 10.1073/pnas.0812666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webber MM, Waghray A, Bello D. Prostate-specific antigen, a serine protease, facilitates human prostate cancer cell invasion. Clin Cancer Res. 1995;1(10):1089–1094. [PubMed] [Google Scholar]
- 34.Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest. 1985;76(5):1899–1903. doi: 10.1172/JCI112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutkowski DM, et al. Growth regulation of prostatic stromal cells by prostate-specific antigen. J Natl Cancer Inst. 1999;91(19):1663–1669. doi: 10.1093/jnci/91.19.1663. [DOI] [PubMed] [Google Scholar]
- 36.Schiavinato A, et al. EMILIN-3, peculiar member of elastin microfibril interface-located protein (EMILIN) family, has distinct expression pattern, forms oligomeric assemblies, and serves as transforming growth factor beta (TGF-beta) antagonist. J Biol Chem. 2012;287(14):11498–11515. doi: 10.1074/jbc.M111.303578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ao M, et al. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67(9):4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 38.Loeys B, et al. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet. 2002;11(18):2113–2118. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- 39.Wlazlinski A, et al. Downregulation of several fibulin genes in prostate cancer. Prostate. 2007;67(16):1770–1780. doi: 10.1002/pros.20667. [DOI] [PubMed] [Google Scholar]
- 40.Bedolla RG, et al. Nuclear versus cytoplasmic localization of filamin A in prostate cancer: immunohistochemical correlation with metastases. Clin Cancer Res. 2009;15(3):788–796. doi: 10.1158/1078-0432.CCR-08-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savoy RM, Ghosh PM. The dual role of filamin A in cancer: can't live with (too much of) it, can't live without it. Endocr Relat Cancer. 2013;20(6):R341–R356. doi: 10.1530/ERC-13-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanaja DK, et al. Hypermethylation of genes for diagnosis and risk stratification of prostate cancer. Cancer Invest. 2009;27(5):549–560. doi: 10.1080/07357900802620794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, et al. Genes regulated by androgen in the rat ventral prostate. Proc. Natl. Acad. Sci. USA. 1997;94:12999–13004. doi: 10.1073/pnas.94.24.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu N, et al. Calreticulin: an intracellular Ca++-binding protein abundantly expressed and regulated by androgen in prostatic epithelial cells. Endocrinology. 1998;139:4337–4344. doi: 10.1210/endo.139.10.6242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.