Abstract
Pseudomonas aeruginosa is a Gram-negative opportunistic human pathogen possessing a type III secretion system (T3SS) which injects toxic effector proteins into mammalian host cells. In previous studies, P. aeruginosa strains lacking all of the known type III effectors were shown to cause cytotoxicity upon prolonged infection time. In this study, we report the identification of a new cytotoxin, nucleoside diphosphate kinase (NDK), which is injected into eukaryotic cells in a T3SS-dependent manner. Injection of NDK is inhibited by the presence of previously known effectors of the T3SS, with an effectorless strain injecting the highest amount, suggesting active competition with the known T3SS effectors. NDK is shown to cause a cytotoxic response when expressed in eukaryotic cells, and P. aeruginosa strains harbouring NDK also show a greater toxicity than strains lacking it. Interestingly, the cytotoxic effect of intracellular NDK is independent of its kinase activity. In previous studies, NDK was shown to be secreted into culture supernatants via a type I secretion system and cause cytotoxicity in a kinase-dependent manner. Therefore, the current study highlights an alternative route of NDK secretion as well as two different cytotoxic mechanisms of NDK, depending on the extra- or intra-cellular location of the protein.
Introduction
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen responsible for causing diseases in immunocompromised individuals, most notably those suffering from severe burns or cystic fibrosis (Lyczak et al., 2000). In order to maintain infection, P. aeruginosa relies on the production of numerous virulence factors, some of which are injected directly into host cells via a cell contact-mediated type III secretion system (T3SS) (Galán & Collmer, 1999; Cornelis & Van Gijsegem, 2000). The T3SS is a proteinaeous needle which translocates proteins, known as effectors, directly from the bacterial cytoplasm into the host cell (Hayes et al., 2010). The effectors secreted by the T3SS of P. aeruginosa – exoenzymes S, T, Y and U – are the major contributors to acute toxicity during the course of an infection (Barbieri & Sun, 2004; Engel & Balachandran, 2009; Hauser, 2009). The majority of P. aeruginosa isolates encode three of the four type III effectors, either STY or UTY (Feltman et al., 2001).
We have recently demonstrated that P. aeruginosa could be utilized to deliver functional nuclear proteins into pluripotent and differentiated cells by using a laboratory strain, known as PAK-J, which displays an elevated secretion of effectors compared to most previously characterized strains of P. aeruginosa (Bichsel et al., 2011, 2013). The strain used to deliver nuclear proteins, PAK-JΔSTY, was devoid of all three known type III secreted exotoxins so as to reduce cytotoxicity to the host cells. Despite the depletion of the type III secreted effectors, cytotoxic effects were still observed in cells after prolonged exposure to the bacteria. In contrast, mutants that are defective in the T3SS display almost no cytotoxicity, suggesting the presence of additional effectors secreted by the T3SS.
In an effort to identify the additional type III secreted effectors, we found that nucleoside diphosphate kinase (NDK) is injected into HeLa cells by a strain lacking all T3SS effectors, including ExoS, ExoT and ExoY. NDK is an ATP-utilizing enzyme that is secreted by the type I secretion system (T1SS) in P. aeruginosa and had previously been shown to cause cytotoxicity when incubated with macrophages (Zaborina et al., 1999; Kamath et al., 2000). In this report, we demonstrate that NDK is translocated into host cells in a T3SS-dependent manner and that intracellular NDK causes HeLa cell lifting independent of its kinase activity. This was further proven by transfection of a plasmid expressing a kinase-defective NDK in HeLa cells. Results from the current study suggest a new mechanism of cytotoxicity for the NDK protein during host–Pseudomonas interactions.
Methods
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Strain PAK-J is a laboratory strain of P. aeruginosa while PAK-JΔSTYN is deleted of the three type III effectors (exoS, exoT & exoY) as well as a negative regulator of T3SS (popN). Strain PAK-JΔSTYN was further deleted of the type II secretion system (xcpQ) and quorum sensing (lasI & rhlI) to result in PAK-JΔ7. Strain PAK-JΔ8 is an ndk deletion derivative of the PAK-JΔ7. Both E. coli and P. aeruginosa were grown in Luria (L) broth or on L agar plates at 37 °C. For plasmid selection, the final concentration of antibiotics used was 150 µg carbenicillin ml−1 for P. aeruginosa and 100 µg of ampicillin ml−1 for E. coli. Primers used to generate various constructs are listed in Table 2. Human and E. coli ndk genes were PCR amplified and cloned into the pUCP20 vector using BamHI and HindIII restriction sites located at the end of each primer. P. aeruginosa ndk was cloned into pUCP20 using EcoRI and PstI restriction sites and was further cloned into the eukaryotic expression vector pCDNA3.1(+) using BamHI and EcoRI sites.
Table 1. Bacterial strains and plasmids.
| Strain or plasmid | Description | Source |
| P. aeruginosa | ||
| PAK-J | PAK derivative with enhanced T3SS | Bichsel et al. (2011) |
| PAK-JΔS | PAK derivative with chromosomal deletion exoS | Bichsel et al. (2011) |
| PAK-JΔT | PAK derivative with chromosomal deletion exoT | Bichsel et al. (2011) |
| PAK-JΔST | PAK derivative with chromosomal deletion of exoS and exoT | Bichsel et al. (2011) |
| PAK-JΔSTY | PAK derivative with chromosomal deletion of exoS, exoT and exoY | Bichsel et al. (2011) |
| PAK-JΔSTYN | PAK-JΔSTY with chromosomal deletion of popN | This study |
| PAK-JΔpscF | PAK derivative with chromosomal deletion of pscF | This study |
| PAK-JΔ7 | PAK-JΔSTYN with chromosomal deletion of xcpQ, lasR-I and rhlR-I | This study |
| PAK-JΔ8 | PAK-JΔ7 with chromosomal deletion of ndk | This study |
| Plasmids | ||
| pUCP20 | Escherichia–Pseudomonas shuttle vector; Apr | West et al. (1994) |
| pPaNDK | FLAG-tagged ndk from P. aeruginosa in pUCP20; Apr | This study |
| pPaNDKH117Q | pPaNDK with kinase null ndk mutant; Apr | This study |
| pPaNDKH117QH54Q | pPaNDKH117Q with additional H54Q mutation; Apr | This study |
| pEcNDK | FLAG-tagged ndk from E. coli in pUCP20; Apr | This study |
| pHuNDK | FLAG-tagged ndk from human origin in pUCP20; Apr | This study |
| pCDNA3.1(+) | Eukaryotic expression vector containing CMV promoter; Apr | Invitrogen |
| pJJ0322 | exoS in pEGFP-C1; Kmr | Jia et al. (2006) |
| pDNNDK | ndk from P. aeruginosa in pCDNA3.1(+); Apr | This study |
| pDNNDKH117Q | pDNNDK with kinase null ndk mutant; Apr | This study |
| pEGFP-1 | Mammalian expression vector with CMV promoter driven egfp; Kmr | BD Clontech |
| pNDKΔ8 | NDK in pPaNDK deleted of C-terminal 8 aa; Apr | This study |
| pCre-NDK | Full-length NDK fusion to Cre in pUCP20; Apr | This study |
| pCre-NDKΔ8 | NDKΔ8 fused to Cre in pUCP20; Apr | This study |
| pExoS54-Cre | ExoS54 fused to Cre in pUCP20; Apr | Bichsel et al. (2011) |
Table 2. List of primers.
| Construct | Primer |
| NDK FLAG constructs | |
| PaNDK upstream | 5′-ggagaattcGCGCCTGGCCATCGCGGCGCAGATGG-3′ |
| PaNDK downstream | 5′-GGACTGCAGTCACTTGTCGTCATCGTCCTTGTAGTCGCGAATGCGCTCGCAGACTTCGGTAGCCGC-3′ |
| EcNDK upstream | 5′-ACCGGATCCCGCGACAGTGAAATTTGTCATGCAATAGTC-3′ |
| EcNDK downstream | 5′-ACCAAGCTTTCACTTGTCGTCATCGTCCTTGTAGTCACGGGTGCGCGGGCACACTTCGCCTTC-3′ |
| Human NDK upstream | 5′-ACCGGATCCCGCGACAGTGAAATTTGTCATGCAATAGTC-3′ |
| Human NDK downstream | 5′-ACCAAGCTTTCACTTGTCGTCATCGTCCTTGTAGTCTTCATAGACCCAGTCATGAGCACAAGA-3′ |
| PaNDK pCDNA3.1 UP | 5′-ACCGGATCCGCCATGGCACTGCAACGCACCCTGTCCATCATC-3′ |
| PaNDK pCDNA3.1 DN | 5′-ACCGAATTCTCAGCGAATGCGCTCGCAGACTTCGGTAGCCGC-3′ |
| NDKΔ8 downstream | 5′-GGAGAGCTCTCACTTGTCGTCATCGTCCTTGTAGTCGAAGAAGTAGGCGATCTCGCGAGCGG-3′ |
| NDK mutations | |
| NDKH117Q Forward | 5′-CGAGAACGCCGTCCAGGGATCCGATTCCGAAGCTTCC-3′ |
| NDKH117Q Reverse | 5′-GGAAGCTTCGGAATCGGATCCCTGGACGGCGTTCTCG-3′ |
| NDKH54Q Forward | 5′-GGCTTCTATGCCGAGCAGAAAGAGCGTCCGTTCTTC-3′ |
| NDKH54Q Reverse | 5′-GAAGAACGGACGCTCTTTCTGCTCGGCATAGAAGCC-3′ |
Cell culture.
HeLa and H1299 cells were cultured in Dulbecco’s Modified Eagle Media (DMEM) supplemented with 10 % FBS and 1 % penicillin/streptomycin (Gibco). Cells were incubated at 37 °C with 5 % CO2.
Protein secretion assay.
Bacterial strains were grown overnight in 1.0 ml of L broth containing carbenicillin at 37 °C. Overnight cultures were then inoculated at 5 % into fresh L broth containing antibiotics for non-type III inducing conditions and L broth plus antibiotics and 5 mM EGTA for type III inducing conditions. P. aeruginosa strains were grown in a shaking incubator at 37 °C for 3 h, after which bacterial cells were centrifuged at 20 000 g for 2 min. Bacterial supernatants were collected, precipitated by 15 % TCA (20× concentration), resuspended in 1× SDS protein sample buffer and boiled for 10 min before SDS-PAGE analysis.
Protein injection assay.
HeLa cells were seeded onto six-well plates at approximately 70 % confluence (8.4×105 cells) in DMEM containing 10 % FBS, penicillin and streptomycin (pen/strep) the night before infection. Two hours prior to infection, cells were washed twice in PBS and replaced with DMEM+10 %FBS without pen/strep. Bacterial strains were grown in L broth supplemented with carbenicillin at 37 °C until the OD600 reached 0.8. For an m.o.i. of 20, 2×107 c.f.u. were incubated with HeLa cells. Following infection, culture medium was removed and the HeLa cells were washed three times with PBS, followed by trypsinization. HeLa cells were centrifuged at 500 g for 5 min and then washed three times with PBS. HeLa cell pellets were suspended in 40 µl of PBS containing 0.25 % Triton X-100 and placed on ice for 10 min, which selectively lyses HeLa cells (Ha & Jin, 2001). Cell lysates were then centrifuged at 20 000 g for 2 min and the supernatants were mixed with an equal volume of 2× SDS protein sample buffer. Samples were boiled for 10 min and stored at −20 °C until use.
Western blotting.
Secretion and injection samples were loaded and separated on 4–20 % gradient SDS-PAGE gels (Bio-Rad). Proteins were transferred onto PVDF membranes and subjected to immunoblotting using an anti-FLAG antibody (mouse M2 monoclonal Ab; Sigma) for NDK and anti-β-actin (Santa Cruz) for actin, with a 1000-fold dilution.
HeLa cell lifting assay.
HeLa cells were infected with P. aeruginosa at the indicated m.o.i. for indicated periods of time using procedures described above. Following infection, HeLa cells were washed three times with PBS to remove non-adhering cells, and then the adhering cells were suspended by incubation with 0.25 % trypsin for 5 min. The number of cells in suspension was counted using a haemocytometer.
Assay for type III injected Cre–NDK fusion protein.
To assess the DNA recombinase activity of the injected Cre-NDK fusion protein, a cell line (TE26) with a lacZ reporter gene was infected with P. aeruginosa strain PAK-JΔSTY harbouring the Cre–NDK fusion construct at an m.o.i. of 50 for 3 h. The TE26 cells were washed with PBS three times and grown for 48 h on DMEM+10 % FBS with 10 µg ciprofloxacin ml−1. The cells were then subjected to staining for β-galactosidase activity as described previously (Bichsel et al., 2013).
Results
Strains lacking known type III secreted effectors are still cytotoxic
Our previous studies have demonstrated that P. aeruginosa is capable of delivering functional Cre recombinase and MyoD into the host cell nucleus when they were fused to the secretion signal sequence of the type III effector ExoS (Bichsel et al., 2011, 2013). We generated a strain PAK-JΔSTY, devoid of all three known type III secreted effectors, to use as the protein delivery strain. Although this strain shows reduced cytotoxicity when incubated with mammalian cells for less than 3 h, observations from longer infection times revealed that this strain possesses residual toxicity. To determine the degree of adverse effects resulting from longer incubation with PAK-JΔSTY, we compared the number of HeLa cells that remained adhered to tissue culture plates following infection by the wild-type strain PAK-J or PAK-JΔSTY. For P. aeruginosa mediated cytotoxicity, a high correlation between the results of the HeLa cell lifting assay and that of the LDH release assay had previously been observed (Bichsel et al., 2011). Fig. 1(a) shows that at 4 h post-infection at an m.o.i. of 50, 50 % of the cells remained adhered to the plate following incubation with PAK-JΔSTY whereas incubation with the wild-type strain resulted in only 5 % of cells still adhering. These results are in agreement with previous cytotoxicity studies, which showed that P. aeruginosa strains lacking type III effectors still caused low levels of LDH release from cells following infection (Lee et al., 2005; Vance et al., 2005; Bichsel et al., 2011). To address whether the residual HeLa cell lifting effect related to the T3SS, a type III defective mutant PAKΔpscF (defective of the type III needle subunit) was also incubated with HeLa cells. PAKΔpscF displayed a dramatically reduced HeLa cell lifting capacity, as there was only a 10 % reduction in the number of adhering cells compared to a non-infected control (Fig. 1a). These results suggested that the T3SS of P. aeruginosa, even in the absence of all known effectors, is still capable of causing toxicity.
Fig. 1.
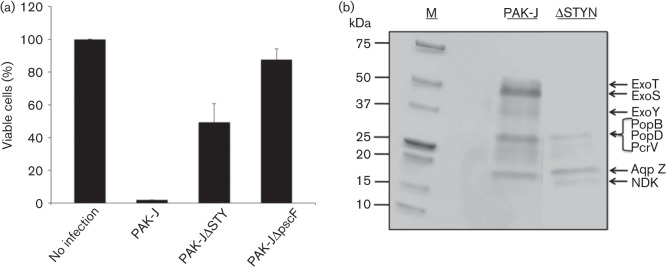
Strains lacking type III effectors are cytotoxic. (a) Toxicity assay of various P. aeruginosa strains. HeLa cells were infected with the indicated strains for 4 h at an m.o.i. of 50, and cells that remained adhered were counted. PAK-J, wild-type; PAK-JΔSTY, deleted of all three type III secreted toxins; PAKΔpscF, T3SS defective mutant. Data represent means of three replicate experiments. Error bars represent SD. (b) Type III protein secretion profiles of wild-type PAK-J and PAK-JΔSTYN mutant. Bacteria were grown in LB with 5 mM EGTA for 3 h, and culture supernatants were concentrated and subjected to Coomassie blue staining.
Thus far, only four type III exotoxins have been identified among P. aeruginosa isolates, and the majority of isolates possess three of the four (Feltman et al., 2001). Therefore, it is possible that additional minor proteins are secreted through the type III needle, especially in the absence of the major effectors. To investigate this possibility, we compared secreted protein patterns of PAK-J (wild-type) and PAK-JΔSTYN (a mutant strain lacking all three known type III secreted effectors and popN), following growth under T3SS inducing conditions. The PopN protein is a regulatory protein whose gene deletion results in a strain that constitutively secretes effector proteins (Sundin et al., 2004; Yang et al., 2007). Bacterial strains were grown in L broth in the presence of 5 mM EGTA for 3 h at 37 °C (a standard type III inducing condition) and the supernatants were TCA precipitated, subjected to SDS-PAGE, and stained with Coomassie blue. As shown in Fig. 1(b), wild-type PAK-J secretes ExoS, T and Y, while strain PAK-JΔSTYN lacks all these effectors as expected. Mass spectrometric analysis of the two bands shared by the two strains indicated a mixture of translocon proteins (PopB, PopD and PcrV) and a water channel protein aquaporin Z (AqpZ) (Fig. 1b). Most interestingly, strain PAK-JΔSTYN secretes an additional small protein about 15 kDa in size, which was identified as a nucleoside diphosphate kinase (NDK). This kinase is known to convert nucleoside diphosphates (NDPs) to nucleotide triphosphates (NTPs) and has previously been shown to be secreted through a P. aeruginosa T1SS (Kamath et al., 2000; Spooner & Yilmaz, 2012).
PAK-J secretes and injects NDK into eukaryotic cells
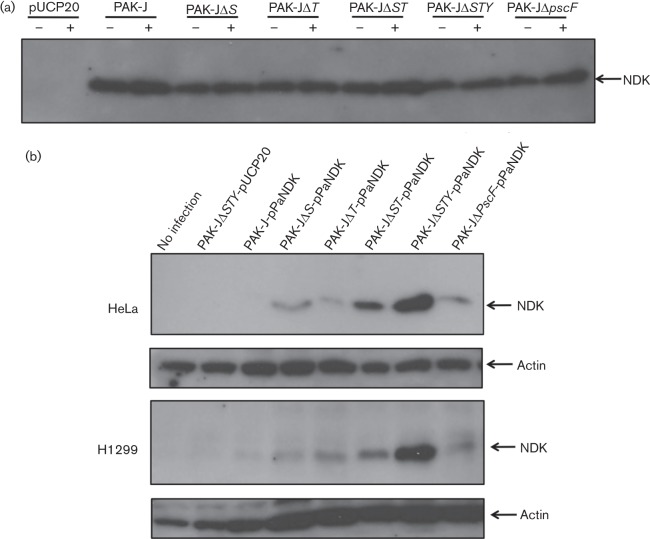
Based on the results shown in Fig. 1(b), we set out to determine if the T3SS plays a role in the secretion of NDK and assess whether the known type III effectors have any role in this. To accomplish this, a plasmid containing a C-terminal FLAG-tagged form of the NDK from P. aeruginosa (pPaNDK) was introduced into various exotoxin deletion derivatives of PAK-J. Protein secretion was determined following bacterial growth in L broth or L broth containing 5 mM EGTA for 3 h at 37 °C. Bacterial culture supernatants were collected, separated on SDS-polyacrylamide gel, and subjected to immunoblotting using an antibody against FLAG tag. As the results shown in Fig. 2(a) all of the strains secreted similar amounts of NDK under type III inducing or non-inducing conditions, suggesting that the T3SS may not play a role in the secretion of the protein. The fact that the type III defective mutant, PAKΔpscF, secretes NDK at amounts similar to strains possessing a functional T3SS further suggests that the T3SS may not be involved in the observed NDK secretion. Additionally, deletion of known type III effectors does not appear to have any effect on the secretion of NDK, as all of the strains secreted NDK at similar levels.
Fig. 2.
Secretion and injection of an NDK–FLAG construct. (a) Detection of NDK–FLAG in the culture supernatant of P. aeruginosa. Bacteria containing pPaNDK were grown under type III inducing (+) and non-inducing (−) conditions, and culture supernatants were directly subjected to Western blotting using an anti-FLAG antibody. (b) HeLa and H1299 cell lysates that were infected with the indicated bacterial strains were subjected to Western blotting using an anti-FLAG antibody. Membranes were stripped and reprobed with an actin antibody to serve as a loading control.
Although the secretion data shown in Fig. 2(a) suggest that the T3SS does not play a role in the secretion of NDK, it was possible that the amount of NDK secreted by the T3SS was so low that no detectable differences could be observed between type I and III secreted proteins in the above assay. If NDK is injected through the type III injectisome, then it should behave like other type III effectors and be injected into eukaryotic cells. We therefore incubated the same deletion strains harbouring FLAG-tagged NDK with HeLa cells for 3 h at an m.o.i. of 20. Following the infection, HeLa cells were lysed and the cell lysates were subjected to immunoblotting with an anti-FLAG antibody. Fig. 2(b) shows that PAK-JΔSTY/pPaNDK injects the highest amount of NDK into HeLa cells and that the amount injected decreased as one or more known type III effector genes were present, with almost none being injected from the wild-type PAK-J strain. Similar results were also obtained when another cell line, human non-small cell lung carcinoma, H1299, was used in place of the HeLa cells (Fig. 2b). Since the amount of NDK injection was negatively influenced by the presence of known type III effectors, the results suggested that NDK might also be injected through T3SS and be competitively inhibited by other effectors. Indeed, infection with the T3SS defective strain (PAKΔpscF) showed no NDK injection, indicating a functional T3SS is required for NDK translocation. Further, no intracellular NDK was detectable following 3 h of incubation of HeLa cells with the culture supernatant of strain PAK-JΔSTY/pPaNDK (data not shown), suggesting that the NDK signals in Fig. 2(b) are the result of T3SS-dependent translocation and not a direct uptake of NDK by the HeLa cells from the culture supernatant. Taken together, the above results suggested that NDK is secreted into the culture supernatant mainly through the T1SS, while a small amount is injected into host cells via a functional T3SS. Also, a higher amount of NDK injection into HeLa cells was observed in bacteria that lack known type III effectors, suggesting that these effectors may compete with NDK for injection through the type III secretion machinery.
Intracellular NDK is cytotoxic to HeLa cells
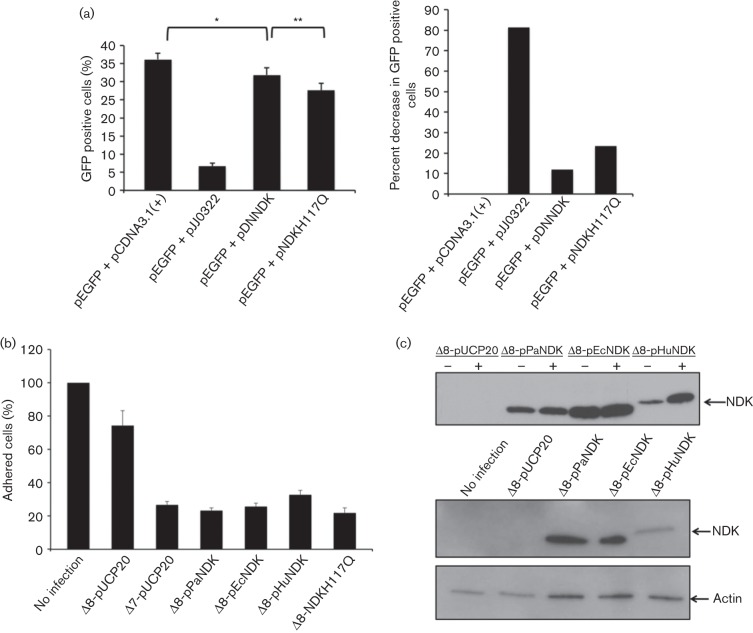
NDK has been reported to cause cytotoxicity in macrophages by disrupting extracellular ATP concentrations (Zaborina et al., 1999); however, it has not been determined whether bacterial NDK is capable of eliciting a cytotoxic response when expressed inside host cells. To examine this, NDK was cloned into a eukaryotic expression vector pCDNA3.1 and co-transfected into HeLa cells with a plasmid expressing GFP (pEGFP). If NDK is toxic to the host cell, the number of GFP positive cells should be lower in samples co-transfected with pEGFP and NDK, compared to cells co-transfected with pEGFP and pCDNA3.1 (empty vector control). Forty-eight hours post-transfection, HeLa cells were collected and analysed by flow cytometry to quantify the number of GFP positive cells. Fig. 3(a) shows transfection with GFP and empty vector (pEGFP+pCDNA3.1+) resulted in 38 % of HeLa cells expressing GFP, whereas co-transfection of GFP and NDK (pEGFP+pDNNDK) resulted in a 12 % reduction in the amount of GFP positive cells compared with the GFP/empty vector transfected cells. The pEGFP was also co-transfected with a plasmid expressing the acute type III secreted cytotoxin ExoS (pJJ0322), resulting in an 80 % decrease in GFP positive cells compared with the GFP/empty vector transfected cells (Fig. 3b). Although NDK caused a significant reduction in the amount of GFP positive cells, the toxicity was not as robust as ExoS. This would suggest that NDK is not an acute toxin, but in the absence of other type III effectors, it does play a significant role in toxicity.
Fig. 3.
Cytotoxicity of strains containing NDK. (a) Cytotoxicity resulting from the expression of NDK in a eukaryotic expression vector. Plasmids containing GFP and NDK were co-transfected into HeLa cells. After 48 h, the number of GFP positive cells was quantified using flow cytometry. Data represent means of three independent replicates. Error bars represent SD. *P = 0.017 and **P = 0.023 using Student’s t-test. (b) Cytotoxicity from strains containing NDK. Bacteria were incubated for 5 h at an m.o.i. of 500 and cells remaining adhered were collected and counted. Data from three independent replicates. (c) Secretion and injection assay with P. aeruginosa expressing various ndk genes. Bacteria were grown under type III inducing and non-inducing conditions and culture supernatants were subjected to Western blotting using an anti-FLAG antibody. HeLa cells were infected at an m.o.i. 20 for 3 h, selectively lysed and subjected to Western blotting with an anti-FLAG antibody.
To further assess NDK-mediated cytotoxicity, we deleted NDK via homologous recombination in a laboratory strain PAK-JΔ7, resulting in a strain PAK-JΔ8. Strain PAK-JΔ7 is a derivative of PAK-JΔSTYN, with additional deletion of the type II secretion system and quorum sensing (Table 1) in an effort to further reduce the bacterial toxicity. Although this strain had the major virulence factors removed, it still shows cytoxicity when incubated with mammalian cells. To determine if NDK expressing strains possess greater toxicity, HeLa cells were infected with PAK-JΔ7, PAK-JΔ8, or PAK-JΔ8 complemented with an ndk expressing plasmid (pPaNDK). Free-floating bacterial cells were then washed off with PBS and fresh media containing ciprofloxacin were applied to the cells to eliminate residual bacteria. Twenty-four hours later, lifted HeLa cells were washed away and the cells that remained adhered were trypsinized and counted using a haemocytometer. As shown in Fig. 3(b), incubation with PAK-JΔ7 at an m.o.i. of 100 for 5 h resulted in a 75 % reduction in the number of adhered cells compared with the uninfected control. However, incubation with PAK-JΔ8 resulted in only a 25 % decrease in adhered cells. Complementation of PAK-JΔ8 with a wild-type ndk gene restored toxicity, causing a similar amount of cell lifting to infection with PAK-JΔ7. NDK containing strains do elicit a toxic response; however, the toxicity is not as robust, and the time necessary to cause toxicity is much longer compared with the well-known type III secreted effector ExoS.
Previous reports have demonstrated that NDK from Mycobacterium tuberculosis is also responsible for causing toxicity when incubated with eukaryotic cells (Chopra et al., 2003, 2004). We were curious as to whether this was a general feature of NDKs or relevant only to P. aeruginosa and M. tuberculosis. To examine this, NDKs from E. coli (pEcNDK) and humans (pHuNDK) were cloned into pUCP20 and introduced into P. aeruginosa. NDK of P. aeruginosa shares 60 % amino acid homology with that of E. coli and 42 % with that of humans (Chakrabarty, 1998). As shown in Fig. 3(c), NDKs from E. coli and human are also secreted into the culture supernatant by P. aeruginosa. Testing these strains for protein injection revealed that NDK from E. coli was injected at amounts similar to that from P. aeruginosa, whereas the human form showed a significantly lower amount of injection (Fig. 3c). The toxicity assay results in Fig. 3(b) reveal that strains possessing E. coli and human NDKs also cause HeLa cells to detach from the monolayer, with 75 % detaching with E. coli NDK and roughly 65 % with the human form. Interestingly, the human form of NDK is secreted at levels similar to the bacterial NDKs; however, the amount injected was much lower, possibly due to the lack of the signal necessary for injection via the type III needle. Although the human NDK causes a cytotoxic response, it is slightly reduced compared with that from E. coli or P. aeruginosa, which may relate to the observation that human NDK is not as readily injected. Based on these observations, it seems that a relatively low amount of injected NDK is sufficient to elicit cytotoxic response. Taken together, these results suggest that NDKs from E. coli and humans are able to generate a cytotoxic response when injected into eukaryotic cells.
Kinase activity of NDK is not required for its toxicity
NDK is responsible for generating nucleotide triphosphates (NTPs) from nuclotide diphosphates (NDPs) by transfer of a terminal phosphate from an NTP to an NDP (Spooner & Yilmaz, 2012). To date, all known prokaryotic and eukaryotic NDKs possess a conserved histidine residue (H117) that becomes phosphorylated during the generation of NTPs (Chakrabarty, 1998). Mutation of H117 to either A or Q has previously been shown to abolish the kinase activity of NDK (Tiwari et al., 2004). We therefore wanted to determine whether the kinase activity was responsible for the toxicity elicited by the NDK. To accomplish this, the histidine H117 of P. aeruginosa NDK was mutated to glutamine (H117Q) through site-directed mutagenesis. As shown in Fig. 3(a), co-transfection of the mutant NDK (pNDKH117Q) with GFP resulted in an 18 % reduction in the amount of GFP positive cells when compared with co-transfection of GFP/empty vector control. Surprisingly, the toxicity was slightly greater in the mutant than in the wild-type, suggesting that the kinase activity is not linked to the toxicity of NDK. Consistent with this, infection with a P. aeruginosa strain harbouring a kinase-null NDK resulted in a toxic effect similar to that of wild-type NDK containing strains (Fig. 3b). These assay results suggest that the toxicity of NDK in mammalian cells is independent of its kinase activity.
There is another conserved histidine in NDK at position 54. We further mutated this site in the background of the NDK (H117Q) mutant. The strain PAK-JΔ8 harbouring the resulting double point mutant NDK caused a similar HeLa cell lifting profile to the single H117Q mutant (data not shown), eliminating the possible involvement of secondary kinase domain causing the toxic effect.
C-terminal peptide of NDK is essential for injection via a functional T3SS
A truncated version of the NDK, lacking the C-terminal 8 aa (Fig. 4a) was generated, which have previously been shown to be essential for the T1SS-dependent NDK secretion (Kamath et al., 2000). The construct was then introduced into the PAK-JΔ8 strain and tested for secretion under type III inducing conditions as well as injection into HeLa cells. Independent of type III inducing conditions, the full-length NDK was secreted into the extracellular media, whereas the truncated form was not detectable in the culture supernatant, although similar levels of protein were produced inside the bacterial cells (Fig. 4b). These results are in agreement with a previous report which demonstrated that NDK secretion through the T1SS is dependent on a DTEV motif located in the last 8 aa of the carboxyl terminus (Kamath et al., 2000). Interestingly, the truncated form of NDK appears to run at a higher molecular mass than the full-length form, although sequencing results confirmed the gene sequence was correct, so the discrepancy may represent a post-translational modification. The NDK of P. aeruginosa has previously been shown to be cleaved from 16 to 12 kDa by elastase (Kamath et al., 1998). It is possible that the C-terminal deletion represents the uncleaved 16 kDa band, implicating the requirement of C-terminal peptide for elastase mediated proteolytic processing.
Fig. 4.
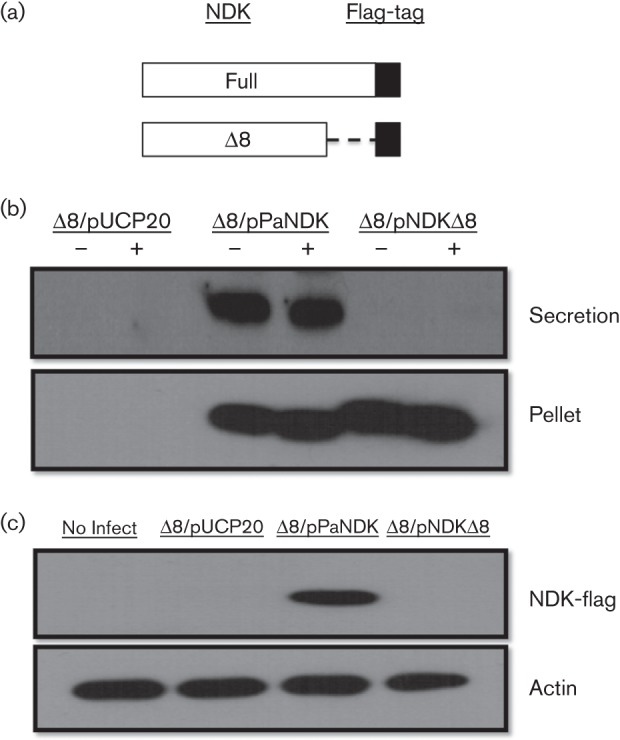
Injection and secretion of truncated NDK. (a) A truncated form of NDK, lacking the last 8 aa, was fused with a FLAG-tag. (b) Detection of truncated NDK from bacterial culture supernatants. Bacteria were grown under type III inducing and non-induction conditions and bacterial culture supernatants were collected for Western blot. (c) Detection of truncated NDK in HeLa cell lysates. Bacteria were incubated with HeLa cells at an m.o.i. of 20 for 3 h, lysed and probed with an antibody against the FLAG-tag. Membrane was stripped and reprobed with actin as a loading control.
To determine if the C-terminal peptide of NDK is essential for injection via the T3SS, bacterial strains containing the two forms of NDK were incubated with HeLa cells at an m.o.i. of 20 for 3 h. As shown in Fig. 4(c), the full-length NDK was readily translocated into HeLa cells while the truncated protein was not detectable, suggesting it is not readily injected into the host cells. The above data suggested that the C-terminal 8 aa are required for both T1SS-dependent secretion and T3SS-dependent injection of NDK. Whether the C-terminal 8 aa function as a secretion signal for both the T1SS and the T3SS, or whether T1SS-dependent secretion is a prerequisite for T3SS-dependent injection is not clear at the present time.
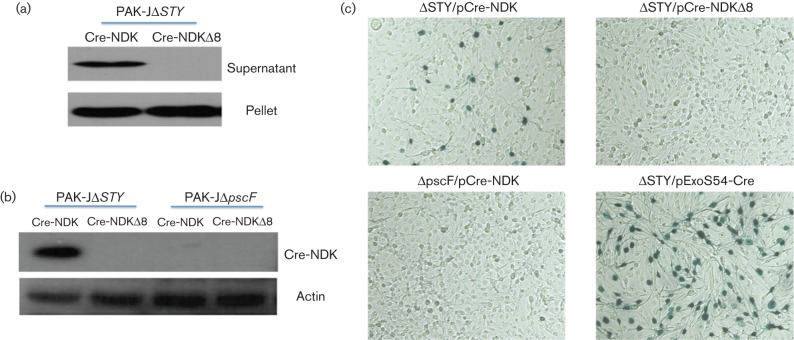
To further confirm the above observations, an alternative protein injection assay system was utilized. We have previously developed an assay system where T3SS-dependent injection of Cre recombinase can efficiently cause LoxP mediated recombination on the host chromosome, leading to activation of lacZ reporter gene expression (Bichsel et al., 2011). NDK or C-terminal truncated NDK (NDKΔ8) was fused to a Cre recombinase which contains a nuclear localization sequence. Upon introduction of the resulting plasmid constructs into PAK-JΔSTY, fusion proteins with the expected molecular mass were detectable at similar amounts from respective bacterial cell lysates (Fig. 5a). In the culture supernatants, however, the full-length Cre–NDK fusion was detectable but not the Cre–NDKΔ8 fusion, indicating that NDK is able to direct the secretion of the Cre–NDK fusion protein through the T1SS where the C-terminal 8 aa is essential for the secretion. Upon infection of a reporter TE26 cell line (human sarcoma derivative) which encodes a lacZ reporter gene whose expression is blocked by a floxed transcriptional terminator from SV40 (Bichsel et al., 2011), the injected intracellular Cre–NDK was clearly detectable by Western blot, but not that of Cre–NDKΔ8 or Cre–NDK in cells infected with a T3SS defective mutant (PAK-JΔpscF/pCre-NDK) (Fig. 5b). Upon colorimetric straining for LacZ enzyme activity 48 h after the bacterial infection, LacZ positive cells were detectable in TE26 cells that were infected with PAK-JΔSTY/pCre-NDK but not in those infected with PAK-JΔSTY/pCre-NDKΔ8 or PAK-JΔpscF/pCre-NDK (Fig. 5c), confirming that Cre–NDK is indeed injected via the T3SS into the TE26 cells and triggered the LoxP-dependent recombination. The percentage of LacZ positive cells was about 5 %, compared with 40 % in TE26 cells infected by PAK-JΔSTY/pExoS54-Cre. These results further support the notion that NDK is a less efficiently translocated effector than the ExoS.
Fig. 5.
Injection of Cre–NDK fusions. (a) Detection of the Cre–NDK and Cre–NDKD8 fusions in bacterial cell lysates as well as culture supernatants by anti-Flag Western blot. (b) Detection of Cre–NDK and Cre–NDKD8 fusions in TE26 cell lysates. TE26 cells were incubated with bacterial cells at m.o.i. 20 for 3 h, lysed and probed with anti-Flag Ab. Membrane was stripped and reprobed for actin as a loading control. (c) β-galactosidase staining of the TE26 cells following delivery of the Cre–NDK, Cre–NDKD8 and ExoS54–Cre by P. aeruginosa strains PAK-JDSTY or Cre–NDK by PAK-JDpscF.
Discussion
Previous studies, along with data presented here, clearly show that prolonged exposure to P. aeruginosa strains lacking all known type III effectors results in significant cytotoxicity (Vance et al., 2005; Bichsel et al., 2011). This toxicity is dependent on the T3SS, as infection with strains lacking a functional T3SS causes almost no cytotoxicity. In a recent study, we reported that the formation of type III translocon pore by the PopB/PopD also contributes to the cytotoxicity (Galle et al., 2012). Therefore, the residual cytotoxicity of the mutant strain PAK-JΔ8 may represent the translocon pore-mediated cytotoxicity (Fig. 1a). To date, only four type III secreted exotoxins have been characterized in P. aeruginosa, which is relatively low in number compared with other T3SS-encoding bacteria, such as Yersinia, Salmonella and Shigella species (Coburn et al., 2007). The goal of this study was to determine if any additional proteins are secreted through the T3SS. Examination of secreted proteins in the culture supernatants from a strain of P. aeruginosa lacking all three known type III secreted effectors as well as popN (PAK-JΔSTYN) revealed that NDK was secreted at a higher level than that by wild-type when grown under type III inducing conditions. The molecular mechanism by which PopN might influence NDK secretion remains to be determined. It should be noted that previous studies had shown that the expression of ndk is under the control of AlgQ and inducible by phosphate starvation while NDK secretion is influenced by levels of Ca2+, Mg2+ as well as the κ-chain of casein (Zaborina et al., 1999; Ambrosi et al., 2005).
Although not as toxic as the well-known type III effector ExoS, results presented in this study as well as previous reports strongly support a delayed type of cytotoxicity caused by NDK. Previous studies had shown that NDK is secreted into the culture supernatant, presumably through the T1SS pathway; however, it had never been linked to the T3SS or direct injection into the host cytosol. A number of key experimental data suggest that NDK is injected into the host cells through a bacterial T3SS rather than the secreted NDK being taken up by the eukaryotic cells. First, our results demonstrate that a functional T3SS is necessary for the injection of NDK into eukaryotic cells. Second, direct incubation of HeLa cells with the culture supernatant of P. aeruginosa strain PAK-JΔSTY/pPaNDK, which contains T1SS-secreted NDK, did not result in detectable amounts of intracellular NDK. Third, the presence of known T3SS effectors inhibits NDK injection, presumably through competition for the T3SS injectisome, also indicating that NDK is a low-affinity substrate for the T3SS.
This is the first report to show that NDK is injected into host cells by the T3SS and that intracellular expression of NDK results in a cytotoxic response. Additionally, toxicity does not appear to be dependent on the kinase activity of this protein, as the kinase defective mutant generates as much toxicity as the wild-type does (Fig. 3a, b). It is therefore possible that NDK from P. aeruginosa possesses additional functional domains that are responsible for the observed toxicity. In the case of M. tuberculosis, in addition to its kinase activity, NDK has also been shown to possess GAP activity for Rho-GTPases (Chopra et al., 2004; Sun et al., 2010). Considering that extracellular NDK causes cytotoxicity in macrophages by disrupting extracellular ATP through its kinase activity (Zaborina et al., 1999), our current findings suggest that NDK causes cytotoxicity through two completely different mechanisms, depending on whether it is in the extracellular or intracellular space. Further studies are required to understand the mechanism of cytotoxicity of intracellular NDK as well as the biological significance of T3SS mediated NDK injection.
NDK toxicity does not appear to be restricted to that of P. aeruginosa, as an NDK defective P. aeruginosa complemented by NDK from E. coli or human caused similar toxicity (Fig. 3b). Although the human form of NDK is not as readily injected as NDKs from P. aeruginosa and E. coli, the toxicity was only slightly reduced (Fig. 3c), suggesting that a small amount of intracellular NDK is sufficient to cause cytotoxicity. Additionally, it is possible that expressing a functional NDK in P. aeruginosa results in the activation of additional unknown virulence mechanisms. Recent data from our laboratories show that P. aeruginosa strains expressing NDK are able to induce pro-inflammatory cytokine expression in human alveolar epithelium cells (U.-H. Ha, unpublished data). These results were seen in strains expressing NDK from P. aeruginosa but not in strains lacking NDK, consistent with the current findings.
NDK does not possess a canonical T3SS signal sequence; however, by making a truncated form of NDK which is defective for T1SS-dependent secretion, it was found that disruption of the type I signal sequence also eliminates injection by the type III needle. A possible explanation for this observation is that the signal sequence for type I secretion overlaps with that for type III secretion. Alternatively, NDK might be secreted into the extracellular milieu first through the T1SS, and then is injected by the type III needle, as such a two-step process is not unprecedented (Vidal & Navarro-García, 2008; Akopyan et al., 2011).
In summary, results from this study have identified NDK as an additional protein injected into eukaryotic cells by the T3SS of P. aeruginosa. Although not as toxic as the known type III effectors, in the absence of said effectors, NDK is able to display significant cytotoxicity with prolonged incubation. We have demonstrated that NDK is injected into mammalian cells in a T3SS-dependent manner, causing a cytotoxic effect independent of its kinase activity. Further efforts are under way to determine the exact mechanism by which NDK causes toxic effects on the host cells.
Acknowledgements
We would like to thank Dr Andrew Wright of Tufts University School of Medicine for the ndk gene clones of human and E. coli. This work was supported by National Institutes of Health (RC1GM091238), Florida Department of Health Biomedical Research Programs (3KB04), National Science Foundation of China (31170128), National Basic Research Program of China (973 Program, 2012CB518700) and Basic Science Research Program (2010-0009047) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
Abbreviations:
- NDK
nucleotide diphosphate kinase
- T1SS
type I secretion system
- T3SS
type III secretion system
References
- Akopyan K., Edgren T., Wang-Edgren H., Rosqvist R., Fahlgren A., Wolf-Watz H., Fallman M. (2011). Translocation of surface-localized effectors in type III secretion. Proc Natl Acad Sci U S A 108, 1639–1644. 10.1073/pnas.1013888108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi C., Tiburzi F., Imperi F., Putignani L., Visca P. (2005). Involvement of AlgQ in transcriptional regulation of pyoverdine genes in Pseudomonas aeruginosa PAO1. J Bacteriol 187, 5097–5107. 10.1128/JB.187.15.5097-5107.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri J. T., Sun J. (2004). Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol 152, 79–92. 10.1007/s10254-004-0031-7 [DOI] [PubMed] [Google Scholar]
- Bichsel C., Neeld D. K., Hamazaki T., Wu D., Chang L. J., Yang L., Terada N., Jin S. (2011). Bacterial delivery of nuclear proteins into pluripotent and differentiated cells. PLoS ONE 6, e16465. 10.1371/journal.pone.0016465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichsel C., Neeld D., Hamazaki T., Chang L., Yang L., Terada N., Jin S. (2013). Trans-differentiation of fibroblasts to myocytes via bacterial injection of MyoD protein. Cell Reprogram 15, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. (1998). Nucleoside diphosphate kinase: role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol Microbiol 28, 875–882. 10.1046/j.1365-2958.1998.00846.x [DOI] [PubMed] [Google Scholar]
- Chopra P., Singh A., Koul A., Ramachandran S., Drlica K., Tyagi A. K., Singh Y. (2003). Cytotoxic activity of nucleoside diphosphate kinase secreted from Mycobacterium tuberculosis. Eur J Biochem 270, 625–634. 10.1046/j.1432-1033.2003.03402.x [DOI] [PubMed] [Google Scholar]
- Chopra P., Koduri H., Singh R., Koul A., Ghildiyal M., Sharma K., Tyagi A. K., Singh Y. (2004). Nucleoside diphosphate kinase of Mycobacterium tuberculosis acts as GTPase-activating protein for Rho-GTPases. FEBS Lett 571, 212–216. 10.1016/j.febslet.2004.06.073 [DOI] [PubMed] [Google Scholar]
- Coburn B., Sekirov I., Finlay B. B. (2007). Type III secretion systems and disease. Clin Microbiol Rev 20, 535–549. 10.1128/CMR.00013-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G. R., Van Gijsegem F. (2000). Assembly and function of type III secretory systems. Annu Rev Microbiol 54, 735–774. 10.1146/annurev.micro.54.1.735 [DOI] [PubMed] [Google Scholar]
- Engel J., Balachandran P. (2009). Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol 12, 61–66. 10.1016/j.mib.2008.12.007 [DOI] [PubMed] [Google Scholar]
- Feltman H., Schulert G., Khan S., Jain M., Peterson L., Hauser A. R. (2001). Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147, 2659–2669. [DOI] [PubMed] [Google Scholar]
- Galán J. E., Collmer A. (1999). Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284, 1322–1328. 10.1126/science.284.5418.1322 [DOI] [PubMed] [Google Scholar]
- Galle M., Jin S., Bogaert P., Haegman M., Vandenabeele P., Beyaert R. (2012). The Pseudomonas aeruginosa type III secretion system has an exotoxin S/T/Y independent pathogenic role during acute lung infection. PLoS ONE 7, e41547. 10.1371/journal.pone.0041547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha U., Jin S. (2001). Growth phase-dependent invasion of Pseudomonas aeruginosa and its survival within HeLa cells. Infect Immun 69, 4398–4406. 10.1128/IAI.69.7.4398-4406.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser A. R. (2009). The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 7, 654–665. 10.1038/nrmicro2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes C. S., Aoki S. K., Low D. A. (2010). Bacterial contact-dependent delivery systems. Annu Rev Genet 44, 71–90. 10.1146/annurev.genet.42.110807.091449 [DOI] [PubMed] [Google Scholar]
- Jia J., Wang Y., Zhou L., Jin S. (2006). Expression of Pseudomonas aeruginosa toxin ExoS effectively induces apoptosis in host cells. Infect Immun 74, 6557–6570. 10.1128/IAI.00591-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath S., Kapatral V., Chakrabarty A. M. (1998). Cellular function of elastase in Pseudomonas aeruginosa: role in the cleavage of nucleoside diphosphate kinase and in alginate synthesis. Mol Microbiol 30, 933–941. 10.1046/j.1365-2958.1998.01121.x [DOI] [PubMed] [Google Scholar]
- Kamath S., Chen M. L., Chakrabarty A. M. (2000). Secretion of nucleoside diphosphate kinase by mucoid Pseudomonas aeruginosa 8821: involvement of a carboxy-terminal motif in secretion. J Bacteriol 182, 3826–3831. 10.1128/JB.182.13.3826-3831.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. T., Smith R. S., Tümmler B., Lory S. (2005). Activities of Pseudomonas aeruginosa effectors secreted by the Type III secretion system in vitro and during infection. Infect Immun 73, 1695–1705. 10.1128/IAI.73.3.1695-1705.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyczak J. B., Cannon C. L., Pier G. B. (2000). Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2, 1051–1060. 10.1016/S1286-4579(00)01259-4 [DOI] [PubMed] [Google Scholar]
- Spooner R., Yilmaz Ö. (2012). Nucleoside-diphosphate-kinase: a pleiotropic effector in microbial colonization under interdisciplinary characterization. Microbes Infect 14, 228–237. 10.1016/j.micinf.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Wang X., Lau A., Liao T. Y., Bucci C., Hmama Z. (2010). Mycobacterial nucleoside diphosphate kinase blocks phagosome maturation in murine RAW 264.7 macrophages. PLoS ONE 5, e8769. 10.1371/journal.pone.0008769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin C., Thelaus J., Bröms J. E., Forsberg A. (2004). Polarisation of type III translocation by Pseudomonas aeruginosa requires PcrG, PcrV and PopN. Microb Pathog 37, 313–322. 10.1016/j.micpath.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Tiwari S., Kishan K. V., Chakrabarti T., Chakraborti P. K. (2004). Amino acid residues involved in autophosphorylation and phosphotransfer activities are distinct in nucleoside diphosphate kinase from Mycobacterium tuberculosis. J Biol Chem 279, 43595–43603. 10.1074/jbc.M401704200 [DOI] [PubMed] [Google Scholar]
- Vance R. E., Rietsch A., Mekalanos J. J. (2005). Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun 73, 1706–1713. 10.1128/IAI.73.3.1706-1713.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J. E., Navarro-García F. (2008). EspC translocation into epithelial cells by enteropathogenic Escherichia coli requires a concerted participation of type V and III secretion systems. Cell Microbiol 10, 1975–1986. 10.1111/j.1462-5822.2008.01181.x [DOI] [PubMed] [Google Scholar]
- West S. E., Schweizer H. P., Dall C., Sample A. K., Runyen-Janecky L. J. (1994). Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148, 81–86. 10.1016/0378-1119(94)90237-2 [DOI] [PubMed] [Google Scholar]
- Yang H., Shan Z., Kim J., Wu W., Lian W., Zeng L., Xing L., Jin S. (2007). Regulatory role of PopN and its interacting partners in type III secretion of Pseudomonas aeruginosa. J Bacteriol 189, 2599–2609. 10.1128/JB.01680-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborina O., Misra N., Kostal J., Kamath S., Kapatral V., El-Idrissi M. E., Prabhakar B. S., Chakrabarty A. M. (1999). P2Z-Independent and P2Z receptor-mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun 67, 5231–5242. [DOI] [PMC free article] [PubMed] [Google Scholar]