Abstract
Drug-drug interactions at transporters present a significant and under-investigated clinical problem. Investigations of specific transporter functions and screening for potential drug-drug interactions, both in vitro and especially in vivo, will require validated experimental probes. Fexofenadine, an approved, well-tolerated drug, is a promising probe for studies of membrane transporter function. Although fexofenadine pharmacokinetics are known to be controlled by transporters, the contributions of individual transporters have not been defined. We have developed a rapid, specific, and sensitive analytical method for quantitation of fexofenadine to support this work. This LC-MS/MS method quantifies fexofenadine in cell lysates from in vitro studies using cetirizine as the internal standard. Cell lysates were prepared for analysis by acetonitrile precipitation. Analytes were then separated by gradient reverse-phase chromatography and analyzed by tandem mass spectrometry using the m/z 502.17/466.2 transition for fexofenadine and m/z 389.02/201.1 for cetirizine. The method exhibited a linear dynamic range of 1–500 ng/mL for fexofenadine in cell lysates. The lower limit of quantification was 1 ng/mL with a relative standard deviation of less than 5%. Intra- and inter-day precision and accuracy were within the limits presented in the FDA guidelines for bioanalysis. We also will validate this method to support not only the quantification of fexofenadine, but also other probe drugs for drug-drug interaction studies. This method for quantification will facilitate the use of fexofenadine as a probe drug for characterization of transporter activity.
Keywords: Fexofenadine, cell lysate, probe drug, transporter
Introduction
Fexofenadine, a non-sedating H1-receptor antagonist, is a substrate of the uptake transporter organic anion transporting polypeptide 1A2 (OATP1A2) and the efflux transporter, P-glycoprotein (P-gp).[1, 2] Additional reports have suggested that OATP1B1, OATP1B3, and OATP2B1 might also transport fexofenadine.[3, 4] Ninety-five percent of fexofenadine is excreted unchanged, with only 1% metabolized prior to excretion[1], thus fexofenadine pharmacokinetics are controlled by transporters, rather than by metabolism. The pharmacokinetics of fexofenadine suggests that it might be a useful probe drug for functional studies of both the OATP family of transporters and P-glycoprotein.[5] However, the precise contributions of individual transporters to fexofenadine pharmacokinetics are still unknown.[5,6,7] This will require careful characterization of fexofenadine transport in model systems to define the relative roles of these various transporters. Both in vitro studies to identify transporters with high affinity for fexofenadine, and in vivo studies to characterize the role of fexofenadine as a selective probe for certain transporter activity, will require a bioanalytical method that is accurate, rapid, sensitive, and selective for this probe drug.
Previous methods for quantification of fexofenadine have been described based on HPLC using fluorescence detection.[8,9] Those methods, however, required relatively long analysis times and provide less sensitivity and specificity than required for our studies. Due to these issues, high selectivity and sensitivity LC-MS/MS methods were developed for the quantification of fexofenadine. However, these methods had relatively long run times (>10 minutes) and were validated for the quantification of fexofenadine in plasma or urine samples.[10,11] To meet the need for the bioanalytical support for cell-based transporter assays, we have developed and validated an LC-MS/MS method for the identification and quantification of this drug in cell culture lysates using cetirizine as the internal standard. This method will be applied to the analysis of fexofenadine in mammalian cell lysates from in vitro transporter studies, and will be developed further to measure other probe drugs to support drug-drug interaction studies in these model systems.
Materials and Methods
Materials
Fexofenadine hydrochloride was obtained from Toronto Research Chemical (Toronto, Ontario, Canada) and cetirizine hydrochloride (internal standard, IS) was obtained from Sigma Aldrich (St. Louis, MO, USA). Chemical structures of these analytes are provided in Figure 1. Ammonium formate, methanol, acetonitrile, and formic acid, all of HPLC or Optima grade, were from Fisher Scientific (Fair Lawn, NJ, USA). Water was from a Millipore Q Water System (Millipore, Bedford, MA, USA). All other chemicals were analytical grade. Cell lysate source was HEK293 cells obtained from American Type Culture Collection (ATCC, Manassas, VA, USA).
Figure 1. Structures of fexofenadine (1A) and cetirizine (1B, internal standard).
Sample Preparation
Fexofenadine and cetirizine (IS) stock solutions (1 mg/mL) were individually prepared in methanol. The internal standard was diluted to 100 ng/mL (working concentration) by diluting the stock solution with and a diluent composed of 7.5 mM ammonium formate, pH 5, methanol, acetonitrile (50:25:25, v/v/v). This same diluent was used for all dilutions and for sample reconstitution.
HEK293 cell culture lysates were spiked with 25 or 50 µL of fexofenadine working solutions to obtain final fexofenadine concentrations of 0, 1, 2, 5, 10, 50, 100, and 500 ng/mL fexofenadine, containing the IS at a concentration of 10 ng/mL. Quality control (QC) samples were prepared independently on separate days at concentrations of 3 (low), 75 (medium), 400 and 500 (high) ng/mL fexofenadine.
For in vitro studies of fexofenadine transport, the matrix was a mammalian cell lysate derived from HEK293 cells transiently transfected with the uptake transporter OATP1A2[12]. Confluent monolayers of HEK293 cells containing OATP1A2 in 24-well plates were washed with uptake buffer (142 mM NaCl, 5 mM KCl, 1 mM KH2PO4, 1.2 mM MgSO4, 1.5 mM CaCl2, 5 mM glucose and 12.5 mM Hepes, and was adjusted to pH 7.4 with Tris base) three times and then frozen overnight. Cells then were thawed and 150 µL of diluent containing the desired concentration of fexofenadine and the internal standard (final concentration 10 ng/mL cetirizine) was added and cells were lysed at room temperature for 20 minutes on a rocker platform. This mixture was centrifuged at 20,000 rpm for 5 minutes to pellet precipitated protein. Lysates were then transferred to a round bottom 96-well plate and 10 µL was injected for analysis.
LC-MS/MS Conditions
Chromatography was performed using a Luna C18 column (5 µm, 50 × 2 mm), fitted with a C18 4 × 2 mm guard column (Phenomenex, Torrance, CA, USA) at 40 °C. The aqueous mobile phase (Solvent A) was 7.5 mM ammonium formate, pH 5, and the organic mobile phase (Solvent B) was acetonitrile and methanol (50:50, v/v). Total flow rate was 0.5 mL/min. The gradient used allows the quantification of fexofenadine as well as the ability to broaden the procedure to include other probe drugs in the future. Elution was performed with a linear gradient from 5% to 90% Solvent B from 0–2 minutes, elution with 90% B for 0.5 minutes, and then a linear decrease to 5% B over 0.75 minutes. Total run time was 5.0 minutes.
Mass spectrometry was performed using a Waters Quattro Premier mass spectrometer (Waters Corporation, Milford, MA, USA) in the positive ion electrospray mode. Source temperature was 120 °C, with a desolvation temperature of 350 °C. Cone gas flow was set at 60 L/hr and a desolvation gas flow of 650 L/hr. The mass spectrometer was operated in the MRM mode with a dwell time of 0.050 seconds per MRM channel. Fexofenadine or cetirizine at a concentration of 100 µg/mL in methanol was infused directly into the source of the mass spectrometer to determine characteristic product ions of the analyte and IS. Resulting mass spectra are shown in Figure 2. The transitions (m/z) chosen for MRM were 502.17/466.2 for fexofenadine and 389.02/201.1 for the internal standard cetirizine. The collision energy was set at 26 eV for the analyte and 20 eV for the internal standard. Cone voltage was set at 30 V for fexofenadine and 40 V for cetirizine. Data acquisition was performed with MassLynx (Version 4.12) and quantitation of results with QuanLynx software.
Figure 2. Mass spectra obtained for fexofenadine (2A) and cetirizine (2B).
Product spectra for fexofenadine and cetirizine were generated by direct infusion into the ESI probe.
Bioanalytical Method Validation
Standards for calibration curves were prepared by spiking aliquots of diluent or cell culture lysate with 10 ng/mL cetirizine (IS) and seven non-zero analyte concentrations, covering a range of 1–500 ng/mL fexofenadine. The calibration curves for standards in diluents and in cell lysates, were generated on consecutive days from samples injected in duplicate using the analyte to IS peak area ratios by weighted (1/x) least squares linear regression. Acceptance criteria for calibration curves were a correlation coefficient (r2) of 0.99 or greater and standard concentrations determined within 15% of the actual value, the exception being the LLOQ which must be within 20% of the actual value. The precision and accuracy of the method was determined by analyzing three sets of QC samples (low, medium, high), with each batch containing five replicates of each concentration level, analyzed on consecutive days. Precision of the method was calculated by determining the coefficient of variation (CV). The acceptable criteria for intra- and inter-day precision were <15% for all non-LLOQ samples and <20% for the LLOQ. Accuracy of the method was considered acceptable if measured values were between 85–115% of the actual value, or 80–120% for the LLOQ.
Extraction recovery of the analyte (fexofenadine) was determined by comparing the raw peak area of fexofenadine isolated from spiked cell culture matrix to the peak area of the analyte at the same concentration in diluent.
Results
Mass Spectrometry
Figure 2 shows the mass spectra obtained for fexofenadine and the internal standard cetirizine. Fragmentation of fexofenadine shows its characteristic product ions at m/z of 131.1, 171.2, 189.0, 233.9, 262.3, and 466.2. The two most abundant ions are 466.2 and 171.2 (m/z). For cetirizine a characteristic product ion at m/z 200.9 was observed. The most sensitive transitions were 502.17/466.2 for fexofenadine and 389.09/201.1 for the internal standard cetirizine.
Chromatography
Chromatographic conditions were optimized by testing aqueous mobile phases that varied in pH from 3 to 8, and by using methanol, acetonitrile, or a 50/50 (v/v) methanol-acetonitrile mixture as the organic phase. Based on sensitivity, peak shape for the analyte and internal standard, and on overall run time, we found that a gradient of ammonium formate (7.5 mM, pH 5) and acetonitrile-methanol (50:50, v/v) achieved the best chromatographic results. This mobile phase allowed elution of fexofenadine at 2.48 minutes and the internal standard at 2.66 minutes with a total run time of less than five minutes. Although ideal for analysis of fexofenadine alone, we also anticipate the need to measure additional analytes as we investigate drug-drug interactions at membrane transporters. To support this future need, we modified the method to include other probe drugs of interest. Use of the same gradient we developed for fexofenadine alone also yielded acceptable retention of the relatively polar probe drug caffeine and the elution of fexofenadine, three other less polar probe drugs (buspirone, dextromethorphan, losartan), and the internal standard within 5 minutes (data not shown).
Selectivity
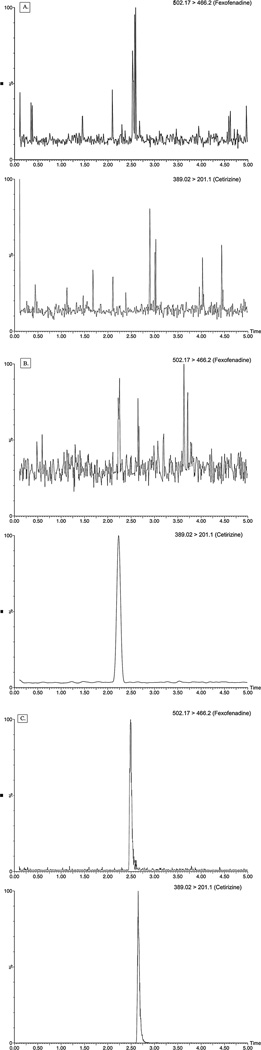
Method selectivity was examined by analyzing blank cell culture matrix samples (Figure 3A) and matrix spiked only with IS (Figure 3B). As seen in Figure 4A, there is no endogenous compound interference at the retention times of the analyte or internal standard. Similarly, the internal standard does not show any crossover in the MRM of the analyte (Figure 3B). The MRM chromatogram in Figure 3C illustrates the result from a sample spiked with 1 ng/mL fexofenadine, demonstrating the sensitivity of the method.
Figure 3. MRM chromatograms for fexofenadine and cetirizine.
Aliquots of HEK293 cell lysates were spiked, prepared by lysis with mobile phase, analyzed by LC-MS/MS, and quantified as described in Materials and Methods. The chromatogram in Panel 3A from analysis of a sample prepared from blank cell lysate, whereas the lysate sample analyzed for 3B contained 10 ng/mL cetirizine, and the sample for 3C was spiked with both the internal standard and with fexofenadine at the LLOQ, 1 ng/mL.
Figure 4. Calibration curve for fexofenadine in HEK293 cell lysate.
Aliquots of HEK293 cell lysates were spiked with the from 0–500 ng/mL fexofenadine and with 10 ng/mL of the internal standard, cetirizine. Samples were prepared by acetonitrile precipitation, analyzed by LC-MS/MS, and quantified as described in Materials and Methods.
Linearity
The seven-point calibration curve for fexofenadine extracted from HEK293 cell lysates was linear over the selected concentration range of 1–500 ng/mL fexofenadine. The best linear fit and linear regression were obtained with a 1/x weighing factor. Figure 4 illustrates a representative standard calibration curve for fexofenadine in cell lysates, with an r2 of 0.99. Table 1 summarizes the calibration curve results, illustrating our ability to both precisely and accurately measure fexofenadine in mobile phase. Fexofenadine quantitation at 1 ng/mL was accomplished with a precision (CV) of 2.6% and a mean accuracy of 106.5%, well within the limits for defining the LLOQ. Similarly, the precision and accuracy of each concentration of fexofenadine in the calibration curve met the FDA criteria for bioanalytical method validation. [14]
Table 1.
Precision and accuracy data for fexofenadine calibration standards from spiked HEK293 cell lysate
| Concentration Added (ng/mL) |
Concentration Found (ng/mL) |
Precision (CV, %) |
Accuracy (%) |
|---|---|---|---|
| 1 | 1.06 | 2.6 | 106.5 |
| 2 | 1.98 | 7.6 | 98.9 |
| 5 | 4.73 | 2.1 | 94.5 |
| 10 | 8.79 | 3.5 | 87.9 |
| 50 | 53.5 | 1.7 | 106.9 |
| 100 | 99.2 | 2.5 | 99.2 |
| 500 | 498.6 | 0.7 | 99.7 |
The inter-day coefficient of variation (CV) for the lowest QC standard (3 ng/mL) in cell lysate is 9.5% and the between batch accuracy was 97.4% (Table 2). Within the batches, the low QC standard had a CV of 8.5% and the accuracy was 91.9%. For the middle and upper quantification levels (ranging from 75–500 ng/mL), the precision (CV) ranged from 2.5–6.2% and the accuracy from 90.1–97.0% within batch (Table 2). Between batches, the precision ranged from 3.4–4.9% and the accuracy from 97.4–103.6% in HEK293 cell lysate.
Table 2.
Intra- and Inter-day Precision and accuracy of the method for determining fexofenadine concentrations in HEK293 cell lysates
| Intra-day (n = 5) | Inter-day (n = 3) | |||||
|---|---|---|---|---|---|---|
| Concentration Added (ng/mL) |
Concentration found (mean) (ng/mL) |
Precision (CV, %) |
Accuracy (%) |
Concentration found (mean) (ng/mL) |
Precision (CV, %) |
Accuracy (%) |
| 3 | 2.76 | 8.5 | 91.9 | 2.92 | 9.5 | 97.4 |
| 75 | 72.8 | 3.5 | 97.0 | 76.3 | 3.4 | 101.7 |
| 400 | 373.9 | 6.2 | 93.5 | 404.5 | 4.9 | 103.6 |
| 500 | 450.6 | 2.5 | 90.1 | 500.8 | 4.3 | 100.2 |
Recovery
Table 3 illustrates the recovery of fexofenadine from HEK293 cell lysate as compared to samples spiked in mobile phase. The average recovery of fexofenadine from spiked cell culture matrix was from 86% to 123%. With the exception of the LLOQ, all concentrations had standard deviations of recovery less than 20%.
Table 3.
Recovery of fexofenadine from HEK293 cell lysates
| Concentration (ng/mL) |
Recovery 1 | Recovery 2 | Average Recovery |
|---|---|---|---|
| 1 | 75.7 | 96.3 | 86.0 |
| 2 | 98.5 | 116.3 | 107.4 |
| 5 | 106.8 | 104.9 | 105.9 |
| 10 | 119.8 | 115.5 | 117.7 |
| 50 | 105.3 | 77.3 | 91.3 |
| 100 | 121.4 | 125.6 | 123.5 |
| 500 | 117.1 | 105.7 | 111.4 |
Discussion
Fexofenadine is a promising probe for transporter function both in vitro and especially in vivo. In order to use fexofenadine as a probe of transporter function and measure fexofenadine transporter activity, a rapid, specific, and sensitive analytical method has been developed to meet this need. Compared to other analytical methods, including HPLC with fluorescence detection [8,9] and existing LC-MS/MS methods [10,11,13], we are able to provide improved selectivity and sensitivity. Our method yields a LLOQ of 1 ng/mL, versus previously published LLOQs of greater than 1 ng/mL. [5,8,10,11,13] While the LLOQ potentially could be extended to concentrations lower than 1 ng/mL fexofenadine, this was not necessary for our studies. Uptake of fexofenadine via transporters like OATP1A2 in transiently transfected cell models, similar to the model we employ, resulted in maximum fexofenadine concentrations of 1–3 µg/mL, [2] therefore, our method provides a level of sensitivity that will allow the study of even low efficiency transporter systems. This method also meets the FDA guidelines for bioanalysis, with demonstrated accuracy within 90–110% of actual concentrations, precision of ± 10%, and recoveries greater than 75% from the HEK293 cell lysates. [13]
The choice of the internal standard for this method was a key step in the development and validation. Our first approach was to use the pro-drug terfenadine as internal standard, since it shares extensive structural identity with fexofenadine. We found, however, that the lack of the carboxyl group present in fexofenadine resulted in profoundly different chromatographic behavior, and that we experienced substantial carryover with terfenadine. These problems ruled out the use of terfenadine as internal standard. We then examined another antihistamine, cetirizine, as a possible internal standard based on similar structure and chemical properties with fexofenadine. Use of cetirizine provided very similar chromatographic properties to those of fexofenadine and we observed no carryover or interference with the MRM channels between compounds. These observations supported our use of cetirizine as the internal standard for this assay.
Although examination of fexofenadine transport is one goal of our studies, an additional goal is to employ fexofenadine as a probe for the detection and characterization of drug-drug interactions at specific transporters. This will require the detection and quantitation of potentially competing drugs from cell lysate samples. Our use of gradient elution is intended to support not only the analysis for fexofenadine, but also additional drugs for interaction studies. We have performed initial studies to expand this assay to include the quantitation of four additional probe drugs: buspirone, caffeine, dextromethorphan, and losartan. Use of a steep chromatographic gradient allows us to analyze five drugs and one internal standard in less than five minutes. Once the method is validated for these additional analytes it will allow us to characterize the kinetics of multiple substrates with transporters in a single sample and analytical run, greatly facilitating our investigations of drug-drug interactions.
This gradient method has been successfully used to characterize the kinetics of fexofenadine transport by HEK293 cells transiently-transfected with OATP1A2. This validated method provides a rapid, sensitive, and specific analytical tool supporting the use of fexofenadine as a probe to study the contributions of individual membrane transporters in uptake and efflux of xenobiotics.
Conclusion
We have presented a fully validated LC-MS/MS method for the quantification of fexofenadine in HEK293 cell lysate from in vitro transporter studies according to the FDA guidelines for bioanalysis.[14] Using a positive electrospray ionization mode, along with a commercially available internal standard (cetirizine), we have shown this method to be precise, accurate, and both sensitive and selective for the quantification of fexofenadine from cell culture uptake studies. An easy and rapid sample preparation method and quick analysis time (less than 5 minutes) makes this suitable for high throughput bioanalysis.
Acknowledgment
This work was supported by NIH grants by grants from NCCAM (R21 AT002907), NIEHS (T32 ES007079), and from NCRR (P20 RR021940).
References
- 1.Glaser H, Bailey DG, Dresser GK, Gregor JC, Schwartz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, Kim RB. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–369. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 2.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the celluar uptake and excretion of fexofenadine. Drug Metab Dispo. 1999:866–871. [PubMed] [Google Scholar]
- 3.Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Investigation. 2003;33:1–5. doi: 10.1046/j.1365-2362.33.s2.5.x. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, Sugiyama Y. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005:1477–1481. doi: 10.1124/dmd.105.004622. [DOI] [PubMed] [Google Scholar]
- 5.Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 6.Hamman MA, Bruce MA, Haehner-Daniels BD, Hall SD. The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther. 2001;69:114–121. doi: 10.1067/mcp.2001.113697. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Hamman MA, Huang SM, Lesko LJ, Hall SD. Effect of St. John’s wort on the pharmacokinetics of fexofenadine. Clin Pharmacol Ther. 2002;71:414–420. doi: 10.1067/mcp.2002.124080. [DOI] [PubMed] [Google Scholar]
- 8.Coutant JE, Westmark PA, Nardella PA, Walter SM, Okerholm RA. Determination of terfenadine and terfenadine acid metabolite in plasma using solid-phase extraction and high performance liquid chromatography with fluorescence detection. J Chromatogr. 1991;570:139–146. doi: 10.1016/0378-4347(91)80208-t. [DOI] [PubMed] [Google Scholar]
- 9.Uno T, Yasui-Furukori N, Takahata T, Sugawara K, Tateishi T. Liquid chromatographic determination of fexofenadine in human plasma with fluorescence detection. J Pharmaceut Biomed. 2004;35:937–947. doi: 10.1016/j.jpba.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann U, Seiler M, Drescher S, Fromm MF. Determination of fexofenadine in human plasma and urine by liquid chromatography-mass spectrometry. J Chromatogr B. 2002;766:227–237. doi: 10.1016/s0378-4347(01)00468-6. [DOI] [PubMed] [Google Scholar]
- 11.Fu I, Woolf EJ, Matuszewski BK. Determination of fexofenadine in human plasma using 96-well solid phase extraction and HPLC with tandem mass spectrometric detection. J Pharmaceut Biomed. 2004;35:837–844. doi: 10.1016/j.jpba.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Roth M, Timmerman BN, Hagenbuch B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab Dispos. 2011;39:920–926. doi: 10.1124/dmd.110.036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamane N, Tozuka Z, Sugiyama Y, Tanimoto T, Yamazaki A, Kumagai Y. Microdose clinical trial: Quantitation determination of fexofenadine in human plasma using liquid chromatography/electrospray ionization tandem mass spectrometry. J Chromatogr B. 2007;858:118–128. doi: 10.1016/j.jchromb.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 14.FDA Guidance for Industry: Bioanalytical Method Validation. 2001 http://www.fda.gov/cder/guidance/4252fnl.pdf.