Abstract
Objective:
Considering the physiologic roles of vitamin D on insulin regulation, the effects of vitamin D treatment on insulin sensitivity and resistance indexes and beta cell function in pre-diabetic vitamin D deficient patients were investigated.
Methods:
In a randomized open clinical trial, 61 pre-diabetic vitamin D deficient patients who were the first degree relatives of type 2 diabetic patients, were enrolled and randomized into three groups (A, B and C). Group A (n = 21) were treated with intramuscular injection of 300,000 units of vitamin D at the beginning of the study and one month later. In group B (n = 20), injection of vitamin D plus 500 mg/d calcium and in group C (n = 20), just calcium was administered for two months. At baseline and two months later, oral glucose tolerance test was done. Homeostasis Model of Assessment-Insulin Resistance (HOMA-IR), insulin resistance index, Homeostasis Model of Assessment-B (HOMA-B) which is a beta cell function index, and Matsuda index, an insulin sensitivity index, were calculated and compared before and after intervention and between three groups.
Findings:
In vitamin D treated groups (A + B), the mean (SD) of HOMA-IR increased from 2.46 (1.36) to 3.1 (2.3) (P = 0.02), and Matsuda index decreased from 11 (3) to 9.0 (2.3) (P = 0.001).
Conclusion:
Injection of vitamin D increased insulin resistance and decreased insulin sensitivity indexes.
Keywords: Vitamin D, pre-diabetic state, diabetes mellitus type 2, oral glucose tolerance test
INTRODUCTION
The presence of vitamin D receptor and vitamin D depended calcium binding protein on the beta cells may express some physiological roles for vitamin D on the regulation of insulin secretion. Direct effect of vitamin D is stimulation of insulin receptor expression on β cells and its indirect action is the regulation of extra cellular calcium and maintaining of normal calcium influx to these cells.[1,2,3,4]
The relationship between low concentration of 25(OH) D and insulin resistance has been previously reported in several studies.[5,6] It is well known that pre-diabetic patients have increased insulin resistance and decreased insulin sensitivity. They are high risk people for progression to type 2 diabetes (T2DM). Therefore, they are a suitable population target for primary prevention program of diabetes.[7] It is expected that vitamin D treatment in vitamin D deficiency pre-diabetic patients can improve insulin sensitivity and decrease insulin resistance indexes.
Considering the role of vitamin D deficiency on insulin resistance, vitamin D treatment may prevent the progression vitamin D deficient pre-diabetic to T2DM patients.
In order to investigate this hypothesis, we administered intramuscular injection of vitamin D with and without oral calcium to vitamin D deficient pre-diabetic patients.
The purpose of this study was to investigate the effects of intramuscular injection of vitamin D with and without calcium, on the insulin sensitivity, insulin resistance and beta cell function indexes in the first degree relatives of type 2 diabetic patients with pre-diabetes.
METHODS
In a randomized open clinical trial, 142 pre-diabetic participants were enrolled the study. They were selected by consecutive patients sampling method from 35-55 years old people. They were the first degree relatives of type 2 diabetic people in Isfahan Diabetes Prevention Program Study. After 10-12 hrs of overnight fasting, a 75g oral glucose was administered and plasma glucose and insulin concentrations were measured at 0, 30, 60 and 120 mine after glucose taking (OGTT). HbA1c, serum calcium, phosphate, albumin and serum plasma 25(OH) D were measured at baseline. Patients with diabetes mellitus (n = 9), pre-diabetes with serum 25(OH) D ≥ 30 ng/ml (n = 12), past history of renal stone (n = 4), history of chronic kidney disease (n = 6), history of metabolic bone disease (n = 2), hyperthyroidism (n = 1), pregnant women (n = 0), taking corticosteroids (n = 12) and hydrochlorothiazide (n = 23), taking vitamin D or calcium products within recent past 2 months (n = 9), and febrile disease within past two weeks (n = 3), were excluded from the study.
Overall, 61 participants with pre-diabetes according to the 2003 American Diabetes Association (ADA) criteria[8] and plasma 25(OH) D < 30 ng/ml were eligible.
All participants were randomized into three groups (A, B and C). In group A (n = 21) people were treated with intramuscular injection of 300,000 units of vitamin D at the beginning of the study and one month later.
In group B (n = 20), participants were treated with intramuscular vitamin D with the same protocol of group A plus oral calcium carbonate, 500 mg per day for two months.
In group C (n = 20), participants were just in taking calcium carbonate, 500 mg per day for two months. No vitamins D supplementations were administered in this group.
All patients were asked not to take any more calcium and or vitamin D supplementation for two next months. There was no especial recommendation for changing the life style and sunlight exposure modification.
Anthropometric parameters including height, weight, waist circumference (WC) and systolic and diastolic blood pressure were measured.
Body mass index (BMI) was calculated by weight (kg) divided by the square of height (m).
Blood pressure (BP) was measured twice in the sitting position after 5 mine of resting with a standard and calibrated mercury sphygmomanometer (ALPK2, Japan).
Plasma glucose and insulin were measured by GHOD-PAP and sandwich chemiluminescence immunoassay methods, respectively. Serum 25(OH) D was measured by direct competitive chemiluminescence. Homeostasis model assessment insulin resistance (HOMA-IR), an insulin resistance index, calculated as glucose (mmol/l)× insulin (μu/mL)/22.5, HOMA-B a beta cell function index, calculated as; fasting insulin (μu/mL)/fasting glucose (mg/dl)-3.5, and Matsuda index, an insulin sensitivity index calculated as; 10,000/[(FPG*FPI)(Gm*Im)]0.5 where FPG and FPI are fasting plasma glucose and fasting plasma insulin, Gm and Im are mean of glucose and insulin during OGTT. In matsuda index glucose and insulin values are in mg/dl and μu/mL, respectively.[9] This study was approved by the Isfahan Endocrine and Metabolism Research Center (IEMRC) Medical Ethics Committee and all patients signed a written consent form to enroll in this clinical trial.
After two months, 2nd OGTT with the same protocol as the beginning of the study, was repeated for all participants (n = 61) and plasma glucose, insulin and 25(OH) D were measured.
HOMA-IR, HOMA-B, Matsuda index, area under the curve (AUC) of insulin and glucose were compared before and after intervention and between three groups.
HOMA-IR, HOMA-B, Matsuda index, area under the curve of insulin and glucose, were compared before and after intervention in the entire participant with vitamin D injection (A + B groups).
Statistical analysis of the data was performed using SPSS version 15 for windows. Data were expressed as mean and standard deviation (SD), if the data were normally distributed, and as median if the distribution was not normal.
Student t test was used for the comparison of quantitative variables. Chi square test was used for comparison of qualitative variable between two groups.
Comparison of data before and after of intervention in each group was done with paired t-test in normally distributed data and with Wilcoxon t-test, if the data distribution was not normal.
P < 0.05 was considered significant.
RESULTS
General demographic and clinical characteristics of all participants are summarized in Table 1. There are no significant differences in these basal characteristics between three groups [Table 1]. Mean age of all participants was 45.7 (8.5) years. Table 2 presents the mean of biochemical parameters, insulin resistance, and insulin sensitivity and beta cell function indexes before intervention in three groups.
Table 1.
Basal demographic and clinical characteristics of pre-diabetic and vitamin D deficient groups (A, B and C)*
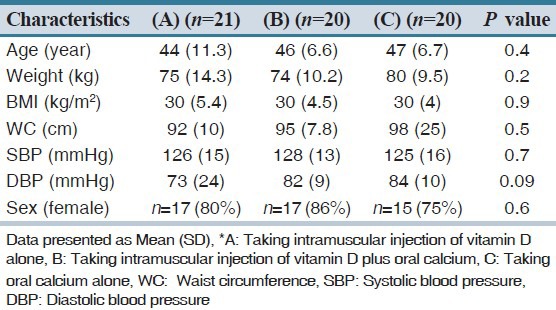
Table 2.
Biochemical, insulin resistance, insulin sensitivity and beta cell function indexes in pre-diabetic and vitamin D deficient groups (A, B and C)*
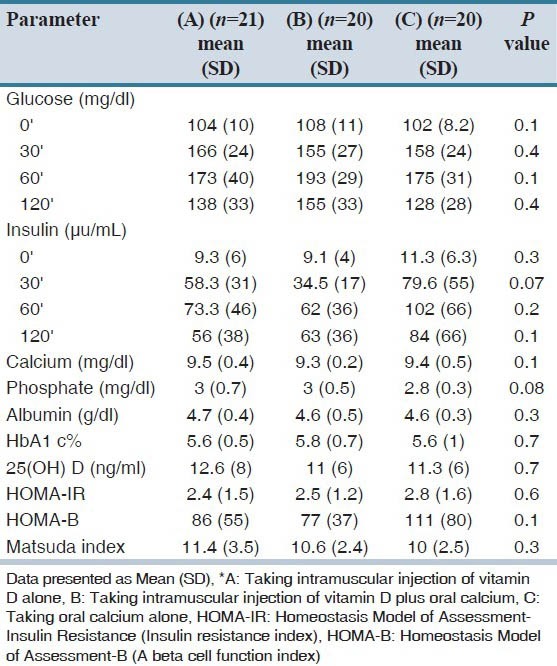
The mean concentrations of 25(OH) D in all groups were low. There was no significant difference between serum vitamin D levels, HOMA-IR, HOMA-B and matsuda index between groups before intervention [Table 2]. Table 3 shows the mean of biochemical parameters, 25(OH) D, insulin resistance, insulin sensitivity, beta cell function indexes and AUC of glucose and insulin in all groups before and 2 months after intervention. In group A, the mean of 25(OH) D increased significantly (P = 0.002) but insulin resistance, insulin sensitivity and beta cell function indexes, AUC of glucose and insulin before and two month after vitamin D injection did not change significantly.
Table 3.
Biochemical, insulin resistance, insulin sensitivity and beta cell function indexes before and two months after intervention in three groups (A, B and C)*
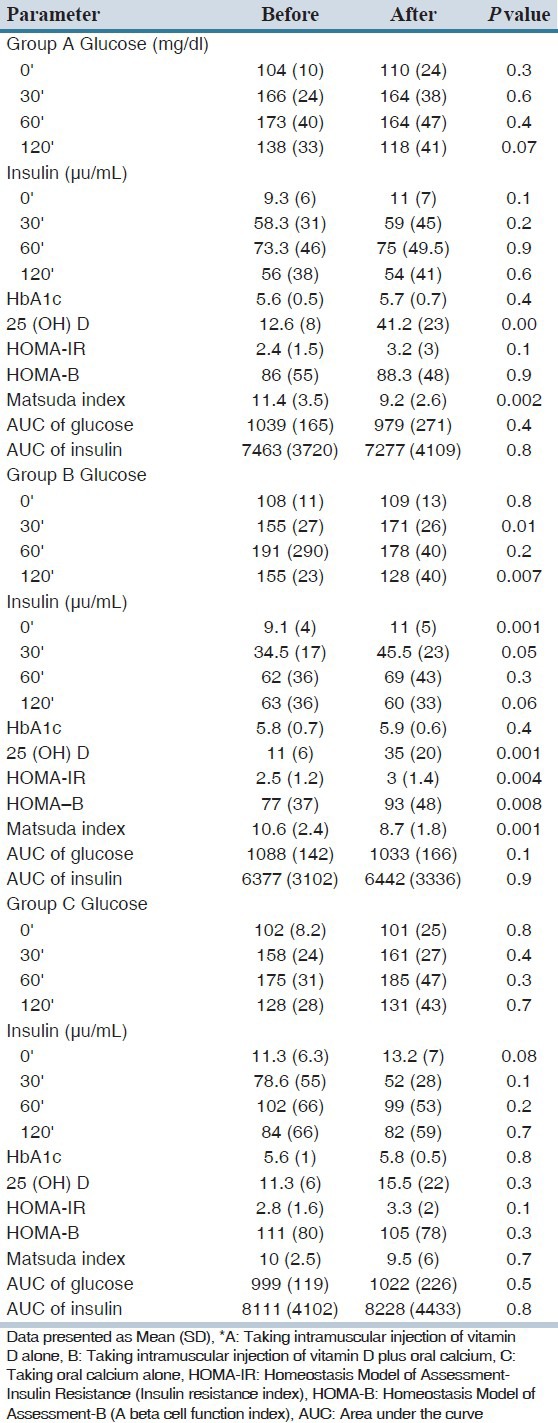
In B group, two month after vitamin D injection plus oral calcium carbonate, plasma glucose 30’ and insulin 0’ increased, significantly (P = 0.01, P = 0.001, respectively). However, glucose 120’ decreased (P = 0.007). In this group with two month of intervention, HOMA-IR and HOMA-B increase and Matsuda index decrease significantly. However, AUC of plasma insulin and glucose in this group did not change. In group C there is no statistically different in all biochemical parameters and indexes before and after calcium administration. Comparing the mean of insulin resistance, insulin sensitivity, and beta cell function indexes, AUC of plasma glucose and insulin in three groups has been presented in Table 4.
Table 4.
Comparison of mean and standard deviation of insulin resistance, insulin sensitivity and beta cell function indexes, area under the curve of plasma glucose and insulin between three pre-diabetic and vitamin D deficient groups*
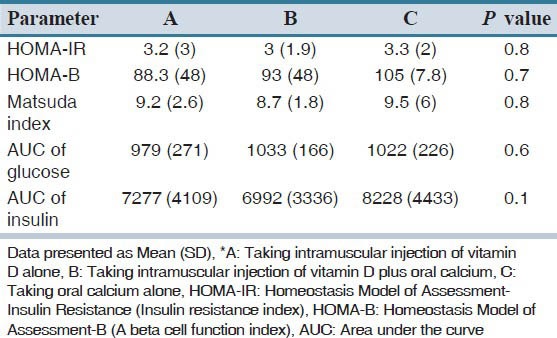
There is no significant difference between three groups in insulin sensitivity, resistance and beta cell function indexes, AUC of plasma glucose and insulin, two months after intervention [Table 4]. Table 5 presents the mean and standard deviation of HOMA-IR, HOMA-B, matsuda index and AUC of glucose and insulin in all of the participant with vitamin D injection (A + B groups) before and after of intervention. The mean (SD) of 25(OH) D levels increased from 11.8 (7) ng/ml to 38. 2 (21) ng/ml in group A + B (P = 0.001).
Table 5.
Comparison of insulin sensitivity, insulin resistance and beta cell function indexes, area under the curve of glucose and insulin in all pre-diabetic and vitamin D deficient groups who were treated with intramuscular injection of vitamin D (A+B groups) before and after intervention
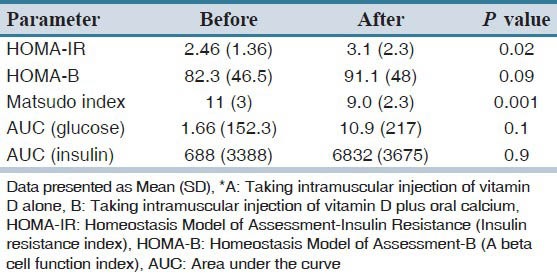
In this comparison, HOMA-IR Increased (P = 0.02) and Matsuda index decreased significantly (P = 0.001), 2 months after intervention in the entire participant who were treated with intramuscular injection of vitamin D.
DISCUSSION
This is a randomized prospective control trial to investigate the possibility of reducing insulin resistance and slowing of progression to type 2 diabetes, in pre-diabetic patients. Two months after intervention, HOMA-IR, HOMA-B and Matsuda indexes did not change in participants with vitamin D injection (group A) in comparison to those who did not take vitamin D (group C) [Table 4]. There was no significant difference in the area under the curve of plasma glucose and insulin between these two groups [Table 4]. In group A, before and after of vitamin D administration, Matsuda index decreased, however, HOMA-IR and HOMA-B did not change.
In those receiving intramuscular injection of vitamin D and oral calcium (group B), before and two months after intervention, HOMA-IR and HOMA-B increased and Matsuda index decreased, significantly [Table 3]. The increase in insulin resistance may not be the results of increased body weight, because it was constant during of study. In group B, the plasma glucose concentration of 30 min during OGTT increased. It can be explained by increased in insulin resistance, beta cell function and decrease in insulin sensitivity. However, the plasma glucose of 120 min during OGTT decreased in this group. It showed that early insulin secretion defect, probably, has a major role in increased plasma glucose level in 30 min. It seems that the combination of intramuscular vitamin D and oral calcium, led to the more decrease in insulin sensitivity and increase insulin resistance indexes than what has been observed by vitamin D injection alone.
Vitamin D decreases production of cytokines such as IL2, IL6 and TNF and exposure of β cells to this cytokines, leading to apoptosis.[10] On other hand, vitamin D has an important role for the homeostasis of glucose with direct effect on β cell function. So that in vitamin D deficiency state, impaired insulin secretion and glucose intolerance occurs. Several study showed that these two defects have been corrected by vitamin D supplementation.[11,12,13,14,15,16,17,18]
In the Nurses’ Health Study, which has been done on 1557 women over a period of 20 years, those who had taking of 1200 mg calcium and more than 800 IU vitamin D3 daily, had 33% decrease in the risk of T2DM in comparison to those who received less than 400IU vitamin D per day.[1]
In Finish Mobile Clinic Study, similar to previous study, high vitamin D status protected again T2DM.[19]
On the other hand, some studies show no effect of vitamin D supplementation in glucose homeostasis and there are, rarely, some studies the negative effect of vitamin D on the insulin resistance. For example, in the Women's Health Initiative Study, intakes of 400 IU of vitamin D per day did not show any protection against diabetes.[20] In Nagpal et al., study on 100 men with central obesity, three oral doses of vitamin D during six, could not make any change in insulin secretion.[21] In Nilasl et al., study, treatment with vitamin D did not show any significant change in body weight or plasma glucose level in post menopausal women.[22] In the Taylor et al. study, vitamin D replacement with 300,000 IU of intramuscular injection in Asian people, increased insulin resistance, significantly.[23] This study was similar to our study from two aspects; one of them is the route of vitamin D administration and the second one the increased insulin resistance by vitamin D injection.
In order to explain our findings some points should be considered. The population of this study was selected from the first degree relatives of T2DM. They have a strong component of insulin resistance and β cell dysfunction, genetically. As mentioned above, most of other studies showed that insulin resistance decreased with vitamin D administration with or without oral calcium. However, in our study, insulin resistance increased. It may reflect the strong genetically, determined insulin resistance predisposition in our patients which could not overcome with vitamin D administration.
The route of vitamin D administration probably has some effects on the insulin resistance. In the most of the previous studies, which reported the decrease in insulin resistance by vitamin D, it had been orally, administered.[1]
A novelty in our study was that the combination of intramuscular vitamin D and oral calcium showed more deteriorating effect on insulin resistance and insulin sensitivity indexes than intramuscular vitamin D alone. It should be studied more in future with a bigger sample size, to make sure if this finding is a persistent result. At this moment, we do not have any explanation for this finding.
In the first degree relatives of T2DM, with pre-diabetes and vitamin D insufficiency and or deficiency, after two months of vitamin D injection alone or its combination with oral calcium, insulin resistance and sensitivity indexes did not change, significantly in comparison with placebo. However, in people who received vitamin D and calcium, insulin resistance and β cell function indexes increased and insulin sensitivity index decreased.
Statistical analysis of the data was performed using SPSS version 15 for windows. Data were expressed as mean and standard deviation (SD), if the data were normally distributed, and as median if the distribution was not normal.
Student t test was used for the comparison of quantitative variables. Chi square test was used for comparison of qualitative variable between two groups.
Comparison of data before and after of intervention in each group was done with paired t-test in normally distributed data and with Wilcoxon t-test, if the data distribution was not normal.
P < 0.05 was considered significant.
AUTHORS’ CONTRIBUTION
All authors had active contributions in all stages of this study including data gathering, data analysis and manuscript preparation.
ACKNOWLEDGMENT
Authors would like to thank Mr. Majid Abyar and Ms. Zahra Khani for their computer technical help and people who accepted our invitation and participated in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pittas AG, Lau j, Hu FB, Dawson-Huges B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S, Davies M, Zakaria Y, Mawer EB, Gordon C, Olukoga AO, et al. Improvement in glucose tolerance and beta-cell function in a patient with vitamin D deficiency during treatment with vitamin D. Postgrad Med J. 1994;70:440–3. doi: 10.1136/pgmj.70.824.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and B cell dysfunction. Am J Clin Nutr. 2004;79:820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 4.Schwolfenberg G. Vitamin D and diabetes: improvement of glycemic control with vitamin D3 repletion. Can Fam Physician. 2008;54:864–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Chertow BS, Sivitz WI, Baranetsky NG, Clark SA, Waite A, Deluca HF. Cellular mechanisms of insulin release: the effects of vitamin D deficiency and repletion on rat insulin secretion. Endocrinology. 1983;113:1511–8. doi: 10.1210/endo-113-4-1511. [DOI] [PubMed] [Google Scholar]
- 6.Code C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 7.Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle intervention to prevent or delay type 2 diabetes in people with impaired glucose tolerance: Systematic review and meta-analysis. BMJ. 2007;334:299. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaccaro O, Riccardi G. Changing the definition of impaired fasting glucose: Impact on the classification of individuals and risk definition. Diabetes Care. 2005;28:1786–8. doi: 10.2337/diacare.28.7.1786. [DOI] [PubMed] [Google Scholar]
- 9.Abdelmannan D, Tahboub R, Genuth S, Ismail-Beigi F. Effect of dexamethasone on oral glucose tolerance in healthy adults. Endocr Pract. 2010;16:770–7. doi: 10.4158/EP09373.OR. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 11.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–8. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozfirat Z, Choudhury TA. Vitamin D deficiency and type 2 diabetes. Postgrad Med J. 2010;86:18–25. doi: 10.1136/pgmj.2009.078626. [DOI] [PubMed] [Google Scholar]
- 13.Mattila C, Knekt P, Männistö S, Rissanen H, Laaksonen MA, Montonen J, et al. Serum 25-hydroxy vitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–70. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005-2006. Diabetes Care. 2009;32:342–7. doi: 10.2337/dc08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwalfenberg G. Vitamin D and diabetes: improvement of glycemic control with vitamin D3 repletion. Can Fam Physician. 2008;54:864–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca VA. Identification and treatment of predibetes to prevent progression to type 2 diabetes. Clinic Cornerstone. 2008;9:51–9. doi: 10.1016/s1098-3597(09)62039-1. [DOI] [PubMed] [Google Scholar]
- 17.Hyppönen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: A cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57:298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 18.Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia. 1995;38:1239–45. doi: 10.1007/BF00422375. [DOI] [PubMed] [Google Scholar]
- 19.Mattila C, Knekt P, Männistö S, Rissanen H, Laaksonen MA, Montonen J, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–70. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- 20.de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care. 2008;31:701–7. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 22.Nilas L, Christianson C. Treatment with vitamin D or its analogues does not change body weight or blood glucose level in postmenopausal women. Int J Obes. 1984;8:407–11. [PubMed] [Google Scholar]
- 23.Taylor AV, Wise PH. Vitamin D replacement in Asians with diabetes may increase insulin resistance. Postgrad Med J. 1998;74:365–6. doi: 10.1136/pgmj.74.872.365. [DOI] [PMC free article] [PubMed] [Google Scholar]


