Abstract
Insulin-like growth factor 2 (Igf2) regulates development, memory and adult neurogenesis in the hippocampus. Calorie restriction (CR) is known to modulate non-neuronal Igf2 expression intergenerationally, but its effect has not been evaluated on brain Igf2. Here, Sprague-Dawley (S) dams underwent moderate CR between gestational days 8–21. To identify parent of origin expression pattern of the imprinted Igf2 gene, their offspring (SS F1) were mated with naïve male or female Brown Norway (B) rats to obtain the second generation (BS and SB F2) progeny. CR did not affect adult hippocampal Igf2 transcript levels in SS F1 males or their BS F2 progeny, but increased it in SS F1 females and their SB F2 offspring. The preferentially maternal Igf2 expression in the SB F2 control male hippocampus relaxed to biallelic with CR, with no effect of grandmaternal diet in any other groups. Thus, allele-specific and total expression of hippocampal Igf2 is affected by maternal, grandmaternal CR in a strain and sex-specific manner.
Keywords: allele-specific expression, imprinting, Sprague-Dawley rat, Brown Norway rat, sex-specific, matrilineal, grandmaternal calorie restriction
Introduction
Insulin-like growth factor 2 (Igf2) is involved in growth and development (for review see Bergman et al. (2013)). Among its effects on the CNS, Igf2 increases the survival of 17–19 days old neurons in the hippocampus while also promoting neural stem cell proliferation (Agis-Balboa et al., 2011; Bracko et al., 2012). Additionally, the effects of Igf2 on adult hippocampal neurogenesis is connected to hippocampus based learning and memory (Agis-Balboa et al., 2011). Upregulation of hippocampal Igf2 plays an important role in extinction of contextual fear memory and increases memory retention in an inhibitory avoidance task (Agis-Balboa et al., 2011; Chen et al., 2011).
Igf2 is an imprinted gene; expressed from the paternal allele under the control of a differentially methylated region (Bergman et al., 2013). Interestingly, though Igf2 is maternally imprinted in the embryonic brain, it becomes paternally imprinted in specific regions of the adult human and mouse brain, i.e. the globus pallidus and hypothalamus (Gregg et al., 2010; Pham et al., 1998). Since changes in imprinting status can alter transcript levels of imprinted genes (Sittig et al., 2011), conditions that change the imprinting status of Igf2 could have lasting effects on learning and memory by affecting the transcript levels. Gestational nutrition might be one such condition, since rodents born of diabetic mothers show altered non-neuronal Igf2 levels with concomitant increased methylation (Ding et al., 2012), while decreased methylation alters non-neuronal IGF2 transcript levels in humans born during famine (Heijmans et al., 2008). Moreover, gestational methylation changes at the Igf2 locus has been shown to be transferrable to the second generation (Ding et al., 2012; Stouder et al., 2011), evoking the possibility of intergenerational inheritance of Igf2-related neurodevelopmental deficits.
Our goal is to determine the intergenerational effect of calorie restriction (CR) on total and allele-specific expression of Igf2 in the rat hippocampus. To achieve this, offspring of Sprague-Dawley (S) dams, with and without CR, were mated with naïve Brown Norway (B) male and female rats (Figure 1) to distinguish the maternal or paternal transmission of grandmaternal CR on hippocampal Igf2 expression in the second generation. Additionally, allele-specific expression of hippocampal Igf2 could be measured on the SB and BS F2 progeny using this mating paradigm. Moderate maternal CR during pregnancy increased hippocampal total expression of Igf2 in the female SS F1 offspring, which was transferred to their SB F2 progeny. Furthermore, the preferentially maternal expression of hippocampal Igf2 relaxed to biallelic expression in SB F2 control male offspring with grandmaternal CR, with no other group showing this effect. Therefore, allele-specific and total expression of hippocampal Igf2 are affected by maternal and grandmaternal CR in a sex-specific manner.
Figure 1. Schematic experimental design.
Sprague Dawley (S) females were mated with S males. From gestational day 8 through 20 these dams were exposed to one of 2 prenatal treatments (Control or Calorie Restricted). The resulting SS F1 males and females were mated with naive female and male Brown Norway (B) rats to generate the BS F2 and SB F2 progeny, respectively.
Materials and Methods
The Northwestern University Animal Care and Use Committee approved all procedures. After mating male and female S rats overnight (Harlan, Indianapolis, IN, USA), sperm positive vaginal smear marked gestational day one (GD1). Pregnant females were divided between control (C), laboratory rat chow and water ad libitum, and CR groups. From GD4-8, CR rats were provided with water and a liquid diet (Lieber-DeCarli ’82; Bio-Serv. Frenchtown, NJ, USA) ad libitum to acclimatize them to the diet as described previously (Harper et al., 2014). From GD8-21, CR rats consumed an average of 21.8 to 26.8 kcal per 100 g−1 of body weight per day. This represents approximately 83–89% of the daily caloric intake of C dams, which is a mild CR with no significant body weight difference between the F1 C vs CR pups (Harper et al., 2014). At all other times regular laboratory chow and water were available ad libitum. One to two rats of each sex/litter/prenatal treatment of SS F1 offspring were mated with naïve B rats (Charles River, Wilmington, MA, USA) to generate SB and BS F2 progeny. B is the most phylogenetically divergent inbred rat strain (Swerdlow et al., 2008), and the B and S genomes have been sequenced by the Rat Genome Project and Celera, respectively. All of which increased the chances that we would be able to identify a single nucleotide polymorphism (SNP) between the B and S cDNAs at the Igf2 locus that we could use to measure allele-specific contribution to the expression of hippocampal Igf2.
Sample preparation and qPCR
At approximately 70 days of age, one-two rats of each sex/litter (N=9–12/group) were sacrificed for tissue collection. Brains were directly placed into RNAlater© (Life technologies, Grand Island, NY). Whole hippocampus was dissected as previously described (Sittig et al., 2011). RNA was isolated by the Trizol© reagent (Life technologies, Grand Island, NY), then converted to cDNA by ABI Reverse Transcription kit (Foster City, CA). For qPCR, SYBR Green PCR Master Mix (ABI, Foster City, CA) was used on the ABI Prism 7300. Reactions were performed in triplicate and ΔΔCt method was employed using 18S (primers from Ambion, Grand Island, NY) as endogenous control. Igf2 primers were; forward CCGTACTTCCGGACGACTTC and reverse CGTCCCGCGGACTGTCT.
Pyrosequencing
A SNP of A/G at Chromosome 1:222725126 bp in the 3′ untranslated region of Igf2 was identified between the B (A) and S (G) strains (rs8143502) by sequencing, and the SNP was confirmed in the SB and BS F2 offspring. Both forward and biotinylated reverse primers, that flank the SNP, were designed by EpigenDx (Worchester, MA, USA). After PCR, the purification and pyrosequencing of the PCR product were carried out by EpigenDx, which gave the percentage of the A vs G allele in the Igf2 (N=3–4/sex/cross/prenatal treatment). In the reciprocal F2 crosses, the maternal contribution to Igf2, “A” in BS F2 and “G” in SB F2, is shown.
Statistical analysis
Data were analyzed by two-way ANOVA (sex and prenatal treatment) for the F1 generation and three-way ANOVA (cross, sex and prenatal treatment) for the F2 generation. The F2 generation was also analyzed by two-way ANOVA for hypothesis testing. When appropriate, Bonferroni post-hoc comparisons were made. p<.05 was considered statistically significant. Statistical analyses were carried out using Systat 11 (Chicago, IL, USA).
Results
Female SS F1 offspring had significantly greater hippocampal Igf2 levels than males (sex: F1, 34=12.25, p<.01; Figures 2A & B), and CR caused further increases in these levels in females (prenatal treatment: F1,34=2.85, p=.10; sex X prenatal treatment: F1,34=3.57, p=.068; female C vs CR p<.05). There was no effect of CR on hippocampal Igf2 levels in SS F1 male progeny.
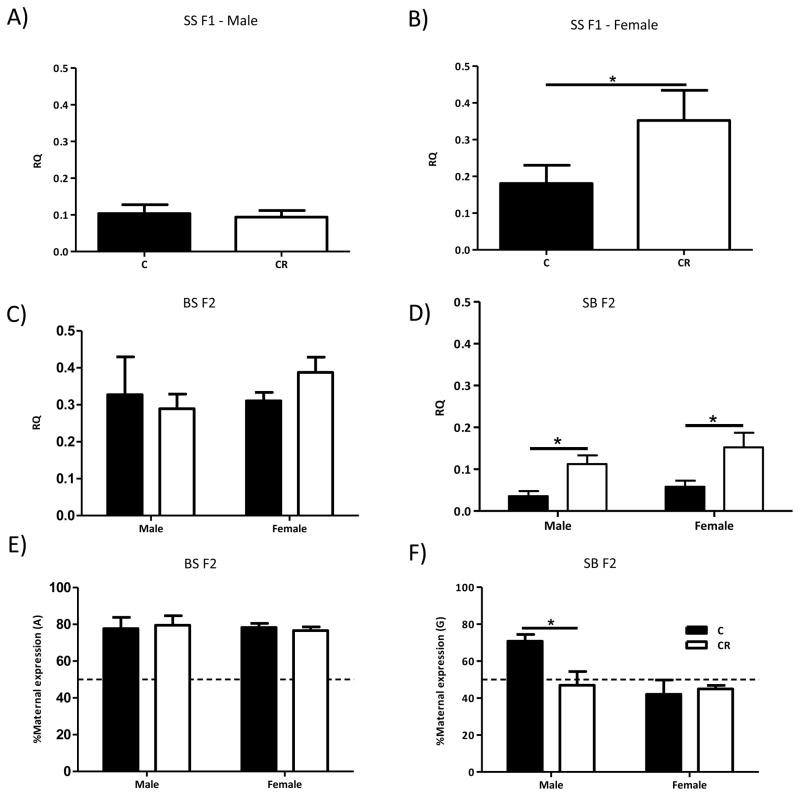
Figure 2. Calorie restriction (CR) during pregnancy alters total and allele-specific expression of hippocampal Igf2 only through the female line.
A) CR has no effect on Igf2 transcript levels in adult SS F1 male offspring, while B) SS F1 female offspring of CR dams have a significant increase in hippocampal Igf2 transcript levels. C) Grandmaternal CR has no effect on hippocampal Igf2 expression in adult BS F2 male and female progeny, and D) Grandmaternal CR results in increased hippocampal Igf2 in SB F2 male and female offspring. E) In BS F2 offspring, hippocampal Igf2 is preferentially expressed from the maternal allele with no changes by grandmaternal CR, but F) Grandmaternal CR affects allele-specific expression only in the male SB F2 animals. Data are represented as mean +/− SEM. qPCR experiments: N=9–12/group; pyrosequencing N=3–4/group.
In the second generation, a significant difference in Igf2 transcript levels was observed between SB vs BS (cross: F1,37=84.83, p<.01), due to higher Igf2 expression in the BS F2 progeny (Figure 2C & D). Grandmaternal treatment affected only the SB F2 progeny (prenatal treatment: F1,37=3.09, p=.09; cross X prenatal treatment: F1,37=1.05, p=.31; sex: F1,37=2.45, p=.13; sex X prenatal treatment: F1,37=2.123, p=.15; sex X cross: F1,37=.001, p=.97; sex X prenatal treatment X cross: F1,37=.50, p=.49). A within cross two-way ANOVAs revealed that grandmaternal CR had no effect on the BS F2 progeny (prenatal treatment: F1,15=.13, p=.73), while SB F2 CR offspring have significantly increased hippocampal Igf2 levels compared to C (prenatal treatment: F1,20=16.17, p<.01; Figure 2D).
By using the A/G SNP, a significant difference in allele-specific expression between the crosses (cross: F1,17=45.74, p<.01) and a trend towards a cross-specific prenatal treatment effect (cross X prenatal treatment X sex F1,17=3.63, p=.074; Figures 2E & F) on hippocampal Igf2 were found. In general, Igf2 showed similar, preferentially maternal expression in both male and female, C and CR BS F2 hippocampi (prenatal treatment: F1,7=2.26e-005, p=1.0; Figure 2E). In contrast, although SB F2 C male hippocampus also exhibited preferentially maternal Igf2 expression, this maternal expression became biallelic in the male SB grandoffspring of CR grandmothers. Moreover, female SB F2 progeny of both C and CR grandmothers showed biallelic hippocampal expression of Igf2 (prenatal treatment X sex: F1,10=5.15, p<.05; Figure 2F).
Discussion
Here we describe for the first time a sex and lineage-specific effect of calorie restriction on hippocampal Igf2 expression. Specifically, only females and their progeny responded by increased expression of hippocampal Igf2 to maternal or grandmaternal CR. Furthermore, the matrilineal male offspring of grandmothers on calorie restricted diet during pregnancy show biallelic hippocampal Igf2 expression in contrast to the preferential maternal expression of control male grandoffspring.
Maternal diet affects fetal growth directly, by determining the amount of nutrients available, and epigenetically modulating gene activity in the fetus. Nutritional changes during critical periods of gestation may have long-lasting effects on progeny. Igf2 is a very important growth-regulatory imprinted gene, and its alteration by maternal nutrition has been studied thoroughly. In humans, famine during pregnancy causes persistent hypomethylation of Igf2, leading to biallelic expression in plasma (Heijmans et al., 2008), which is still present 60 years after famine exposure. In contrast, a high sugar/fat diet during pregnancy increases placental Igf2 expression (Sferruzzi-Perri et al., 2013). These results indicate that various nutritional changes during pregnancy can alter imprinting of Igf2, and consequentially Igf2 transcript levels in various tissues in the offspring.
To our knowledge, this is the first report of adult hippocampal Igf2 imprinting, which is consistent with the 80% preferential maternal expression found in the adult mouse hypothalamic preoptic area and medial prefrontal cortex (Gregg et al., 2010). In the present study, however, we found that both cross and sex affects allelic expression of hippocampal Igf2, such that only SB females exhibited biallelic expression. Such sex and cross-specific parent of origin effect is found for hippocampal Dio3 gene in the BS and SB (F1) offspring (Sittig et al., 2011). In that case, the cross and sex specific changes in the imprinting of Dio3 led, in the adults, to behavioral vulnerabilities to adverse prenatal conditions, which might occur in response to changes in Igf2 imprinting status as well.
Our results have implications for nutritional effects on hippocampus based learning and memory. Increases in hippocampal Igf2 expression immediately after training in both the avoidance learning task and contextual fear memory lead to increased memory retention (Chen et al., 2011), but during extinction training in the contextual fear memory task it allows for normal extinction (Agis-Balboa et al., 2011). Additional behavioral changes, due to increased hippocampal Igf2 expression, are possible. For example, a placental knockout mouse model of Igf2 with limited nutrition in utero shows increased anxiety in adulthood (Mikaelsson et al., 2013). However, a disconnect between decreased hippocampal Igf2 expression and anxiety-like behavior has also been shown in a study using a mouse model of a schizophrenia-associated disorder. Still, deficits in adult neurogenesis and working memory in this model are restored by Igf2 administration (Ouchi et al., 2013). Thus, higher basal Igf2 expression could be beneficial for the retention of short-term memory, and thus female offspring, and their progeny, of CR dams might show superior performance on hippocampal based learning and memory tasks, such as contextual fear conditioning. Interestingly, moderate CR in baboons during gestation and lactation has been shown to improve cognitive performance in female offspring (Rodriguez et al., 2012), which is in agreement with our predictions based on the Igf2 expression changes in female offspring. It is important to emphasize that both the baboon study and the present one employed CR and not a nutritional restriction. In CR, animals are maintained on the proper nutrients and vitamins needed for a healthy diet, but have a reduced daily calorie consumption.
The prevalence of metabolic disorders, including obesity and hyperglycemia, in women of childbearing years is on the rise in the US (Ramos and Olden, 2008), indicating an increase in pregnancies at risk for altered intrauterine environments. Although we administered only a moderate CR during pregnancy, we still saw a positive effect on hippocampal Igf2 expression in a sex specific manner. Should our future research confirm that natural prenatally programmed changes in hippocampal Igf2 levels are beneficial to the mother and her progeny, the present data would significantly contribute to our understanding of how early epigenetic changes effect adult cognition and behavior.
Acknowledgments
We would like to thank NM for giving us helpful advice, and BA and TU for assisting in setting up initial breeding.
Funding
Funding for this research provided by National Institute on Alcohol Abuse and Alcoholism grant AA017978.
Footnotes
Conflict of Interest
The authors have no conflict of interest.
Contributors
Conceived and designed the experiments: EER. Identified and confirmed SNP: LBKH. Performed the experiments: KMH, ETO, ENG. Analyzed the data: KMH. Wrote the manuscript: KMH. Edited and revised manuscript: KMH, ETO, ENG, EER, LBKH. Approved final version of the manuscript: KMH, ETO, ENG, EER, LBKH
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agis-Balboa RC, Arcos-Diaz D, Wittnam J, Govindarajan N, Blom K, Burkhardt S, Haladyniak U, Agbemenyah HY, Zovoilis A, Salinas-Riester G, Opitz L, Sananbenesi F, Fischer A. A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J. 2011;30:4071–4083. doi: 10.1038/emboj.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman D, Halje M, Nordin M, Engstrom W. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology. 2013;59:240–249. doi: 10.1159/000343995. [DOI] [PubMed] [Google Scholar]
- Bracko O, Singer T, Aigner S, Knobloch M, Winner B, Ray J, Clemenson GD, Jr, Suh H, Couillard-Despres S, Aigner L, Gage FH, Jessberger S. Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J Neurosci. 2012;32:3376–3387. doi: 10.1523/JNEUROSCI.4248-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding GL, Wang FF, Shu J, Tian S, Jiang Y, Zhang D, Wang N, Luo Q, Zhang Y, Jin F, Leung PC, Sheng JZ, Huang HF. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61:1133–1142. doi: 10.2337/db11-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KM, Tunc-Ozcan E, Graf EN, Redei EE. Intergenerational Effects of Prenatal Ethanol on Glucose Tolerance and Insulin Response. Physiol Genomics. 2014 doi: 10.1152/physiolgenomics.00181.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaelsson MA, Constancia M, Dent CL, Wilkinson LS, Humby T. Placental programming of anxiety in adulthood revealed by Igf2-null models. Nat Commun. 2013;4:2311. doi: 10.1038/ncomms3311. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Banno Y, Shimizu Y, Ando S, Hasegawa H, Adachi K, Iwamoto T. Reduced adult hippocampal neurogenesis and working memory deficits in the Dgcr8-deficient mouse model of 22q11.2 deletion-associated schizophrenia can be rescued by IGF2. J Neurosci. 2013;33:9408–9419. doi: 10.1523/JNEUROSCI.2700-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham NV, Nguyen MT, Hu JF, Vu TH, Hoffman AR. Dissociation of IGF2 and H19 imprinting in human brain. Brain Res. 1998;810:1–8. doi: 10.1016/s0006-8993(98)00783-5. [DOI] [PubMed] [Google Scholar]
- Ramos RG, Olden K. The prevalence of metabolic syndrome among US women of childbearing age. Am J Public Health. 2008;98:1122–1127. doi: 10.2105/AJPH.2007.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JS, Bartlett TQ, Keenan KE, Nathanielsz PW, Nijland MJ. Sex-dependent cognitive performance in baboon offspring following maternal caloric restriction in pregnancy and lactation. Reprod Sci. 2012;19:493–504. doi: 10.1177/1933719111424439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Vaughan OR, Haro M, Cooper WN, Musial B, Charalambous M, Pestana D, Ayyar S, Ferguson-Smith AC, Burton GJ, Constancia M, Fowden AL. An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. FASEB J. 2013;27:3928–3937. doi: 10.1096/fj.13-234823. [DOI] [PubMed] [Google Scholar]
- Sittig LJ, Herzing LB, Shukla PK, Redei EE. Parent-of-origin allelic contributions to deiodinase-3 expression elicit localized hyperthyroid milieu in the hippocampus. Mol Psychiatry. 2011;16:786–787. doi: 10.1038/mp.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouder C, Somm E, Paoloni-Giacobino A. Prenatal exposure to ethanol: a specific effect on the H19 gene in sperm. Reprod Toxicol. 2011;31:507–512. doi: 10.1016/j.reprotox.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Breier M, Mora AB, Ko D, Shoemaker JM. A novel rat strain with enhanced sensitivity to the effects of dopamine agonists on startle gating. Pharmacol Biochem Behav. 2008;88:280–290. doi: 10.1016/j.pbb.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]