Summary
The cell of origin of pancreas tumors has been hotly debated. Studies of mouse models of pancreas tumorigenesis have begun to resolve this conundrum, with most pointing to the differentiationally plastic acinar cell as the likely culprit.
Abstract
Pancreatic ductal adenocarcinoma (PDA) is especially deadly due to its recalcitrance to current therapies. One of the unique qualities of PDA that may contribute to this resistance is a striking plasticity of differentiation states starting at tumor formation and continuing throughout tumor progression, including metastasis. Here, we explore the earliest steps of tumor formation and neoplastic progression and how this results in a fascinating cellular heterogeneity that is probably critical for tumor survival and progression. We hypothesize that reinforcing differentiation pathways run awry or targeting morphologically and molecularly distinct tumor stem-like cells may hold promise for future treatments of this deadly disease.
Introduction
Pancreatic ductal adenocarcinoma (PDA) is an almost universally lethal disease. Its dismal 6% 5-year survival rate is probably due, in part, to the lack of early detection. However, it is becoming increasingly clear that PDA is distinctive in its ability to resist conventional therapies. The most effective treatment for PDA remains surgery, but only 20% of patients present with resectable disease and, even with successful resection, the cancer returns in 80% of those patients (1). One unique quality of PDA that probably contributes to its remarkable resistance to therapy is that the tumor epithelium demonstrates a striking plasticity in its differentiation status, which manifests at every stage of progression. For instance, in tumorigenesis, ‘terminally’ differentiated acinar cells can give rise to ductal tumors. After tumor formation, histopathologically well-differentiated pancreatic intraepithelial neoplasms (PanINs) can undergo an epithelial-to-mesenchymal transition, potentially seeding metastases at a stage long before detection of the primary tumor is possible (2). Even PDA metastases themselves can have the chameleon-like ability to take on the differentiation qualities of a primary tumor type that had originated in the distant organ they now occupy (3). With this apparently fast and loose relationship with differentiation states probably comes an equally ethereal adaptivity when faced with the survival challenges posed by therapy.
The cellular plasticity of PDA is reflected by a similar plasticity observed in normal and injured tissue that has only begun to be fully appreciated (4–6). It being an intrinsic quality of normal pancreatic cells suggests that exposing the true identity of the normal cell(s) of origin of PDA will teach us much about how to approach the disease. As our genetic tools become more sophisticated, we are able to explore the process of epithelial plasticity and the resulting cellular heterogeneity present within even the earliest stages of PDA progression. Ideally, new therapies designed to constrain, or even reverse this aberrant differentiation, may present a novel approach to treatment. In this review, we will discuss what is currently known about the cells of origin of PDA together with early tumor cell heterogeneity and speculate on how this knowledge may contribute to our ongoing efforts to eradicate this devastating disease.
Appearances can be deceiving: ductal histology implies ductal origin
Although numerous histologically distinct pancreatic cancers have been defined, the morphologically distinct variant, ductal adenocarinoma, accounts for >85% of cases of pancreatic cancer and is the most deadly. As its name implies, PDA was initially characterized and described by its ductal, glandular morphology and has been hypothesized to progress through a series of histologically distinct precursor lesions, known as PanIN. PanIN also possess a ductal morphology and express ductal lineage genes (7). The PanIN progression model suggests that PDA progresses through changes in cellular morphology and a sequential set of genetic mutations, beginning with oncogenic mutations in Kras (8,9). The earliest lesions, PanIN-1, are comprised of mucinous tall columnar epithelium with basally oriented nuclei, in contrast to the non-mucinous cuboidal or low columnar appearance of the normal duct, suggesting a cellular reprogramming associated with transformation. As the cells progress through histologically defined stages (PanIN-2 and PanIN-3), they express more supranuclear mucin and display nuclear atypia and papillary projections. In the context of PanIN-3 lesions, also classified as carcinoma in situ, cells are often visualized shedding into the PanIN lumen (9). Two other neoplastic lesions have the capacity to develop into pancreatic cancers: intraductal papillary mucinous neoplasia (IPMN) and mucinous cystic neoplasia (MCN). IPMNs are pancreatic neoplasias that grow in the main or large branch duct lumens and produce abundant mucin (10). IPMNs can develop into invasive pancreatic neoplasias and require strict monitoring upon diagnosis. MCN are generally considered to be disconnected from the pancreatic ductal system and are usually defined by mucin-producing columnar epithelium with an ‘ovarian-like, stromal component (10,11). To date, mouse models have most reliably recapitulated the PanIN-PDA model of pancreatic cancer initiation and progression (12,13), with a few that give rise to the cystic mucinous neoplasms (14–16). However, the latter are genetic modifications of the PanIN models, suggesting that an early alteration in tumor differentiation, rather than a distinct cell of origin, may be responsible for their formation in these models. Currently, faithful animal models of IPMN are lacking.
Despite the ductal histological classification of PDA, the evidence supporting duct cells as the cell of origin for PanIN and PDA remains inadequate. The most compelling data regarding the cell of origin for pancreatic cancer has been generated using murine models designed to conditionally express oncogenic Kras (henceforth, referred to as Kras*), using Cre/Lox technology (17). The initial mouse modeling experiments used the Pdx1-Cre or Ptf1a Cre/+ mouse driver lines to initiate Kras* expression in embryonic pancreatic progenitor cells (12,18). These seminal experiments definitively demonstrate that murine models could effectively recapitulate many aspects of human PDA, including the PanIN progression model (referred to as mPanIN in the context of mouse models), along with desmoplastic and inflammatory stromal responses. However, because both models rely on embryonic activation of Kras*, essentially all parenchymal cell types in the adult tissue express Kras* and thus fail to address the issue of cell of origin.
To better distinguish the cell type or types that could give rise to PanIN, several investigators have utilized inducible Cre driver lines that more selectively target the duct, islet and acinar cell compartments, independently. Although the driver-line options that target ducts have been relatively limited, some recent successful studies have come to the surprising conclusion that adult duct cells are remarkably resistant to transformation by Kras*. Using the CK19 Cre/+ driver, Ray et al. (19) interrogated the ability of adult ductal cells to develop mPanIN in response to inducible Kras* expression. The CK19 Cre/+ driver effectively induced recombination throughout the ductal compartment with <1% recombination in acinar cells and islets. When used to drive Kras* throughout the ductal compartment, only 1% of the cross-sectional area evaluated was occupied by mPanIN. Although the tumors in these animals did not progress to invasive and metastatic PDA, the resultant lesions expressed abundant supranuclear mucin, as assessed by alcian blue staining, and expression of Claudin-18, a marker specific for human PanIN (20). More recently, Kopp et al. used the Sox9CreER T2 driver line, also specifically targeting the duct and centroacinar cells, to test their capacity to form mPanIN. Consistent with the results of Ray et al., these experiments empirically determined that Sox9-expressing ductal cells are intrinsically limited in their capacity for mPanIN formation in response to Kras* expression. The few mPanIN that did form were usually associated with large ducts, possibly giving rise to a more IPMN-like tumor (21). Although these data suggest that the centroacinar cells are also somewhat recalcitrant to Kras* transformation, more precise Cre drivers targeting this putative stem cell population need to be developed to solidify this conclusion.
A true testament to the plasticity of the adult pancreas was demonstrated by Gidekel Friedlander et al. (22), who targeted Kras* expression in insulin-producing islet cells, using the RipCreER TM-inducible Cre driver. Initially, as might be expected, this did not lead to the induction of mPanIN. However, in the context of cerulein-mediated chronic inflammation, expression of Kras* in the insulin-producing cells resulted in mPanIN lesion formation.
Efforts to express Kras* in the adult acinar cell lineage were initiated using a variety of cell-specific-inducible CreER driver lines. The NestinCre ER driver activates Kras* in the exocrine progenitors and their acinar cell descendants (23), leading to mPanIN formation. Although this was one of the first studies to limit Kras* expression to a more defined cell population, it still targeted a developmental progenitor rather than simulating the acquisition of the oncogenic mutation in the adult tissue. Two studies that do target Kras* expression to adult acinar cells, using acinar cell-specific elastase and carboxypeptidase promoter-based Cre drivers, show resistance to spontaneous mPanIN formation, requiring the additional insult of experimental pancreatitis to drive tumorigenesis (22,24). However, other studies utilizing a variety of other Cre drivers that specifically activate Kras* in adult acinar cells (24–26) demonstrate spontaneous formation of mPanIN. Habbe et al. (26) described effective mPanIN formation using both the Mist1 CreERT2/+ and Elastase-CreER T2 inducible Cre drivers to specifically target Kras* expression in acinar cells. Expression of Kras* in the adult acinar cells led to the formation of mPanIN histologically similar to human PanIN. Habbe et al. also reported the entire spectrum of mPanIN at 12 months after the expression of Kras* using the Elastase-CreER T2 model. We have recently reported in the context of the Mist1 CreERT2/+ driver that histologic acinar-to-ductal metaplasia (ADM) and early mPanIN are present as early as 3 weeks after the onset of Kras* expression in acinar cells. The percentage of the pancreas occupied by PanIN in this model significantly increases as a function of time after the expression of Kras* (27), similar to the data described previously by Habbe et al.
In the study previously mentioned by Kopp et al., the investigators directly compared the capacity of acinar and duct cells to form spontaneous mPanIN. Using the Ptf1aCre ERT2 driver in age-matched, tamoxifen-dose-controlled experiments, the acinar cells were over 100 times more efficiently transformed than the Sox9Cre ERT2-targeted duct cells. It remains unclear why some acinar targeting systems lead to spontaneous mPanIN formation and others require pancreatitis, although some possibilities include specific Kras mutations, robustness of Cre drivers and even animal housing environments. However, it is not under debate that experimental pancreatitis greatly enhances the degree and the rate of transformation in all systems with acinar cell Kras* expression. The explanation appears to lie in the efficiency of the requisite reprogramming of acinar differentiation required for them to form morphologically ductal tumors.
Gatekeepers of acinar cell differentiation are modulators of tumorigenesis
The premise that acinar cells are a probable source of what is phenotypically ductal adenocarcinoma immediately suggests a reprogramming of their normal differentiation state that precedes or accompanies transformation. In fact, the concept of ADM, the coincident disappearance of acinar cells and appearance of ‘metaplastic duct lesions’ (MDL) in their place, has been commonly associated with PDA and chronic pancreatitis. Unlike normal ducts in the adult organ, MDL are highly proliferative and express progenitor cell markers (28). These qualities have led many to hypothesize that they may serve as PanIN precursors.
The association of MDL with pancreatitis immediately suggests an explanation for the enhancement of tumorigenesis in acinar-cell-specific models by experimental pancreatitis. It has been long known that exposure of acinar cells to ectopic epidermal growth factor receptor ligands induces acinar-to-ductal transdifferentiation (29–31) and enhances the efficiency of Kras-driven transformation (16,32). We and others have shown recently that genetic ablation of the endogenous epidermal growth factor receptor protects mice from ADM during experimental pancreatitis, as well as from both pancreatitis-induced and spontaneous pancreatic tumorigenesis, even in models where recombination is induced during pancreatic development (33,34). In complementary observations, although Kopp et al. describe a limited capacity for normal Sox9+ ductal epithelium to form mPanIN in response to Kras* expression, they also find that Sox9 is expressed in metaplastic acinar cells in response to oncogenic Kras. Deletion of the Sox9 gene impedes ADM and mPanIN formation in Kras *-expressing acinar cells (21). Together these observations support a model where suppression of ADM suppresses transformation on the whole.
Consistent with ADM being a prerequisite for acinar-cell-derived PDA, recent mouse studies have shown that genes required for the active maintenance of adult acinar cell differentiation are tumorigenesis suppressors. One such gene that falls into this category is Mist1, a basic helix-loop-helix transcription factor uniquely expressed in acinar cells. Mist1 KO mice are defective in acinar cell organization and by 12 months of age, isolated lesions are observed, characterized by a reduction in amylase levels, and a build up of the active form of carboxypeptidase A, indicative of extensive intracellular degradation. Furthermore, electron micrographs show ultrastructural defects, intracellular digestion of individual organelles and distended apical lumens (35). In another set of experiments by Shi et al. (36), loss of Mist1 significantly accelerated Kras*-driven mPanIN development and Mist1 KO mice had an increased propensity to undergo ADM. The authors show that this metaplasia resulting from Mist1 ablation is regulated by the Notch and epidermal growth factor receptor signaling pathways. Gain-of-function experiments demonstrated that expression of Mist1 in a mouse model of ADM/PanIN significantly attenuated tumorigenesis upon acinar-specific expression of Kras* (37).
A second factor shown to be important for maintaining acinar cell differentiation is nuclear receptor 5 subtype a2 (Nr5a2). Recent work by Flandez et al. and von Figura et al. has revealed that Nr5a2 is required for the maintenance of acinar cell identity in the adult animal and for pancreatic regeneration (38,39). These investigators studied the role of Nr5a2 using the PdxCre Late, ElastasteCre ERT2 and Pft1a Cre mouse models. Both publications report that loss of Nr5a2 did not affect the completion of pancreatic exocrine differentiation but did significantly affect the severity of acute pancreatitis and the reestablishment of acinar cell identity during the recovery phase of cerulein-mediated pancreatitis. Flandez et al. demonstrated that loss of one allele of Nr5a2 sensitizes acinar cells to Kras G12V-driven, pancreatitis-induced pancreatic tumorigenesis and von Figura et al. showed that a complete knockout of Nr5a2 greatly enhanced spontaneous tumorigenesis when Kras* was expressed specifically in adult acinar cells. Perhaps most importantly, genome-wide association studies identified Nr5a2 as a significant susceptibility locus for human pancreatic cancer (40,41), strongly suggesting that the transdifferentiating acinar cell is a cell of origin in human PDA and is not a phenomenon confined to mouse models.
Acinar cell reprogramming: Kras* takes cell fate into its own hands
After the initial transformation of pancreatic epithelia, the resulting metaplasia and neoplasia continue to display extreme flexibility in their differentiation states. The advent of lineage tracing approaches to indelibly label Cre-recombined, Kras*-expressing cells with fluorescent and colorimetric markers has revealed new insights regarding how Kras* expression influences the differentiation state of early tumors in ways that may greatly affect progression and treatment. Using such techniques, Rhim et al. (2) reported that continual Kras* expression directed to acinar cells can induce delamination and an epithelial to mesenchymal transition (EMT) in early ‘well-differentiated’ mPanINs, as evidenced by loss of E-cadherin and increased abundance in mPanIN-associated stroma. Furthermore, recent publications by our laboratory groups independently confirm that in response to oncogenic Kras, a population of acinar cells has the capacity to transdifferentiate into highly specialized epithelial cells called tuft cells (27,42). The acinar cell-tuft cell transdifferentiation occurs just weeks after Kras* expression in acinar cells and tuft cells are abundant in ADM and early mPanIN. Furthermore, clonogenic experiments indicate these doublecortin-like kinase 1 (Dclk1) expressing cells, when isolated from mPanIN epithelium, possess the distinctive qualities of a PanIN stem cell. These seminal findings are significant, as they implicate a specific cellular component of the metaplastic duct capable of progenitor cell function previously unappreciated in the PanIN progression model.
Tuft cells were originally described over 50 years ago in the hollow viscera of the intestine (43). They are a type of solitary chemosensory cell located in multiple organs and they are characterized by the presence of elongated microvillae that extend into the apical lumen of epithelial cells. Tuft cells were recently characterized by the expression of acetylated alpha tubulin and Dclk1 with thick bundles of actin microfilaments jutting into the prominent apically oriented microvillae (44,45). Their robust expression of taste receptors and associated signal transduction machinery, such as TRPM5 and α-gustucin, strongly suggest that tuft cells play an active role in sampling their immediate environment, whereas their expression of proteins such as Cox1, Cox2 and hematopoietic prostaglandin-D synthase cast them into a role of important modifiers of this environment by regulating inflammation (44). In an effort to determine if Dclk1-expressing cells in the intestine marked a differentiated cell, a normal stem cell or a tumor stem cell, Nakanishi et al. (46) performed lineage tracing experiments using Dclk1 CreERT2/CreERT2 mice to substantiate that Dclk1-expressing cells are not normal stem cells in the intestine but are tumor stem cells in the context of Apc min/+ mice. Recent work by Westphalen et al. (47) revealed that Dclk1+ cells present in the intestine are derived from Lgr5+ cells and proliferate in response to neuronal signals. These data are the first to specifically identify a unique niche that may be responsible for tuft cell function. In organoid cultures, organoids grew larger when cocultured with tuft cells and nerves and the tuft cells proliferated in response to Wnt3a signals. Furthermore, ablation of Dclk1+ cells from the intestinal epithelium significantly increased morbidity and mortality in irradiation or dextran sulphate sodium models of gastrointestinal injury (47).
In the pancreas, the question of whether Dclk1-expressing cells are normal stem cells remains to be answered. Unlike organs such as the intestine or the bile duct, tuft cells in the normal pancreas are found very rarely, if at all. But their prominence in Kras*-expressing pancreata combined with our data showing that sorted Dclk1+ Kras*-expressing cells demonstrate a highly increased efficiency of sphere formation compared with their non-tuft cell, Kras*-expressing counterparts, strongly suggests that we have discovered a previously unidentified unique PanIN stem cell (48).
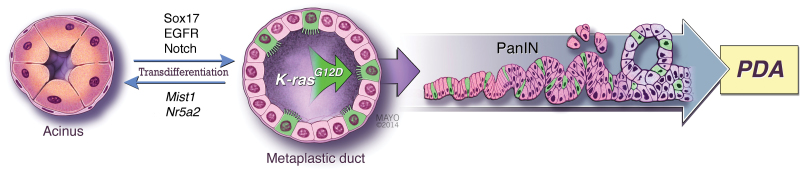
Another revelation of our discovery of acinar-to-tuft cell transdifferentiation is the nature of the Kras*-expressing MDL itself. Given the general lack of tuft cells in the pancreas and their abundance in the developmentally related bile duct and intestine, we hypothesized that MDL were not simply mimicking pancreatic progenitor cells, as previously suggested by several of the studies cited above. Using co-expression of the transcription factors Sox17 and Pdx1 as a marker of a pancreatobiliary progenitor cell (49), we discovered that tuft-cell-containing metaplasia and neoplasia induced by acinar cell expression of Kras were Sox17+/Pdx1+. In fact, Sox17 expression in pancreatic acinar cells induced a phenotype similar to chronic pancreatitis, including tuft-cell-containing metaplasia. Consistent with Sox17’s ability to reprogram acinar cell differentiation, co-expression of Kras* and Sox17 in adult acinar cells led to complete replacement of normal pancreatic epithelium by Pdx1+ Dclk1+ PanIN epithelium, consistent with our observations that the tuft cells can act as PanIN stem cells (50). A model for acinar-cell-derived PanIN is shown in Figure 1.
Fig. 1.
A model depicting the importance of acinar cell differentiation in pancreas tumorigenesis. The differentiation of acinar cells is actively maintained by factors such as Mist1 and Nr5a2, among others. Acinar cell differentiation can be counteracted by expression of Sox17 or activation of the EGFR or Notch pathways, driving transdifferentiation into the tuft-cell-containing (shown in green) metaplastic duct. If a transdifferentiated tuft cell should acquire or have a pre-existing oncogenic mutation in Kras, it can act as a tumor-initiating cell and seed the formation of PanIN, a precancerous lesion that can progress to PDA. Illustration used with permission of the Mayo Foundation for Medical Education and Research.
Currently, it is unknown exactly how Kras* expression distinguishes the ‘PanIN stem’ tuft cell from the normal tuft cell. Just like normal tuft cells, Kras *-expressing tuft cells express taste cell-signaling molecules and prostaglandin synthesis factors (51). Microarray data from fluorescence-activated cell-sorted Dclk1+ versus Dclk1 cells from human pancreatic cancer cell lines revealed Plectin as one of the most highly expressed genes in the Dclk1+ fraction (27). Interestingly, Plectin is a known marker for PDA (52) that is an important mediator of exosome formation (53). Tuft cells possibly being a major source of exosomes are consistent with a 'sense and respond' function that we hypothesize coordinate signals that drive pancreatic cancer progression. Whether tuft cells in the pancreas have a similar response to neuronal signals remains to be answered, but given that they are a known source of β-endorphin (51), this would suggest the intriguing possibility that tuft cells may act as a node of tumor cell/nerve cell crosstalk.
IPMN origins: ducts amok?
Despite the resistance by ductal cells to oncogenic transformation in mouse models, clinical evidence in humans implicates a ductal cell of origin for pancreatic cancer in the context of cystic lesions in the pancreas. Depending on the type of cyst diagnosed, patients may be at an increased risk for developing pancreatic cancer. Two main classes of cysts have been shown clinically to have the potential to progress into pancreatic adenocarcinomas: IPMNs and MCNs. IPMNs are epithelial cystic neoplasms of the main pancreatic duct, or in one of the associated large branched ducts. IPMNs that occur in the main pancreatic duct are at increased risk for progression to invasive pancreatic cancer than are IPMNs arising in the branched ductal epithelium. Clinically relevant pathologic features in IPMNs include the degree of dysplasia and presence or absence of an associated invasive carcinoma (54). Cytological atypia is further used to subclassify IPMNs into low-grade dysplasia, intermediate dysplasia and high-grade dysplasia. Approximately one-third of IPMNs are associated with an invasive carcinoma. The invasive carcinomas are usually colloid or ductal adenocarcinomas. The ductal adenocarcinomas are associated with pancreatobiliary lesions that express Muc1 (55,56).
Conflicting data regarding cell of origin exists with regard to the genetic mutations associated with IPMNs and MCN. A number of studies have shown a variety of genetic alterations that are distinct in IPMN versus PanIN. Furthermore, on the whole, the incidence of Kras* mutations is significantly lower in IPMN relative to PanIN and IPMNs have been shown to have a distinct cytogenetic profile to that of PDA (57–59). Refined genetic evaluation of preinvasive IPMN versus adenocarcinoma-associated IPMN will prove beneficial in understanding ductal cells as a cell of origin in human PDA. Molecular analysis of MCN has shown that Kras* mutations are present even in the lowest grade lesions, with the accumulation of mutations in TP53 and SMAD4 occurring in more advanced dysplasias (60,61). Muc1 expression is present in the invasive MCN and the Notch pathway is active in MCN epithelium, indicating a potential therapeutic treatment option for patients with MCN (62,63). Refined mouse models that mimic IPMN and MCN preneoplastic disease will help resolve the question of whether the ductal epithelium can serve as a cell of origin for pancreatic cancer.
Therapeutic implications of the cell of origin
The obvious question that derives from myriad studies dedicated to uncovering the cell of origin of pancreatic cancer is, is the cell of origin relevant to our fervent attempts to treat the disease? An early answer to this question may have been provided very recently by Collins et al. (64), who showed that inhibition of MEK, a downstream target of Kras*, leads to regression of early mPanIN by forcing re-differentiation of the neoplasia to acinar cells. Thus, at least at this stage of progression, epigenetic reprogramming of the neoplastic epithelium has not initiated a permanent ‘amnesia’ as to cell of origin, despite the wildly aberrant alteration in differentiation status. Besides being an important observation on its own, it also implies that PanIN, and possibly PDA, will be susceptible to alternative methods of inducing re-differentiation, possibly taking great advantage of the wealth of information we have on biliary and pancreas development, as well as acinar cell differentiation during organogenesis.
Although an exciting consideration, perhaps even more benefit can be derived from our identification of the unique cellular heterogeneity induced by oncogenic Kras’ hijacking of stable acinar cell differentiation. Furthermore, the general rarity of the tuft cell within the healthy pancreas compared with their abundance in neoplasia immediately suggests their possible targeting for imaging, possibly by taking advantage of unique extracellularly accessible epitopes of Dclk1 and acetylated α-tubulin. These same epitopes may also provide a gateway to poisoning these PanIN stem cells. Like any therapy, this would probably have detrimental effects on normal tissues, specifically those that rely on tuft cell function, and may impact tissue regeneration. Furthermore, our observations show that tuft cell numbers gradually decrease during progression to PDA, so therapies based on them will only be possible when imaging technologies are able to detect the earliest neoplasms. But as these technologies advance, our increasing understanding of the cellular heterogeneity of PanIN, together with the contribution of various subpopulations of cells to disease pathology, will prepare us for rapid intervention at the disease’s presumably most curable stage.
Funding
National Institute of Health grants (R01CA159222, R01CA136754 to H.C.C.); Mayo Clinic Pancreas SPORE (P50CA102701); Pancreatic Cancer Action Network-American Association for Cancer Research pathway to Leadership Award (NCI 5F32 CA157044 to J.M.B).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- ADM
acinar-to-ductal metaplasia
- Dclk1
doublecortin-like kinase 1
- IPMN
intraductal papillary mucinous neoplasia
- MCN
mucinous cystic neoplasia
- MDL
metaplastic duct lesions
- Nr5a2
nuclear receptor 5 subtype a2
- PanIN
pancreatic intraepithelial neoplasm
- PDA
pancreatic ductal adenocarcinoma.
References
- 1. Paulson A.S., et al. (2013). Therapeutic advances in pancreatic cancer. Gastroenterology, 144, 1316–1326 [DOI] [PubMed] [Google Scholar]
- 2. Rhim A.D., et al. (2012). EMT and dissemination precede pancreatic tumor formation. Cell, 148, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yachida S., et al. (2009). The pathology and genetics of metastatic pancreatic cancer. Arch. Pathol. Lab. Med., 133, 413–422 [DOI] [PubMed] [Google Scholar]
- 4. Strobel O., et al. (2007). In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology, 133, 1999–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stanger B.Z., et al. (2013). Control of cell identity in pancreas development and regeneration. Gastroenterology, 144, 1170–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziv O., et al. (2013). The plastic pancreas. Dev. Cell, 26, 3–7 [DOI] [PubMed] [Google Scholar]
- 7. Hruban R.H., et al. (2001). Molecular pathology of pancreatic cancer. Cancer J., 7, 251–258 [PubMed] [Google Scholar]
- 8. Feldmann G., et al. (2007). Molecular genetics of pancreatic intraepithelial neoplasia. J. Hepatobiliary. Pancreat. Surg., 14, 224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maitra A., et al. (2003). Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod. Pathol., 16, 902–912 [DOI] [PubMed] [Google Scholar]
- 10. Katabi N., et al. (2008). Intraductal papillary mucinous neoplasms of the pancreas: clinical and pathological features and diagnostic approach. J. Clin. Pathol., 61, 1303–1313 [DOI] [PubMed] [Google Scholar]
- 11. Klimstra D.S. (2005). Cystic, mucin-producing neoplasms of the pancreas: the distinguishing features of mucinous cystic neoplasms and intraductal papillary mucinous neoplasms. Semin. Diagn. Pathol., 22, 318–329 [DOI] [PubMed] [Google Scholar]
- 12. Hingorani S.R., et al. (2003). Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell, 4, 437–450 [DOI] [PubMed] [Google Scholar]
- 13. Guerra C., et al. (2003). Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell, 4, 111–120 [DOI] [PubMed] [Google Scholar]
- 14. Izeradjene K., et al. (2007). Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell, 11, 229–243 [DOI] [PubMed] [Google Scholar]
- 15. Mazur P.K., et al. (2010). Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc. Natl Acad. Sci. USA, 107, 13438–13443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siveke J.T., et al. (2007). Concomitant pancreatic activation of Kras(G12D) and Tgfa results in cystic papillary neoplasms reminiscent of human IPMN. Cancer Cell, 12, 266–279 [DOI] [PubMed] [Google Scholar]
- 17. Jackson E.L., et al. (2001). Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev., 15, 3243–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aguirre A.J., et al. (2003). Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev., 17, 3112–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ray K.C., et al. (2011). Epithelial tissues have varying degrees of susceptibility to Kras(G12D)-initiated tumorigenesis in a mouse model. PLoS One, 6, e16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karanjawala Z.E., et al. (2008). New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am. J. Surg. Pathol., 32, 188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kopp J.L., et al. (2012). Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell, 22, 737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gidekel Friedlander S.Y., et al. (2009). Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell, 16, 379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carrière C., et al. (2007). The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc. Natl Acad. Sci. USA, 104, 4437–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guerra C., et al. (2007). Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell, 11, 291–302 [DOI] [PubMed] [Google Scholar]
- 25. De La O J.P., et al. (2008). Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl Acad. Sci. USA, 105, 18907–18912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Habbe N., et al. (2008). Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc. Natl Acad. Sci. USA, 105, 18913–18918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bailey J.M., et al. (2014). DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology, 146, 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lardon J., et al. (2005). Metaplasia in the pancreas. Differentiation., 73, 278–286 [DOI] [PubMed] [Google Scholar]
- 29. Means A.L., et al. (2005). Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development, 132, 3767–3776 [DOI] [PubMed] [Google Scholar]
- 30. De Lisle R.C., et al. (1990). Pancreatic acinar cells in culture: expression of acinar and ductal antigens in a growth-related manner. Eur. J. Cell Biol., 51, 64–75 [PubMed] [Google Scholar]
- 31. Sandgren E.P., et al. (1990). Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell, 61, 1121–1135 [DOI] [PubMed] [Google Scholar]
- 32. Ray K.C., et al. (2014). Heparin-binding epidermal growth factor-like growth factor eliminates constraints on activated Kras to promote rapid onset of pancreatic neoplasia. Oncogene, 33, 823–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ardito C.M., et al. (2012). EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell, 22, 304–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Navas C., et al. (2012). EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell, 22, 318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pin C.L., et al. (2001). The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J. Cell Biol., 155, 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi G., et al. (2009). Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology, 136, 1368–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suzuki A., et al. (2004). Expression of nitric oxide and inducible nitric oxide synthase in acute renal allograft rejection in the rat. Int. J. Urol., 11, 837–844 [DOI] [PubMed] [Google Scholar]
- 38. von Figura G., et al. (2014). Nr5a2 maintains acinar cell differentiation and constrains oncogenic Kras-mediated pancreatic neoplastic initiation. Gut, 63, 656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flandez M., et al. (2014). Nr5a2 heterozygosity sensitises to, and cooperates with, inflammation in KRas(G12V)-driven pancreatic tumourigenesis. Gut, 63, 647–655 [DOI] [PubMed] [Google Scholar]
- 40. Petersen G.M., et al. (2010). A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat. Genet., 42, 224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li D., et al. (2012). Pathway analysis of genome-wide association study data highlights pancreatic development genes as susceptibility factors for pancreatic cancer. Carcinogenesis, 33, 1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Delgiorno K.E., et al. (2014). Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology, 146, 233–44.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sato A. (2007). Tuft cells. Anat. Sci. Int., 82, 187–199 [DOI] [PubMed] [Google Scholar]
- 44. Gerbe F., et al. (2011). Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell Biol., 192, 767–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gerbe F., et al. (2009). DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology, 137, 2179–80; author reply 2180 [DOI] [PubMed] [Google Scholar]
- 46. Nakanishi Y., et al. (2013). Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat. Genet., 45, 98–103 [DOI] [PubMed] [Google Scholar]
- 47. Westphalen C.B., et al. (2014). Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Invest., 124, 1283–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bailey J.M., et al. (2014). DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology, 146, 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spence J.R., et al. (2009). Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev. Cell, 17, 62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Delgiorno K.E., et al. (2014). Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology, 146, 233–44.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Delgiorno K.E., et al. (2014). Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology, 146, 233–44.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bausch D., et al. (2011). Plectin-1 as a novel biomarker for pancreatic cancer. Clin. Cancer Res., 17, 302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shin S.J., et al. (2013). Unexpected gain of function for the scaffolding protein Plectin due to mislocalization in pancreatic cancer. Proc. Natl Acad. Sci. USA, 110, 19414–19419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Matthaei H., et al. (2011). Cystic precursors to invasive pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol., 8, 141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Adsay N.V. (2008). Cystic neoplasia of the pancreas: pathology and biology. J. Gastrointest. Surg., 12, 401–404 [DOI] [PubMed] [Google Scholar]
- 56. Adsay N.V., et al. (2004). Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am. J. Surg. Pathol., 28, 839–848 [DOI] [PubMed] [Google Scholar]
- 57. Fritz S., et al. (2009). Global genomic analysis of intraductal papillary mucinous neoplasms of the pancreas reveals significant molecular differences compared to ductal adenocarcinoma. Ann. Surg., 249, 440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schönleben F., et al. (2007). BRAF and KRAS gene mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/IPMC) of the pancreas. Cancer Lett., 249, 242–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schönleben F., et al. (2008). PIK3CA, KRAS, and BRAF mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/C) of the pancreas. Langenbecks. Arch. Surg., 393, 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jimenez R.E., et al. (1999). Sequential accumulation of K-ras mutations and p53 overexpression in the progression of pancreatic mucinous cystic neoplasms to malignancy. Ann. Surg., 230, 501–9; discussion 509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Iacobuzio-Donahue C.A., et al. (2000). Dpc4 protein in mucinous cystic neoplasms of the pancreas: frequent loss of expression in invasive carcinomas suggests a role in genetic progression. Am. J. Surg. Pathol., 24, 1544–1548 [DOI] [PubMed] [Google Scholar]
- 62. Lüttges J., et al. (2002). The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am. J. Surg. Pathol., 26, 466–471 [DOI] [PubMed] [Google Scholar]
- 63. Fukushima N., et al. (2004). Characterization of gene expression in mucinous cystic neoplasms of the pancreas using oligonucleotide microarrays. Oncogene, 23, 9042–9051 [DOI] [PubMed] [Google Scholar]
- 64. Collins M.A., et al. (2014). MAPK signaling is required for dedifferentiation of acinar cells and development of pancreatic intraepithelial neoplasia in mice. Gastroenterology, 146, 822–834.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]