Abstract
Physical activity influences inflammation, and both affect brain structure and Alzheimer’s disease (AD) risk. We hypothesized that older adults with greater reported physical activity intensity and lower serum levels of the inflammatory marker tumor necrosis factor α (TNFα) would have larger regional brain volumes on subsequent magnetic resonance imaging (MRI) scans. In 43 cognitively intact older adults (79.3 ± 4.8 years) and 39 patients with AD (81.9 ± 5.1 years at the time of MRI) participating in the Cardiovascular Health Study, we examined year-1 reported physical activity intensity, year-5 blood serum TNFα measures, and year-9 volumetric brain MRI scans. We examined how prior physical activity intensity and TNFα related to subsequent total and regional brain volumes. Physical activity intensity was measured using the modified Minnesota Leisure Time Physical Activities questionnaire at year 1 of the study, when all subjects included here were cognitively intact. Stability of measures was established for exercise intensity over 9 years and TNFα over 3 years in a subset of subjects who had these measurements at multiple time points. When considered together, more intense physical activity intensity and lower serum TNFα were both associated with greater total brain volume on follow-up MRI scans. TNFα, but not physical activity, was associated with regional volumes of the inferior parietal lobule, a region previously associated with inflammation in AD patients. Physical activity and TNFα may independently influence brain structure in older adults.
Keywords: tumor necrosis factor (TNFα), exercise, MRI, supramarginal gyrus, inferior parietal lobule, Alzheimer’s disease
INTRODUCTION
Alzheimer’s disease (AD), the most common form of dementia, is characterized by the presence of amyloid plaques (composed of amyloid beta; Aβ) and neurofibrillary tangles (composed of hyperphosphorylated tau protein). However, ~37–44% of older adults who are cognitively normal also show significant AD pathology at autopsy (Bennett et al., 2006) or on amyloid imaging (Mielke et al., 2012). This suggests that other factors, such as baseline differences in brain health when pathology develops, may influence whether disease symptoms are manifest (Stern, 2012). Higher levels of physical activity have been associated with a lower risk of developing AD (Luck et al., 2013). The main mechanisms for this reduced risk are unclear. Physical activity may be associated with lower brain amyloid levels in humans (Liang et al., 2010; Head et al., 2012; Brown et al., 2013), contributing to this effect. In transgenic mouse models of AD, exercise has been associated with lower amyloid deposition and better Aβ clearance as well as reductions in tau phosphorylation. In addition to possible effects on AD pathology, physical activity also promotes human brain regeneration, including in the hippocampus (Pajonk et al., 2010; Erickson et al., 2011), cingulate gyrus and prefrontal cortex (Colcombe et al., 2006; Floel et al., 2010) – regions that are vulnerable in AD. This association between physical activity and larger regional brain volumes in older humans (Colcombe et al., 2006; Erickson et al., 2010; Erickson et al., 2011; Benedict et al., 2013; Boyle et al., 2013) may help protect active older adults from developing AD symptoms.
The effects of physical activity on brain volume may stem from other factors that arise from exercise and are likewise associated with brain integrity. For instance, exercise promotes better sleep (Dzierzewski et al., 2014) and cardiorespiratory fitness (such as the maximum volume of oxygen consumption), both of which are associated with a lower risk of dementia (Defina et al., 2013; Benedict et al., 2014; Di Meco et al., 2014) and with larger regional brain volumes including in frontal and parietal cortices (Altena et al., 2010; Hayes et al., 2013). Exercise also reduces obesity, inflammation, and modulates levels of the stress hormone cortisol (Tsukui et al., 2000; Mastorakos et al., 2005; Cotman et al., 2007; Rasmussen et al., 2011), all of which are implicated in AD (Gustafson et al., 2003; Kivipelto et al., 2005; Razay and Vreugdenhil, 2005; Whitmer et al., 2005; Doorduin et al., 2009; Fitzpatrick et al., 2009; Lee et al., 2010; Xu et al., 2011; Hinterberger et al., 2013; Krstic and Knuesel, 2013) and associated with smaller regional brain volumes (Marsland et al., 2008; Ho et al., 2010a; Ho et al., 2011; Rajagopalan et al., 2012; Kesler et al., 2013) (Fig. 1).
Fig. 1.
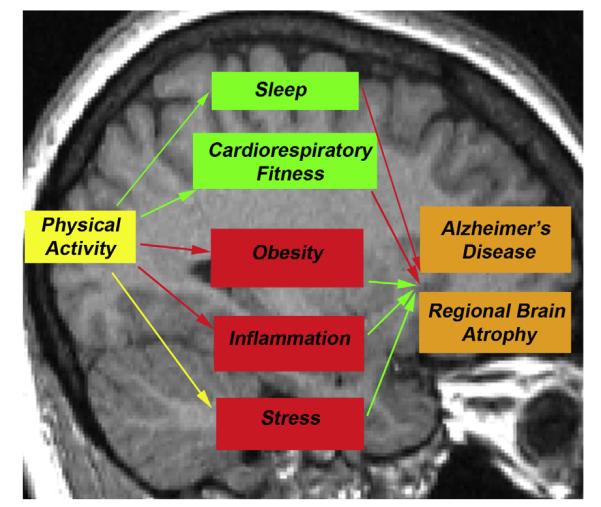
In physical activity intervention studies, physical activity over a period of time has numerous effects in the body. These effects have been previously associated with both lower regional brain atrophy and lower risk of developing AD. Red arrows indicate a decreased result; green arrows indicate an increased result. The yellow arrow between physical activity and stress demonstrates the complex relationship between the two in which acute physical activity increases cortisol levels, but over time, decreases the body’s reactivity to stress (Mastorakos et al., 2005). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Physical activity reduces pro-inflammatory conditions in the brain (Cotman et al., 2007), which link to poorer cognition in rodents, and less hippocampal gray matter in humans (Marsland et al., 2008). Inflammation contributes to AD (Lee et al., 2010) symptoms, and several confirmed AD risk genes (Harold et al., 2009; Lambert et al., 2009; Hollingworth et al., 2011; Morgan, 2011; Naj et al., 2011) relate to inflammatory processes. Interleukin 6 (IL-6) is a measure of systemic inflammation, typically acting as a pro-inflammatory cytokine (Rasmussen et al., 2011). Higher serum IL-6 levels relate to greater hippocampal inflammation and poorer cognition in rodents, and less hippocampal gray matter in humans (Marsland et al., 2008). Paradoxically though, IL-6 may also reduce inflammation, making a high measure of IL-6 difficult to interpret. In response to exercise, active muscles and the brain release IL-6, and its mRNA expression is up-regulated in the hippocampus (Rasmussen et al., 2011). It then down-regulates tumor necrosis factor α (TNFα), another pro-inflammatory cytokine, reducing peripheral and hippocampal inflammation in rodents (Schindler et al., 1990; Funk et al., 2011). TNFα is both pro-inflammatory and decreased with exercise (Schindler et al., 1990; Funk et al., 2011), so high values can be consistently interpreted as detrimental. Higher peripheral IL-6 levels are associated with lower hippocampal gray matter in healthy middle-aged adults (Marsland et al., 2008), but in breast cancer survivors, higher IL-6 levels are associated with larger left hippocampal volumes (Kesler et al., 2013). In contrast, higher serum TNFα was associated with smaller hippocampal volumes in breast cancer survivors (Kesler et al., 2013).
Here, we investigate whether lower systemic inflammation contributes to the effect of physical activity on brain volume in older adults who have AD or are cognitively intact. The level of physical activity intensity was assessed for each subject based on self-reported recent activities upon admission into the Cardiovascular Health Study (CHS; see Experimental procedures). We included these physical activity intensity measures and serum TNFα levels in a model that predicts brain volume, while controlling for other factors known to be associated with regional brain volumes, including body mass index (BMI; a measure of obesity) (Raji et al., 2010; Ho et al., 2010a; Ho et al., 2010b). We examine brain volume in two ways: whole-brain volume as a percentage of intracranial volume (listed as “total brain volume” for the remainder of this paper) and voxelwise volume assessed using tensor-based morphometry (TBM), a method for mapping profiles of atrophy in 3D throughout the brain. We hypothesized that greater physical activity intensity would be associated with greater brain volume on a follow-up magnetic resonance imaging (MRI) scan. We further hypothesized that variability in systemic inflammation, as measured by serum TNFα, would explain part of this effect.
EXPERIMENTAL PROCEDURES
Participants
Subjects were selected from the Cardiovascular Health Study (Fried et al., 1991; Lopez et al., 2003b), a population-based longitudinal study of coronary heart disease and stroke in adults aged 65 and older. Subjects were recruited from a Medicare database, and all were ambulatory and living outside an institution. Those included here had neurological and neuropsychological exams in 1998–99 (year 9 of the study). Of those with T1-weighted volu-metric brain MRI scans at that time, we identified 517 without a history of infarct but with measures of baseline exercise intensity available. Of these, 85 had available serum TNFα levels at year 5. Three of the 85 had scans that were unusable due to severe artifact. The remaining 82 subjects examined in this study were all cognitively intact at baseline. These cognitively intact subjects included 43 older adults who remained cognitively intact for the duration of the study (controls) (79.3 ± 4.8 SD years of age; 22 F/21 M), and 39 who developed AD in the course of the study before the time of MRI scanning (81.9 ± 5.1 SD years of age; 24 F/15 M) (Table 1). Included in this study were 72 subjects who identified their race as primarily White, and 10 who identified their race as primarily African-American/Black.
Table 1.
Summary participant data
| ADa | Controlsa | p-Valuesb | |
|---|---|---|---|
| Subjects (#) | 39 | 43 | |
| Sex (# Males/Females) | 15 M/24 F | 21 M/22 F | 0.34 |
| Age (years) | 81.9±5.1 (73–95) | 79.3±4.8 (69–89) | 0.02 d |
| Education (years) | 13.6±2.9 (7–17) | 14.0±2.8 (7–17) | 0.49 |
| APOE4± (#)c | 15+/18− | 10+/28− | 0.09 |
| BMI – year 5 | 25.4±5.5 (18.5–42.0) | 25.4±4.2 (17.8–36.9) | 0.99 |
| BMI - year 9c | 24.9±5.2 (17.8–43.0) | 25.8±4.7 (16.5–37.3) | 0.41 |
| CESD depression score – year 5 | 5.4±4.2 (0–19) | 4.4±3.5 (0–15) | 0.23 |
| CESD depression score – year 10 | 6.4±5.4 (1–24) | 4.5±3.8 (0–14) | 0.06 |
| 3MSE scores – year 5 | 92.1±5.5 (81–99) | 94.3±3.8 (85–100) | 0.03 d |
| 3MSE scores – year 10 | 82.0±10.8 (50–99) | 95.7±4.2 (79–100) | 3.8 × 10 −11 d |
| TNFα – year 2 (pg/ml)g | 2.1±0.7 (1.0–5.0) | 2.2±0.9 (1.2–5.5) | 0.83 |
| TNFα – year 5 (pg/ml) | 2.2±0.8 (1.0–4.6) | 2.2±0.9 (1.0–5.6) | 0.80 |
| Physical activity intensity – year 1 | 1.5±0.7 (0–3) | 1.9±0.9 (0–3) | 0.03 d |
| Physical activity intensity – year 9 | 1.5±0.8 (0–3)f | 1.8±0.8 (0–3) | 0.16 |
Modified Mini-Mental State Examinations (3MSE) (Teng and Chui, 1987) is a test of global cognition similar to the Mini-Mental State Exam, but modified to include a broader array of cognitive functions and span a wider range of difficulty levels.
Values are mean±standard deviation unless otherwise indicated; for all values listed here, diagnostic groups represent diagnoses made within 1 year of the MRI in study year 9.
Differences between diagnostic groups were evaluated using two-tailed unpaired t-tests to evaluate differences for all measures except for sex and the presence or absence of an APOE4 allele, which were evaluated using χ2 test.
BMI measures were missing for three AD patients and one control; APOE genotypes were missing for six AD patients and five controls. This table does not include imputed values for the missing data.
Values are significantly different between diagnostic groups (p < 0.05).
Information was not available for two subjects.
Information was not available for nine AD and eight controls.
Diagnoses
CHS participants undergo a neuropsychiatric exam battery to test premorbid intelligence, memory, language, visuo-perceptual/visuo-constructional, psychomotor speed, executive function, and fine motor skills. Annual Modified Mini-Mental State Examinations (3MSE) (Teng and Chui, 1987), Digit Symbol Substitution Tests (Wechsler, 1981), and Benton Visual Retention Tests (Benton, 1967) are also performed (Lopez et al., 2006). Technicians at each site were trained to perform neuropsychological testing under the supervision of a neuropsychologist (Lopez et al., 2003b). The 3MSE was used as it measures a greater variety of cognitive function and is more sensitive across cognitive abilities (Teng and Chui, 1987), than the commonly used Mini-Mental State Exam (MMSE) (Folstein et al., 1975). Local neurologists examined all participants, assessing motor and sensory functions, and mental status as described previously. Data were sent to a single neurologist who performed diagnoses based on neuropsychological testing, neurological exams, medical records, physician questionnaires and informant interviews (Lopez et al., 2003a).
APOE genotype
Apolipoprotein E allele ε4 (APOE4) is a known genetic risk factor for late onset AD (Corder et al., 1993), while allele ε 2 reduces AD risk (Corder et al., 1994). We controlled for APOE genotype in all analyses. In our sample, there were no 2/2 genotypes; eight people had a 2/3 genotype (coded as ‘2’ in analyses); two people were 2/4 (coded as ‘3’); 38 people were 3/3 (coded as ‘4’); 21 were 3/4 (coded as ‘5’); and two were 4/4 (coded as ‘6’). APOE genotype was not available for 11 subjects. We imputed the missing genotypes to the most common genotype (3/3) and performed the analyses with and without the imputed data to ensure that the imputed values did not unduly influence our results.
Collection and analysis of plasma samples
As described previously (Vallejo et al., 2011), morning blood samples were collected after fasting. Plasma was processed the same day of collection and plasma aliquots were stored at −80 °C until use. As needed, plasma sam ples were thawed on ice and used immediately (no more than two freeze/thaw cycles). We used a human Cytokine 17-plex panel kit (BioRad) to perform TNFα assays according to the manufacturer’s specifications, and the Luminex 100 system (Luminex Corp) to obtain concentrations (Vallejo et al., 2011).
MRI scan acquisition
Each subject underwent 1.5-Tesla MRI scanning at one of the four coordinated scanning sites, as detailed elsewhere (Bryan et al., 1994). The scanning protocol included a sagittal T1-weighted spoiled gradient-recalled whole-brain volumetric scan with 1.5-mm thickness/0-mm interslice gap.
Physical activity intensity
We examined baseline subject-reported physical activity intensity assessed ~9 years before MRI scanning, when all subjects were still cognitively intact. Physical activity intensity was assessed as described previously (Siscovick et al., 1997) using the modified Minnesota Leisure Time Physical Activities questionnaire (Taylor et al., 1978; Geffken et al., 2001). This details frequency and duration of 15 physical activities over the previous 2 weeks. These activities included swimming, hiking, aerobics, tennis, jogging, racquetball, walking, gardening, mowing, raking, golfing, bicycling, dancing, calisthenics, and riding an exercise cycle (Geffken et al., 2001). Subjects also provided information about their typical walking pace outside the home. The intensity of these activities was established and validated previously (Taylor et al., 1978). Based on the highest intensity activity reported over the previous 2 weeks, physical activity intensity was rated as none, low, moderate, or high (Siscovick et al., 1997). We compared baseline physical activity intensity measures with year-9 numbers to assess stability of the measure.
Brain measurement
We initially removed non-brain matter from the images automatically using the Skull Stripping Meta-Algorithm (SSMA) software (Leung, 2011). One person manually refined the masks to exclude non-brain matter while retaining cerebrospinal fluid (CSF) within and around the brain. We used FSL FAST software to adjust for spatial intensity variations (bias field inhomogeneities), and segmented the skull-stripped images into brain matter versus CSF.
Minimal deformation template (MDT)
Using a template brain derived from scans in the same study reduces bias that may be introduced when transforming scans into a template space. We created a study-specific MDT from 20 AD and 20 control subjects in the current study, matched by AD diagnosis for age and sex. To do this, we first used FSL FLIRT software (Jenkinson et al., 2002) to linearly align (with 9 degrees of freedom) our skull-stripped, bias field-corrected scans to one brain in our sample that had already been moved into standard template space (ICBM53; International Consortium for Brain Mapping standard average brain template) (Mazziotta et al., 2001), and resampled to an 220 × 220 × 200 voxel space for 1-mm3 voxels. This linear alignment brought all scans into a common space in which the MDT could be created. To create the MDT, we took a voxelwise average of the linearly aligned scans that served as an affine template. We then iteratively nonlinearly normalized individual brain images to the affine template (Yanovsky et al., 2008; Yanovsky et al., 2009). The resulting displacement fields are 3-D vector arrays describing the displacement of each voxel in the source brain to the equivalent voxel in the target brain. The MDT was then computed using the geometric centroid of the displacement fields (Kochunov et al., 2002; Kochunov et al., 2005). In other words, the MDT is a template brain created by minimizing the distance between the target brain and all the brains in the study.
Voxelwise analysis
We used a validated TBM tool developed in our laboratory and reported previously in detail (Gutman et al., 2013; Hua et al., 2013) to perform a voxelwise analysis of regional brain volumes that we then related to exercise intensity levels and serum measures of TNFα. TBM evaluates differences in brain structure based on the gradients of deformation fields (i.e., the description of the extent to which each voxel must be deformed) to warp one brain to another. In this case, each brain was warped to the MDT, and the resulting Jacobian maps (i.e., measures of regional brain volumes) were included in statistical analyses to evaluate regional structural brain differences among subjects.
Statistical analysis
We used total brain volume (i.e., whole-brain volume as a percentage of intracranial volume) as our dependent variable in multiple regression analyses. We investigated the relationship between total brain volume at year 9, serum TNFα at year 5, and baseline intensity of physical activity. In all comparisons we controlled for eight covariates: age, sex, years of education completed, diagnosis (AD versus control), APOE genotype, clinic where the MRI was acquired, BMI near the time of scanning, and Center for Epidemiological Studies – Depression Scale (CESD) rating (possible scores 0–60) near the time of scanning. BMI was calculated as (weight in pounds × 703)/(height in inches)2. Our serum TNFα measures were somewhat skewed. To ensure that this skew did not affect our results, we performed the same analysis using the log10 of the TNFα values in place of the raw values. BMI values were not available for four subjects (three AD patients; one control) and APOE genotype (see APOE genotype section) was not available for 11 subjects. One subject lacked both measures. We used imputed data – a mean BMI value or a 3/3 APOE genotype – for 14 subjects for whom these values were absent. To ensure that these substitutions were not responsible for our significant findings, we also performed our primary analysis without these 14 subjects.
To further evaluate our significant results, we performed two additional analyses. First, we included in the same multiple regression model both explanatory variables (physical activity intensity, TNFα) that significantly contributed to total brain volume. This new analysis also included the same eight covariates as in our initial analyses and was designed to determine whether the effects of our explanatory variables were independent of one another. Next, to better identify brain regions in which regional volumes were most strongly related to our variables of interest, we included each significant variable separately and both together in a regression using the voxelwise TBM data, again controlling for the same eight covariates as in the initial analyses. To evaluate how BMI contributed to these results, we also performed this analysis without controlling for BMI.
RESULTS
All subjects included here were cognitively intact when the study began. As evaluated using a two-tailed Student’s t test, those who developed AD by the time of year-9 MRI scanning were significantly older than controls (p = 0.02). As expected, AD patients had significantly lower year-10 3MSE scores (p = 0.03). AD patients also had lower physical activity intensity reported at baseline (p = 0.03). AD patients and controls did not differ significantly in educational level, year-10 BMI or depression score, or year-5 TNFα score (Table 1).
Of our 82 subjects, 65 had TNFα measures at years 2 and 5 of the study. To assess the stability of this measure over time, we compared the TNFα values at years 2 and 5. In all subjects (r = 0.70; p = 1.22 × 10−10) and in controls alone (r = 0.93; p = 1.53 × 10−16), TNFα was highly correlated between years 2 and 5 in the subset of subjects examined. When only those who were AD patients at year 9 were considered, there was still a strong trend for correlation between TNFα values measured at years 2 and 5 (r = 0.36; p = 0.053).
Serum TNFα at year 5 of the study was not significantly correlated with year-5 serum IL-6 in the 81 subjects who had both measures. However, the IL-6 numbers were highly skewed. Log10 serum measures of TNFα and IL-6 were significantly correlated in all subjects (r = 0.23; p = 0.04).
We also evaluated how stable the physical activity intensity measure was over time. In the 43 control subjects who had physical activity intensity values at both time points, the physical activity intensity relationship between the start of the study and year 9 was significantly correlated (r = 0.63; p = 0.00001). In the 37 subjects who had AD at year 9 and had physical activity intensity values at both time points, the physical activity intensity relationship between the start of the study and year 9 was also significantly correlated (r = 0.49; p = 0.002). However, as this is a self-reported measure that relies on recent memory, it is unclear how accurate it is at year 9 in those who were demented at that point.
When considered alone, baseline physical activity intensity was significantly correlated with total brain volume (overall p = 1.17 × 10−8; physical activity partial p = 0.03). Diagnosis (partial p = 0.001) and age (partial p = 0.000001) also contributed significantly to the relationship. TNFα was also significantly associated with total brain volume when considered alone (overall p = 5.19 × 10−9; TNFα partial p = 0.01), and diagnosis (partial p = 0.0002) and age (partial p = 0.000004) contributed significantly to that relationship as well.
When exercise intensity and TNFα were both included in the same model, both continued to be significantly associated with total brain volume (overall p = 5.14 × 10−9; TNFα partial p = 0.014; physical activity partial p = 0.018). Diagnosis (partial p = 0.0006), APOE genotype (partial p = 0.0497), and age (partial p = 0.000008) also contributed significantly to the relationship, with a diagnosis of AD, older age, and possession of APOE4 all associated with smaller total brain volumes. The relationship of baseline physical activity intensity, TNFα, and total brain volume was still significant when TNFα log10 values were used (overall p = 9.76 × 10−9; TNFα partial p = 0.031; physical activity partial p = 0.015), suggesting that the statistical residuals were not skewed, and that the skewness of the TNFα data did not drive our results. We therefore used raw TNFα numbers for the remainder of the analyses. The relationship between total brain volume, TNFα, and physical activity was also significant when the 14 subjects with imputed APOE genotypes or BMI values were not included in the analyses (overall p = 1.50 × 10−6; TNFα partial p = 0.04; physical activity partial p = 0.03).
We performed an additional test to determine whether baseline or year-9 physical activity intensity correlated better with total brain volume. Year-9 physical activity intensity (overall p = 1.69 × 10−7; physical activity partial p = 0.13) was not significantly correlated with total brain volume at year 9 in the 80 subjects for whom year-9 values were available.
Physical activity intensity and TNFα were not significantly associated with each other (overall model p = 0.02; physical activity partial p = 0.55). Using TBM, TNFα, but not physical activity, was significantly associated with regional brain volume in the left inferior parietal lobule (supramarginal gyrus) (FDR critical p value = 3. 0 × 10−5) (Fig. 2). When we performed the same voxelwise analysis without controlling for BMI, results were identical in scope and location (FDR critical p value = 3.0 × 10−5).
Fig. 2.
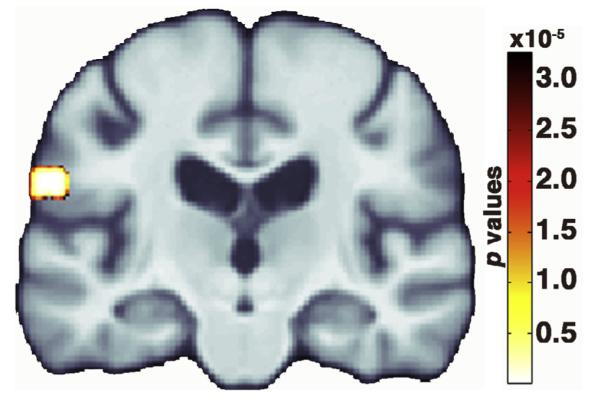
Colored voxels in the inferior parietal lobule indicate the p values where serum TNFα levels at year 5 of the study were significantly associated with year-9 voxelwise brain volume after adjusting for year-1 physical activity intensity, age, sex, education, diagnosis, apolipoprotein E genotype, scanner location, body mass index, and depression rating. The false discovery rate method (Benjamini and Hochberg, 1995) controlled for multiple comparisons such that significant p values were those below the critical p value of 3.0 × 10−5. Data are presented in neurological orientation (left = left). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
DISCUSSION
Lower physical activity intensity at baseline was significantly associated with developing AD at year 9, consistent with many prior studies reporting a strong relationship between physical activity and risk of later cognitive impairment; see Erickson et al. (2012) for a review. Lower physical activity intensity was also associated with lower total brain volume 9 years later in older adults, after controlling for other factors that influence brain atrophy, such as age, sex, AD diagnosis, and BMI. These results are consistent with prior studies in which physical activity was associated with greater regional brain volume in older adults (Erickson et al., 2010; Boyle et al., 2013). Some studies found that aerobic exercise training may actually increase regional brain volume in older adults (Colcombe et al., 2006; Erickson et al., 2011).
All of our subjects were cognitively intact at the beginning of the study, but 48% had AD by year 9 when the MRI scanning took place. We evaluated the relationship between brain volume and reported physical activity intensity at baseline, so the accuracy of reported physical activity was not affected by dementia status. However, physical activity intensity values at baseline and year 9 were highly correlated across all subjects suggesting that it is a stable measure. When evaluated as an additional test after our primary analysis, year-9 physical activity values were not significantly associated with total brain volume. It is possible that imperfect reporting of physical activity by AD patients contributed to this weakened effect. It is also possible that long-term effects of physical activity are more important to total brain volume than immediate effects. This is consistent with prior reports that physical activity throughout life was associated with lower risk of AD, but the strongest correlation was with earlier life activity (Middleton et al., 2010).
Increased physical activity in interventional studies may result in a variety of effects including, but not limited to, improved resting cerebrovascular reactivity to hypercapnia (Murrell et al., 2013), reduced arterial pressure (Vicente-Campos et al., 2012), lower total cholesterol and triglycerides (Vicente-Campos et al., 2012), lower BMI and blood pressure (Stewart et al., 2013), better glycemic control (Roberts et al., 2013), reduced chronic low-grade inflammation (Nimmo et al., 2013), increased growth factor levels (Cotman and Berchtold, 2002), better sleep (Dzierzewski et al., 2014), and greater brain neurogenesis (Cotman and Berchtold, 2002). Many of these effects relate to health factors that may modulate AD risk (Launer et al., 2000; Del Bo et al., 2009; Solomon et al., 2009; Lee et al., 2010; Matsuzaki et al., 2011; Piriz et al., 2011; Vargas et al., 2011; Crane et al., 2013; Tolppanen et al., 2013; Di Meco et al., 2014). Additionally, physical activity (Benedict et al., 2013), obesity (Debette et al., 2010; Raji et al., 2010; Cole et al., 2013), and blood glucose (Mortby et al., 2013) have all been associated with brain volume in cognitively intact adults.
Some effects of physical activity are immediate and short lived. For instance, in some cases, exercise-related changes to insulin sensitivity took place after only 38–48 h (Burstein et al., 1985). Other effects, such as anti-inflammatory effects may be evident after months, but disappear within a few weeks after the end of the exercise intervention (Thompson et al., 2010). We controlled for BMI, decreasing the probability that our physical activity effects are related to weight control, although differences in fat-to-muscle ratios that result from exercise could still play a role; recently correlations have been noted between brain atrophy and markers of adiposity and body fat (Rajagopalan et al., 2013).
Ongoing physical activity may have longer lasting effects. For instance, physical activity may be related to brain amyloid levels. In some animal models of AD, exercise is associated with less brain amyloid deposition and better clearance of amyloid beta, the primary component of amyloid plaques, from the brain (Stranahan et al., 2012). Thus far, no exercise intervention study has evaluated the relationship between physical activity and brain amyloid in humans. However, one research group found associations in their full subject sample between physical activity and brain amyloid in vivo (Liang et al., 2010; Head et al., 2012). Two studies have found that a sedentary lifestyle was significantly associated with greater brain amyloid in APOE4 carriers, but not in non-carriers. Another study did not detect a relationship between physical activity and brain amyloid (Landau et al., 2012). In the two studies in which no relationship between physical activity and brain amyloid was found in the full subject sample, physical activity was assessed using questionnaires that measured only recent physical activity (Landau et al., 2012; Brown et al., 2013) whereas the research group that found significant main effects of physical activity on brain amyloid used questionnaires that assessed physical activity over the previous 10 years (Liang et al., 2010; Head et al., 2012). Possibly, only physical activity performed for longer periods of time – or earlier in life – significantly affects brain amyloid. However, there are other methodological differences in studies including statistical tests used, evaluation of physical activity as a continuous or categorical variable, mean subject age, and differences in covariates (Liang et al., 2010; Head et al., 2012; Landau et al., 2012; Brown et al., 2013). As our physical activity measures were stable over time, it is hard to assess whether the relationship we found between physical activity and brain volume related to short- or long-term effects of physical activity or both.
We found in all subjects that blood serum measures of TNFα were correlated with total brain volume five years later. Our results are consistent with previous findings associating higher serum TNFα levels with smaller hippocampal volumes in breast cancer survivors (Kesler et al., 2013). Inflammation has previously been implicated in AD, and some anti-inflammatory treatments have been proposed for AD (Ross et al., 2012; Krstic and Knuesel, 2013; Meraz-Rios et al., 2013). When both baseline physical activity intensity and year-5 TNFα were included in the same model, both were still significantly associated with year-9 total brain volume. Furthermore, physical activity and serum TNFα were not significantly correlated with each other. This suggests that, contrary to our hypothesis, the two effects were independent and that the TNFα effect did not help explain the physical activity effect. The effect of physical activity on the total brain volume must stem from other effects of physical activity that are known to modulate brain health.
When we created a single model that evaluated the relationship of physical activity intensity and serum TNFα to voxelwise brain volume, higher TNFα, but not physical activity intensity, was associated with lower regional brain volume in the left inferior parietal lobule. The inferior parietal lobule has been shown previously to have greater levels of inflammation in AD patients versus controls, as measured using positron emission tomography and a putative biomarker for inflammation (Kreisl et al., 2013).
We benefit from having a long-term longitudinal study with measures of brain volume, inflammation, and exercise intensity available for each of our subjects. However, a limitation of this study is that it used self-reported measures of physical activity, whose accuracy may be affected by memory ability and by social desirability bias. Self-reported measures also do not consider unintentional activities such as fidgeting or pacing (Erickson et al., 2012). Future studies that evaluate physical activity more objectively in the short and long term may provide important information about the effects of physical activity on the brain.
CONCLUSIONS
We found that measures of physical activity intensity and inflammation were associated with brain volume assessed years later, particularly in the inferior parietal lobule. These results suggest that physical activity and inflammation both contribute independently to brain health status in older adults. The ability to assess how the individual, modifiable effects of exercise affect the brain may help to focus future treatment and prevention efforts of AD.
Acknowledgments
The research reported in this article was supported in part by funds from contract numbers N01-HC-80007, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional funds were provided by the National Institute on Aging to O.L.L. (AG020098), L.H.K. (AG15928), P.T. (AG040060), and the University of Pittsburgh (AG05133), and by subcontract (N01-HC-055222) to J.T.B. Funding to M.N.B was also provided by the UCLA Older Americans Independence Center (OAIC) and UCLA Clinical and Translational Science Institute (CTSI) Rapid Pilot Grant. A full list of principal CHS investigators and institutions can be found at www.chs-nhlbi.org/pi.htm.
GLOSSARY
- Aβ
Amyloid beta is a peptide of varying length that is processed by cleaving of the amyloid precursor protein. It is the primary component of amyloid plaques, one of the pathological hallmarks of Alzheimer’s disease.
- AD
Alzheimer’s disease is a type of dementia marked by memory and other cognitive deficits and massive loss of brain tissue and connectivity.
- APOE4
Apolipoprotein E allele ε 4 is the strongest known genetic risk factor for late-onset Alzheimer’s disease.
- BMI
Body mass index is a measure of body fat based on height and weight. BMI = (weight in kilograms)/(height in meters)2.
- CESD
Center for Epidemiologic Studies Depression scale is a measure of depressive feelings and behaviors during the previous week.
- CSF
Cerebrospinal fluid is a bodily fluid that surrounds the brain and spinal cord, acting as mechanical and chemical protection for those structures.
- IL-6
Interleukin 6 is a measure of systemic inflammation that typically acts as a pro-inflammatory cytokine.
- MDT
Minimal deformation template is an average template brain created from brains in the study such that the transformations needed to bring all brains into a common space are minimized.
- MRI
Magnetic resonance imaging is a non-invasive medical imaging technique that uses magnetic fields and radiofrequency pulses to create images of the brain and other internal structures.
- mRNA
Messenger ribonucleic acid is an RNA molecule that is synthesized in the nucleus using a DNA template. It transports the genetic information out of the nucleus and into the cytoplasm where the resulting proteins are synthesized.
- TBM
Tensor-based morphometry is a means for comparing anatomical structures on a voxelwise basis over time or between individuals. Differences are characterized by the spatial transformations that must be performed to co-register each brain to a given template brain.
- TNFα
Tumor necrosis factor α is a measure of systemic inflammation that acts as a pro-inflammatory cytokine.
- 3MSE
Modified Mini-Mental State Examinations are tests of cognitive function typically used to screen for dementia.
Footnotes
DISCLOSURE STATEMENT No author has any real or perceived conflict of interest with the work presented here.
REFERENCES
- Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67:182–185. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Benedict C, Brooks SJ, Kullberg J, Nordenskjold R, Burgos J, Le Greves M, Kilander L, Larsson EM, Johansson L, Ahlstrom H, Lind L, Schioth HB. Association between physical activity and brain health in older adults. Neurobiol Aging. 2013;34:83–90. doi: 10.1016/j.neurobiolaging.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Benedict C, Cedernaes J, Giedraitis V, Nilsson EK, Hogenkamp PS, Vagesjo E, Massena S, Pettersson U, Christoffersson G, Phillipson M, Broman JE, Lannfelt L, Zetterberg H, Schioth HB. Acute sleep deprivation increases serum levels of neuron-specific enolase (NSE) and S100 calcium binding protein B (S-100B) in healthy young men. Sleep. 2014;37:195–198. doi: 10.5665/sleep.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate -a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Benton AL. The visual retention test as a constructional praxis task. Confin Neurol. 1967;29:1–16. doi: 10.1159/000104348. [DOI] [PubMed] [Google Scholar]
- Boyle C, Raji CA, Erickson KI, Lopez O, Becker JT, Gach M, Longstreth WT, Teverovskiy L, Kuller L, Carmichael O, Thompson PM. Physical activity is correlated with regional brain volumes in normal aging and Alzheimer’s disease. Organization for human brain mapping, annual meeting Seattle; Washington, USA. 2013. [Google Scholar]
- Brown BM, Peiffer JJ, Taddei K, Lui JK, Laws SM, Gupta VB, Taddei T, Ward VK, Rodrigues MA, Burnham S, Rainey-Smith SR, Villemagne VL, Bush A, Ellis KA, Masters CL, Ames D, Macaulay SL, Szoeke C, Rowe CC, Martins RN. Physical activity and amyloid-beta plasma and brain levels: results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol Psychiatry. 2013;18:875–881. doi: 10.1038/mp.2012.107. [DOI] [PubMed] [Google Scholar]
- Bryan RN, Manolio TA, Schertz LD, Jungreis C, Poirier VC, Elster AD, Kronmal RA. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the cardiovascular health study. AJNR Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Polychronakos C, Toews CJ, MacDougall JD, Guyda HJ, Posner BI. Acute reversal of the enhanced insulin action in trained athletes. Association with insulin receptor changes. Diabetes. 1985;34:756–760. doi: 10.2337/diab.34.8.756. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol Ser A. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Cole JH, Boyle CP, Simmons A, Cohen-Woods S, Rivera M, McGuffin P, Thompson PM, Fu CH. Body mass index, but not FTO genotype or major depressive disorder, influences brain structure. Neuroscience. 2013;252C:109–117. doi: 10.1016/j.neuroscience.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369:540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Beiser A, Hoffmann U, Decarli C, O’Donnell CJ, Massaro JM, Au R, Himali JJ, Wolf PA, Fox CS, Seshadri S. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68:136–144. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defina LF, Willis BL, Radford NB, Gao A, Leonard D, Haskell WL, Weiner MF, Berry JD. The association between midlife cardiorespiratory fitness levels and later-life dementia: a cohort study. Ann Intern Med. 2013;158:162–168. doi: 10.7326/0003-4819-158-3-201302050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bo R, Ghezzi S, Scarpini E, Bresolin N, Comi GP. VEGF genetic variability is associated with increased risk of developing Alzheimer’s disease. J Neurol Sci. 2009;283:66–68. doi: 10.1016/j.jns.2009.02.318. [DOI] [PubMed] [Google Scholar]
- Di Meco A, Joshi YB, Pratico D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Dzierzewski JM, Buman MP, Giacobbi PR, Jr, Roberts BL, Aiken-Morgan AT, Marsiske M, McCrae CS. Exercise and sleep in community-dwelling older adults: evidence for a reciprocal relationship. J Sleep Res. 2014;23:61–68. doi: 10.1111/jsr.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood: the cardiovascular health study. Neurology. 2010;75:1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer’s disease. Arch Med Res. 2012;43:615–621. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Ruscheweyh R, Kruger K, Willemer C, Winter B, Volker K, Lohmann H, Zitzmann M, Mooren F, Breitenstein C, Knecht S. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? Neuroimage. 2010;49:2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- Funk JA, Gohlke J, Kraft AD, McPherson CA, Collins JB, Jean Harry G. Voluntary exercise protects hippocampal neurons from trimethyltin injury: possible role of interleukin-6 to modulate tumor necrosis factor receptor-mediated neurotoxicity. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Gutman BA, Hua X, Rajagopalan P, Chou YY, Wang Y, Yanovsky I, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM. Maximizing power to track Alzheimer’s disease and MCI progression by LDA-based weighting of longitudinal ventricular surface features. NeuroImage. 2013;70:386–401. doi: 10.1016/j.neuroimage.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Hayes JP, Cadden M, Verfaellie M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front Aging Neurosci. 2013;5:31. doi: 10.3389/fnagi.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol. 2012;69:636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterberger M, Zehetmayer S, Jungwirth S, Huber K, Krugluger W, Leitha T, Krampla W, Tragl KH, Fischer P. High cortisol and low folate are the only routine blood tests predicting probable Alzheimer’s disease after age 75-results of the Vienna Transdanube aging study. J Am Geriatr Soc. 2013;61:648–651. doi: 10.1111/jgs.12178. [DOI] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, Lee S, Hibar D, Dinov ID, Stein JL, Jack CR, Jr, Weiner MW, Toga AW, Thompson PM. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging. 2010a;31:1326–1339. doi: 10.1016/j.neurobiolaging.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, Dinov ID, Toga AW, Saykin AJ, Shen L, Foroud T, Pankratz N, Huentelman MJ, Craig DW, Gerber JD, Allen AN, Corneveaux JJ, Stephan DA, DeCarli CS, DeChairo BM, Potkin SG, Jack CR, Jr, Weiner MW, Raji CA, Lopez OL, Becker JT, Carmichael OT, Thompson PM. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci U S A. 2010b;107:8404–8409. doi: 10.1073/pnas.0910878107. PMCID 2889537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Saharan P, DeGiorgio A, Madsen SK, Hibar DP, Stein JL, Becker JT, Lopez OL, Toga AW, Thompson PM. Hippocampal volume is related to body mass index in Alzheimer’s disease. NeuroReport. 2011;22:10–14. doi: 10.1097/wnr.0b013e3283412868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ER, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Ruther E, Schurmann B, Heun R, Kolsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Gallacher J, Hull M, Rujescu D, Giegling I, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, van Duijn CM, Breteler MM, Ikram MA, Destefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, Berr C, Campion D, Epelbaum J, Dartigues JF, Tzourio C, Alperovitch A, Lathrop M, Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Bjornsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossu P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O’Donovan M, Amouyel P, Williams J. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Hibar DP, Ching CR, Boyle CP, Rajagopalan P, Gutman BA, Leow AD, Toga AW, Jack CR, Jr, Harvey D, Weiner MW, Thompson PM. Unbiased tensor-based morphometry: Improved robustness and sample size estimates for Alzheimer’s disease clinical trials. NeuroImage. 2013;66C:648–661. doi: 10.1016/j.neuroimage.2012.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(Suppl.):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, Fox P. An optimized individual target brain in the Talairach coordinate system. Neuroimage. 2002;17:922–927. [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Hardies J, Thompson PM, Woods RP, Cody JD, Hale DE, Laird A, Fox PT. Mapping structural differences of the corpus callosum in individuals with 18q deletions using targetless regional spatial normalization. Hum Brain Mapp. 2005;24:325–331. doi: 10.1002/hbm.20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, Corona W, Morse CL, Zoghbi SS, Pike VW, McMahon FJ, Turner RS, Innis RB. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013;136:2228–2238. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic D, Knuesel I. Deciphering the mechanism underlying late-ons et al zheimer disease. Nat Revi Neurol. 2013;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanche H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O’Neil JP, Wilson RS, Jagust WJ. Association of lifetime cognitive engagement and low beta-amyloid deposition. Arch Neurol. 2012;69:623–629. doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Han SB, Nam SY, Oh KW, Hong JT. Inflammation and Alzheimer’s disease. Arch Pharm Res. 2010;33:1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- Leung KT-K. Principal ranking meta-algorithms Los Angeles. University of California; Los Angeles: 2011. [Google Scholar]
- Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003a;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003b;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Jagust WJ, Fitzpatrick A, Carlson MC, DeKosky ST, Breitner J, Lyketsos CG, Jones B, Kawas C, Kuller LH. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry. 2006;77:159–165. doi: 10.1136/jnnp.2004.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck T, Riedel-Heller SG, Luppa M, Wiese B, Kohler M, Jessen F, Bickel H, Weyerer S, Pentzek M, Konig HH, Prokein J, Ernst A, Wagner M, Mosch E, Werle J, Fuchs A, Brettschneider C, Scherer M, Maier W. Apolipoprotein E epsilon 4 genotype and a physically active lifestyle in late life: analysis of gene-environment interaction for the risk of dementia and Alzheimer’s disease dementia. Psychol Med. 2013:1–11. doi: 10.1017/S0033291713001918. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, Chrousos GP. Exercise and the stress system. Hormones (Athens) 2005;4:73–89. [PubMed] [Google Scholar]
- Matsuzaki T, Sasaki K, Hata J, Hirakawa Y, Fujimi K, Ninomiya T, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T. Association of Alzheimer disease pathology with abnormal lipid metabolism: the Hisayama study. Neurology. 2011;77:1068–1075. doi: 10.1212/WNL.0b013e31822e145d. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V. Inflammatory process in Alzheimer’s disease. Front Integr Neurosci. 2013;7:59. doi: 10.3389/fnint.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc. 2010;58:1322–1326. doi: 10.1111/j.1532-5415.2010.02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Roberts RO, Geda YE, Swenson-Dravis DM, Boeve BF, Senjem ML, Vemuri P, Petersen RC, Jack CR., Jr Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012;79:1570–1577. doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K. Commentary: the three new pathways leading to Alzheimer’s disease. Neuropathol Appl Neurobiol. 2011 doi: 10.1111/j.1365-2990.2011.01181.x. [DOI] [PubMed] [Google Scholar]
- Mortby ME, Janke AL, Anstey KJ, Sachdev PS, Cherbuin N. High “Normal” blood glucose is associated with decreased brain volume and cognitive performance in the 60s: the PATH through life study. PLoS ONE. 2013;8:e73697. doi: 10.1371/journal.pone.0073697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell CJ, Cotter JD, Thomas KN, Lucas SJ, Williams MJ, Ainslie PN. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: effect of age and 12-week exercise training. Age (Dordr) 2013;35:905–920. doi: 10.1007/s11357-012-9414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JS, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, George-Hyslop PS, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, Decarli C, Dekosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin LW, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Parisi JE, Perl DP, Peskind E, Petersen RC, Poon WW, Quinn JF, Rajbhandary RA, Raskind M, Reisberg B, Ringman JM, Roberson ED, Rosenberg RN, Sano M, Schneider LS, Seeley W, Shelanski ML, Slifer MA, Smith CD, Sonnen JA, Spina S, Stern RA, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Williamson J, Woltjer RL, Cantwell LB, Dombroski BA, Beekly D, Lunetta KL, Martin ER, Kamboh MI, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Montine TJ, Goate AM, Blacker D, Tsuang DW, Hakonarson H, Kukull WA, Foroud TM, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-ons et al zheimer’s disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo MA, Leggate M, Viana JL, King JA. The effect of physical activity on mediators of inflammation. Diabetes Obes Metab. 2013;15(Suppl. 3):51–60. doi: 10.1111/dom.12156. [DOI] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Muller S, Oest M, Meyer T, Backens M, Schneider-Axmann T, Thornton AE, Honer WG, Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67:133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46:96–99. doi: 10.1016/j.exger.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Rajagopalan P, Gutman B, Toga AW, Jack CR, Weiner MW, Thompson PM. Plasma cortisol is associated with accelerated brain atrophy: an Alzheimer’s disease neuroimaging initiative (ADNI) study. Society for neuroscience annual meeting New Orleans, LA; USA. 2012. [Google Scholar]
- Rajagopalan P, Toga AW, Jack CR, Weiner MW, Thompson PM. Fat-mass-related hormone, plasma leptin, predicts brain volumes in the elderly. NeuroReport. 2013;24:58–62. doi: 10.1097/WNR.0b013e32835c5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. PMCID 2826530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P, Vedel JC, Olesen J, Adser H, Pedersen MV, Hart E, Secher NH, Pilegaard H. In humans IL-6 is released from the brain during and after exercise and paralleled by enhanced IL-6 mRNA expression in the hippocampus of mice. Acta Physiol (Oxf) 2011;201:475–482. doi: 10.1111/j.1748-1716.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- Razay G, Vreugdenhil A. Obesity in middle age and future risk of dementia: midlife obesity increases risk of future dementia. Br Med J. 2005;331:455. doi: 10.1136/bmj.331.7514.455. author reply 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CK, Little JP, Thyfault JP. Modification of insulin sensitivity and glycemic control by activity and exercise. Med Sci Sports Exerc. 2013;45:1868–1877. doi: 10.1249/MSS.0b013e318295cdbb. [DOI] [PubMed] [Google Scholar]
- Ross J, Thompson PM, Tariot P, Reiman E, Schneider LS, Frigerio E, Fiorentini F, Giardino L, Calza L, Norris D, Cirirello H, Casula D, Imbimbo BP. Primary and secondary prevention trials in subjects at risk of developing Alzheimer’s disease: the GEPARDAD (genetically enriched population at risk of developing Alzheimer’s disease) studies. Clinical trials conference on Alzheimer’s disease Monte Carlo; Monaco. 2012. [Google Scholar]
- Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–47. [PubMed] [Google Scholar]
- Siscovick DS, Fried L, Mittelmark M, Rutan G, Bild D, O’Leary DH. Exercise intensity and subclinical cardiovascular disease in the elderly. The cardiovascular health study. Am J Epidemiol. 1997;145:977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AD, Rolland C, Gryka A, Findlay S, Smith S, Jones J, Davidson IM. Morphological and health-related changes associated with a 12-week self-guided exercise programme in overweight adults: a pilot study. J Sports Sci. 2013 doi: 10.1080/02640414.2013.812791. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Martin B, Maudsley S. Anti-inflammatory effects of physical activity in relationship to improved cognitive status in humans and mouse models of Alzheimer’s disease. Curr Alzheimer Res. 2012;9:86–92. doi: 10.2174/156720512799015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Thompson D, Markovitch D, Betts JA, Mazzatti D, Turner J, Tyrrell RM. Time course of changes in inflammatory markers during a 6-mo exercise intervention in sedentary middle-aged men: a randomized-controlled trial. J Appl Physiol. 2010;108:769–779. doi: 10.1152/japplphysiol.00822.2009. [DOI] [PubMed] [Google Scholar]
- Tolppanen AM, Ngandu T, Kareholt I, Laatikainen T, Rusanen M, Soininen H, Kivipelto M. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimer Dis. 2013 doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- Tsukui S, Kanda T, Nara M, Nishino M, Kondo T, Kobayashi I. Moderate-intensity regular exercise decreases serum tumor necrosis factor-alpha and HbA1c levels in healthy women. Int J Obes Relat Metab Disord. 2000;24:1207–1211. doi: 10.1038/sj.ijo.0801373. [DOI] [PubMed] [Google Scholar]
- Vallejo AN, Hamel DL, Jr, Mueller RG, Ives DG, Michel JJ, Boudreau RM, Newman AB. NK-like T cells and plasma cytokines, but not anti-viral serology, define immune fingerprints of resilience and mild disability in exceptional aging. PLoS ONE. 2011;6:e26558. doi: 10.1371/journal.pone.0026558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas T, Martinez-Garcia A, Antequera D, Vilella E, Clarimon J, Mateo I, Sanchez-Juan P, Rodriguez-Rodriguez E, Frank A, Rosich-Estrago M, Lleo A, Molina-Porcel L, Blesa R, Gomez-Isla T, Combarros O, Bermejo-Pareja F, Valdivieso F, Bullido MJ, Carro E. IGF-I gene variability is associated with an increased risk for AD. Neurobiol Aging. 2011;32:556, e553–556, e511. doi: 10.1016/j.neurobiolaging.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Vicente-Campos D, Mora J, Castro-Pinero J, Gonzalez-Montesinos JL, Conde-Caveda J, Chicharro JL. Impact of a physical activity program on cerebral vasoreactivity in sedentary elderly people. J Sports Med Phys Fitness. 2012;52:537–544. [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale – revised. The Psychological Corporation; San Antonio: 1981. [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Br Med J. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky I, Thompson P, Osher S, Leow AD. Asymmetric and symmetric unbiased image registration: statistical assessment of performance. IEEE computer society workshop on mathematical methods in biomedical image analysis; 2008. pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky I, Leow AD, Lee S, Osher SJ, Thompson PM. Comparing registration methods for mapping brain change using tensor-based morphometry. Med Image Anal. 2009;13:679–700. doi: 10.1016/j.media.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


