Abstract
Reductions in skeletal muscle function occur during the course of healthy aging as well as with bed rest or diverse diseases such as cancer, muscular dystrophy, and heart failure. However, there are no accepted pharmacologic therapies to improve impaired skeletal muscle function. Nitric oxide may influence skeletal muscle function through effects on excitation‐contraction coupling, myofibrillar function, perfusion, and metabolism. Here we show that augmentation of nitric oxide‐cyclic guanosine monophosphate signaling by short‐term daily administration of the phosphodiesterase 5 inhibitor sildenafil increases protein synthesis, alters protein expression and nitrosylation, and reduces fatigue in human skeletal muscle. These findings suggest that phosphodiesterase 5 inhibitors represent viable pharmacologic interventions to improve muscle function.
Keywords: translational research, exercise, metabolism, protein S‐nitrosylation
Introduction
Reductions in skeletal muscle function occur during the course of healthy aging as well as with bed rest or diverse diseases such as cancer and heart failure. These decrements in function can limit activities of daily living and, when severe enough, contribute to death.1, 2, 3 Muscle dysfunction is characterized by reduced force or power production or an increased susceptibility to fatigue, the decline in muscle performance that occurs during repeated contractions. Changes in both muscle mass and muscle qualities, such as protein complement, metabolic state, and neural activation strategies, can contribute to these impairments. Apart from exercise training, there are few options, and no universally accepted pharmacologic therapies, for improving human skeletal muscle function, despite intense interest among scientists, clinicians, and the public. Thus, there is a need for identification of new strategies for improving skeletal muscle function.
An emerging body of evidence suggests promise of strategies targeting signaling initiated by nitric oxide (NO). In addition to its role as an important mediator of skeletal muscle hemodynamics,4 NO has been shown to augment anabolic responses to insulin or amino acids in older individuals5, 6 and to be essential for the hypertrophic response to muscle overload in mice.7 NO also promotes muscle regeneration8, 9 and mitochondrial biogenesis.10 Impairments in one or more of these NO‐mediated processes are thought to contribute to the reduced muscle performance observed in a variety of settings, such as aging,5, 6, 11, 12 cachexia,13, 14 or Becker or Duchenne‐type muscular dystrophies.4, 15, 16 In addition, mice with deficient skeletal muscle NO production exhibit increased in situ skeletal muscle fatigability.17
Phosphodiesterase 5 inhibitors augment some responses to NO by inhibiting degradation of the downstream mediator cyclic GMP (cGMP). Chronic treatment of mdx mice (a murine model of Duchenne muscular dystrophy) with phosphodiesterase 5 inhibitors reduces muscle fibrosis18 and increases in vitro force production,18 whereas acute treatment improves muscle perfusion and increases post‐exercise activity levels.19 Similarly, acute treatment of muscular dystrophy patients with phosphodiesterase 5 inhibitors improves perfusion of active muscles during exercise.4 Although these studies provide proof‐of‐concept support for potential in vivo efficacy of phosphodiesterase 5 inhibitors to improve muscle health in a select human patient population, acute responses in skeletal muscle of otherwise healthy humans are unknown, as are chronic skeletal muscle responses in patients in which muscle function impairment occurs by different mechanisms (e.g., cancer cachexia, bedrest, and sarcopenia) or in healthy individuals, despite widespread use of these drugs (more than 37 million prescriptions as of 2008).20
Accordingly, we administered the phosphodiesterase 5 inhibitor sildenafil (Viagra) to generally healthy males, who receive the vast majority of phosphodiesterase 5 inhibitor prescriptions, to test the hypothesis that sildenafil would increase skeletal muscle function and protein synthesis (study design, Figure 1). The outcome variables examined were skeletal muscle function (strength and repetitions to fatigue), skeletal muscle protein synthesis, and protein expression and cysteinyl‐S‐nitrosylation. The rationale for measurement of the latter was previous work from us21 and others22, 23, 24 demonstrating an important role for S‐nitrosylation in muscle physiology, as well as emerging evidence for modulation of NO synthase activity via cGMP‐mediated signaling mechanisms.25, 26, 27
Figure 1.
Study time line.
Methods
Subjects
The study was approved by The University of Texas Medical Branch (UTMB) Institutional Review Board and complied with the Declaration of Helsinki. Written informed consent was obtained from all subjects. Two groups of men were studied over 15 days, including a baseline period (the week preceding the treatment period) in which subjects were familiarized with dynamometry testing, underwent baseline glucose tolerance and indirect calorimetry testing (see below), and baseline dynamometry testing occurred (day 0, the day prior to beginning treatment and 6 days after familiarization), and a subsequent treatment period (days 1–8) in which they received either daily low‐dose (25 mg) sildenafil (N = 5) or placebo (N = 6) in a randomized, double‐blinded fashion, with treatments dispensed by the UTMB Investigational Drug Service research pharmacy, and glucose tolerance, indirect calorimetry, and dynamometry testing were repeated. Subjects underwent cardiopulmonary stress tests at the UTMB heart station as part of the screening process. The men were healthy, based on their history and screening results, represented a wide age range, and the majority (4/6 placebo, 4/5 sildenafil) were overweight based on their BMI (subject characteristics are presented in Table 1). Exclusion criteria included cardiac, liver, kidney, pulmonary, autoimmune or vascular disease; hypo‐ or hypercoagulation disorders, diabetes, cancer, or infectious diseases. Subjects taking nitrates, anabolic steroids, or corticosteroids were also excluded. Subjects were instructed to continue regular activities of daily living and maintain their usual diet during the study.
Table 1.
Baseline subject characteristics
| Placebo | Sildenafil | ||||
|---|---|---|---|---|---|
| Characteristic | Mean ± SE [range] | N | Mean ± SE [range] | N | p Value |
| Age (years) | 44 ± 9 [20–68] | 6 | 55 ± 11 [26–76] | 5 | 0.436 |
| BMI (kg/m2) | 25 ± 1 [20–29] | 6 | 28 ± 1 [24–32] | 5 | 0.212 |
| %Body fat | 27 ± 3 [15–39] | 6 | 35 ± 2 [29–40] | 5 | 0.101 |
| Lean body mass | |||||
| Total (kg) | 56.2 ± 2.2 [51–65] | 6 | 54.3 ± 2.5 [48–61] | 5 | 0.580 |
| Leg (kg) | 10.1 ± 0.5 [9.3–12.2] | 6 | 9.8 ± 0.6 [8.4–10.9] | 4 | 0.726 |
| WBC (/uL) | 6.2 ± 0.5 [4.2–7.8] | 6 | 7.2 ± 0.9 [4.9–9.5] | 5 | 0.339 |
| RBC (/uL) | 5.2 ± 0.2 [4.6–5.8] | 6 | 4.7 ± 0.2 [4.0–5.4] | 5 | 0.096 |
| Hemoglobin (g/dL) | 15.0 ± 0.5 [13.0–16.6] | 6 | 14.1 ± 0.5 [12.8–15.4] | 5 | 0.257 |
| Hematocrit (%) | 44.8 ± 1.2 [40–48] | 6 | 41.7 ± 1.2 [38–45] | 5 | 0.121 |
| Glucose (mg/dL) | 90.0 ± 2.9 [80–99] | 6 | 89.0 ± 2.5 [83–98] | 5 | 0.803 |
| Total cholesterol (mg/dL) | 194.0 ±7.1 [169–217] | 6 | 183.0 ± 11.5 [143–208] | 5 | 0.420 |
| Triglycerides (mg/dL) | 119.7 ± 33.1 [45–241] | 6 | 165.0 ± 30.3 [103–240] | 5 | 0.347 |
| HDL (mg/dL) | 50.3 ±3.5 [35–60] | 6 | 44.4 ± 4.2 [33–58] | 5 | 0.300 |
| LDL (mg/dL) | 119.7 ± 7.2 [98–147] | 6 | 105.4 ± 13.3 [63–137] | 5 | 0.347 |
| VLDL (mg//dL) | 24.0 ± 6.6 [9–48] | 6 | 33.2 ± 6.0 [21–48] | 5 | 0.339 |
| Isometric MVC (Nm) | 167 ± 17 [123–216] | 6 | 150 ± 22 [90–194] | 5 | 0.561 |
| Isokinetic MVC (W) | 274 ± 35 [142–377] | 6 | 266 ± 44 [159–401] | 5 | 0.891 |
| Resting HR (beats/min) | 64 ± 5 [48–77] | 6 | 73 ± 2 [66–80] | 5 | 0.149 |
| Max achieved HR (beats/min) | 180 ± 9 [142–206] | 6 | 161 ± 12 [126–187] | 5 | 0.218 |
| Max Predicted HR (beats/min) | 176 ± 9 [153–200] | 6 | 165 ± 11 [145–194] | 5 | 0.435 |
| Resting SBP (mm Hg) | 119 ± 2 [112–124] | 6 | 118 ± 4 [104–128] | 5 | 0.798 |
| Resting DBP (mm Hg) | 78 ± 1 [74–84] | 6 | 70 ± 4 [54–80] | 5 | 0.105 |
| Max SBP (mm Hg) | 163 ± 5 [146–176] | 6 | 171 ± 12 [140–212] | 5 | 0.554 |
| Max DBP (mm Hg) | 80 ± 2 [74–90] | 6 | 66 ± 5 [48–80] | 5 | 0.029 |
| METs achieved | 13 ± 2 [5–18] | 6 | 10 ± 2 [5–13] | 5 | 0.321 |
| Oxygen uptake (VO2) achieved (mL/kg/min) | 46± 9 [19–62] | 6 | 34 ± 5 [19–47] | 5 | 0.321 |
Dynamometry
Maximal isometric torque production, maximal isokinetic power production, and skeletal muscle fatigue of the knee extensors were determined on a Biodex® 4 dynamometer (Biodex Medical Systems, Inc. Shirley, NY, USA) located in the Acute Care for Elders (A.C.E.) Unit at UTMB. On days 1 and 8 of the treatment period, sildenafil or placebo ingestion occurred approximately 1 hour before dynamometry testing. Briefly, subjects were tested in a seated position, with straps across the hips, chest, and thigh to reduce movement. A warm‐up set of 30 low‐resistance contractions was completed as an activity‐specific warm‐up prior to maximal testing. For isometric testing, the knee was positioned at 90° of flexion and contractions held for 5 seconds. Maximal isometric torque production was considered the average of the peak torques generated during the final 2 of 3 maximal efforts. Similarly, maximal dynamic (isokinetic) power production was considered the average of the maximum power generated from the final 2 of 3 maximal efforts. Muscle fatigue development was assessed during repeated maximal voluntary efforts at 120° per second, with contractions occurring approximately once every 2 seconds, performance quantified as the number of successful repetitions (power ≥60% of initial), and subjects given strong verbal encouragement.
Blood and skeletal muscle sampling
Screening and posttreatment blood samples were obtained under fasting conditions (apart from glucose tolerance testing described below) at the UTMB Institute for Translational Sciences (ITS). For posttreatment sampling, subjects were admitted to the ITS‐Clinical Research Center on the penultimate day of treatment and fasted overnight through the end of the study the following day. Following collection of a blood sample for the determination of background enrichment, a primed (2 μmol/kg), continuous (0.05 μmol/kg/min) infusion of L‐[ring‐d 5] phenylalanine, dissolved in 0.9% saline and filtered through 2‐μm filters, was started in the morning on day 8 and continued until the completion of the study. Venous blood samples were collected hourly for the first 2 hours after starting isotope infusion and approximately every 15 minutes following pill ingestion, which occurred after the 2‐hour blood sample. A muscle biopsy (∼100–200 mg) was taken from the vastus lateralis, 15–20 cm above the knee, approximately 1 hour following pill ingestion. Muscle samples were processed and analyzed for protein expression and nitrosylation (below) and isotope enrichment.28
Skeletal muscle protein expression and S‐nitrosylation
Analyses of the skeletal muscle proteome and S‐nitrosoproteome (via SNOFlo) were performed as described previously.29 In brief, after amino acid analysis to quantify cysteine content (cysteic acid), samples in denaturing buffer were split into two aliquots: one was labeled with Bodipy Fl‐maleimide (BD), and the second treated with ascorbate to reverse the S‐nitrosylation. Both sets of samples were dialyzed against denaturing buffer, with the second then undergoing BD labeling. After two‐dimensional gel electrophoresis, spot quantification by fluorescence imaging, and image analysis, protein abundance was measured by calculating the ratio of spot volumes of sildenafil versus control spots of the ascorbate‐treated samples. The ratios for the samples not treated with ascorbate were calculated similarly. The levels of S‐nitrosylation were then expressed as the ratio of the two ratios (ratio of ratios), with the nonascorbate‐treated ratios (representing the sum of S‐nitrosylated and abundance differences) normalized against the ascorbate‐treated ratios (abundance difference only). A negative ratio of ratios indicates increased cysteinyl S‐nitrosylation, and a positive ratio of ratios decreased S‐nitrosylation. Note that in the case of the ascorbate‐untreated gels, an observed change in fluorescence intensity of a spot could be either due to a change in protein abundance, or a change in the degree of S‐nitrosylation, or both. The ratio of ratios enables the calculation of change in the degree of S‐nitrosylation even when accompanied by an abundance change. Comparisons of differential protein abundance or S‐nitrosylation between treatment groups were performed as previously described.29 Determination of canonical or functional pathways differing between treatment groups was determined using Ingenuity Pathways Analysis software.
Lean body mass
Lean body mass was determined using dual energy x‐ray absorptiometry. In one subject, leg lean mass was not determined.
Skeletal muscle protein synthesis
Fasting rates of mixed muscle skeletal muscle protein synthesis were determined using the precursor‐product approach.28
Oral glucose tolerance
Oral glucose tolerance testing was conducted during the baseline week and on the seventh day of the treatment period, before that day's pill ingestion (i.e., after 6 days of treatment). Briefly, after reporting to the ITS Clinical Research Center in the morning after an overnight fast and resting quietly for approximately 30 minutes, a baseline blood sample was obtained from an antecubital vein. Subjects then ingested an oral glucose solution (0.75 g/kg Fisherbrand Glucose Tolerance Test Beverage 401009FB), followed by blood sampling at 15, 30, 60, 90, and 120 minutes following glucose ingestion. Glucose determinations were made using a commercial glucose analyzer (YSI 2300 STAT Plus, Yellow Springs, OH, USA).
Indirect calorimetry
Resting oxygen consumption was determined during the baseline week and on the seventh day of the treatment period, prior to glucose tolerance testing. Briefly, in the fasted state and after resting quietly for approximately 15 minutes, oxygen consumption was measured over 15 minutes using a VMax Encore 20 metabolic cart (Carefusion, San Diego, CA, USA), with the last 5 minutes averaged for the determination of resting oxygen consumption.30
Statistical Analysis
With the exception of proteomic and nitrosylation data, statistical differences between groups were assessed using unpaired t‐tests, either in Microsoft Excel (Microsoft, Redmond, WA, USA) (for the subject characteristics in Table S1) or GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) (for the data presented in Figures 1, 2, 3), with an α level of 0.05. Basal metabolic rate before and after treatment was compared using paired t‐tests in GraphPad Prism 5.
Figure 2.
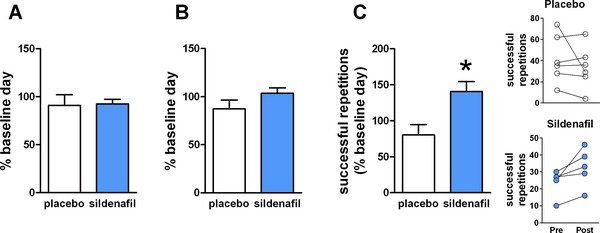
Effects of sildenafil treatment on skeletal muscle function. (A) Isometric strength of knee extensors (mean percent baseline day ± standard error (SE)) after 8 days of treatment, determined using dynamometry. (B) Isokinetic (120° per second) strength of knee extensors (mean percent baseline day ± SE) after 8 days of treatment, determined using dynamometry. (C) Successful repetitions (mean percent baseline day ± SE) during fatiguing isokinetic (120° per second) contractions after 8 days of treatment. *p = 0.016 vs. placebo, unpaired t‐test, N = 6 placebo, 5 sildenafil. Individual numbers of successful repetitions before (pre) and after (post) treatment for those receiving placebo (upper panel) and sildenafil (lower panel) are shown at right.
Figure 3.
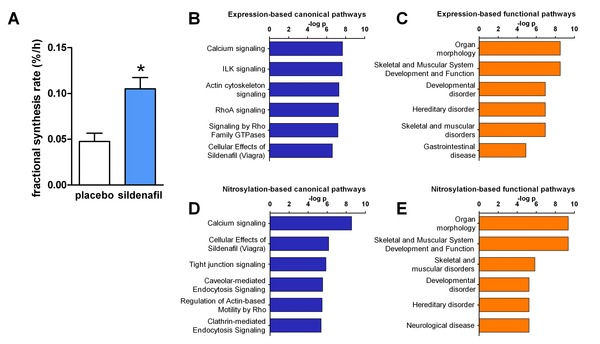
Effects of sildenafil treatment on skeletal muscle proteome. (A) Skeletal muscle protein synthesis (mean ± SE) after 8 days of treatment, determined using the precursor‐product approach to determine fractional synthesis rate. *p = 0.004 vs. placebo, unpaired t‐test, N = 6 placebo, 5 sildenafil. Canonical (B) and functional (C) pathways differentially affected by sildenafil and placebo, determined using Ingenuity Pathways Analysis (IPA) of protein expression in skeletal muscle biopsy samples (top 6 pathways shown). Canonical (D) and functional (E) pathways differentially affected by sildenafil and placebo, determined using IPA of protein S‐nitrosylation in skeletal muscle biopsy samples (top six pathways shown).
Results
Sildenafil reduces muscle fatigue
Maximal isometric torque production (Figure 2A) and maximal isokinetic (120° per second) power (Figure 2B) of the knee extensors were stable over the course of the study and did not vary according to treatment. Skeletal muscle fatigue on the initial day of treatment (day 1) was not statistically different from baseline or different between treatment groups (data not shown). However, following 8 days of treatment, subjects in the sildenafil group completed significantly more successful repetitions relative to baseline than those receiving placebo during repeated maximal isokinetic contractions (Figure 2C).
Sildenafil remodels the skeletal muscle proteome
Analyses of skeletal muscle biopsy samples identified 30 and 42 proteins, respectively, exhibiting differential abundance or S‐nitrosylation in individuals receiving sildenafil versus those receiving placebo (Table S1), suggesting that skeletal muscle remodeling occurs in response to short‐term sildenafil administration. Consistent with this notion, skeletal muscle protein synthesis was significantly higher in those receiving sildenafil (Figure 3A).
Functional and canonical pathways associated with sildenafil treatment
Ingenuity Pathways Analysis (Ingenuity Systems, Inc., Redwood City, CA, USA) identified canonical and functional pathways differentially affected by treatment (Figure 3B–E) using the differentially abundant or nitrosylated proteins which met threshold criteria for protein identification. The functional analysis suggested the differences in abundance and nitrosylation were linked to differences in morphology, development, and function of skeletal muscle, in agreement with the observed changes in protein synthesis and fatigue. Calcium signaling was identified as the canonical pathway most different between the placebo and sildenafil groups, using either the protein expression or the protein nitrosylation data.
Discussion
In this study we found that short‐term treatment with the phosphodiesterase 5 inhibitor sildenafil reduces skeletal muscle fatigue and stimulates skeletal muscle protein synthesis while altering muscle protein expression and nitrosylation. Our findings thus provide evidence that sildenafil remodels human skeletal muscle in a functionally adaptive manner.
As a determinant of the amount and composition of skeletal muscle proteins, protein synthesis can affect skeletal muscle function through changes in both the mass and quality of muscle. The differences between treatment groups in protein abundance and S‐nitrosylation observed in this study suggest that changes in myofibrillar function and actin dynamics may have contributed to an increase in muscle quality, manifested as increased fatigue resistance, in individuals receiving sildenafil. Notably, the approximate doubling of skeletal muscle protein synthesis observed in response to sildenafil is of similar magnitude to that observed in response to 100–200 mg/week testosterone injection,31, 32, 33 an intervention which increases skeletal muscle mass and strength32, 33, 34 but does not reduce muscle fatigability,32, 35 with long‐term administration. Whether the short‐term responses of protein synthesis and expression eventually manifest as a change in muscle mass or simply reflect an adaptive qualitative change in muscle protein composition will be important to determine in future longer‐term studies.
NO‐cGMP signaling has been proposed to mediate skeletal muscle mitochondrial biogenesis,36 a hallmark adaptation to chronic endurance‐type training37, 38 thought to contribute to skeletal muscle fatigue resistance, as well as skeletal muscle glucose uptake.39, 40, 41, 42 However, glucose tolerance in this group of healthy men, who were prescreened for normal fasting glucose concentrations, did not change in either treatment group over the course of the study (Figure S1 A and B). Similarly, the proteomic analyses did not suggest that mitochondrial biogenesis contributed to the reduced skeletal muscle fatigability in the sildenafil group following treatment. These findings, along with the fact that the short intervention period likely represents the lower threshold of time for the occurrence or detection of changes in mitochondrial abundance, argue against mitochondrial biogenesis being a major contributor to the reduced fatigability observed in response to short‐term sildenafil therapy. Notably, NO‐cGMP signaling has also been associated with mitochondrial biogenesis in brown adipocytes10 and short‐term high‐dose sildenafil treatment has been reported to induce “browning” of white adipocytes in mice.43 Longer‐term (12 weeks) treatment at the same dose increased metabolic rate and reduced weight gain during high‐fat feeding, although changes in brown fat mitochondria were not observed.44 In this study, resting metabolic rate was significantly increased in response to sildenafil treatment, whereas in the control group metabolic rate did not change (Figure S1C and D). The relative contributions of the increased rate of protein synthesis, an energetically expensive process,45, 46 or a potential phenotypic remodeling of adipose tissue to the sildenafil‐induced increase in metabolic rate cannot be determined from our data but may be important to consider in future studies in the context of obesity.
Several limitations of the study, as well as ramifications for possible future application in clinical settings, should be considered when interpreting the results of this study. As noted above, the short‐term nature of the study prevents conclusions regarding possible effects of chronic sildenafil therapy on muscle mass or mitochondrial biogenesis. Such endpoints, as well as the possibility of reduced effectiveness with chronic use (tachyphylaxis), will need to be addressed in longer‐term studies. On the other hand, the response observed to approximately one week of sildenafil therapy suggests sildenafil may have potential for use in short‐term rehabilitation settings. Mechanistically, sildenafil may have induced changes in other mediators of fatigue, such as excitation‐contraction coupling, redox status, and muscle perfusion, which could have contributed to the current findings and warrant investigation in future studies. In addition, it is worth noting that the observed reduction in muscle fatigability occurred during exercise of a relatively small muscle mass (the knee extensors); it cannot be extrapolated based on the findings of this study that performance or fatigability will necessarily be improved by sildenafil during exercise types in which maximal cardiac output is a limiting factor. Finally, although significant effects were observed with low‐dose (25mg) sildenafil in this study of healthy males, dose–response relationships in healthy and diseased populations of men and women may be different.
Conclusion
Despite massive outlays of human and economic resources, skeletal muscle dysfunction has persisted as an intractable hallmark of numerous inherited (e.g., Duchenne or Becker type muscular dystrophies) or acquired (e.g., cachexia, sarcopenia, dynapenia, bedrest, steroid) myopathies, with few options (apart from exercise) for treatment. As a drug already approved and with an excellent safety record, the findings from this study suggest that sildenafil, and possibly other phosphodiesterase 5 inhibitors,4 represents a potential pharmacologic strategy to improve skeletal muscle function.
Supporting information
Supporting Information Table
Supporting Information Figure
Acknowledgments
This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch (supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health), a pilot grant (to W.J.D.) from the Claude D. Pepper Older Americans Independence Center (5P30‐AG024832), and grants from the National Institutes of Health/National Institute on Aging (R01 AG21539, to M.S.M.) and National Cancer Institute (5R01CA127971, to M.S.M.).
References
- 1. Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, Guralnik JM, Ferrucci L. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009; 64(3): 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006; 61(1): 72–77. [DOI] [PubMed] [Google Scholar]
- 3. Manini, TM , Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012; 67(1): 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin EA, Barresi R, Byrne BJ, Tsimerinov EI, Scott BL, Walker AE, Gurudevan SV, Anene F, Elashoff RM, Thomas GD, et al. Tadalafil alleviates muscle ischemia in patients with becker muscular dystrophy. Sci Transl Med. 2012; 4(162): 162ra155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, Drummond MJ, Sheffield‐Moore M, Rasmussen BB, Volpi E. Pharmacological vasodilation improves insulin‐stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010; 59(11): 2764–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dillon EL, Casperson SL, Durham WJ, Randolph KM, Urban RJ, Volpi E, Ahmad M, Kinsky MP, Sheffield‐Moore M. Muscle protein metabolism responds similarly to exogenous amino acids in healthy younger and older adults during NO‐induced hyperemia. Am J Physiol Regul Integr Comp Physiol. 2011; 301(5): R1408–R1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito N, Ruegg UT, Kudo A, Miyagoe‐Suzuki Y, Takeda S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med. 2013; 19: 101–106. [DOI] [PubMed] [Google Scholar]
- 8. Brunelli S, Sciorati C, D'Antona G, Innocenzi A, Covarello D, Galvez BG, Perrotta C, Monopoli A, Sanvito F, Bottinelli R, et al. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci U S A. 2007; 104(1): 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buono R, Vantaggiato C, Pisa V, Azzoni E, Bassi MT, Brunelli S, Sciorati C, Clementi E. Nitric oxide sustains long‐term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring Vangl2 and cyclic GMP. Stem Cells. 2012; 30(2): 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 2003; 299(5608): 896–899. [DOI] [PubMed] [Google Scholar]
- 11. Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, Aranda JM, Sandesara BD, Pahor M, Manini TM. et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high‐ and low‐functioning elderly individuals. Aging Cell. 2012; 11(5): 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, Debeer J, Tarnopolsky MA. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS One. 2010; 5(5): e10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dillon EL, Volpi E, Wolfe RR, Sinha S, Sanford AP, Arrastia CD, Urban RJ, Casperson SL, Paddon‐Jones D, Sheffield‐Moore M. Amino acid metabolism and inflammatory burden in ovarian cancer patients undergoing intense oncological therapy. Clin Nutr. 2007; 26(6): 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. Muscle oxidative capacity during IL‐6‐dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2011; 300(2): R201–R211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase‐deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 2000; 97(25): 13818–13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995; 82(5): 743–752. [DOI] [PubMed] [Google Scholar]
- 17. Percival JM, Anderson KN, Gregorevic P, Chamberlain JS, Froehner SC. Functional deficits in nNOSmu‐deficient skeletal muscle: myopathy in nNOS knockout mice. PLoS One. 2008; 3(10): e3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol. 2012; 228(1): 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, et al. Sarcolemma‐localized nNOS is required to maintain activity after mild exercise. Nature. 2008; 456(7221): 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giuliano F, Jackson G, Montorsi F, Martin‐Morales A, Raillard P. Safety of sildenafil citrate: review of 67 double‐blind placebo‐controlled trials and the postmarketing safety database. Int J Clin Pract. 2010; 64(2): 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Durham WJ, Aracena‐Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, Galvan DL, Gilman CP, Baker MR, Shirokova N, et al. RyR1 S‐nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell 2008; 133(1): 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 2009; 15(3): 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, et al. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A 2008; 105(6): 2198–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samengo G, Avik A, Fedor B, Whittaker D, Myung KH, Wehling‐Henricks M, Tidball JG. Age‐related loss of nitric oxide synthase in skeletal muscle causes reductions in calpain S‐nitrosylation that increase myofibril degradation and sarcopenia. Aging Cell. 2012; 11(6): 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butt E, Bernhardt M, Smolenski A, Kotsonis P, Frohlich LG, Sickmann A, Meyer HE, Lohmann SM, Schmidt HH. Endothelial nitric‐oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide‐dependent protein kinases. J Biol Chem. 2000; 275(7): 5179–5187. [DOI] [PubMed] [Google Scholar]
- 26. Gebska MA, Stevenson BK, Hemnes AR, Bivalacqua TJ, Haile A, Hesketh GG, Murray CI, Zaiman AL, Halushka MK, Krongkaew N, et al. Phosphodiesterase‐5A (PDE5A) is localized to the endothelial caveolae and modulates NOS3 activity. Cardiovasc Res. 2011; 90(2): 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakurada M, Shichiri M, Imamura M, Azuma H, Hirata Y. Nitric oxide upregulates dimethylarginine dimethylaminohydrolase‐2 via cyclic GMP induction in endothelial cells. Hypertension. 2008; 52(5): 903–909. [DOI] [PubMed] [Google Scholar]
- 28. Wolfe RR. C.D.: Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. 2nd edn New York: Wiley‐Liss; 2005. [Google Scholar]
- 29. Wiktorowicz JE, Stafford S, Rea H, Urvil P, Soman K, Kurosky A, Perez‐Polo JR, Savidge TC. Quantification of cysteinyl S‐nitrosylation by fluorescence in unbiased proteomic studies. Biochemistry. 2011; 50(25): 5601–5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Compher C, Frankenfield D, Keim N, Roth‐Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006; 106(6): 881–903. [DOI] [PubMed] [Google Scholar]
- 31. Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998; 275(5 Pt 1): E864–E871. [DOI] [PubMed] [Google Scholar]
- 32. Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995; 269(5 Pt 1): E820–E826. [DOI] [PubMed] [Google Scholar]
- 33. Sheffield‐Moore M, Dillon EL, Casperson SL, Gilkison CR, Paddon‐Jones D, Durham WJ, Grady JJ, Urban RJ. A randomized pilot study of monthly cycled testosterone replacement or continuous testosterone replacement versus placebo in older men. J Clin Endocrinol Metab. 2011; 96(11): E1831–E1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, et al. Testosterone dose‐response relationships in healthy young men. Am J Physiol Endocrinol Metab 2001; 281(6): E1172–81. [DOI] [PubMed] [Google Scholar]
- 35. Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, Casaburi R, Bhasin S. Testosterone dose‐dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab 2003; 88(4): 1478–85. [DOI] [PubMed] [Google Scholar]
- 36. Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci U S A 2004; 101(47): 16507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fitts RH, Booth FW, Winder WW, Holloszy JO. Skeletal muscle respiratory capacity, endurance, and glycogen utilization. Am J Physiol 1975; 228(4): 1029–33. [DOI] [PubMed] [Google Scholar]
- 38. Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 1984; 56(4): 831–8. [DOI] [PubMed] [Google Scholar]
- 39. Balon TW, Nadler JL. Nitric oxide mediates skeletal glucose transport. Am J Physiol 1996; 270(6 Pt 1): E1058–9. [DOI] [PubMed] [Google Scholar]
- 40. Baron AD. The coupling of glucose metabolism and perfusion in human skeletal muscle. The potential role of endothelium‐derived nitric oxide. Diabetes 1996; 45 Suppl 1: S105–S109. [DOI] [PubMed] [Google Scholar]
- 41. Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon‐like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide‐dependent mechanism. Diabetes. 2012; 61(4): 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, et al. Impaired insulin signaling in endothelial cells reduces insulin‐induced glucose uptake by skeletal muscle. Cell Metab. 2011; 13(3): 294–307. [DOI] [PubMed] [Google Scholar]
- 43. Mitschke MM, Hoffmann LS, Gnad T, Scholz D, Kruithoff K, Mayer P, Haas B, Sassmann A, Pfeifer A, Kilic A. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 2013; 27(4): 1621–1630. [DOI] [PubMed] [Google Scholar]
- 44. Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat‐fed conscious mice. Diabetes 2007; 56(4): 1025–1033. [DOI] [PubMed] [Google Scholar]
- 45. Biggar KK, Storey KB. The emerging roles of microRNAs in the molecular responses of metabolic rate depression. J Mol Cell Biol. 2011; 3(3): 167–175. [DOI] [PubMed] [Google Scholar]
- 46. Reeds PJ, Wahle KW, Haggarty P. Energy costs of protein and fatty acid synthesis. Proc Nutr Soc 1982; 41(2): 155–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table
Supporting Information Figure


