Summary
Approximately 2 billion people are infected with Mycobacterium tuberculosis, the etiological agent of tuberculosis (TB), and an estimated 1.5 million individuals die annually from TB. Presently, Mycobacterium bovis BCG remains the only licensed TB vaccine; however, previous studies suggest its protective efficacy wanes over time and fails in preventing pulmonary TB. Therefore, a safe and effective vaccine is urgently required to replace BCG or boost BCG immunizations. Our previous studies revealed that mycobacterial proteins are released via exosomes from macrophages infected with M. tuberculosis or pulsed with M. tuberculosis culture filtrate proteins (CFP). In the present study, exosomes purified from macrophages treated with M. tuberculosis CFP were found to induce antigen-specific IFN-γ and IL-2-expressing CD4+ and CD8+ T cells. In exosome-vaccinated mice there was a similar TH1 immune response but a more limited TH2 response compared to BCG vaccinated mice. Using a low-dose M. tuberculosis mouse aerosol infection model, exosomes from CFP-treated macrophages were found to both prime a protective immune response as well as boost prior BCG immunization. The protection was equal to or superior to BCG. In conclusion, our findings suggest that exosomes might serve as a novel cell-free vaccine against an M. tuberculosis infection.
Keywords: Mycobacterium tuberculosis, Exosomes, Vaccine
Introduction
Currently, more than 2 billion individuals have been infected with M. tuberculosis and about 5%–10% those infected will develop active TB disease during their lifetime. In 2011, there was an estimated 8.7 million new cases of TB (13% co-infected with HIV) and among the approximate 1.5 million individuals who died from TB, 430,000 were HIV-positive [1]. In 1921, the vaccine Mycobacterium bovis BCG, developed by Albert Calmette and Camille Guérin, was first used in humans [2, 3]. Currently M. bovis BCG has been administrated to over 4 billion people and remains the only licensed anti-TB vaccine worldwide [4]. Routine BCG vaccination involves the intradermal administration of BCG to infants shortly after birth and it has been found to partially protect against some childhood forms of tuberculosis such as TB meningitis and military TB [1, 5]. However, a number of studies have demonstrated that the efficacy of BCG against TB wanes over time and provides little or no protection against pulmonary TB in adolescents and adults [6]. Furthermore, according to the WHO recommendation, BCG vaccination should not be given to HIV-infected infants because of a high risk of disseminated infection [7, 8]. Therefore, a novel, safe and effective vaccine against TB for both HIV-negative and HIV-positive individuals is urgently needed. For pre-exposure, two main approaches are currently being evaluated [6, 9]. The first approach involves generating modified mycobacteria that would be more effective than BCG with present examples including VPM 1002, rBCG30 and MIP [6]. The second approach relies on the development of a “prime-boost” vaccination strategy consisting of a primary BCG vaccination in newborns and a follow-up booster subunit vaccine, such as recombinant mycobacterial proteins formulated in adjuvants (M72, Hybrid-1, Hyvac 4, H56 and ID93), and recombinant viral vectors expressing mycobacterial proteins (MVA85A, Aeras-402 and AdAg85A). In the case of post-exposure, subunits vaccines would be built as immunotherapeutic agents in combination with antibiotics.
Exosomes are 50 to 150 nm membrane vesicles originating from multivesicular bodies (MVBs) by inward budding of endosomal membranes and are released by hematopoietic and non-hematopoietic cells via the fusion of the limiting membrane of MVBs to the plasma membrane [10, 11]. These membrane vesicles were originally defined as a mechanism to eliminate surface membrane receptors such as the transferrin receptor from maturing reticulocytes [12, 13]. Subsequently it was determined that Epstein-Barr virus-transfomed B lymphocytes release exosomes containing major histocompatibility complex (MHC) class II molecules with bound peptides, which were able to activate antigen-specific T cells in vivo. This suggests a role for exosomes in promoting an acquired immune response [14]. The feasibility of using antigen-containing exosomes as a novel cell-free tumor vaccine has been investigated in some detail [15–18]. Our previous studies determined that cultured macrophages infected with M. tuberculosis or pulsed with M. tuberculosis CFP released exosomes containing mycobacterial components including antigenic proteins and lipids, and were capable of priming a mycobacterial antigen-specific T cell response in mice [19–21]. However, it remained unclear whether these exosomes were able to protect against an M. tuberculosis infection. In this study, we investigated the vaccine efficacy of exosomes against TB in both naïve and prior BCG-immunized mice.
Results
Immune responses induced by CFP exosomes
The M. tuberculosis CFP produced through Colorado State University’s TB Vaccine Testing and Research Materials Contract contains a number of mycobacterial proteins and other components which are secreted/released from the bacteria during liquid culture (Fig.1A and 1B). In our previous proteomic study 29 mycobacterial proteins were identified in/on exosomes released from macrophages treated with M. tuberculosis CFP (CFP exosomes) [21]. Interestingly, the majority of proteins identified including the antigen 85 complex and GroES have been recognized as T-cell antigens in either human TB patients, animal models or both [22–24]. In order to determine if CFP exosomes could be used as an effective vaccine in a mouse TB infection model, we treated Raw 264.7 cells with CFP and isolated the exosomes from the culture media 24 hours post-treatment. The quality of the purified exosomes was evaluated by particle tracking using a NanoSight LM10 and by Western blot. Particle tracking measurements illustrated that purified vesicles were mainly located in a range of 50–150 nm which is consistent with the size of exosomes released from macrophages (Data not shown) [25]. Additionally, Western blot analysis detected LAMP-1 as a host exosomal marker and the 19-kDa Lipoprotein as the M. tuberculosis exosomal marker (Fig. 1C). However, although the purified vesicles contained exosomal markers and were filtered through a 0.22 µm filter to remove larger microvesicles, we cannot completely rule out that there may be other types of extracellular vesicles in our preparation.
Figure 1. Exosomes from CFP-treated macrophages contains both host and mycobacterial components.
(A) Purified CFP analyzed by SDS-PAGE gel (15%) and stained with Coomassie. (B) Western blot analysis of CFP using a polyclonal anti-serum made against the M. tuberculosis culture filtrate proteins. (C) Exosomes released from CFP-treated (CFP exosomes) or untreated (UT exosomes) macrophages were analyzed by Western blot for the mycobacterial 19-KDa lipoprotein and the host exosomal marker LAMP1.
To investigate the efficacy of the CFP exosomes as primary anti-TB vaccines, groups of naïve C57BL/6 mice were intranasally immunized with purified CFP exosomes without adjuvant at a dose of either 20 µg/mouse or 40 µg/mouse. Exosomes were also purified from untreated macrophages and used to vaccinate mice at the same concentrations. BCG and PBS served as positive and negative controls, respectively. Mice were immunized as described in the Materials and Methods and 2 weeks after the final exosome vaccination, mice were sacrificed and the CD4+ and CD8+ T cells from the spleen and lungs were evaluated for IFN-γ, IL-2 and CD69 expression ex-vivo following incubation with M. tuberculosis cell lysate. As shown in figure 2A and B, immunization with CFP-exosomes lead to a measurable number of antigen-specific CD4+ and CD8+ T cells expressing IFN-γ in both lung and spleens. CFP exosomes elicited a comparable level of antigen-specific IFN-γ expressing T cells as BCG. Moreover, IFN-γ levels in the culture supernatant of splenocytes or lung cells following stimulation with M. tuberculosis protein lysate were similar between mice immunized with high dose of CFP exosomes or with BCG (Fig. 2E). IL-2 production by CD4+ and CD8+ T cells were similarly elevated in mice immunized with CFP exosomes (Fig. 2C, Dand F). As expected, mice vaccinated with exosomes from uninfected cells did not induce M. tuberculosis antigen-specific CD4+ or CD8+ T cell activation. We also observed an increased population of CD69-positive CD4+ and CD8+ T cells in mice immunized with CFP exosomes, further supporting the effective immunogenic activity of the exosomes (data not shown).
Figure 2. Antigen-specific immune response in the lungs and spleens of mice primarily vaccinated with exosomes containing mycobacterial proteins.
(A–D) Flow cytometric analysis of antigen-specific cytokine – expressing CD4+ and CD8+ T cells isolated from the spleens and lungs of vaccinated or non-vaccinated mice. The frequency of INF-γ-positive CD4+ (A) or CD8+ (B) T cells as well as the frequency of IL-2 positive CD4+ (C) or CD8+ (D) T cells after ex vivo stimulation with M. tuberculosis antigens. (E and F) IFN-γ and IL-2 ELISA using spleen and lung cells isolated from vaccinated and non-vaccinated mice. The results are expressed as the cytokine concentration after ex vivo stimulation of 1×106 cells with M. tuberculosis antigens. The data shown +/− standard error of pooled lung or spleen homogenate from 5 mice per given condition run in duplicate and is the representative of three independent experiments. *, p < 0.05 compared to PBS control (one-way ANOVA with Tukey post-test. UT20 and UT40, exosomes (20 or 40µg) isolated from untreated marcophages; CFP20 and CFP40, exosomes (20 or 40µg) isolated from macrophages pulsed with M. tuberculosis CFP.
Limited TH2 immune response generated by CFP exosome vaccination
T-helper (TH1) CD4+ cells expressing INF-γ play a critical role in controlling M. tuberculosis infection in humans as well as in various animal models [26–28]. However, the protective efficacy of TH1 CD4+ cells might be attenuated by a TH2 cell response. Recently, it was found that antigen-containing exosomes can drive a predominate TH1 immune response against parasite infection or tumor progression in mice [29–31]. To determine whether CFP exosome vaccination generates both a TH1 and TH2 immune response, the expression of IL-4, a marker for TH2-mediated immunity, was investigated by intracellular cytokine staining followed by FACS analysis. BCG but not CFP exosome vaccination induced expression of IL-4 positive CD4+ cells following ex-vivo stimulation (Fig. 3). To evaluate this TH1/TH2 balance further, mycobacterial antigen-specific antibody isotypes in serum were defined 2 weeks post-vaccination. Both BCG and CFP-exosome vaccinated mice produced antigen-specific IgG (Fig. 4A). However, CFP exosomes induced a greater antigen-specific IgG2c antibody, an indicator of a TH1 mediated immune response, compared to BCG (Fig. 4B). In contrast, antibody titers for IgG1, which is an indicator of TH2-mediated immune response, was higher in mice immunized with BCG compared to those receiving CFP exosomes (Fig. 4C). The relative ratio (IgG2c/IgG1) against specific antigens is used as an indicator of the balance between a TH1 or TH2 immune response (Fig. 4D). Our results suggest that mice vaccinated with CFP exosomes produce a more predominate TH1 immune response compared to BCG vaccinated mice.
Figure 3. BCG but not CFP-exosome vaccination induces IL-4-expressing CD4+ T cells.
Splenic and lung cells were harvested 2 weeks after the final exosome vaccination and ex vivo stimulated with M. tuberculosis antigens followed by flow cytometry staining for CD4 and IL-4 expression. The data shown is the representative of three independent experiments. *, p < 0.05 (one-way ANOVA with Tukey post-test).
Figure 4. Exosomes from CFP-treated macrophages induce a limited TH2 immune response in mice compared to BCG vaccinated mice.
Antigen-specific (A) IgG, and subtypes (B) IgG2c and (C) IgG1 endpoint titers were defined using mouse serum which was collected 2 weeks after the final vaccination with exosomes. Mean reciprocal dilutions are used as the endpoint titer (log10). The data shown +/− standard error of pooled lung or spleen homogenate from 5 mice per given condition run in duplicate and is the representative of three independent experiments. *, p < 0.05 compared to the BCG group (one-way ANOVA with Tukey post-test).
Vaccine efficacy of CFP exosomes in a mouse TB infection model
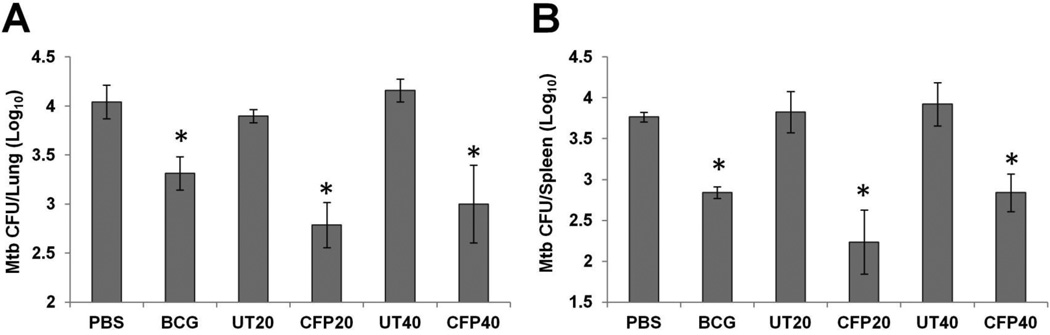
To measure the exosome’s ability to protect against an M. tuberculosis infection, mice were vaccinated with CFP exosomes or exosomes from uninfected macrophages at a dose of 20µg or 40µg per mouse as described in Materials and Methods. As a positive control, mice were vaccinated intranasally with M. bovis BCG. 4 weeks after the last exosome vaccination, all mice were subjected to a low-dose aerosol challenge with virulent M. tuberculosis H37Rv using the Glas-Col Inhalation Exposure System. Initial infection dose was approximately 100 CFU. After a 6-week infection, mycobacterial load in the lungs and spleens were determined. In CFP exosome-vaccinated mice, M. tuberculosis burden decreased significantly in the spleens when compared with unvaccinated mice or mice vaccinated with exosomes from uninfected cells (Fig. 5). We did not observe a statistical difference between the 20 and 40 µg CFP exosome doses. Of note, the CFP exosomes generated a comparable protection to BCG vaccination and showed a half log better protection than BCG in the lung, although this was only statistically different for the 20 µg vaccine dose (Fig. 5).
Figure 5. Mice vaccinated with exosomes from CFP-treated macrophages were partially protected against a low-dose aerosolized M. tuberculosis infection.
Mice (n=4) were infected with virulent M. tuberculosis H37Rv by aerosol challenge 4 weeks after the final exosome vaccination. Six weeks postinfection, all mice were sacrificed and mycobacterial counts in the lungs (A) and spleens (B) were determined by plating. Results are expressed as the means ± standard errors of 4 mice per group. The data shown is the representative of three independent experiments. *, p < 0.05 compared to results with PBS control groups (one-way ANOVA with Tukey post-test).
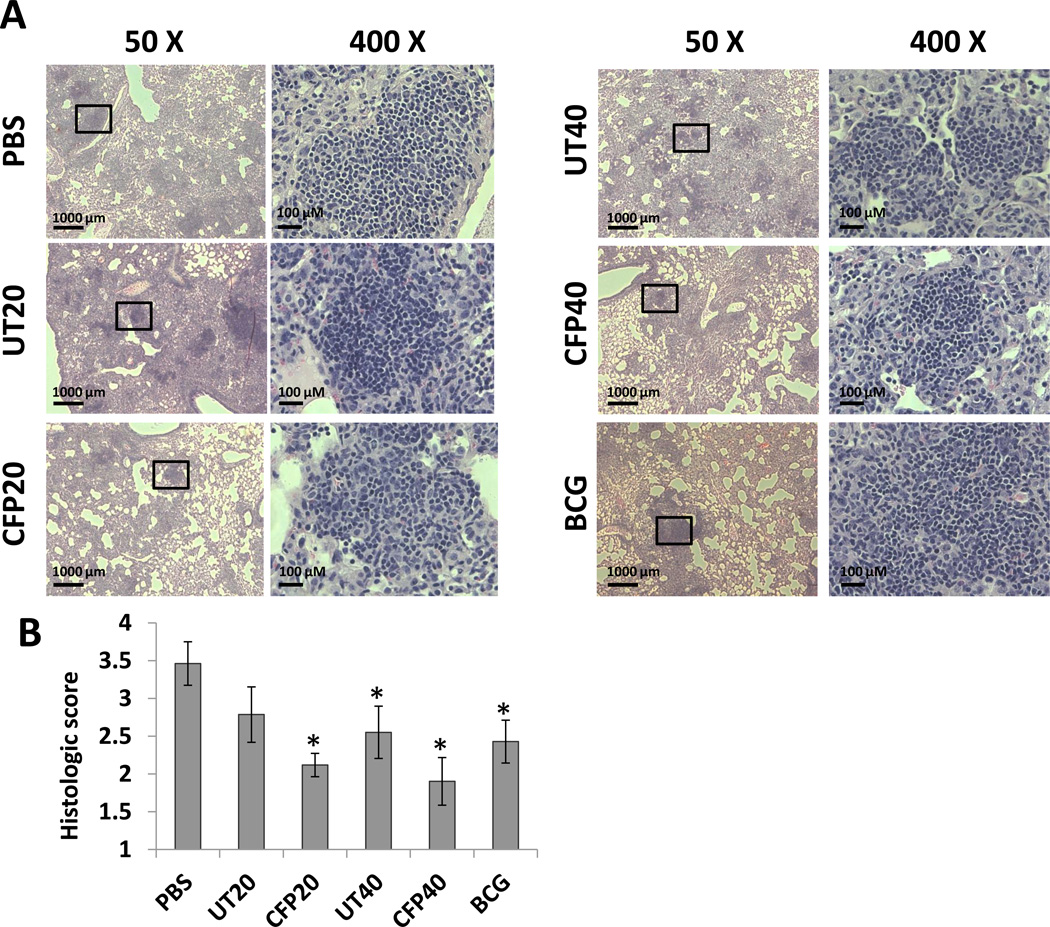
As the primary infection site after aerosol challenge, M. tuberculosis causes pathological changes in the lungs of mice defined by the accumulation of leukocytes. Analysis of the infected lungs by Hematoxylin and Eosin straining revealed lymphocyte infiltration for all infected mice. In the non-vaccinated mice or those vaccinated with exosomes from uninfected cells, lung sections displayed more abundant and larger inflammatory lesions which were characterized by mononuclear infiltration. Inversely, pulmonary lesions were discrete and surrounded by largely normal lung areas with minimal interstitial involvement in BCG and CFP exosome-vaccinated mice (Fig. 6A). The level of inflammation was quantified using the procedures described by Sweeny et al. [32]. The quantitative results indicated that both BCG and CFP exosome vaccinations significantly restricted the progression of inflammation in the lungs compared to the control PBS group (Fig. 6B). Interestingly, inflammation was also decreased in infected mice when using the higher dose of exosomes from uninfected cells suggesting that exosomes alone may have some anti-inflammatory activity under these experimental conditions.
Figure 6. Lung histopathology in M. tuberculosis-infected mice.
H&E staining of lung sections from vaccinated and non-vaccinated mice following infection with virulent M. tuberculosis H37Rv. (A) The lung sections were analyzed under low (50X) and high (400X) magnification. (B) Histopathological scores of lung sections. PBS and BCG were used as a negative and positive control, respectively. Sections are representatives of lung sections from 4 mice per group and from one of three independent experiments. *, p<0.05 compared to the PBS group (one-way ANOVA with Tukey post-test).
Boosting prior BCG vaccination with CFP exosomes
To evaluate whether CFP exosomes could also provide effective protection against an M. tuberculosis infection in a prime-boost vaccination model, C57BL/6 mice were vaccinated S.C. with BCG followed by an 8 month rest period and then re-vaccinated intranasally with exosomes or BCG. To confirm that the initial BCG vaccination was eliciting an antigen-specific immune response, a group of mice were sacrificed 2 weeks post vaccination. Similar to what is shown in figure 2, the BCG vaccinated mice contained antigen-specific IFN-γ producing CD4+ and CD8+ T cells (data not shown). Eight months after the original BCG vaccination, when the immune response induced by the initial BCG vaccination had waned; mice were boosted with exosomes, BCG or left untreated. In mice boosted with CFP exosomes, we observed an increased number of antigen-specific IFN-γ positive CD4+ and CD8+ T cells compared to the BCG primed vaccinated mice (Fig. 7A and B). A similar trend was observed with IL-2 production although the differences in cytokine production were more modest then for IFN-γ (Fig. 7C and D). ELISA analysis following ex-vivo stimulation of lung cells or splenocytes with M. tuberculosis lysate showed a significant increase in IFN-γ and IL-2 levels in mice vaccinated with CFP exosomes compared to BCG boost vaccinated mice. In addition, both groups showed higher IFN-γ and IL-2 levels compared to BCG primed or non-vaccinated mice (Fig. 7E and F). CD69 expression on both lung and spleen CD4+ and CD8+ T cells following CFP exosomes vaccination and comparable to levels observed for BCG prime/BCG boost vaccinated mice (data not shown). In summary, the CFP exosomes induced a TH1-mediated T cell response when used as a boost vaccine in mice previously vaccinated with M. bovis BCG. However, in contrast to the prime vaccination with CFP exosomes, in the prime-boost mouse model, the ratio of IgG1 and IgG2c in serum were comparable between the exosome and BCG vaccinated mice (Fig. S1).
Figure 7. Antigen-specific immune response in the lungs and spleens of mice after prime-boost vaccination.
(A–D) Flow cytometric analysis of antigen-specific cytokine – expressing CD4+ and CD8+ T cells isolated from the spleens and lungs of non-vaccinated mice or mice vaccinated with BCG as the prime vaccine only or with BCG plus a booster vaccine with BCG or exosomes. The frequency of INF-γ-positive CD4+ (A) or CD8+ (B) T cells as well as the frequency of IL-2 positive CD4+ (C) or CD8+ (D) T cells after ex-vivo stimulation with M. tuberculosis antigens. (E and F) IFN-γ and IL-2 ELISA using spleen and lung cells isolated from vaccinated and non-vaccinated mice. The results are expressed as cytokine concentration after ex vivo stimulation of 1 × 106 cells with M. tuberculosis antigens. The data shown +/− standard error of pooled lung or spleen homogenate from 5 mice per given condition run in duplicate and is the representative of three independent experiments. *, p < 0.05 compared to PBS control (one-way ANOVA with Tukey post-test). UT20 and UT40, exosomes (20 or 40µg) isolated from untreated macrophages; CFP20 and CFP40, exosomes (20 or 40µg) isolated from macrophages pulsed with M. tuberculosis CFP.
Protective activity against M. tuberculosis infection in prior BCG-vaccinated mice boosted with CFP exosomes
To determine if we could protect mice against an M. tuberculosis infection using CFP exosome in a prime-boost model, mice were again S.C. vaccinated with BCG, rested for 8 months then followed by a booster vaccination with exosomes or a second vaccination with BCG intra-nasally. Mice were given a low dose aerosol infection with M. tuberculosis H37Rv six weeks after the last exosome booster vaccination. Six weeks later, all mice were sacrificed and mycobacterial counts were measured in lungs and spleens. As shown in figure 8, the mice given only the prime BCG vaccination gave little to no significant protection. In contrast, the mycobacterial load in the BCG/CFP exosome vaccinated mice was significantly reduced both in the lungs and spleens in comparison with non-vaccinated or BCG primed vaccinated mice. Interestingly, mycobacterial numbers were significantly lower in the lungs of mice vaccinated with the high dose (40 µg/mouse) CFP exosomes compared to BCG prime/boost vaccinated mice. This same trend was observed in the spleen but the decrease was not statistically different (Fig. 8). Again, vaccination with exosomes isolated from uninfected macrophages gave no protection.
Figure 8. BCG vaccinated mice boosted with exosomes from CFP-treated macrophages were protected against a low-dose aerosolized M. tuberculosis infection.
Six weeks after the final exosome vaccination mice were infected with virulent M. tuberculosis H37Rv by aerosol challenge and 6 weeks later the mycobacterial burden in the lungs (A) and spleens (B) was determined by plating the tissue homogenates on 7H10 agar plates. Results are expressed as the means ± standard error for 4 mice per group and the data shown is the representative of two independent experiments. *, p < 0.05 compared to PBS control; **, P < 0.05 compared to BCG primed/BCG boost vaccinated mice; as determined by Student paired t-test.
Discussion
There are currently 12 TB vaccine candidates in various phases of clinical trial. These vaccine candidates fall under three broad categories: 1) recombinant BCG or other mycobacteria species, 2) viral vectors expressing various mycobacterial proteins, and 3) recombinant mycobacterial proteins in conjugation with robust adjuvants. At present it remains unclear whether these vaccine candidates will provide the effectiveness required for TB control [6]. However, recent data indicates that the MVA85A does not provide efficacious protection when used as a booster vaccine in HIV-negative infants previously immunized with BCG [33]. Herein, we hypothesize that exosomes may provide a novel approach for TB vaccine development. Exosomes have a number of advantages including: (i) stable conformational conditions for the proteins, (ii) effective molecular distribution due to the ability of microvesicles to recirculate in body fluids and reach distal organs and (iii) a more efficient association of antigen with target cells [10]. The potential for using exosomes as a cell-free vaccine against TB has its roots in previous cancer vaccine studies. Three exosome-based vaccine candidates have already accomplished phase I clinical trials in the late-stage cancer patients, indicating that exosomes are safe in humans. One candidate is currently undergoing a phase II clinical trial for non-small cell lung cancer patients. [15–18]. However, these studies were performed using exosomes obtained from autologous cells, a process which would not be feasible for a TB vaccine. In the context of infectious diseases, exosomes purified from DC pulsed with antigens of parasites, Toxoplasma gondii, Leishmania major or Eimeria tenella, induced antigen-specific cellular and humoral immune responses against the corresponding pathogen in mouse models or chickens [34–38]. Moreover, reticulocytes infected with Plasmodium yoelii released exosomes capable of activating a protective anti-malaria immune response in naïve mice in an adjuvant-independent manner [39]. Our present data, demonstrating the protective efficacy of exosomes in controlling an M. tuberculosis infection, supports the potential application for this type of cell-free vaccine.
Unexpectedly we did not see much protection with the BCG nine months after vaccination. Examination of the data suggests that the BCG vaccinated mice showed only a slightly lower CFU compared to unvaccinated mice (i.e. PBS control vs. BCG, or BCG plus exosomes from untreated macrophages). However, the 0.3 log drop in spleen CFU between BCG vaccinated and non-vaccinated mice was statistically significant. In a number of published studies there was protection by the initial BCG vaccination even in the absences of a booster vaccine. In most of these studies a shorter window between BCG vaccination and boosting was used [40, 41]. Nevertheless, in some studies where protection with the primary BCG vaccination was observed, the intervals between BCG vaccination and M. tuberculosis infection were on the same time frame as in our study [42]. Interestingly, in the study by Dietrich et al. a similar ~0.3 log drop in spleen CFU was observed when comparing unvaccinated mice to those vaccinated with BCG 8 months prior to M. tuberculosis infection [42]. These results suggest that in some cases the protection may be minimal after a long interval between vaccination and infection.
The incomplete protection we observed is likely due to limited antigen-specific memory T cells available for reactivation 9 months after the initial BCG vaccination (see figure 7). It is unclear why we see this limited immune/protective response but one hypothesis is that our BCG strain failed to survive in vivo for the time necessary to induce a potent long-term memory response. Previous studies of BCG vaccinated mice treated with antibiotics suggest that viable BCG is required for vaccine efficacy [43].
For most individuals M. tuberculosis infection induces a protective TH1-mediated immune response characterized by the development of antigen-specific CD4+ and CD8+ lymphocytes producing INF-γ and other TH1-type cytokines [28]. During the subunit vaccine studies it was evident that the control of a M. tuberculosis infection required an adjuvant that induces a robust TH1 but limited TH2 immune response [44, 45]. It has been demonstrated that exosomes carrying parasitic or tumor antigens could generate a strong antigen-specific TH1 immune responses resulting in control of the parasitic infection or in limiting tumor progression [29–31]. Our previous studies indicated that exosomes released from M. bovis BCG-infected macrophages, when used as an intranasal vaccine, stimulated a TH1 immune response as defined by antigen-specific IFN-γ production [20]. This response was not dependent on the addition of adjuvant as the immune response was similar using exosomes +/− CpG; a potent adjuvant. Exosomes released from macrophages treated with CFP gave a similar immune response [21]. Our present study also indicates that vaccinating with CFP exosomes stimulates a TH1 immune response but, based on the IgG2c/IgG1 ratio and IL-4 data, it induces a more limited TH2 response compared to BCG. However, in the prime-boost mouse model, there was no difference in the IgG2c/IgG1 ratio or IL-4 production between BCG-exosome and BCG-BCG vaccinated mice. This may be due to CFP exosomes boosting both the TH1 and TH2 response initially induced by prior BCG immunization, a process that would not have been observed in the prime vaccination studies.
Another important consideration is the mechanism by which the mycobacterial antigens are being presented to T cells for their activation. The MHCs haplotypes differ between the exosomes and the mouse strain used for these studies, suggesting that in vivo, the exosomes are being endocytosis by antigen presenting cells and the antigens subsequently presented by the host MHC. This is supported by our previous studies where we determined that exosomes carrying mycobacterial antigens when added to sensitized T cells were very limited in their ability to activate the cells and that exosomes could only induce a strong T cell response in the presence of antigen presenting cells [20].
Previously we identified 29 mycobacterial proteins on exosomes released by macrophages pulsed with M. tuberculosis CFP [21]. Importantly, among them were mycobacterial antigens 85A and 85B; key antigens contained in a number of subunit vaccines currently under clinical trials. Furthermore, the majority of identified proteins are known T cell antigens verified in TB patients or animal models, indicating a high immunogenic activity of CFP exosomes [22–24]. Another advantage of exosomes over live BCG vaccine is the limited risk associated with using a non-living vaccine. The use of BCG is not recommended in HIV patients due to the high risk of disseminated BCG. One main goal of current anti-TB vaccine development is to create an effective immunotherapeutic vaccine as an adjuvant in combination with chemotherapy. There are now two distinct vaccine candidates under clinical trial, whole heat-killed Mycobacterium vaccae and RUTI, mycobacterial fragments prepared from M. tuberculosis grown under stress conditions [46, 47]. As to the development of post-exposure vaccine against TB, there is some concern that these vaccines would lead to the “Koch phenomenon” in which M. tuberculosis components cause necrotic reaction and severe progression of active TB in M. tuberculosis-infected individuals [48, 49]. The limited repertoire of mycobacterial antigens present on the CFP exosomes compared to what would be part of the M. vaccae or RUTI vaccine may make the possibility of the “Koch phenomenon” less likely when using exosomes as a immunotherapeutic vaccine.
In summary, our results indicated that exosomes released from macrophages pulsed with M. tuberculosis CFP can provide protection against an M. tuberculosis infection both as a primary vaccine as well as a booster of a BCG-induced immune response. Further studies are needed to define which antigens within the CFP are providing the protection and to develop cell lines which express and release these specific antigens on exosomes.
Materials and Methods
Animals
All wild type C57BL/6 mice were housed at the institutional animal facility under specific-pathogen-free conditions during the experiment. M. tuberculosis infection was carried out in the biosafety level 3 laboratory. The University of Notre Dame is accredited through the Animal Welfare Assurance (#A3093-01). All animal procedures were approved by the Institutional Animal Care and Use Committee.
Bacteria and macrophage cell line
Wild type M. tuberculosis H37Rv and M. bovis BCG (Pastuer) strains were grown in Middlebrook 7H9 broth medium (Difco, Becton-Dickinson) supplemented with 10% OADC (oleic acid/albumin/dextrose/catalase), 0.2% glycerol and 0.05% Tween 80 until exponential phase and then aliquoted and stored at −70 °C until use. Mouse macrophage cell line RAW 264.7 was maintained in Dulbecco modified Eagle's minimal essential medium (DMEM, Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum (Hyclone, South Logan, Utah), 25 mM Na-HEPES (ThermoScientific, Rockford, IL), 1 mM Sodium pyruvate (Lonza, Walkersville, MD), 100 U/mL penicillin and 100 U/mL streptomycin (Hyclone, South Logan, Utah) at 37 °C with 5% CO2.
Preparation of exosomes
Exosomes were purified as described previously [25]. Briefly, exosome-free FBS was prepared by centrifuging at 100,000 ×g, 4 °C for 16 hours. Monolayer of RAW 264.7 mouse macrophage cell line with a cell confluence of 70–80% in DMEM containing 10% exosome-free FBS were untreated (UT) or treated with CFP (BEI Resources, NR-14825) with a final concentration of 20 µg/ml at 37°C and 5% CO2. After 20 h, culture supernatant was harvested and centrifuged at 350 ×g, 4 °C for 10 min to remove cell debris and free cells, and then collected culture supernatant was passed through a 0.22 µm polythersulfone filter (Corning, NY, USA). Filtrated supernatant was ultracentrifuged at 100, 000 ×g, 4 °C for 1 h to spin down expected exosomes. The pellets were resuspended in 11 ml PBS and washed thrice with PBS. Finally, the pellets were resuspended in 0.5 ml PBS and purified using ExoQuick (System BioSciences, Mountain View, CA). The purified exosomes were resuspended in PBS and the concentration was determined by a Micro BCA assay (Pierce, Rochford, IL). Before use, all purified exosomes were stored at −80°C.
Western Blots
Exosomes were run on an SDS-PAGE gel and transferred to a PVDF membrane as described previously [25]. The membranes were probed with mouse monoclonal antibody against M. tuberculosis 19-kDa Lipoprotein (IT-12) and rat monoclonal antibody against mouse LAMP1 (SC-19992, Santa Cruz Biotechnology, Tx), and then incubated with goat anti-mouse and anti-rat HRP-conjugated IgG (ThermoScientific, Rockford, IL), respectively. For mycobacterial CFP, the membrane was probed with rabbit polyclonal antibodies made against M. tuberculosis CFP (BEI Resources, NR-13809) and then incubated with goat anti-rabbit HRP-conjugated IgG as described above. IT-12 and NR-13809 were obtained from Colorado State University, Colorado, USA, Under TB Vaccine Testing and Research Material Contract.
Immunization
In exosome-priming experiments, mice were immunized via an intranasal route with a final injection volume of 30 µl (15 µl/nostril) as described previously [21]. Briefly, 5 mice per group were anaesthitzed with isoflurane and administered with PBS alone or with purified exosomes isolated from CFP-treated or untreated macrophages, at a dose of 20 µg/mouse or 40 µg/mouse. The mice were immunized three times at an interval of 2 weeks between vaccinations. Two weeks after final exosome vaccination mice were sacrificed and used to measure antigen-specific T cell activation and 4 weeks after final vaccination a separate set of mice were infected with M. tuberculosis. As a positive control, M. bovis BCG (1×106 CFU/mouse, Pasteur strain) was given intra-nasally as a single dose 8 weeks prior to M. tuberculosis infection.
For BCG-priming and exosome-boosting experiments, 5 mice per group were first S.C. immunized with a single dose of M. bovis BCG (1×106 CFU/mouse, Pasteur strain) in 50 µl of PBS and subsequently rested for 8 months before boosting. Exosome booster immunization was administrated twice intra-nasally at 2 weeks intervals as described above. Another set of BCG vaccinated mice were also boosted with BCG intra-nasally at 1 × 106 CFU at the same time as the first exosome boost vaccination. Mice were sacrificed to measure antigen-specific immune responses or infected with M. tuberculosis H37Rv as described for the exosome-priming experiments.
Mycobacterial Challenge
Six weeks following the final vaccination of exosomes, mice were challenged with M. tuberculosis H37Rv using an Inhalation Exposure System (Glas-Col, Terre haute, IN). Four M. tuberculosis infected mice per group were humanely sacrificed 1 day after infection to determine the bacterial load in the lungs and spleens. The amount of M. tuberculosis used in the infection was calculated to give approximately 50 to 150 CFU/lung in mice. For all other infections mice were euthanized six weeks after mycobacterial challenge and the lungs and spleens were removed and homogenized in phosphate-buffered saline (PBS) containing 0.05% (vol/vol) Tween 80. The tissue homogenate was appropriated diluted in the same buffer, and then 50 µl of the diluted homogenate was spread on Middlebrook 7H11 agar plates with 10% OADC, 0.5% glycerol and 0.05% Tween 80, and containing a cocktail of fungizone (Hyclone, South Logan, Utah) and PANTA (polymixin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin; BD, Sparks, MD). M. tuberculosis colonies were counted after 3–4 weeks of incubation at 37 °C and expressed as log10 CFU per organ.
Lymphocyte isolation and culture
Lymphocytes were isolated from the lungs and spleens of mice two weeks after the final exosome injection as described previously [21]. For splenic lymphocytes, the organ was removed and perfused in pre-cold RPMI-1640 medium (DMEM, Cellgro, Manassas, VA) using 10mL syringe fitted with 26G needle and then filtrated through a 70 µM nylon mesh followed by a centrifuge at 300 ×g, 4 °C for 10 min. For lung lymphocytes, the tissue was homogenized in 5 ml of sterile complete RPMI-1640 medium with sterile glass homogenizer and subsequently incubated at 37 °C for 2 h in the presence of type IV collagenase (125 to 150 U/ml) and DNase I (50–60 U/ml). The incubated cell suspension was passed through a 70 µM nylon mesh followed by a centrifuge at 300 ×g, 4 °C for 10 min. The red blood cells in cell suspension were lysed by hypotonic shock with 3 ml ACK lysis buffer (Gibco, Grand Island, New York) for 5 min in ice. The cells were then washed with RPMI-1640 medium 3X to remove lysed RBCs and lysis buffer.
Flow Cytometry
Cells were isolated from the lungs and spleens of mice as described above. For the staining of intracellular cytokines, cells (1×106 cells/well) were stimulated with 5 µg/ml M. tuberculosis whole cell lysate (WCL) (BEI Resources, NR-14822) for 6 h and subsequentally incubated for another 6 h in the presence of 2 µM monensin (Biolegend, San Diego, CA) at 37 °C and 5 % CO2. The cells were gently washed with Dulbecco’s PBS and blocked in FACS buffer (0.1% BSA and 0.02% sodium azide in PBS) plus 10% normal mouse serum (NMS, eBioSciences, San Diego, CA) for 30 min in ice, and then stained with PE-conjugated anti-mouse CD4 (Biolegend, San Diego, CA) and PE-Cy5-conjugated anti-mouse CD8 (Biolegend, San Diego, CA) antibodies for 30 min on ice and in the dark. The pre-stained cells were washed in FACS buffer 3X and then fixed and permeated with fixation and permeabilization wash buffers (Biolegend, San Diego, CA), respectively, according to the manufacturer’s protocol. Afterwards, cells were stained with FITC-conjugated anti-mouse INF-γ, IL-2 or IL-4 antibodies (Biolegend, San Diego, CA) and washed with FACS buffer 3X before analyzed on a Beckman Coulter FC500 flow cytometer.
For in vitro T cell activation assay, 1×106 cells were seeded into each well in 24-well plates and treated with 5 µg/ml M. tuberculosis WCL for 72 h at 37 °C and 5 % CO2. After blocking on ice with 10% NMS cells were stained with FITC-conjugated anti-mouse CD69 (Biolegend, San Diego, CA), PE-conjugated anti-mouse CD4 (Biolegend, San Diego, CA) or PE-Cy5-conjugated anti-mouse CD8 (Biolegend, San Diego, CA) antibodies and fixed in fixation buffer. Finally, cells were resuspended in FACS buffer and measured as described above.
Antibody endpoint titers
Mouse blood was collected 2 weeks after the final exosome vaccination and antigen-specific Ab titers for IgG1, Ig2c and total IgG were performed as described previously [50]. Briefly, Nunc Polysorp plates were coated with M. tuberculosis WCL at 2µg/ml in 0.1 M bicarbonate solution at 4 °C overnight and subsequently blocked at 0.05% PBS-tween 20/1% BSA for 2 h at room temperature. The prepared mouse sera were then added to the plates and incubated at 4 °C overnight. Plates were washed and treated with HRP-conjugated secondary Antibodies: rat anti-mouse IgG1 HRP (ebioScience, San Diego, CA), goat anti-mouse IgG2C HRP (SouthernBiotech, Birmingham, AL) or goat anti-mouse IgG HRP (ThermoScientific, Rockford, IL) for 1 h at room temperature. Plates were washed and developed with tetramethylbenzidine (ebioScience, San Diego, CA) and the reaction was stopped by adding 1 N H2SO4. Finally, plates were read using a microplate ELISA reader (Spectramax M5, Molecular Devices, Sunnyvale, CA) at 450 nm and soft Max Pro 5 software (Molecular Devices, Sunnyvale, CA) with a cutoff of 0.1 absorbance value.
Cytokine ELISA
Spleen and lung cells (1×106 cells/well) were seeded into 24-well tissue culture plates in 500 µL of RPMI-1640 medium supplemented with 10% FBS, 25mM Na-HEPES, 2mM L-glutamine, 1mM sodium pyruvate, 100 U/mL penicillin and 100 U/mL streptomycin, and subsequently treated with 5 µg/ml M. tuberculosis WCL at 37°C with 5% CO2. After 72 h, cell-free culture supernatant was collected and analyzed for INF-γ and IL-2, by ELISA (eBioScience, San Diego, CA) according to the manufacturer’s instruction
Histopathology
Six weeks after the M. tuberculosis infection, small sections of the right and left lung, removed prior to harvesting the tissue for CFU determination, were fixed in 10% neutral buffered formalin (Fisher Scientific, Fair Lawn, NJ) at room temperature overnight, and then embedded in paraffin (Leica, Richmond, IL). The sections were taken at 4 µm thickness and stained with hematoxylin and eosin for microscopic analysis. To determine histopathological changes, all sections were scored for severity by scanning entire fields in 3 sections of each tissue per mouse based on the extent of granulomatous inflammation as described [32]: 0= no lesion, 1= minimal lesion (1–10% area of tissue in section involved), 2= mild lesion (11–30% area involved), 3= moderate lesion (31–50% area involved), 4= marked lesion (50–80% area involved), 5= severe lesion (>80% area involved).
Statistical methods
The data obtained was analyzed by ANOVA and Student paired t-test. Differences between means were assessed for significance by Tukey’s test. A value of p ≤ 0.05 was considered significant. The computer program GraphPad PRISM 5 was used for the analysis.
Supplementary Material
Supplementary Figure 1. Determination of antigen-specific IgG1, IgG2c and IgG in vaccinated mice. Antigen-specific (A) IgG, and subtypes (B) IgG2c and (C) IgG1 endpoint titers were defined using mouse serum which was collected 2 weeks after the final vaccination with exosomes. Mean reciprocal dilutions are used as the endpoint titer (log10). The data shown is the representative of three independent experiments. *, p < 0.05 compared to the BCG group (one-way ANOVA with Tukey post-test).
Acknowledgements
This work was supported through the grant RO1AI052439 from the National Institute of Allergy and Infectious Diseases
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.WHO. Geneva: WHO; 2012. Global Tuberculosis Report 2012. [Google Scholar]
- 2.Calmette A. Sur la vaccination préventive des enfants nouveau-nés contre la tuberculose par le BCG. Ann. Inst. Pasteur. 1927;41:201–232. [Google Scholar]
- 3.Kaufmann SH. Envisioning future strategies for vaccination against tuberculosis. Nat. Rev. Immunol. 2006;6:699–704. doi: 10.1038/nri1920. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann SH. Future vaccination strategies against tuberculosis: thinking outside the box. Immunity. 2010;33:567–577. doi: 10.1016/j.immuni.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 6.Brennan MJ, Thole J. Tuberculosis vaccines: a strategic blueprint for the next decade. Tuberculosis (Edinb) 2012;92(Suppl 1):S6–S13. doi: 10.1016/S1472-9792(12)70005-7. [DOI] [PubMed] [Google Scholar]
- 7.Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey-Faussett P, Beyers N. The risk of disseminated Bacille Calmette-Guerin (BCG) disease in HIV-infected children. Vaccine. 2007;25:14–18. doi: 10.1016/j.vaccine.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Hesseling AC, Johnson LF, Jaspan H, Cotton MF, Whitelaw A, Schaaf HS, Fine P, et al. Disseminated bacille Calmette-Guerin disease in HIV-infected South African infants. Bull World Health Organ. 2009;87:505–511. doi: 10.2471/BLT.08.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahomed H, Fourie PB. Clinical trials of TB vaccines: harmonization and cooperation. Tuberculosis (Edinb) 2012;92(Suppl 1):S21–S24. doi: 10.1016/S1472-9792(12)70008-2. [DOI] [PubMed] [Google Scholar]
- 10.Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 12.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 14.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J. Transl. Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 19.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One. 2008;3:e2461. doi: 10.1371/journal.pone.0002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giri PK, Kruh NA, Dobos KM, Schorey JS. Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics. 2010;10:3190–3202. doi: 10.1002/pmic.200900840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vita R, Zarebski L, Greenbaum JA, Emami H, Hoof I, Salimi N, Damle R, et al. The immune epitope database 2.0. Nucleic Acids Res. 2010;38:D854–D862. doi: 10.1093/nar/gkp1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deenadayalan A, Heaslip D, Rajendiran AA, Velayudham BV, Frederick S, Yang HL, Dobos K, et al. Immunoproteomic identification of human T cell antigens of Mycobacterium tuberculosis that differentiate healthy contacts from tuberculosis patients. Mol. Cell Proteomics. 2010;9:538–549. doi: 10.1074/mcp.M900299-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho YS, Dobos KM, Prenni J, Yang H, Hess A, Rosenkrands I, Andersen P, et al. Deciphering the proteome of the in vivo diagnostic reagent "purified protein derivative" from Mycobacterium tuberculosis. Proteomics. 2012;12:979–991. doi: 10.1002/pmic.201100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh PP, Smith VL, Karakousis PC, Schorey JS. Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J. Immunol. 2012;189:777–785. doi: 10.4049/jimmunol.1103638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wangoo A, Sparer T, Brown IN, Snewin VA, Janssen R, Thole J, Cook HT, et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous lung disease. J. Immunol. 2001;166:3432–3439. doi: 10.4049/jimmunol.166.5.3432. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann SH. Tuberculosis vaccine development: strength lies in tenacity. Trends Immunol. 2012;33:373–379. doi: 10.1016/j.it.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect. Immun. 2004;72:4127–4137. doi: 10.1128/IAI.72.7.4127-4137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qazi KR, Gehrmann U, Domange Jordo E, Karlsson MC, Gabrielsson S. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009;113:2673–2683. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- 31.Rountree RB, Mandl SJ, Nachtwey JM, Dalpozzo K, Do L, Lombardo JR, Schoonmaker PL, et al. Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy. Cancer Res. 2011;71:5235–5244. doi: 10.1158/0008-5472.CAN-10-4076. [DOI] [PubMed] [Google Scholar]
- 32.Sweeney KA, Dao DN, Goldberg MF, Hsu T, Venkataswamy MM, Henao-Tamayo M, et al. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat. Med. 2011;17:1261–1268. doi: 10.1038/nm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I. A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes Infect. 2007;9:1614–1622. doi: 10.1016/j.micinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Beauvillain C, Juste MO, Dion S, Pierre J, Dimier-Poisson I. Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine. 2009;27:1750–1757. doi: 10.1016/j.vaccine.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Schnitzer JK, Berzel S, Fajardo-Moser M, Remer KA, Moll H. Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine. 2010;28:5785–5793. doi: 10.1016/j.vaccine.2010.06.077. [DOI] [PubMed] [Google Scholar]
- 37.Del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, Sanchez-Acedo C. Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine. 2011;29:3818–3825. doi: 10.1016/j.vaccine.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Silverman JM, Reiner NE. Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol. 2011;13:1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Jaular L, Nakayasu ES, Ferrer M, Almeida IC, Del Portillo HA. Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS One. 2011;6:e26588. doi: 10.1371/journal.pone.0026588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cervantes-Villagrana AR, Hernandez-Pando R, Biragyn A, Castaneda-Delgado J, Bodogai M, Martinez-Fierro M, et al. Prime-boost BCG vaccination with DNA vaccines based in beta-defensin-2 and mycobacterial antigens ESAT6 or Ag85B improve protection in a tuberculosis experimental model. Vaccine. 2013;31:676–684. doi: 10.1016/j.vaccine.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.You Q, Wu Y, Wei W, Wang C, Jiang D, Yu X, et al. Immunogenicity and protective efficacy of heterologous prime-boost regimens with mycobacterial vaccines and recombinant adenovirus- and poxvirus-vectored vaccines against murine tuberculosis. Int. J. Infect. Dis. 2012;16:e816–e825. doi: 10.1016/j.ijid.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, Andersen P. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J. Immunol. 2006;177:6353–6360. doi: 10.4049/jimmunol.177.9.6353. [DOI] [PubMed] [Google Scholar]
- 43.Olsen AW, Brandt L, Agger EM, van Pinxteren LA, Andersen P. The influence of remaining live BCG organisms in vaccinated mice on the maintenance of immunity to tuberculosis. Scand. J. Immunol. 2004;60:273–277. doi: 10.1111/j.0300-9475.2004.01471.x. [DOI] [PubMed] [Google Scholar]
- 44.Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J. Immunol. 2012;188:2189–2197. doi: 10.4049/jimmunol.1102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agger EM, Cassidy JP, Brady J, Korsholm KS, Vingsbo-Lundberg C, Andersen P. Adjuvant modulation of the cytokine balance in Mycobacterium tuberculosis subunit vaccines; immunity, pathology and protection. Immunology. 2008;124:175–185. doi: 10.1111/j.1365-2567.2007.02751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanford J, Stanford C, Grange J. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis. Front Biosci. 2004;9:1701–1719. doi: 10.2741/1292. [DOI] [PubMed] [Google Scholar]
- 47.Cardona PJ. RUTI: a new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis (Edinb) 2006;86:273–289. doi: 10.1016/j.tube.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 48.Doherty TM, Andersen P. Vaccines for tuberculosis: novel concepts and recent progress. Clin. Microbiol. Rev. 2005;18:687–702. doi: 10.1128/CMR.18.4.687-702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orme IM. Safety issues regarding new vaccines for tuberculosis, with an emphasis on post-exposure vaccination. Tuberculosis (Edinb) 2006;86:68–73. doi: 10.1016/j.tube.2005.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Determination of antigen-specific IgG1, IgG2c and IgG in vaccinated mice. Antigen-specific (A) IgG, and subtypes (B) IgG2c and (C) IgG1 endpoint titers were defined using mouse serum which was collected 2 weeks after the final vaccination with exosomes. Mean reciprocal dilutions are used as the endpoint titer (log10). The data shown is the representative of three independent experiments. *, p < 0.05 compared to the BCG group (one-way ANOVA with Tukey post-test).