Abstract
Objective
Perimenopausal and postmenopausal women frequently report midlife onset of impairments of attention, organization, and short-term memory. We sought to determine whether these cognitive symptoms in healthy women in the menopause transition without a history of attention-deficit/hyperactivity disorder (ADHD) would respond to treatment with atomoxetine (ATX), a medication demonstrated to be effective in reducing similar cognitive impairments in adults with ADHD.
Methods
Sixteen healthy women with complaints of midlife-onset subjective difficulties in memory and concentration/attention and without a history of ADHD or other psychiatric disorders were enrolled in a double-blind, placebo-controlled crossover study of ATX 80 mg/day. Treatment arms were 6 weeks long, separated by a 4-week washout. The Brown Attention Deficit Disorder Scale (BADDS) was used to systematically elicit self-report of perceived cognitive difficulties in executive function. Participants also underwent neuropsychological testing, behavioral assessments, and vital signs monitoring.
Results
Mean baseline BADDS scores were 37.9 for all 16 participants and 42.3 for the 12 who completed both arms of the study. Total BADDS scores decreased significantly from baseline during ATX treatment but not placebo treatment. ATX treatment was superior to placebo in reducing the BADDS working memory cluster score, whereas there was a trend for ATX superiority for the BADDS attention/concentration cluster score. ATX did not differ from placebo with respect to effects on neuropsychological tests, behavioral assessments, or cardiac vital signs.
Conclusions
Perimenopausal and postmenopausal women presenting with midlife-onset subjective cognitive difficulties may experience significant subjective improvement in memory and attention/concentration with ATX treatment.
Keywords: Atomoxetine, Menopause, Memory, Concentration, Aging
In clinical practice, midlife onset of decline in cognitive functions, particularly short-term memory, sustaining attention, and activating/organizing for work, is a frequent complaint of perimenopausal and postmenopausal-aged women. Mitchell and Woods1 reported that 60% of their sample of women transitioning through menopause reported increased memory problems; others have reported similar concerns.2,3 Such impairments occurring for the first time during midlife can cause affected women difficulties in their professional and personal lives; they can also cause unwarranted fears of early-onset dementia.
However, despite the high frequency of such clinical complaints, research on links between menopause and cognitive impairments has yielded contradictory results. Several studies found no differences cross-sectionally among premenopausal, perimenopausal, and postmenopausal women in their performance on cognitive tests.4-6 In contrast, others have reported significant differences among these groups on one or more tests of cognitive performance.7,8 Luetters et al6 speculated that such contradictory findings may be due to variation in the menopausal stages compared, inadequate assessment of covariates, and/or use of a wide variety of cognitive tests, each of which taps discrete cognitive functions.
This study assessed perimenopausal and postmenopausal women who reported midlife onset of impairments of concentration/attention, memory, and work organization. For our primary measure, we used a normed, self-report rating scale used to assess cognitive impairments of attention-deficit/ hyperactivity disorder (ADHD) in adults, which has been demonstrated to be effective in capturing similar cognitive impairments in adults with ADHD. In addition to this primary measure, this study also used a number of neuropsychological tests used in previous studies to assess cognition in postmenopausal women.
It has been proposed that a menopause-related decline in estrogen input to the prefrontal cortex9,10 may reduce the effectiveness of neurotransmitters implicated in attention, arousal regulation, organization, and working memory, aspects of cognition often referred to as executive functions.11-14 Although there is a wealth of preclinical evidence demonstrating the neuroprotective and cognitive enhancing role of estradiol,15-18 data from clinical populations have been conflicting and thus difficult to interpret.19-22 Moreover, even if estrogen therapy (ET) were unambiguously helpful in improving menopausal-onset cognitive difficulties, a substantial proportion of perimenopausal and postmenopausal women are wary of ET and/or have medical contraindications that limit their ability to use hormone therapy (HT).22 Thus, an alternative to HT for these midlife-onset cognitive concerns could be useful.
Atomoxetine (ATX; Strattera, Eli Lilly & Company, Indianapolis, IN), a selective norepinephrine reuptake inhibitor,23,24 has demonstrated efficacy in the treatment of ADHD symptoms, including cognitive impairments in children and adults.25-27 Acute and chronic administration of ATX increases both extracellular norepinephrine and dopamine levels in the prefrontal cortex, but not the striatum.23,28 We examined the effects of ATX on subjective reports of executive function and cognitive performance on neuropsychological tests in perimenopausal and postmenopausal women who had no history of ADHD but were distressed by what they perceived as deterioration in their short-term memory, organizational skills, and ability to sustain attention to tasks after onset of the menopausal transition.
METHODS
Participants
Perimenopausal and postmenopausal women were recruited by fliers, paid advertising, and word of mouth to the Yale Program for Women's Reproductive Behavioral Health. Recruitment material indicated that women between the ages of 45 and 60 years who were “worried about their memory and concentration” may be eligible to participate in a “nonhormonal medication study of cognitive difficulties in menopausal women.” Cognitive symptoms had to represent a subjective change from the woman's previous norm and coincide with her transition to menopause. All participants were English speaking and signed a written informed consent form for this study, which was approved by the Yale University School of Medicine Human Investigations Committee. Women whose last menstrual period (LMP) was less than 12 months were required to have menstrual irregularity for at least 6 months, a cycle duration of 21 or fewer days or 35 or more days, and a serum follicle-stimulating hormone (FSH) level of greater than 20 IU/L. Otherwise, all women had FSH levels greater than 35 IU/L. Women who reported ET, HT, or psychotropic medication use within the previous 12 months were excluded.
Participants underwent physical examination and electrocardiogram. Women with hypertension, cardiac disease, electrocardiographic abnormalities, neurological abnormalities, or serious medical illness were excluded. A Mini Mental Status Examination score of 26 or lower, a Wechsler Abbreviated Scale of Intelligence score of 80 or lower, and a psychiatric or substance abuse disorder within the previous year, as assessed with the Structured Clinical Interview for Diagnosis, Non-patient Version,29 were grounds for exclusion. The Kiddie Schedule for Affective Disorders and Schizophrenia30 module for ADHD, a structured interview segment often used to assess history of ADHD in adults,31 was administered. Participants with a history of ADHD were excluded so we could assess midlife onset of these cognitive impairments in women with no previous history of such cognitive problems.
Cognitive symptom assessment
The Brown Attention Deficit Disorder Scale (BADDS) for Adults, our primary measure of cognitive impairments, is a normed and validated measure of ADHD-related executive function impairments,32 many of which are similar to the cognitive problems reported by perimenopausal and postmenopausal women. Comparative validity and reliability and utility of the BADDS were tested and reported by Kooij et al.33 The BADDS has been found to be effective in assessing ADHD in adults and has been used successfully in several clinical trials of various medications for ADHD.34-36 This 40-item measure elicits self-report data on the frequency and severity of five clusters of executive functions often impaired in ADHD. Individuals are asked to respond to each question using a scale of 0 to 3, with anchors being “no problem or never” and “kind of a big problem or almost every day.” The five clusters include the following:
1. Organizing and Activating for Work: nine items tap for excessive difficulties in getting organized and getting started on work-related tasks or undue problems in self-activating for daily routines.
2. Sustaining Attention and Concentration: nine items query for chronic problems in sustaining attention to work-related tasks (eg, excessive daydreaming or distractibility when listening or when doing required reading) or repeatedly losing track and needing to re-read assigned material.
3. Sustaining Alertness, Effort, and Processing Speed: nine items assess problems in keeping up consistent alertness and effort for work-related tasks, daytime drowsiness, slow processing of information, or inadequate task completion.
4. Managing Affective Interference: seven items assess difficulties with mood and sensitivity to criticism, apparent lack of motivation, excessive frustration, or discouragement.
5. Using Working Memory and Accessing Recall: six items inquire about forgetfulness in daily tasks and routines, problems in recall of learned material, and losing track of needed items.
The BADDS was administered by a member of the research staff at baseline and upon completion of each treatment arm. Staff members were blind to the status of participants throughout the data collection process. The reference period for the BADDS was the past month. Total BADDS scores were reported as total raw scores (range, 0-120). Raw scores for clusters 1, 2, and 3 range from 0 to 27; for cluster 4, the range is 0 to 21; and for cluster 5, the range is 0 to 18. Raw scores for the BADDS clusters are converted to t scores, which range from 50 to 99. The mean (SD) total raw score in the nonclinical standardization sample for the BADDS was 30.9 (15.8), whereas the mean total raw score for the clinical ADHD sample was 77.9 (16.3).32
Neuropsychological testing
Cognitive performance was also assessed with a battery of neuropsychological tests administered at baseline and after each phase of treatment; we used three different versions of each task to minimize the effects of practice on performance. Verbal learning and memory were assessed with the Verbal Paired Associates Task, whereas the Digit Symbol Coding and Symbol Search Tasks from the Wechsler Adult Intelligence Scale-III37 gave measures of cognitive processing speed and attention/focus. Verbal working memory was tested using the Letter-Number Sequencing subtest from the Wechsler Memory Scale Revised.38 The Controlled Oral Word Association Test provided a measure of verbal fluency. These tasks were chosen because they probe for domains of possible cognitive impairments, which have been frequently studied in adults with ADHD39 and in postmenopausal women in various hormonal states.6,40-44
Behavioral assessments
Depression and anxiety symptoms are common in perimenopausal women, particularly those in the early and mid menopausal transition periods.45 Although women with a current psychiatric diagnosis were excluded, we were interested in the potential effects of ATX administration on subclinical mood symptoms, as depression and anxiety are known to have adverse effects on cognitive performance.46 Women completed the Beck Depression Inventory,47 the Beck Anxiety Inventory,48 and the Profile of Mood States (POMS)49 at baseline and then again after ATX and placebo treatments.
Randomization and medication treatment
Upon completion of baseline assessments, participants were randomized in a counterbalanced, double-blind fashion to the ATX or the placebo group. After 6 weeks, all participants were withdrawn over the course of 4 days from medication treatment and remained drug-free for 4 weeks before being crossed over to the other treatment condition for 6 weeks. During the ATX phase of the study, participants were started on 40 mg/day for the first week and then increased to 80 mg/day. A similar change occurred in the placebo group in that they were taking one look-alike capsule the first week and two capsules the second week. Weight, blood pressure, and heart rate were measured at baseline and at the end of each treatment phase.
Statistical analysis
The effects of ATX on behavioral measures, neuro-psychological tests, blood pressure, heart rate, and weight were evaluated using the nonparametric method for repeated measures by Brunner et al,50 where the data were first ranked and then fitted using a mixed-effects model with an unstructured variance–covariance matrix and P values were adjusted for analysis of varianceYtype statistics (ATS). The non-parametric approach was chosen based on the small sample size and deviations from normality. Models included treatment (baseline, placebo, and ATX) as a within-subjects explanatory variable and random subject effects. Potential order effects were also tested but were not significant. The analyses were performed on an intent-to-treat basis for the 14 participants who were randomized and had at least one postrandomization visit where behavioral measures were assessed. We further explored the impact of treatment on the five BADDS clusters in the sample of study completers (n = 12), using the same models described previously. Data were analyzed using SAS, version 9.1.
RESULTS
Participants
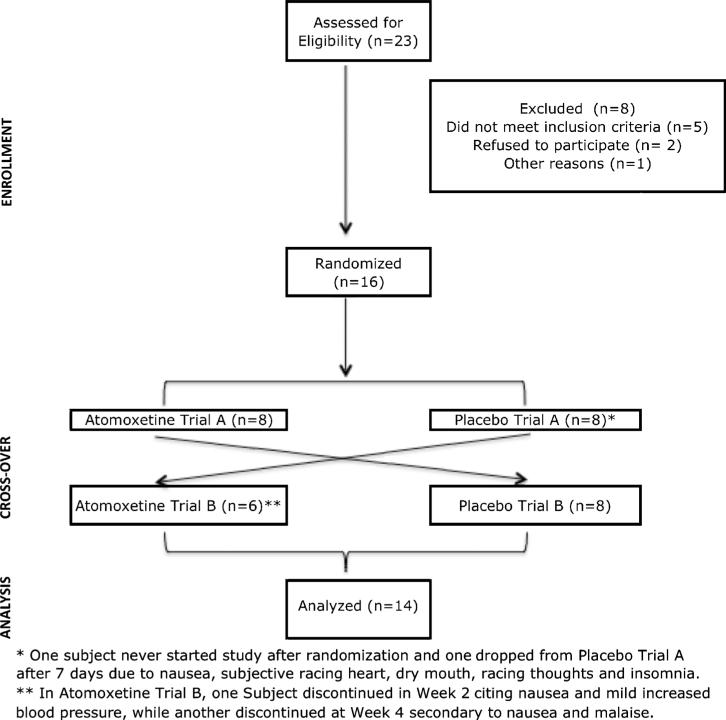
Of 23 women who appeared, through telephone screen, to meet the study criteria, 16 proceeded to randomization, and 14 had data from at least one postrandomization visit (Fig. 1). None were excluded on the basis of meeting criteria for ADHD. Participant characteristics, including mean age, years of education, number of months since LMP, menopause status, and baseline FSH and estradiol levels, are depicted in Table 1. One 58-year-old woman had a partial hysterectomy at the age of 38 years; therefore, LMP could not be used to confirm menopause status. FSH and estradiol level indicated that she was postmenopausal. All participants were white, except for one African American participant.
FIG. 1.
Study participant flow.
TABLE 1.
Participant characteristics
| Mean (SD) | Range | |
|---|---|---|
| Age, y | 54.0 (2.8) | 50-58 |
| Education, y | 16.4 (3.2) | 12-20 |
| LMP, mo | 29.3 (20.5) | 2-60 |
| FSH, IU/L | 76.0 (33.8) | 24-163 |
| Estradiol, pg/mL | 31.9 (32.9) | <20-128a |
| WASI-Full Scale IQ | 115.1 (14.3) | 94-141 |
| BADDS baseline | 38.6 (20.2) | 8-71 |
| n (%) | ||
| Perimenopausal | 4/14 (28.6) | |
| Postmenopausal | 10/14 (71.4) | |
| Previous OCP use | 11/14 (78.6) | |
| Previous HT use | 4/14 (28.6) |
LMP, last menstrual period; FSH, follicle-stimulating hormone; WASI, Wechsler Abbreviated Scale of Intelligence; BADDS, Brown Attention Deficit Disorder Scale; OCP, oral contraceptive pill; HT, hormone therapy.
Estradiol levels <20 pg/ml were converted to 19 pg/ml to compute mean circumference.
Self-reported cognitive symptoms
Although exclusion criteria ensured that all participants were without any history of ADHD and any current history of other psychiatric diagnoses, baseline mean ± SD BADDS scores were 37.9 ± 20.1 for all 16 enrolled women and 42.3 ± 19.1 for the 12 completers. Scores ranged from 8 to 71, with more than half of women reporting symptom severity above the normed mean of 31. Of the 12 study completers, 3 had baseline scores greater than 50, indicating a degree of impairment in executive function similar to that in adults with ADHD. Baseline BADDS scores were 24, 24, 8, and 43 in the four women who did not complete the entire study.
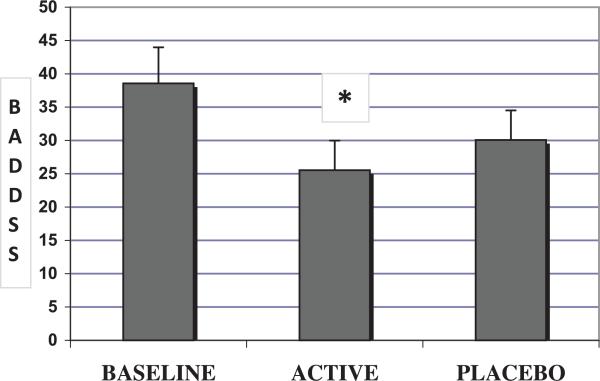
With respect to total BADDS scores (mean ± SD), there was a significant treatment effect (numerator degrees of freedom [num df ] = 2, ATS = 3.52, P = 0.03), demonstrating that ATX treatment significantly reduced BADDS scores from a baseline mean of 38.6 (±20.2) to 25.5 (±16.0; num df = 1, ATS = 6.8, P = 0.009). In our study, BADDS scores decreased to 30.1 (+/− 16.0) in the placebo treatment arm; this change from baseline was not statistically significant (num df = 1, ATS = 1.67, P = 0.20) (Fig. 2).
FIG. 2.
Mean total Brown Attention Deficit Disorder Scale (BADDS) scores at baseline, and at the end of each treatment arm for the intent to treat group (n=14). Line bars represent standard errors. *Mean BADDS scores were significantly reduced from baseline at the end of atomoxetine treatment (num df = 1, ATS = 6.8, P = 0.009).
At baseline, there were three clusters of ADHD-related cognitive symptoms, as measured using the BADDS, for which participants reported the greatest level of impairment. These were cluster 1, Organizing and Activating for Work; cluster 2, Sustaining Attention and Concentration; and cluster 5, Using Working Memory and Accessing Recall. Nonparametric tests for repeated measures revealed a significant group effect (num df = 1.74, ATS = 9.7, P = 0.001) for the working memory cluster, with women reporting improved memory with ATX treatment compared with both baseline (num df = 1, ATS = 23.5, P < 0.0001) and placebo (num df = 1, ATS = 11.0, P = 0.0009). There was a trend (num df = 1.7, ATS = 2.88, P = 0.06) for a treatment effect on the attention/concentration cluster, with ATX significantly reducing symptoms from baseline (num df = 1, ATS = 9.88, P = 0.002). There was no significant improvement in attention/concentration during placebo treatment (num df = 1, ATS = 1.17, P = 0.28). Women scored lower on the affective interference cluster during both ATX (num df = 1, ATS = 10.4, P = 0.001) and placebo (num df = 1, ATS = 10.1, P = 0.002) treatments compared with baseline. Results are displayed in Table 2.
TABLE 2.
Baseline and posttreatment t scores for the BADDS clusters
| Baseline | ATX | Placebo | Num df | ATS | P | |
|---|---|---|---|---|---|---|
| Organizing/activating | 60.7 (11.6) | 55.6 (8.9) | 56.3 (8.9) | 1.61 | 1.68 | 0.19 |
| Attention/concentration | 58.8 (8.7) | 52.1 (4.0)a | 56.4 (7.1) | 1.70 | 2.88 | 0.06 |
| Alertness/effort/processing | 57.4 (10.0) | 54.2 (5.5) | 54.7 (7.9) | 1.68 | 0.82 | 0.42 |
| Managing affect interference | 55.3 (7.9) | 51.8 (4.5)b | 51.6 (3.8)c | 1.95 | 6.28 | 0.002 |
| Working memory/recall | 61.3 (10.0) | 52.4 (5.3)d | 59.6 (10.3)e | 1.74 | 9.70 | 0.0001 |
Effect of treatment condition is reported, as well as significant post hoc comparisons. Baseline, ATX, and Placebo data given as mean (SD). BADDS, Brown Attention Deficit Disorder Scale; ATX, atomoxetine; ATS, analysis of variance-type statistics.
ATX versus baseline (ATS, 9.88; P < 0.002).
ATX versus baseline (ATS, 10.35; P < 0.001).
Placebo versus baseline (ATS, 10.1; P < 0.002).
ATX versus baseline (ATS, 23.5; P < 0.0001).
ATX versus placebo (ATS, 11.01; P < 0.001).
Neuropsychological testing
There were no significant effects of treatment condition (ATX vs placebo) on any of the following tasks: Symbol Search, Letter-Number Sequencing, Digit Symbol Coding, or Controlled Oral Word Association Test (Table 3). There was a significant effect of time on Verbal Paired Associates Task performance (num df = 1, ATS = 8.0, P = 0.0004), indicating considerable improvement with practice regardless of treatment condition. These results are consistent with reports that neuropsychological tests are not generally very sensitive to cognitive impairments of ADHD because they tend to have low ceilings and do not adequately address the complexity of managing tasks of daily life.51,52
TABLE 3.
Baseline and posttreatment raw scores for neuropsychological, behavioral, and physiological measures
| Baseline | Atomoxetine | Placebo | Num df | ATS | P | |
|---|---|---|---|---|---|---|
| Neuropsychological tasks | ||||||
| Verbal Paired Associates | 17.3 (9.0) | 21.2 (8.0) | 22 (6.5) | 1.89 | 8.04 | 0.0004 |
| Digit Symbol Coding | 65.5 (16.5) | 69.8 (16.1) | 73.1 (21.5) | 1.98 | 1.90 | 0.15 |
| Symbol Search | 30.1 (6.9) | 34.8 (9.5) | 32.6 (10.2) | 1.91 | 2.12 | 0.12 |
| COWAT | 44.8 (12.9) | 44.9 (13.1) | 44.9 (12.7) | 1.77 | 0.13 | 0.85 |
| Letter-Number Sequencing | 11.8 (3.0) | 12.3 (2.1) | 12.8 (2.2) | 1.61 | 1.27 | 0.28 |
| Behavioral assessments | ||||||
| Beck Depression | 6.53 (4.2) | 4.5 (4.2) | 5.4 (4.9) | 1.66 | 1.29 | 0.27 |
| Beck Anxiety | 5.3 (5.3) | 6.5 (6.4) | 7.0 (6.3) | 1.57 | 0.72 | 0.46 |
| POMS-Tension | 2.93 (1.9) | 5.1 (7.9) | 4.4 (4.5) | 1.49 | 0.04 | 0.92 |
| POMS-Depression | 3.29 (5.2) | 3.6 (10.1) | 1.7 (2.6) | 1.64 | 0.38 | 0.91 |
| POMS-Anger | 1.79 (3.0) | 3.2 (7.5) | 1.4 (3.2) | 1.61 | 0.33 | 0.67 |
| POMS-Fatigue | 4.14 (4.9) | 4.0 (7.3) | 4.1 (4.6) | 1.31 | 1.19 | 0.29 |
| POMS-Vigor | 13.7 (5.5) | 14.9 (7.8) | 14.3 (7.3) | 1.91 | 0.61 | 0.54 |
| POMS-Confusion | 4.64 (2.6) | 4.1 (4.6) | 3.8 (2.2) | 1.84 | 0.80 | 0.44 |
| Physiological monitoring | ||||||
| Systolic BP, mm Hg | 118.8 (12.5) | 116.7 (11.3) | 120.3 (8.5) | 1.83 | 0.62 | 0.52 |
| Diastolic BP, mm Hg | 74 (10.0) | 69.8 (7.7) | 70.7 (5.2) | 1.66 | 0.62 | 0.51 |
| Heart rate, beats/min | 63 (3.2) | 68.8 (8.2) | 66.3 (5.5) | 1.76 | 1.26 | 0.28 |
| Weight, lb | 160.1 (41.8) | 158.1 (44.4) | 159.8 (42.6) | 1.91 | 0.85 | 0.42 |
Baseline, Atomexetine, and Placebo data given as mean (SD). ATS, analysis of variance-type statistics; COWAT, Controlled Oral Word Association Test; POMS, Profile of Mood States; BP, blood pressure.
Behavioral measures
There was no significant effect of ATX on depression or anxiety as measured with the Beck Depression Inventory and Beck Anxiety Inventory, respectively. Similarly, there was no effect of treatment on Profile of Mood States subscores for tension, depression, anger, fatigue, vigor, and confusion.
Cardiovascular and weight effects
Although one woman dropped from the study during the ATX arm out of concern regarding an increase in blood pressure (baseline, 110/80 mm Hg; placebo, 124/60 mm Hg; and ATX, 138/88 mm Hg), there was no significant effect of ATX treatment on blood pressure or heart rate for the group as a whole. Likewise, participant weight was stable across the study (Table 3).
DISCUSSION
The principal finding of this pilot study is that ATX significantly reduced subjective appraisal of short-term working memory difficulties in women presenting with concerns about their memory and concentration during the menopausal transition. Although women reported improvement in attention and concentration during the ATX treatment arm and not the placebo arm, the improvement with ATX versus placebo on that cluster did not reach significance in this small study. In these healthy, nondepressed perimenopausal and postmenopausal women, ATX had no observable effect on levels of depression or anxiety.
Results from this study indicating an effect of ATX over placebo on subjective working memory difficulties but no measurable effect on neuropsychological tasks in women with midlife-onset cognitive concerns are consistent with previous research on assessing cognitive impairments in adults. Shallice53 demonstrated that individuals with frontal lobe damage were unable to perform adequately everyday errands that require planning and multitasking, even though they achieved average or well above-average scores on traditional neuropsychological tests. Burgess and Rabbit54 argued that neuropsychological tests tend to be insufficiently sensitive to cognitive impairments in attention, organization and getting started on tasks, utilization of working memory, and others that are reported by older adults. Other investigators demonstrated that patient report provided more adequate data on real-world cognitive impairments of ADHD than did neuro-psychological tests.55 Biederman et al52 additionally showed that neuropsychological tests assess different impairments of cognitive functions in ADHD than do self-report rating scales. Brown32 has discussed the reasons that self-report measures are more likely to capture cognitive impairments identified as “executive functions” than are neuropsychological tests.
Although we did not observe objective effects of ATX treatment (ie, enhanced performance on neuropsychological tests), results from this pilot study suggest that subjective difficulties with working memory and possibly attention/ concentration reported by perimenopausal and postmenopausal women may be alleviated significantly by ATX, a medication approved by the Food and Drug Administration for the treatment of ADHD. This is not to say that these women had ADHD, a syndrome involving symptoms that usually become apparent in childhood or early adolescence.56 Rather, these findings suggest that some women report a syndrome of cognitive difficulties emerging in midlife that bear strong resemblance to cognitive impairments commonly reported by adults with ADHD and that may be significantly alleviated when treated with a medication demonstrated to be effective for treatment of ADHD. Our study also showed that treatment with ATX in this sample of women was reasonably well tolerated. One participant experienced an increase in blood pressure that contributed to her discontinuation in the study, and two others experienced mild nausea. Although we did not observe an effect of ATX on blood pressure in the group overall, this is a small study, and blood pressure monitoring in future studies would be prudent.57 Subjective improvement in reported executive functions with relatively few adverse effects suggests that ATX could significantly improve the quality of life of middle-aged women with new-onset concerns regarding their memory and concentration.
Limitations of this pilot study include the small sample size, the relatively short duration of treatment, and the relatively low dose of ATX. Research reported subsequent to initiation of this study has shown that some adult individuals with ADHD benefit from higher doses of ATX than were administered in this study and that the full benefits of ATX for alleviation of ADHD symptoms are sometimes not fully apparent until up to 3 months of treatment.58 Whether dose and duration of ATX treatment are crucial for midlife women who do not meet criteria for ADHD is unknown. Another limitation is that relying on women's recall to distinguish the onset of “cognitive or memory” complaints with relation to the menopausal transition has obvious limitations. That we included women who were largely within 5 years of their LMP reduces, but does not negate, this concern.
CONCLUSIONS
Despite the limitations of this study, these pilot data suggest that further research on midlife onset of cognitive impairments in perimenopausal and early postmenopausal women is warranted. Such research could be useful to test whether self-report elicited with ADHD rating scales may capture midlife-onset cognitive impairments of women that may not be captured adequately by neuropsychological tests. Further research with higher doses of ATX and studies using other medications approved for treatment of ADHD in adults might also be useful in developing an effective protocol for women reporting midlife onset of distressing memory and concentration difficulties that subjectively impair their daily functioning.
Acknowledgments
We thank Ms. Sara Beyor, BS, and Ms. Deborah Ward-O'Brien, APRN, for their contribution to participant recruitment and data collection/management throughout this study.
Funding/support: This study was funded by an investigator-initiated grant from Eli Lilly & Company (C.N.E.) and in part by the National Institute of Mental Health K02MH73090 (C.N.E.).
Footnotes
Financial disclosure/conflicts of interest: Dr. Brown has the following relationships to disclose: Eli Lilly—consultant, research support, speaker and advisory board; Shire—consultant, research support, speaker and advisory board; Novartis—consultant; and Shionoga—consultant. Dr. Brown is also the author of the Brown Attention Deficit Disorder Scale. Dr. Epperson has the following relationships to disclose: Eli Lilly— research support, speaker; GlaxoSmithKline—consultant; Johnson & Johnson—stocks. Other authors do not have any disclosures.
REFERENCES
- 1.Mitchell ES, Woods NF. Midlife women's attributions about perceived memory changes: observations from the Seattle Midlife Women's Health Study. J Womens Health Gend Based Med. 2001;10:351–362. doi: 10.1089/152460901750269670. [DOI] [PubMed] [Google Scholar]
- 2.Minkin MJ, Wright CV, editors. A Woman's Guide to Menopause and Perimenopause. 1st ed. Yale University Press; New Haven, CT: 2004. [Google Scholar]
- 3.Brizendine L, editor. The Female Brain. 1st ed. Broadway; New York, NY: 2006. [Google Scholar]
- 4.Fuh JL, Wang SJ, Lee SJ, Lu SR, Juang KD. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53:447–453. doi: 10.1016/j.maturitas.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Henderson VW, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Estrogen exposures and memory at midlife: a population-based study of women. Neurology. 2003;60:1369–1371. doi: 10.1212/01.wnl.0000059413.75888.be. [DOI] [PubMed] [Google Scholar]
- 6.Luetters C, Huang MH, Seeman T, et al. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the Study of Women's Health Across the Nation (SWAN). J Womens Health. 2007;16:331–344. doi: 10.1089/jwh.2006.0057. [DOI] [PubMed] [Google Scholar]
- 7.Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe S. Possible acceleration of age effects on cognition following menopause. J Psychiatr Res. 1995;29:153–163. doi: 10.1016/0022-3956(95)00005-p. [DOI] [PubMed] [Google Scholar]
- 8.Kok HS, Kuh D, Cooper R, et al. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13:19–27. doi: 10.1097/01.gme.0000196592.36711.a0. [DOI] [PubMed] [Google Scholar]
- 9.Krug R, Born J, Rasch B. A 3-day estrogen treatment improves prefrontal cortexYdependent cognitive function in postmenopausal women. Psychoneuroendocrinology. 2006;31:965–975. doi: 10.1016/j.psyneuen.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Stevens MC, Clark VP, Prestwood KM. Low-dose estradiol alters brain activity. Psychiatry Res. 2005;139:199–217. doi: 10.1016/j.pscychresns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Voytko ML, Murray R, Higgs CJ. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J Neurosci. 2009;29:10362–10370. doi: 10.1523/JNEUROSCI.1591-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wegesin DJ, Stern Y. Effects of hormone replacement therapy and aging on cognition: evidence for executive dysfunction. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:301–328. doi: 10.1080/13825580600802893. [DOI] [PubMed] [Google Scholar]
- 13.Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23:33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 15.Simpkins JW, Singh M. More than a decade of estrogen neuroprotection. Alzheimer Dement. 2008;4:S131–S136. doi: 10.1016/j.jalz.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 17.Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- 18.Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 20.Zandi PP, Carlson MC, Plassman BL. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 21.Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- 22.Shifren JL, Schiff I. Role of hormone therapy in the management of menopause. Obstet Gynecol. 2010;115:839–855. doi: 10.1097/AOG.0b013e3181d41191. [DOI] [PubMed] [Google Scholar]
- 23.Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 24.Gehlert DR, Schober DA, Hemrick-Luecke SK, et al. Novel halogenated analogs of atomoxetine that are potent and selective inhibitors of norepinephrine uptake in brain. Neurochem Int. 1995;26:47–52. doi: 10.1016/0197-0186(94)00113-9. [DOI] [PubMed] [Google Scholar]
- 25.Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11:203–226. doi: 10.2165/00148581-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 26.Kelsey DK, Sumner CR, Casat CD, et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including and assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics. 2004;114:e1–e8. doi: 10.1542/peds.114.1.e1. [DOI] [PubMed] [Google Scholar]
- 27.Adler LA, Spencer T, Brown TE, et al. Once-daily atomoxetine for adult attention-deficit/hyperactivity disorder: a 6-month, double-blind trial. J Clin Psychopharmacol. 2009;29:44–50. doi: 10.1097/JCP.0b013e318192e4a0. [DOI] [PubMed] [Google Scholar]
- 28.Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem. 2010;114:259–270. doi: 10.1111/j.1471-4159.2010.06750.x. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0) American Psychiatric Press; Washington, DC: 1990. [Google Scholar]
- 30.Orvaschel H, Puig-Antich J. Schedule for Affective Disorder and Schizophrenia for School-Age Children—Epidemiologic, 4th Version. Nova University Center for Psychological Study; Ft Lauderdale, FL: 1987. [Google Scholar]
- 31.Seidman LJ, Biederman J, Weber W, et al. Neuropsychological function in adults with attention-deficit hyperactivity disorder. Biol Psychiatry. 1998;44:260–268. doi: 10.1016/s0006-3223(97)00392-2. [DOI] [PubMed] [Google Scholar]
- 32.Brown TE. Brown Attention Deficit Disorders Scale for Adolescents and Adults. The Psychological Corporation; Toronto, Ontario, Canada: 1996. [Google Scholar]
- 33.Kooij JS, Boonstra AM, Swinkels SHN, Bekker EM, Nooord I, Buitelaar JK. Reliability, validity and utility of instruments for self-report and informant report concerning symptoms of ADHD in adult patients. J Atten Disord. 2008;11:445–458. doi: 10.1177/1087054707299367. [DOI] [PubMed] [Google Scholar]
- 34.Spencer TJ, Adler LA, Weisler RH, Youcha SH. Triple-bead mixed amphetamine salts (SPD465), a novel, enhanced extended-release amphetamine formulation for the treatment of adults with ADHD: a randomized, double-blind, multicenter, placebo-controlled study. J Clin Psychiatry. 2008;69:1437–1448. doi: 10.4088/jcp.v69n0911. [DOI] [PubMed] [Google Scholar]
- 35.Brown TE, Quinlan D. Assessing OROS methylphenidate effects on executive functions in adults with ADHD.. Paper presented at: Institute on Psychiatric Services Annual Meeting; Atlanta, GA. October 6–10, 2004. [Google Scholar]
- 36.Brown TE, Holdnack J, Saylor K, et al. Effect of atomoxetine on executive function impairments in adults with ADHD [e-pub]. J Atten Disord. 2009 doi: 10.1177/1087054709356165. [DOI] [PubMed] [Google Scholar]
- 37.Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 38.Wechsler D. Weschler Memory Scale. 3rd ed. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 39.Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18:485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- 40.Lokken KL, Ferraro FR. The relationship between menopausal status, phase of menstrual cycle, and replacement estrogen on cognition in healthy women without dementia. J Psychol. 2006;140:533–547. doi: 10.3200/JRLP.140.6.533-547. [DOI] [PubMed] [Google Scholar]
- 41.Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology. 2007;69:1322–1330. doi: 10.1212/01.wnl.0000277275.42504.93. [DOI] [PubMed] [Google Scholar]
- 42.Alhola P, Polo-Kantola P, Erkkola R, Portin R. Estrogen therapy and cognition: a 6-year single-blind follow-up study in postmenopausal women. Neurology. 2006;67:706–709. doi: 10.1212/01.wnl.0000230135.10179.86. [DOI] [PubMed] [Google Scholar]
- 43.Amin Z, Gueorguieva R, Cappiello A, et al. Estradiol and tryptophan depletion interact to modulate cognition in menopausal women. Neuropsychopharmacology. 2006;31:2489–2497. doi: 10.1038/sj.npp.1301114. [DOI] [PubMed] [Google Scholar]
- 44.Epperson CN, Amin Z, Naftolin F, et al. The resistance to depressive relapse in menopausal women undergoing tryptophan depletion: preliminary findings. J Psychopharmacol. 2007;21:414–420. doi: 10.1177/0269881106067330. [DOI] [PubMed] [Google Scholar]
- 45.Freeman EW. Associations of depression with the transition to menopause. Menopause. 2010;17:823–827. doi: 10.1097/gme.0b013e3181db9f8b. [DOI] [PubMed] [Google Scholar]
- 46.Bierman EJM, Comijs HC, Jonker C, Beekman A. Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry. 2005;13:686–693. doi: 10.1176/appi.ajgp.13.8.686. [DOI] [PubMed] [Google Scholar]
- 47.Beck AT, Steer RA. Manual for the Beck Depression Inventory. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 48.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 49.McNair ML, Lorr M, Droppleman LF. POMS Manual: Profile of Mood States. EdITS; San Diego, CA: 1981. [Google Scholar]
- 50.Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. John Wiley & Sons; New York, NY: 2002. [Google Scholar]
- 51.Brown TE. Executive functions and attention deficit hyperactivity disorder: implications of two conflicting views. Int J Disabil Dev Educ. 2006;53:35–46. [Google Scholar]
- 52.Biederman J, Petty CR, Fried R. Discordance between psychometric testing and questionnaire-based definitions of executive function deficits in individuals with ADHD. J Atten Disord. 2008;12:92–102. doi: 10.1177/1087054707305111. [DOI] [PubMed] [Google Scholar]
- 53.Shallice T. Higher-order cognitive impairments and frontal lobe lesions in man. In: Levin HS, editor. Frontal Lobe Function and Dysfunction. Oxford University Press; New York, NY: 1991. pp. 125–138. [Google Scholar]
- 54.Burgess PW, Rabbit P. Theory and methodology in executive function research. In: Rabbitt P, ed. Methodology of Frontal and Executive Function. Psychology Press Publishers; East Sussex, UK: 1997. pp. 81–116. [Google Scholar]
- 55.Stavro GM, Ettenhofer ML, Nigg JL. Executive functions and adaptive functioning in young adult attention-deficit/hyperactivity disorder. J Int Neurol Soc. 2007;13:324–334. doi: 10.1017/S1355617707070348. [DOI] [PubMed] [Google Scholar]
- 56.Faraone SV, Biederman J, Spencer TJ, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry. 2006;163:1720–1729. doi: 10.1176/ajp.2006.163.10.1720. [DOI] [PubMed] [Google Scholar]
- 57.Shibao C, Raj SR, Gamboa A, et al. Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension. 2007;50:47–53. doi: 10.1161/HYPERTENSIONAHA.107.089961. [DOI] [PubMed] [Google Scholar]
- 58.Virani AS, Bezchulibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 18th ed. Hogrefe & Huber; Gottingen, Germany: 2009. p. 224. [Google Scholar]