Abstract
Galanin-like peptide (GALP) neurons participate in the metabolic control of reproduction and are targets of insulin and leptin regulation. Phosphoinositide 3-kinase (PI3K) is common to the signaling pathways utilized by both insulin and leptin. Therefore, we investigated whether PI3K signaling in neurons expressing GALP plays a role in the transcriptional regulation of the GALP gene and in the metabolic control of luteinizing hormone (LH) release. To this end, we deleted PI3K catalytic subunits, p110α and p110β via conditional gene targeting (cKO) in mice (GALP-p110α/β cKO). To monitor PI3K signaling in GALP neurons these animals were also crossed with Cre-dependent FoxO1GFP reporter mice. Compared to insulin-infused control animals, the PI3K-Akt-dependent FoxO1GFP nuclear exclusion in GALP neurons was abolished in GALP-p110α/β cKO mice. We next used food deprivation to investigate if the GALP-neuron specific ablation of PI3K activity affected the susceptibility of the gonadotropic axis to negative energy balance. Treatment did not affect LH levels in either sex. However, a significant genotype effect on LH levels was observed in females. In contrast, no genotype effect on LH levels was observed in males. A sex-specific genotype effect on hypothalamic GALP mRNA was observed, with fed and fasted GALP-p110α/β cKO males having lower GALP mRNA expression compared to WT fed males. Finally, the effects of gonadectomy and steroid hormone replacement on GALP mRNA levels were investigated. Compared to vehicle-treated mice, steroid hormone replacement reduced MBH GALP expression in WT and GALP-p110α/β cKO animals. In addition, within the castrated and vehicle-treated group and compared to WT, LH levels were lower in GALP-p110α/β cKO males. Double immunofluorescence using GALP-Cre/R26-YFP mice showed androgen and estrogen receptor co-localization within GALP neurons. Our data demonstrate that GALP neurons are direct targets of steroid hormones and that PI3K signaling regulates hypothalamic GALP mRNA expression and LH levels in a sex-specific fashion.
Keywords: GALP, LH, PI3K, metabolism, hypothalamus
Introduction
Galanin-like peptide (GALP) belongs to a growing list of neuropeptides that play a role in the central metabolic control of the reproductive axis. GALP producing neurons are exclusively located in the arcuate nucleus of the hypothalamus (ARC) and in the median eminence (ME), which are pivotal areas for metabolic control and for regulating pituitary function, respectively [1–3]. Along with other neuropeptides such as kisspeptin, GALP can rescue or prevent the detrimental effects of negative energy balance on pubertal maturation and gonadotropin release [4]. For example, intracerebroventricular (icv) infusion of GALP reverses the suppressive effects of streptozotocin (STZ)-induced diabetes on luteinizing hormone (LH) levels and reproductive behavior in male rats [5]. Recent studies confirmed that the effects of GALP on puberty onset and gonadotropin release depend on the metabolic status of the animal. For instance, whereas icv GALP treatment does not affect puberty onset in male or female rats, GALP administration rescues pubertal delay in food-restricted weanling rats of both sexes [6].
The role of GALP in the interplay between metabolism and reproduction is also supported by the fact that models of nutrient deprivation are accompanied by a significant reduction in hypothalamic GALP mRNA expression [7]. In rats, STZ-induced type 1 diabetes and short-term food deprivation served as models to investigate the effects of a sharp reduction in the levels of peripheral metabolic cues, such as insulin and leptin, on GALP expression. In these models, insulin or leptin treatment not only normalizes LH levels and reproductive behavior, but also reverses the reduction of GALP mRNA expression caused by nutrient deprivation or diabetes [7, 8] [9]. A decrease in GALP mRNA levels is also observed in obese animal models in which leptin receptor signaling is impaired such as the Zucker obese rat [10], db/db mice [1], and the leptin deficient, ob/ob mice [1]. In the latter, leptin treatment increases the number of GALP expressing cells to levels similar to those in WT animals [1]. These studies support the role of insulin and leptin in maintaining hypothalamic GALP mRNA expression.
In addition to their stimulatory effects on GALP mRNA expression, leptin and insulin also share many of GALP’s neuroendocrine actions on the metabolic and reproductive axes. These include their effect on food intake (anorexic), support of the reproductive axis (i.e., increase in LH levels), and activation of the sympathetic nervous system [4, 11]. While these studies support GALP’s role as part of a hypothalamic relay system that conveys information about metabolic status to the reproductive axis, to date the molecular mechanisms underlying these effects are unknown. However, the common actions of insulin and leptin on GALP expression and their shared neuroendocrine functions have led to the proposal that a common signaling pathway might be responsible for the actions of leptin and insulin on GALP mRNA expression [12]. This signaling pathway may in turn contribute to GALP’s neuroendocrine effects on metabolism and reproduction. In the hypothalamus, the phosphoinositide 3-kinase (PI3K) signaling pathway is a primary example of a signal transduction pathway shared by insulin and leptin to regulate feeding, glucose homeostasis, and neuropeptide gene expression [13, 14].
PI3Ks are phospholipid enzymes that utilize phosphatidylinositol 4,5-biphosphate (PIP2) as the main substrate to generate the second messenger phosphatidylinositol 3,4,5-triphosphate (PIP3) [14]. Subsequently, plasma membrane PIP3 recruits and activates the serine/threonine kinase Akt which then activates or inhibits a number of cytosolic and nuclear proteins including transcription factors important for the control of insulin signaling and glucose metabolism. Class 1A PI3Ks are composed of a regulatory/adapter subunit (p85α, p85β, and p55γ) associated with a catalytic subunit (p110α, p110β, and p110δ) [14]. Deletion of the genes encoding class I PI3K catalytic or regulatory subunits in specific hypothalamic neurons such as pro-opiomelanocortin (POMC), agouti related peptide (AgRP), and SF1-expressing neurons, has confirmed the role of this enzyme as a key integrator of the central effects of insulin and leptin on feeding and metabolic control [15, 16].
In the present study we sought to determine whether PI3K signaling in GALP neurons is part of the downstream signaling mechanism(s) activated by peripheral metabolic cues that influence reproduction, such as insulin. In addition, we investigated the role of GALP-neuron-specific PI3K signaling in the regulation of hypothalamic GALP mRNA expression and in the metabolic control of LH release in male and female mice. To monitor the PI3K-Akt signaling pathway in GALP neurons, we crossed GALP-Cre mice with FoxO1GFP reporter mice. The transcription factor FoxO1 is target of the PI3K-Akt pathway and shuttles between the nucleus and the cytoplasm in response to inhibition and activation of Akt, respectively [17]. Thus, we monitored activation of PI3K in GALP neurons by insulin-induced nuclear export of this transcription factor fused to GFP. Then, we used conditional gene targeting to eliminate the expression of PI3K catalytic subunits p110α and p110β in GALP neurons (GALP-p110α/β cKO) and examined the metabolic and reproductive phenotype of male and female GALP-p110α/β cKO mice. To determine whether ablation of PI3K signaling in GALP neurons affects the susceptibility of the gonadotropic axis to negative energy balance, male and female GALP-p110α/β cKO mice were subjected to fasting and their metabolic and gonadotropic response compared to wild type (WT) littermates. Finally, because some of the phenotypes observed in GALP-p110α/β cKO animals were sex-specific, we also investigated if hypothalamic GALP mRNA levels were regulated by gonadectomy and steroid hormone replacement.
Materials and Methods
Animals
Animals were housed at Stony Brook University, Division of Laboratory Animal Resources (DLAR) under a 12-hr light, 12-hr dark cycle and had access to water and rodent chow ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at Stony Brook University Medical Center in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Generation of GALP-p110α/β cKO
All animals used were of a 129Sv-C57BL/6 mixed genetic background. To generate mice in which the genes for the PI3K catalytic subunits p110α (PIK3CA) and p110β (PIK3CB), are specifically deleted in GALP neurons, double floxed mice, p110αflx/flx / p110βflx/flx were mated with mice carrying the Cre transgene under the control of the GALP promoter (GALP-Cre) [18]. Double floxed animals bear loxP sites flanking exon 1 of the PIK3CA gene and exons 3 and 4 of the PIK3CB gene [19]. Mice generated by the first breeding were then intercrossed to generate GALPCre-p110αflx/flx /p110βflx/flx and GALPCre+p110αflx/flx /p110βflx/flx animals, referred to here as WT and GALP-p110α/β cKO, respectively. Animals were screened for the presence of Cre and floxed p110α and p110β by PCR of isolated genomic tail DNA as described previously [19, 20]. In addition, using genomic DNA from various tissues, including the hypothalamus, the presence or absence of Cre-mediated recombination was detected via PCR. Bands indicating deletion of both the p110α and the p110β alleles were gel extracted, subcloned and sequenced, to verify that they were indeed the expected PCR products.
Generation of FoxO1GFP-GALP mice
To monitor the activity of the PI3K-Akt signaling pathway in GALP neurons, FoxO1GFP reporter mice were crossed with GALP-CRE+ mice. FoxO1GFP is expressed under the control of a ubiquitous promoter that is silenced by a loxP flanked transcriptional blocker [21]. Thus, the resulting FoxO1GFP-GALP-CRE+ animals expressed the FoxO1GFP protein in GALP-CRE+ neurons only. To confirm that the deletion of PI3K catalytic subunits ablated PI3K activity, FoxO1GFP-GALP-CRE+ mice were crossed with GALP-p110α/β cKO animals (GALPCre+p110αflx/flx /p110βflx/flx). The resulting heterozygous animals were intercrossed until Cre+ mice homozygous for all three floxed alleles (FoxOGFP, p110α, and p110β) were obtained: FoxO1GFP-GALP/p110α/β cKO. The genotype of mice was obtained by PCR as described previously [21].
Effects of icv infusion of insulin on FoxO1GFP subcellular localization in GALP neurons
Adult FoxO1GFP-GALP mice were anesthetized with ketamine (80 mg/kg ip; Butler Schein Animal Health, Dublin, OH) and xylazine (32 mg/kg ip; LLOYD Laboratories, Shenandoah, IA), and stereotaxic surgery was used to target a guide cannula (Plastics One, Roanoke, VA) to the right lateral ventricle (coordinates: 0.34 mm caudal to Bregma, 2.5 mm ventral to the skull, 0.9 mm lateral). Six days later, correct placement of the cannula was verified by observing their response to icv infusion of 10 ng of angiotensin II. Mice were fasted overnight with ad libitum access to water. The following day animals were infused with human insulin (100 pmol, Humulin R; Eli Lilly Corp., Indianapolis, IN) or saline. Thirty minutes later, mice were intracardially perfused with 0.1M PBS followed by 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde overnight, followed by immersion in 30% sucrose cryoprotectant. Then, 25 μm sections were cut in series of 3 using a cryostat and placed in tissue culture wells containing cryoprotectant solution and stored at −20°C until processed for staining.
Immunofluorescence
Free floating sections were washed several times in PBS, then incubated for 48 hours at 4°C in a rabbit anti-GFP primary antibody (1:20,000, Invitrogen, Carlsbad, CA) with 0.25% Triton X-100 in 0.1M PBS. After washing in PBS, the tyramine signal amplified fluorescence method was used as previously described [22] using streptavidin conjugated Alexa 488 (Invitrogen).
To investigate estrogen receptor alpha (ERα) and androgen receptor (AR) expression in GALP neurons, GALP-Cre mice were mated with ROSA26-YFP mice (kindly provided by S. Srinivas, University of Oxford, Oxford, United Kingdom). Animals were intracardially perfused and the brains were processed as described above. Coronal sections (14-μm) were obtained using a cryostat. Then, sections were blocked in 1X PBS, 0.025% TX-100, 5% horse serum, and then treated with rabbit anti-estrogen receptor α (ERα) (1:1,000; Millipore, MA) antiserum or with rabbit anti- AR (1:1000, Santa Cruz, Dallas, TX) overnight at 4 °C, followed by Cy3-donkey anti-rabbit IgG (1:500; Jackson Labs, Birmingham, AL) for 1 hr at room temperature. Sections were then coverslipped with Fluoromount-G (Southern Biotech).
Image analysis
Fluorescence images were acquired using an ApoTome imaging system (Imager Z1; Zeiss, Thornwood, NY) with a 20X objective. Quantification of nuclear and cytoplasmic fluorescence was done as previously described [21]. Briefly, fluorescent intensity (pixel intensity) was measured within the cytoplasm and the nuclear region, as well as outside the cell (background) using the AxioVision 4.1 software. After correction for background, the mean pixel intensity was used to determine the nuclear:cytoplasmic (N:C) ratio of fluorescence intensity. Neurons with an N:C ration of <1:2 were classified as having cytoplasmic FoxO1GFP staining, an N:C ratio of 2:1 or more signified nuclear, whereas an N:C ratio between 1:2 and 2:1 meant that the neuron had both nuclear and cytoplasmic FoxO1GFP staining. The mean N:C ratio was determined from 100 neurons in the ARC for each treatment. After analysis of neurons from both sides of the ARC (bilateral), the data were plotted as the percentage of neurons with cytoplasmic FoxO1GFP (# of neurons with a cytoplasmic N:C ratio / total # of neurons).
The effects of 48 hr fasting on serum LH levels
WT and GALP-p110α/β cKO adult (5 months) males were randomly assigned to either a 48 hr fast or ad libitum fed (control group). At the end of the experiment animals were weighed and their glucose levels recorded. Animals were killed in the morning between 9:00 and 10:30 am and their blood was collected via cardiac puncture. Plasma was obtained and stored at −20° C for LH, insulin, and leptin measurements. Brains were removed, and the mediobasal hypothalamus (MBH) was dissected and immediately frozen in dry ice.
The effects of 24 hr fasting on serum LH levels
In this experiment each animal served as its own control. Initial body weight and glucose levels were recorded from adult (3 months) WT and GALP-p110α/β cKO females. Between 9:00 and 10:30 am 100–150 μl of blood was collected from the facial vein using the Lancet method [23]. Seven days later, animals were fasted for 24 hours beginning at 9:00 am. After the fast, blood and tissue was collected. Serum was stored at −80°C until assays were performed. A separate cohort of age-matched females was used to obtain mediobasal hypothalamus (MBH) for GALP RT-PCR studies.
Gonadectomies and steroid hormone replacement
Adult female mice from each genotype were randomly assigned to one of two treatment groups: ovariectomy (OVX) + vehicle, or OVX + E2, (n=6–7 / genotype / treatment). Surgery was performed under isoflurane inhalation anesthesia. Immediately after OVX, animals received subcutaneous SILASTIC capsules (1.5 cm in length plugged with silicone adhesive on each end to leave 1 cm to fill with treatment, inner diameter 1.47 mm; outer diameter 1.95 mm) containing sesame oil (OVX + V) or SILASTIC capsules containing 1mg/ml E2 in sesame oil (OVX + E2) [24]. Seven days after surgeries, animals were killed between 9:00 – 10:30 am and tissue and brains obtained. Serum was assayed for E2 and LH levels.
Male mice of both genotypes were castrated under isoflurane anesthesia. Immediately after surgery, animals received subcutaneous SILASTIC capsules (inner diameter 1.02; outer diameter 2.16 mm) pack with 5 mm of testosterone (T) (Sigma-Aldrich, St. Louis, MO), or left empty. Animals were killed 7 days after surgery between 9:00 – 10:30 am and tissue and brains obtained. Serum was assayed for LH and T.
GALP real-time RT-PCR
To measure GALP mRNA, we dissected the MBH with the aid of a precision brain slicer for adult mouse brain (Braintree Scientific, Braintree, MA). We obtained coronal sections of brain tissue (2 mm) in which the posterior edge of the optic chiasm and the beginning of the mammillary bodies served as rostral and caudal boundaries, respectively. Then, under a dissection microscope two bilateral parasagittal cuts, each 1.5 mm lateral to the third ventricle, and one horizontal cut 2.0 mm dorsal to the ventral surface were made. These margins were chosen to ensure that each tissue sample contained the entire GALP neuronal population from the ARC of each animal.
Total RNA was extracted using Trizol (Invitrogen) according to the manufacturer instructions. RNA (2.5 μg) was reverse-transcribed to cDNA using the SuperScript VILO cDNA synthesis kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed at the Genomics core facility, Stony Brook University, with an ABI 7300 Real-Time PCR system (Applied Biosystems) using TaqMan probe-based gene expression analysis and primer sequences specific for the Galp gene (Id: Mm00626135-m1, Applied Biosystems). A 100 ng total RNA equivalent cDNA was used for Taqman PCR (Applied Biosystems, TaqMan Universal Master Mix). Samples were run in duplicates to obtain an average threshold value (Ct). Quantities of GALP mRNA were normalized to 18s ribosomal RNA gene (Id: Mm03928990_g1). To determine the PCR reaction efficiencies for the Galp and the 18S gene, we ran a standard curve for each gene, using a 1:5 dilution of RNA. The difference between average target gene (Galp) Ct and average control gene (18S) Ct (ΔCt) for each dilution was plotted against the log input cDNA amounts. Because the PCR efficiencies of the target gene (Galp) and control gene (18S) reactions were different (slope of the curve more than 0.1), we used the standard curve method for relative quantification of the transcript level. Samples prepared without reverse transcriptase were used as negative controls.
Hormone assays
Serum measurements of LH from male animals used in the 48 hr fast experiment and mice used in the E2 and T studies were conducted at the University of Virginia Core Facility. Plasma LH levels were determined by RIA using reagents obtained from the National Institute of Diabetes and Digestive and Kidney Diseases (National Institutes of Health, Bethesda, MD), including LH reference (RP-3) and antirat LH antibody (S-11). The assay had a lower limit of detection of 0.2 ng/ml. Intraassay and interassay coefficients of variance were 8.28 and 9.66%, respectively. Serum T levels were measured by ELISA (R&D Systems, Minneapolis, MN), sensitivity of 0.041 ng/mL. Serum E2 was measured by ELISA (Calbiotech, Spring Valley, CA) with sensitivity of <3 pg/ml. Serum leptin levels were measured by a solid phase sandwich ELISA (R&D Systems), sensitivity range of 62.5 – 4,000 pg/mL. Serum insulin levels were measured by ELISA (Millipore, Billerica, MA), with a sensitivity range of 0.2–12.8 ng/mL. For experiments using fasted females, serum LH levels were measured using a Milliplex MAP immunoassay (mouse panel; Millipore) in the Luminex 200.
Evaluation of glucose homeostasis
Glucose measurements were obtained from tail blood using a glucometer (One-Touch Ultra Mini, Lifescan, Milpitas, CA). For glucose tolerance tests, mice were fasted overnight and blood glucose levels were measured immediately before (t=0) and 30, 60, and 120 min after injection with D-glucose (2 g/kg BW). For insulin tolerance tests 4 hr-fasted male mice were injected ip. with 1mU/g human insulin and female mice received 0.5 mU/g. Blood glucose was measured before and 15, 30, 60, 90, and 120 min after insulin injection.
Energy homeostasis phenotype: metabolic chambers
Food intake, ambulatory activity, O2 consumption, CO2 production, and heat production, were simultaneously recorded using a combined indirect calorimetry system (Comprehensive Lab Animal Monitoring System, Oxymax Columbus Instruments, Columbus, OH). Animal motion (ambulatory movements) was detected using a triple action (infrared) IR photocell technology, in which interruption of an IR beam will accrue one “count”. The respiratory exchange ratio (amount of VCO2 consumed/VO2 produced) was also provided. Food and water were provided ad libitum. Mice were housed individually at room temperature under a 12 hr light, 12 hr dark cycle. All parameters were recorded for at least 96 hours, and data obtained in the last 24 hours were used in the final analysis.
Statistical analyses
Data are expressed as mean ± SEM unless stated otherwise. Statistical analysis was done using Sigma Plot 10 (San Jose, CA). Differences between WT and GALP-p110α/β cKO were analyzed using a two-way ANOVA, followed by Holm-Sidak, multiple comparison method. Two-way repeated measures ANOVA was used to analyze data obtained using mice as their own control. GraphPad Prism 4 (Graph-Pad, San Diego, CA) was used for graphic illustrations. For all statistical analyses, P < 0.05 was considered significant.
Results
Deletion of insulin-induced PI3K-Akt signaling in GALP neurons
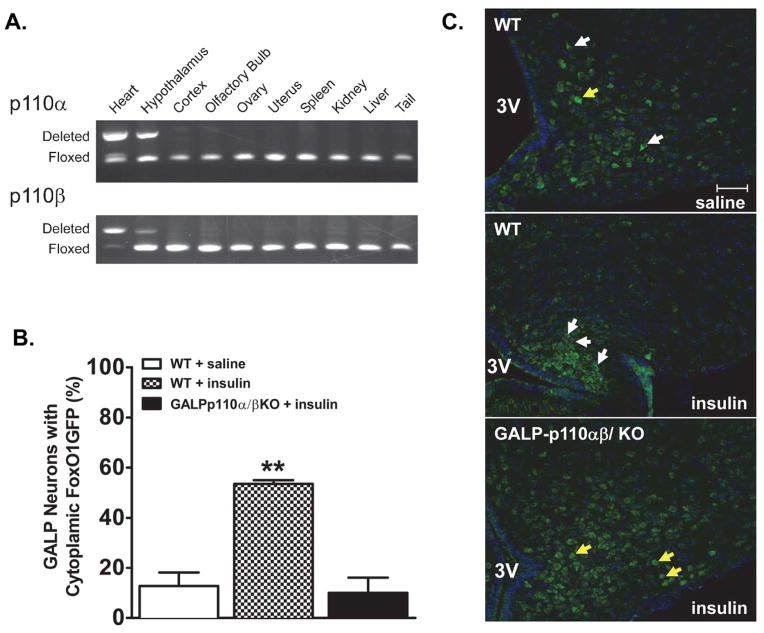
To monitor the PI3K-Akt signaling pathway in GALP neurons, we first crossed GALP-Cre mice in which Cre is expressed in hypothalamic GALP neurons with FoxO1GFP reporter mice (FoxO1GFP-GALP mice). We then used the Akt-dependent nuclear export of FoxO1GFP as an indicator of the activity of PI3K-Akt pathway in GALP neurons after icv infusion of insulin in fasted mice. Single label immunohistochemistry for GFP on sections through the MBH showed that FoxO1GFP was selectively expressed in the ARC-ME in accordance with previous reports for hypothalamic GALP expression in mice [1]. Thirty minutes after insulin infusion, FoxO1GFP was excluded from the nucleus of >55% GALP neurons (Fig. 1B, 1C). This is in contrast to nuclear exclusion of signal in <12% of GALP neurons from saline-treated FoxO1GFP-GALP animals. To confirm that deletion of PI3K catalytic subunits in GALP neurons abolished PI3K activity, we crossed FoxO1GFP-GALP reporter mice with GALP-p110α/β cKO animals, in which both catalytic subunits were deleted in GALP neurons. Icv infusion of insulin failed to exclude FoxO1GFP from the nucleus of GALP neurons from GALP-p110α/β cKO animals (Fig. 1B, 1C; and Supp. Fig. 1 for higher (100X) magnification views).
Fig. 1.
The GALP-neuron specific deletion of PI3K catalytic subunits p110α and p110β abolishes the insulin-induced nuclear export of FoxO1GFP within GALP neurons. (A) PCR products showing site of Cre-mediated DNA recombination in tissues from a Cre positive mouse. Deletion of p110α and p110β was detected in the hypothalamus. DNA from the heart of heart-specific p110α/β cKO mouse served as a positive control (first lane [19]). (B) Quantification of FoxO1GFP nuclear translocation in saline-treated WT, insulin-treated WT, and insulin-treated FoxO1GFP-GALP-p110α/β cKO animals (n = 3–4). Subcellular localization of FoxO1GFP is plotted as the percentage of neurons with cytoplasmic FoxO1GFP ** P < 0.001, One-way ANOVA, followed by Newman-Keuls multiple comparison test (C) Subcellular localization of FoxO1GFP in GALP neurons from saline and insulin-treated FoxO1GFP-GALP and FoxO1GFP-GALP-p110α/β cKO animals. After an overnight fast, mice were infused with saline or insulin (100 pmol, 30 min). White and yellow arrows point to GALP neurons with cytoplasmic and nuclear FoxO1GFP localization, respectively. 3V, third ventricle. Scale bar, 50 μm.
The selective deletion of p110α and p110β in GALP neurons was also validated through PCR of genomic DNA from various tissues of Cre+ animals. The p110α and p110β alleles were deleted from the genome in the MBH, but not from GALP-negative tissue such as the tail, liver, ovary, and uterus (Fig. 1A).
GALP-p110α/β cKO does not affect pubertal onset or fertility
We recorded puberty onset in GALP- p110α/β cKO and WT control mice using external markers such as preputial separation in males and day of vaginal opening and first day of estrus in females. The time of preputial separation was not significantly different between GALP- p110α/β cKO and WT males (27.7 ± 0.7 days vs. 26.2 ± 0.4 GALP- p110α/β cKO and WT, respectively, n = 13, P > 0.05). Similarly, the day of vaginal opening was not significantly different between GALP- p110α/β cKO and WT females (27.2 ± 0.6 v. 27.4 ± 0.6 GALP- p110α/β cKO and WT, respectively, (n = 14). In addition, genotype did not affect the first day of estrus (41.9 ± 1.2 vs. 41.4 ± 1.2 GALP- p110α/β cKO and WT, respectively, n = 14)
Assessment of cyclicity through vaginal cytology indicated that adult GALP- p110α/β cKO females have estrous cycles similar to those of their WT littermates (data not shown). Furthermore, GALP- p110α/β cKO females when paired with a WT male were able to become pregnant, deliver healthy litters, and raise them to weaning age. Likewise, GALP-p110α/β cKO males successfully impregnated WT females.
Hypothalamic GALP mRNA levels are reduced in fasted and non-fasted GALP-p110α/β cKO males
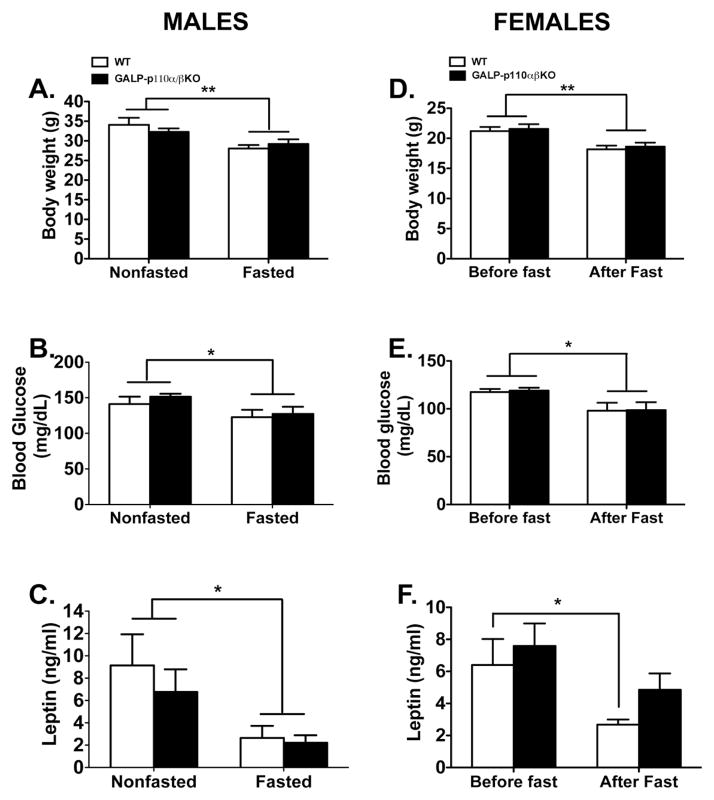
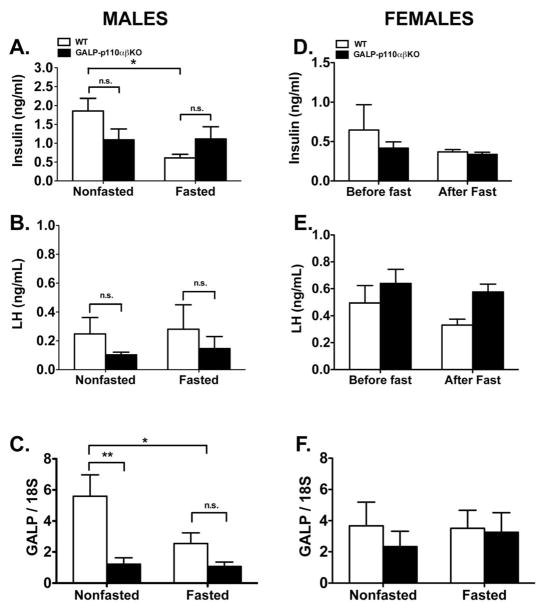
We hypothesized that ablation of PI3K signaling in GALP neurons will impair their ability to respond to changes in peripheral metabolic signals such as insulin. Hence, we reasoned that compared to WT, the gonadotropic axis of GALP-p110α/β cKO males will be more susceptible to the negative effects of fasting. To test this hypothesis we randomly assigned WT and GALP-p110α/β cKO males to either a fed group or a group fasted for 48 hours. There were no significant differences in the body weight of GALP-p110α/β cKO and WT mice that were fed ad libitum (Fig 2A). Males subjected to a 48 hr fast weighed significantly less than fed males, and there was no effect of genotype on weight loss (Fig 2A). Similarly, compared to non-fasted controls, a 48 hr fast significantly decreased blood glucose and serum leptin levels in animals of both genotypes (Fig. 2B and Fig. 2C). There was no significant effect of genotype on serum glucose or leptin levels (ANOVA: genotype, P = 0.42 and 0.45 for glucose and leptin levels, respectively). However, there was a significant interaction between genotype and treatment on serum insulin levels (ANOVA: P = 0.03). Whereas a 48 hr fast decreased serum insulin levels in WT animals, serum insulin levels were not different between fed and fasted GALP-p110α/β cKO mice (Fig. 3A). No effect of food manipulation or genotype was observed on serum LH concentrations in males (2-way ANOVA; P > 0.05; Fig. 3B). Serum T levels were not different between nonfasted WT and nonfasted GALP-p110α/β cKO animals (4.8 ± 2.2 vs. 4.9 ± 1.3 ng/ml, GALP-p110α/β cKO vs. WT, respectively).
Fig. 2.
Effects of a 48-hr and of a 24-hr fast on metabolic parameters of WT and GALP-p110α/β cKO male and female mice, respectively. Fasting reduced body weight (A, D) and glucose (B, E) levels in WT and GALP-p110α/β cKO mice of both sexes. A 48 hr fasting reduced serum leptin (C) levels in WT and GALP-p110α/β cKO males, whereas a 24 hr fasting reduced leptin levels in WT females (F). (Males: 2-Way ANOVA; Females: 2-way ANOVA with repeated measures, *P < 0.05, **P < 0.001); n = 7–8 genotype/treatment. There was no significant effect of genotype or the interaction between food manipulation and metabolic parameters.
Fig. 3.
Effects of a 48-hr and of a 24-hr fast on serum insulin (A, D), serum LH levels (B, E), and on hypothalamic GALP mRNA expression (C, F) in WT and GALP-p110α/β cKO male and female mice, respectively. (A) In males, there was a significant interaction between food manipulation and genotype on serum insulin levels (2-way ANOVA, P < 0.05); multiple comparison test revealed a significant effect of fasting on serum insulin levels in WT animals (* P < 0.05) but not in GALP-p110α/β cKO males (D) A 24 hr fast did not affect serum insulin levels in females of either genotype. (B) A 48 hr fast did not affect serum LH levels in WT or in GALP-p110α/β cKO males and no genotype effect was observed in males subjected to either food manipulation. (E) A 24 hr fast did not affect serum LH levels in female animals of both genotypes. However, compared to WT littermates, GALP-p110α/β cKO females have higher serum LH levels, (2-way ANOVA, repeated measures, P < 0.05). (C) There was a significant genotype effect on hypothalamic GALP mRNA levels in males (2-way ANOVA, **P < 0.01); compared to fed WT mice, GALP mRNA levels from the MBH of GALP-p110α/β cKO males were significantly lower regardless of the food treatment, (Holm-Sidak multiple comparison test, P < 0.05). (F) Hypothalamic GALP mRNA levels were similar in fasted vs. nonfasted females of either genotype. Data were expressed as ± SEM.
Deletion of p110α and p110β in GALP neurons resulted in a 5.2-fold decrease in MBH GALP mRNA levels (2-way ANOVA, genotype, P < 0.001; Fig. 3C). However, no significant interaction between genotype and treatment on GALP mRNA expression in the MBH was observed (P > 0.05). Fasting decreased MBH GALP mRNA expression in WT animals (P < 0.05), whereas fasting did not further reduce GALP mRNA levels in the MBH of GALP-p110α/β cKO.
LH levels are significantly higher in GALP-p110α/β KO females before and after fasting
Previous studies have suggested that females are more susceptible to nutritional stressors than males [13]. Therefore, we chose a 24-hr fasting model to investigate whether GALP-p110α/β cKO females have increased susceptibility to the suppressive effect of negative energy balance on the gonadotropic axis. There was no significant difference in initial body weight between GALP-p110α/β cKO and WT female mice (Fig. 2D). The 24 hr fast caused a similar and significant weight loss in females of both genotypes (Fig. 2D, ANOVA, P < 0.001).
Fasting significantly decreased serum glucose (2-way ANOVA: fed vs. fasted, P < 0.05; Fig. 2E). There was no significant effect of genotype on serum glucose levels (ANOVA: genotype, P = 0.86). Initial leptin concentrations did not differ between GALP-p110α/β cKO and WT females (Fig. 2F). After 24 hr of fasting leptin concentrations were significantly lower in WT female mice (Fig. 2F). However, serum leptin levels were not significantly lower after the fast in the GALP-p110α/β cKO females. Similarly, initial insulin levels were not different between GALP-p110α/β cKO and WT mice (Fig. 3D). However, 24 hr of fasting failed to significantly decrease serum insulin levels in mice of either genotype (ANOVA: treatment, P = 0.47).
In WT females LH levels declined after a 24 hr fast, but the decrease was not statistically significant (0.50 ± 0.13 ng/ml before vs. 0.33 ± 0.04 ng/ml after the fast). However, there was a significant genotype effect on serum LH concentrations (Fig. 3E, ANOVA: genotype, P < 0.05). Mean serum LH levels were higher in GALP-p110α/β cKO mice. However, statistical comparisons for factor (genotype) within each treatment group were not significant (P > 0.05). In addition, there was no significant genotype effect on GALP mRNA levels from the MBH of females that were fasted for 24 hours or in a separate cohort of intact nonfasted female mice (Fig. 3F). In the latter group, serum E2 levels were within the physiological range and no genotype effect was observed (9.85 ± 1.8 vs. 10.6 ± 1.6 pg/ml, GALP-p110α/β cKO vs. WT, respectively).
Hypothalamic GALP mRNA expression in gonadectomized WT and GALP- p110α/β KO mice
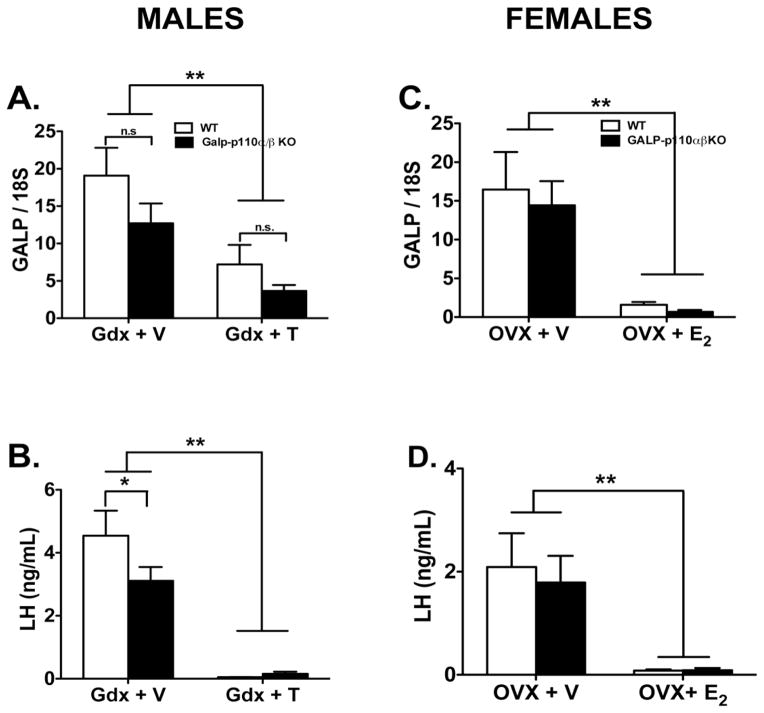
The regulation of hypothalamic GALP mRNA levels by steroid hormones was investigated using gonadectomy of male and female animals. Compared to vehicle-treated castrated mice, T treatment decreased MBH GALP mRNA levels in males of both genotypes (Fig. 4A). However, GALP mRNA levels in GALP-p110α/β cKO were not significantly different from their WT controls in each treatment group (ANOVA genotype, P = 0.09). As expected, compared to Gdx + T treated group, serum LH levels are significantly higher in the Gdx + V treated males of both genotypes (Fig. 4B). The difference was significant by treatment (P < 0.001) but not genotype. However, within the Gdx + V group, serum LH levels are significantly different between WT and GALP-p110α/β cKO mice (Fig. 4B, P = 0.03, Holm-Sidak multiple comparison test). Measurements of serum T levels also confirmed the effectiveness of the treatment; mice that were castrated and implanted with T filled capsules had elevated T levels relative to castrated animals treated with empty capsules (2.1 ± 0.1 and 1.8 ± 0.1, Gdx + empty capsule, in GALP-p110α/β cKO and WT mice, respectively; 6.5 ± 0.4 ng/ml and 7.0 ± 0.4, in Gdx + T-treated GALP-p110α/β cKO and WT mice, respectively).
Fig. 4.
Hypothalamic GALP mRNA expression (A, C), and serum LH levels (B, D), in WT and GALP-p110α/β cKO mice that were gonadectomized (Gdx) and treated with vehicle- (GdX + V for males, OVX + V, females,), or Gdx and treated with steroid hormone (Gdx + T males, OVX + E2, females) for 7 days. (A) GALP mRNA levels in the MBH of males that were castrated and treated with vehicle were significantly higher than those in the castrated and T-treated group of either genotype (2-way ANOVA, **P < 0.001). (C) GALP mRNA levels in the MBH of females that were OVX and treated with vehicle were significantly higher than those in the OVX + E2 treated group of either genotype (2-way ANOVA, P < 0.001). No significant effect of genotype or a significant interaction between genotype and steroid treatment was found in males or females. Compared to Gdx + vehicle treated mice, serum LH levels were increased after Gdx in males (B) and in females (D) of both genotypes, (ANOVA, **P < 0.001). In addition, within the Gdx + V treated group and compared to WT, serum LH levels were significantly lower in GALP-p110α/β cKO males. Data were expressed as ± SEM.
Similar to the effects of T in males, E2 treatment significantly reduced GALP expression in the MBH of WT and GALP- p110α/β cKO females when compared to vehicle-treated controls (Fig. 4C). Serum levels of LH in OVX + V treated mice were elevated relative to those in OVX + E2-treated mice of both genotypes (Fig. 4D). ANOVA showed a significant treatment effect on LH levels (P < 0.0001) whereas no genotype effect was observed (P = 0.62). Serum E2 levels were elevated in OVX mice treated with E2-filled capsules, further confirming the effectiveness of the E2 treatment (12.8 ± 1.9 and 11.0 ± 1.5 in Gdx + vehicle-treated GALP-p110α/β cKO and WT mice, respectively; 46.6 ± 3.5 and 40.8 ± 3.6 in Gdx + E2-treated GALP-p110α/β cKO and WT mice, respectively).
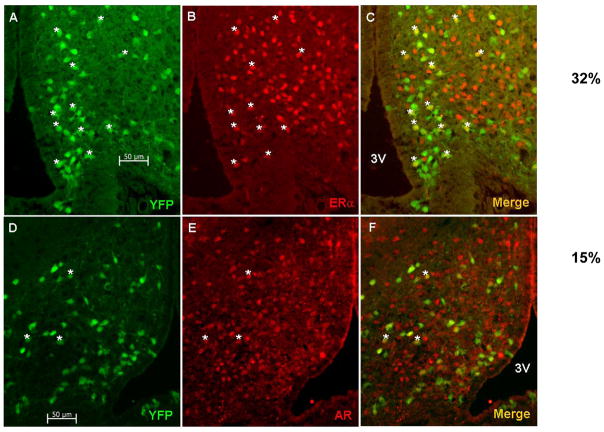
To investigate whether GALP neurons in the ARC express steroid hormone receptors we performed immunofluorescence analysis on ARC sections obtained from GALP-Cre/Rosa-YFP mice. In intact WT GALP-Cre/Rosa-YFP mice approximately 32% of GALP cell bodies colabeled with anti-ERα antiserum (32 ± 1.4%; n = 2) and approximately 15% co expressed ARs (14.7% ± 1.8%; n = 3) (Fig. 5).
Fig. 5.
ERα and AR expression in GALP neurons. Immunofluorescence analysis of ARC sections from GALP-CRE/R26-YFP mice using antibodies against ERα and AR show that a subset of GALP neurons (green) expresses ERα (red, A–C) or AR (red, D–F). Scale bar 50 μm.
GALP-p110α/β cKOs have normal metabolic regulation and tolerance to insulin and glucose
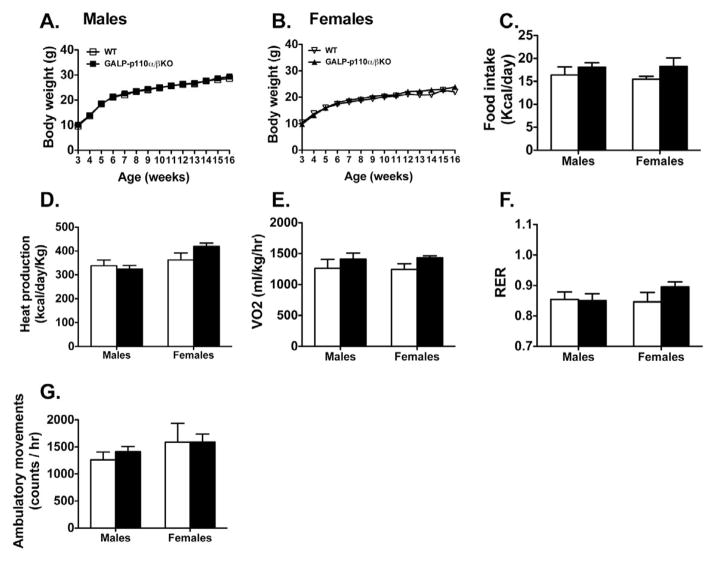
Deletion of PI3K signaling in GALP neurons did not affect postweaning body weight in male or females (Fig. 6A and 6B). Because GALP plays a role in the neuroendocrine regulation of feeding, body weight, and body temperature, weight-matched GALP-p110α/β cKO and WT mice were placed in metabolic chambers. No genotype effect was observed on food intake or activity levels over a 24 hr period in either sex (Fig. 6C and 6G). In addition, GALP- p110α/β cKO and WT mice of both sexes had similar energy expenditures as measured by O2 consumption, heat production, and respiratory exchange ratio (Fig. 6D, 6E, and 6F). Food intake, ambulatory activity, heat production, and oxygen consumption were not different between GALP-p110α/β cKO and WT mice when data were analyzed during 12 hr dark cycle and 12 hr light cycle (data not shown).
Fig. 6.
Deletion of PI3K catalytic subunits, p110α and p110β, in GALP neurons does not affect body weight gain or metabolic parameters in male and female mice. Postweaning body weight gain in GALP-p110α/β cKO males (A) and females (B) and WT littermates on normal chow (P > 0.05, n =13–15). (C) Food intake (D) heat production, (E) volume of oxygen consumed (VO2), (F) respiratory exchange ratio (RER), and (G) ambulatory movement of 5-month old chow-fed GALP-p110α/β cKO male (n = 9) and female (n = 8) mice, and their littermate controls (6–11) were measured by a combined Oxymax indirect calorimetric system over a 24-hr time period. Values are mean ± SEM.
Glucose tolerance tests (GTT) and calculated areas under the curve (AUC) did not show differences between GALP-p110α/β cKO (n = 12–14) and WT (n = 12–13) animals of either sex (data not shown). There were no differences in fasting glucose levels at the start of the GTTs. In addition, the insulin tolerance test (ITT) showed that GALP-p110α/β cKO mice display insulin sensitivity comparable to WT littermates at 5 months of age (data not shown).
Discussion
Our study adds GALP to the list of hypothalamic neuropeptides whose gene expression is regulated by PI3K under normal and negative energy balance conditions. The GALP-specific ablation of PI3K catalytic subunits, p110α and p110β, reduced hypothalamic GALP mRNA levels in males. However, body weight, glucose, leptin, and LH levels were similarly affected by fasting in WT and in GALP-p110α/β cKO males. Only a mild genotype and treatment interaction on peripheral insulin levels was observed in males. In contrast, whereas no genotype effect on hypothalamic GALP gene expression or on any metabolic parameter was found in female mice, there appears to be a genotype effect on serum LH, with GALP-p110α/β cKO females showing higher levels compared to WT. Furthermore, we showed that at least in mice, steroid hormones regulate GALP gene expression, providing a physiological explanation for the known sex-specific effects of GALP on neuroendocrine function and the sex-specific phenotypes observed after deletion of PI3K catalytic subunits in GALP neurons. In line with previous studies, our data do not support an essential role of GALP for the reproductive axis or the maintenance of metabolic homeostasis. Our results further demonstrate that PI3K signaling in GALP neurons has a more prominent role during metabolic challenges such as during states of low nutrient availability.
PI3K signaling and regulation of neuropeptide gene expression
As reported by others [7, 9], we showed that fasting decreased GALP mRNA expression in WT males. However, compared to WT littermates and regardless of food manipulation, hypothalamic GALP mRNA levels were significantly lower in GALP-p110α/β cKO males. Hence, PI3K signaling is important to maintain basal GALP gene expression in the hypothalamus of male mice. Due to the lack of a GALP-specific antibody, one caveat of our study is the inability to investigate whether the decrease in GALP mRNA levels observed in GALP- p110α/β cKO males is accompanied by changes in GALP protein expression. On the other hand, genetic and pharmacological studies have linked PI3K signaling to the regulation of other genes encoding neuropeptides important for the control of food intake and metabolism. For example, icv pretreatment of male rats with the PI3K selective inhibitor LY-294002 prevented the increases of Npy and AgRP expression induced by fasting [25]. Central inhibition of PI3K also prevented the reduction of Npy and AgRP induced by leptin [25]. Similarly, a reduction in Npy mRNA levels was observed in mice with an AgRP-specific deletion of p110α [15] Anorexigenic GALP neurons are part of a broader hypothalamic network of neuropeptides including AgRP, NPY, and POMC, utilized by insulin and leptin to regulate food intake and body weight [4]. Hence, in the hypothalamus PI3K might be a common signaling mechanism utilized by peripheral metabolic factors to control the gene expression of neuropeptides in the ARC under basal or under altered metabolic conditions like negative energy balance.
Even though food deprivation significantly reduced body weight, glucose levels, and leptin levels similarly in males of both genotypes, there was a significant interaction between genotype and treatment on serum insulin levels. Fasting significantly reduced insulin levels in WT males. In contrast, serum insulin levels were almost identical between GALP-p110α/β cKO males that were fasted and GALP- p110α/β cKO males that were fed. At least in rats, insulin and leptin treatment have additive effects on hypothalamic GALP mRNA expression under conditions of negative energy balance such as Type 1 diabetes [8]. Therefore, it is possible that the lower insulin levels observed in GALP- p110α/β cKO males might have contributed to the decrease in GALP mRNA expression. The possibility that PI3K signaling in GALP neurons may regulate peripheral insulin levels is intriguing. However, the central effects of GALP on insulin levels have not been reported. In mice, icv GALP increases autonomic activity in adipose tissue [26] and in rats central infusion of GALP increases leptin levels [27]. Whether GALP regulates insulin levels through similar mechanisms remains to be addressed.
GALP neurons express the LepR; thus, the stimulatory effects of leptin on GALP mRNA expression are thought to be the result of direct actions of leptin on GALP neurons [7]. Even though insulin also up-regulates GALP gene expression, a direct effect on these neurons was assumed but not demonstrated. FoxO1 is a transcription factor downstream of insulin that participates in the transcriptional regulation of orexigenic and anorexigenic neuropeptides such as AgRP, NPY, and POMC [28, 29]. The activation of PI3K by insulin receptors leads to the phosphorylation and activation of the downstream kinase Akt, which translocates to the nucleus, phosphorylating and inactivating FoxO1. Using a FoxO1GFP transgenic mouse we have shown that insulin directly acts on GALP neurons of the ARC to activate PI3K signaling. In contrast, FoxO1 in GALP neurons from GALP-p110α/β cKO mice failed to respond to insulin treatment. Activation of FoxO1 in the hypothalamus increases food intake through up-regulation in the gene expression of orexigenic peptides like AgRP and/or NPY and the inhibition of anorexigenic POMC gene expression [28–31]. A decrease in GALP mRNA levels in GALP-p110α/β cKO mice not only suggests that the PI3K signaling pathway participates in the regulation of GALP gene expression but also that FoxO1 might participate as a downstream target of this signaling pathway in the transcriptional regulation of GALP.
GALP-cell-specific PI3K signaling and reproductive function
Their capacity to serve a dual role in the control of metabolism and LH release has made GALP neurons a target to study the mechanism by which metabolic signals such as insulin and leptin regulate the reproductive axis. These metabolic cues, which are highly important to reproductive maturation and fertility, are known activators of PI3K activity in hypothalamic neurons. Hence, we considered it important to investigate the effects of deletion of PI3K signaling in GALP neurons on the gonadotropic response under both normal and negative energy balance conditions. We had hypothesized that ablation of PI3K activity in GALP neurons would result in increased susceptibility to the effects of negative energy balance on LH levels. Forty-eight hours of fasting did not reduce serum LH levels in WT or in GALP- p110α/β cKO males. Similarly, serum LH levels in WT females after a 24 hr fast were lower than LH levels before the fast, but this effect was not significant. We attribute the lack of a significant treatment effect to low basal LH levels in our control groups, which could have made it difficult to detect a statistically significant effect of fasting. Contrary to our prediction, there was no genotype effect on serum LH levels in males that were fasted or in males fed ad libitum. However, compared to WT littermates, GALP- p110α/β cKO females appeared to have higher LH levels before and after a 24 hr fast. This was surprising because central GALP stimulates gonadotropin release and conditions of negative energy balance, such as fasting, decrease GALP mRNA expression [9, 32]. On the other hand, an increased sensitivity to exogenous GALP has been observed after food deprivation and in animal models with defective LepR signaling such as the Zucker rat. We noticed that after fasting, serum leptin levels in GALP- p110α/β cKO females tended to be higher compared to fasted leptin levels in WT females. In addition, there was no significant genotype effect on fasted hypothalamic GALP mRNA levels in females. We speculate that the deletion of PI3K signaling in GALP neurons might have increased sensitivity to the effects of leptin and/or GALP, resulting in higher LH levels.
The molecular mechanisms by which GALP and other hypothalamic neurons relay information about the metabolic status to the gonadotropic axis, e.g., gonadotropin releasing hormone (GnRH) neurons, remain unknown. PI3K signaling is part of the mechanism utilized by neurons to detect changes in peripheral energy stores, which in turn is reflected by changes in serum leptin and insulin levels. The fact that in the GALP- p110α/β cKO females LH levels remained high even after the fast, suggest that the absence of PI3K signaling in GALP neurons alters their ability to receive input from peripheral metabolic cues, and in turn impairs communication to GnRH neurons. Thus, we propose that PI3K signaling in GALP neurons serves as an integrator of metabolic cues that in turn relay metabolic status to the reproductive axis.
GALP regulation by steroid hormones
The effects of GALP on the reproductive axis, such as stimulation of gonadotropin release and sexual behavior, can be sex-specific [27, 33]. For example, adult male rats but not females respond to central administration of GALP, which increases sexual behavior and LH levels [33]. It has been suggested that the effects of GALP on LH levels in females might be steroid hormone dependent unlike in males. However, sex steroid hormones do not appear to regulate GALP gene expression in rats or in other species such as macaques [9, 34]. Hence, the physiological explanation for the sex-specific effects of GALP on the neuroendocrine axis has not been clear. We report that in mice, GALP gene expression is not solely regulated by peripheral metabolic signals, but that both T and E2 reduce hypothalamic GALP mRNA levels. However, T and E2 decrease the expression of GALP gene in both the WT and the GALP- p110α/β cKO mice, indicating that steroid hormones act independent of the PI3K pathway to regulate GALP expression. Furthermore, a subpopulation of GALP neurons coexpressed receptors for E2 and T, suggesting that GALP neurons are direct targets of steroid hormones.
We observed significant genotype effects on serum LH levels in intact females and in gonadectomized and vehicle-treated males. It is likely that the pulsatile nature of LH release and the variability that is characteristic of in vivo studies, made it difficult to observe stronger effects within other treatment groups. On the other hand, the genotype effects on LH levels were different in each sex (higher levels in females and lower levels in males). GALP mRNA expression in the MBH mirrored the effects of gonadectomy and steroid hormone replacement on LH levels in mice of both genotypes, strongly suggesting that steroid hormones regulate GALP mRNA levels. Previous studies have shown that the congenital deletion of GALP in males results in a significantly attenuated refeeding response after an overnight fast, whereas no genotype effect was observed in GALP-KO females [36]. Therefore, at least in mice GALP effects on metabolism and perhaps on the reproductive axis might be under the control of steroid hormones. In addition, our findings also support previous observations indicating that the mechanisms and pathways utilized by GALP can be species specific. For example, whereas icv infusion of GALP in male rats increases sexual behavior, the opposite effect is observed in male mice [35].
Although many studies have shed light into the peripheral signals that regulate GALP and various hypothalamic neuropeptides that link reproduction and metabolism, ours is the first study linking a specific intracellular signaling pathway governing the regulation of GALP expression and function. Thus, we propose that PI3K signaling in GALP neurons is part of a broader network of ARC nucleus neuropeptides such as NPY [15], AgRP [15], and POMC [28, 30] in which PI3K signaling serves as an integrator of metabolic cues that in turn relay metabolic status to the reproductive axis.
Supplementary Material
Acknowledgments
The authors thank Dr. Todd Miller and Dr. Tom White for sharing resources; Dr. Andrew Wolfe for technical assistance; Dr. Anne Etgen, Dr. Kelly Warren, and Dr. Cheryl Park for comments on the manuscript; Dr. Leon Moore and Dr. Mary Kritzer for their input on statistical outcomes. This work was supported by the Eunice Kennedy Shriver National Institute of Child and Human Development Grant, 5R00HD055446-04 and the Office of the Vice President for Research at Stony Brook. DM and BR’s work is supported by a VA Merit Review Award to DM and by the Institute of Molecular Cardiology at Stony Brook University.
References
- 1.Jureus A, et al. Distribution and regulation of galanin-like peptide (GALP) in the hypothalamus of the mouse. Endocrinology. 2001;142(12):5140–4. doi: 10.1210/endo.142.12.8542. [DOI] [PubMed] [Google Scholar]
- 2.Takatsu Y, et al. Distribution of galanin-like peptide in the rat brain. Endocrinology. 2001;142(4):1626–34. doi: 10.1210/endo.142.4.8089. [DOI] [PubMed] [Google Scholar]
- 3.Takenoya F, et al. Neural interaction between galanin-like peptide (GALP)- and luteinizing hormone-releasing hormone (LHRH)-containing neurons. Peptides. 2006;27(11):2885–93. doi: 10.1016/j.peptides.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence C, Fraley GS. Galanin-like peptide (GALP) is a hypothalamic regulator of energy homeostasis and reproduction. Frontiers in neuroendocrinology. 2011;32(1):1–9. doi: 10.1016/j.yfrne.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoyanovitch AG, et al. Galanin-like peptide rescues reproductive function in the diabetic rat. Diabetes. 2005;54(8):2471–6. doi: 10.2337/diabetes.54.8.2471. [DOI] [PubMed] [Google Scholar]
- 6.Mohr MA, Leathley E, Fraley GS. Hypothalamic galanin-like peptide rescues the onset of puberty in food-restricted weanling rats. Journal of neuroendocrinology. 2012;24(11):1412–22. doi: 10.1111/j.1365-2826.2012.02351.x. [DOI] [PubMed] [Google Scholar]
- 7.Jureus A, et al. Galanin-like peptide (GALP) is a target for regulation by leptin in the hypothalamus of the rat. Endocrinology. 2000;141(7):2703–6. doi: 10.1210/endo.141.7.7669. [DOI] [PubMed] [Google Scholar]
- 8.Fraley GS, et al. Effects of diabetes and insulin on the expression of galanin-like peptide in the hypothalamus of the rat. Diabetes. 2004;53(5):1237–42. doi: 10.2337/diabetes.53.5.1237. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham MJ, et al. Galanin-like peptide as a possible link between metabolism and reproduction in the macaque. The Journal of clinical endocrinology and metabolism. 2004;89(4):1760–6. doi: 10.1210/jc.2003-031628. [DOI] [PubMed] [Google Scholar]
- 10.Kumano S, et al. Changes in hypothalamic expression levels of galanin-like peptide in rat and mouse models support that it is a leptin-target peptide. Endocrinology. 2003;144(6):2634–43. doi: 10.1210/en.2002-221113. [DOI] [PubMed] [Google Scholar]
- 11.Shiba K, et al. Galanin-like peptide and the regulation of feeding behavior and energy metabolism. The FEBS journal. 2010;277(24):5006–13. doi: 10.1111/j.1742-4658.2010.07933.x. [DOI] [PubMed] [Google Scholar]
- 12.Gottsch ML, Clifton DK, Steiner RA. Galanin-like peptide as a link in the integration of metabolism and reproduction. Trends in endocrinology and metabolism: TEM. 2004;15(5):215–21. doi: 10.1016/j.tem.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Acosta-Martinez M. PI3K: An Attractive Candidate for the Central Integration of Metabolism and Reproduction. Frontiers in endocrinology. 2011;2:110. doi: 10.3389/fendo.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanhaesebroeck B, et al. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends in biochemical sciences. 2005;30(4):194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Al-Qassab H, et al. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell metabolism. 2009;10(5):343–54. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, et al. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell metabolism. 2010;12(1):88–95. doi: 10.1016/j.cmet.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends in endocrinology and metabolism: TEM. 2005;16(4):183–9. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Eberhard N, et al. Distribution of alarin immunoreactivity in the mouse brain. Journal of molecular neuroscience : MN. 2012;46(1):18–32. doi: 10.1007/s12031-011-9546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Z, et al. Loss of cardiac phosphoinositide 3-kinase p110 alpha results in contractile dysfunction. Circulation. 2009;120(4):318–25. doi: 10.1161/CIRCULATIONAHA.109.873380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosati B, et al. Robust L-type calcium current expression following heterozygous knockout of the Cav1.2 gene in adult mouse heart. The Journal of physiology. 2011;589(Pt 13):3275–88. doi: 10.1113/jphysiol.2011.210237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda M, et al. Monitoring FoxO1 localization in chemically identified neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(50):13640–8. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman GE, Le WW, Sita LV. The importance of titrating antibodies for immunocytochemical methods. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience / editorial board. Unit 2.12. Chapter 2. 2008. [DOI] [PubMed] [Google Scholar]
- 23.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab animal. 2005;34(9):39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 24.Gottsch ML, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(29):9390–5. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison CD, et al. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. American journal of physiology Endocrinology and metabolism. 2005;289(6):E1051–7. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- 26.Hansen KR, et al. Activation of the sympathetic nervous system by galanin-like peptide--a possible link between leptin and metabolism. Endocrinology. 2003;144(11):4709–17. doi: 10.1210/en.2003-0748. [DOI] [PubMed] [Google Scholar]
- 27.Rich N, et al. Sex differences in the effect of prepubertal GALP infusion on growth, metabolism and LH secretion. Physiology & behavior. 2007;92(5):814–23. doi: 10.1016/j.physbeh.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belgardt BF, et al. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell metabolism. 2008;7(4):291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Ropelle ER, et al. Inhibition of hypothalamic Foxo1 expression reduced food intake in diet-induced obesity rats. The Journal of physiology. 2009;587(Pt 10):2341–51. doi: 10.1113/jphysiol.2009.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plum L, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nature medicine. 2009;15(10):1195–201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iskandar K, et al. PDK-1/FoxO1 pathway in POMC neurons regulates Pomc expression and food intake. American journal of physiology Endocrinology and metabolism. 2010;298(4):E787–98. doi: 10.1152/ajpendo.00512.2009. [DOI] [PubMed] [Google Scholar]
- 32.Krasnow SM, et al. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology. 2003;144(3):813–22. doi: 10.1210/en.2002-220982. [DOI] [PubMed] [Google Scholar]
- 33.Castellano JM, et al. Effects of galanin-like peptide on luteinizing hormone secretion in the rat: sexually dimorphic responses and enhanced sensitivity at male puberty. American journal of physiology Endocrinology and metabolism. 2006;291(6):E1281–9. doi: 10.1152/ajpendo.00130.2006. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham MJ, et al. Regulation of galanin-like peptide gene expression by pituitary hormones and their downstream targets. Journal of neuroendocrinology. 2004;16(1):10–8. doi: 10.1111/j.1365-2826.2004.01118.x. [DOI] [PubMed] [Google Scholar]
- 35.Kauffman AS, et al. Effects of galanin-like peptide (GALP) on locomotion, reproduction, and body weight in female and male mice. Hormones and behavior. 2005;48(2):141–51. doi: 10.1016/j.yhbeh.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Dungan Lemko HM, et al. Altered response to metabolic challenges in mice with genetically targeted deletions of galanin-like peptide. American journal of physiology Endocrinology and metabolism. 2008;295(3):E605–12. doi: 10.1152/ajpendo.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.