Abstract
IMPORTANCE
Reports of neuromyelitis optica spectrum disorder (NMOSD) occurring in the setting of neoplasia suggest that aquaporin-4 autoimmunitymay in some cases have a paraneoplastic basis.
OBSERVATIONS
In this case report, we describe a patient with NMOSD whose test results were seropositive for aquaporin-4 IgG and who had a hepatic metastasis from a small-bowel neuroendocrine tumor. The tumor cells expressed aquaporin-4 immunoreactivity. She presented to the Neurology Department at Wayne State University with bilateral leg weakness, ascending paresthesias, and decreased sensation.
CONCLUSIONS AND RELEVANCE
This case extends the context of NMOSD as a paraneoplastic disorder.
Paraneoplastic autoimmune neurological disorders are a remote manifestation of a patient’s immune response initiated by onconeural antigens expressed in a cancer that is often occult.1 Paraneoplastic autoantibody detection aids the neurological diagnosis and generally predicts a limited number of potentially associated cancer types but not a specific neurological presentation.2 Antibodies reactive with plasmalemmal channels and receptors have the potential to cause the neurological dysfunction, while antibodies reactive with neural nuclear and cytoplasmic autoantigens are surrogate markers for cytotoxic T cells specific for peptides derived from intracellular autoantigens.1,3 The difficulty with imaging cancers in the context of neurological autoimmunity attests to the efficacy of the antitumor response. In a minority of cases, no cancer is found.
Neuromyelitis optica (NMO) is a severe relapsing autoimmune inflammatory demyelinating disease that preferentially affects the optic nerves and spinal cord, thus mimicking multiple sclerosis, from which it is distinguished by a serum autoantibody specific for the astrocytic water channel, aquaporin-4 (AQP4). There is compelling evidence that AQP4 IgG is pathogenic in NMO.4,5 Neuromyelitis optica and NMO spectrum disorders (NMOSDs, unified by AQP4-IgG seropositivity) have been reported in a paraneoplastic context.6,7 In 2 cases, AQP4 was found in the tumor tissue.8,9 Here we describe a patient with a small-bowel neuroendocrine tumor who presented with NMOSD. The serum test result was positive for AQP4 IgG, and metastatic cells in the patient’s liver expressed AQP4 immunoreactivity.
Report of a Case
A 48-year-old woman with a history of sticky platelet syndrome, antiphospholipid syndrome, and increased von Willebrand factor activity had multiple episodes of deep venous thrombosis and a pulmonary embolus more than 5 years prior to her first neurological symptoms. Ten months before coming to our attention, she presented elsewhere with painless left-sided partial vision loss of sudden onset, which was diagnosed as a stroke; 3 months later, she experienced more than 2 days’ decreased sensation and paresthesias involving the entire right leg. Examination revealed reduced pinprick and light touch perception in that leg; motor function and reflexes were normal. The findings from head magnetic resonance imaging (MRI), magnetic resonance angiography of the head and neck, echocardiogram, and routine cerebrospinal fluid studies were normal. Five months later, findings from cervical and thoracic spine MRI, without and with gadolinium infusion, were normal. Vision assessment noted acuity limited to hand motion, pale discs, sluggish pupillary response to light, and left afferent pupillary defect. The remaining neurological examination findings were normal apart from mild residual sensory changes and brisk patellar reflexes. She presented to the Neurology Department at Wayne State University with bilateral leg weakness, ascending paresthesias, and decreased sensation. Within days, she was unable to walk without assistance. She experienced frequent micturition for 4 months prior to presentation but no other bladder/bowel symptoms. There were no symptoms or signs of systemic lupus erythematosus or Sjögren syndrome. Examination revealed severe paraparesis and a sensory level at T10.
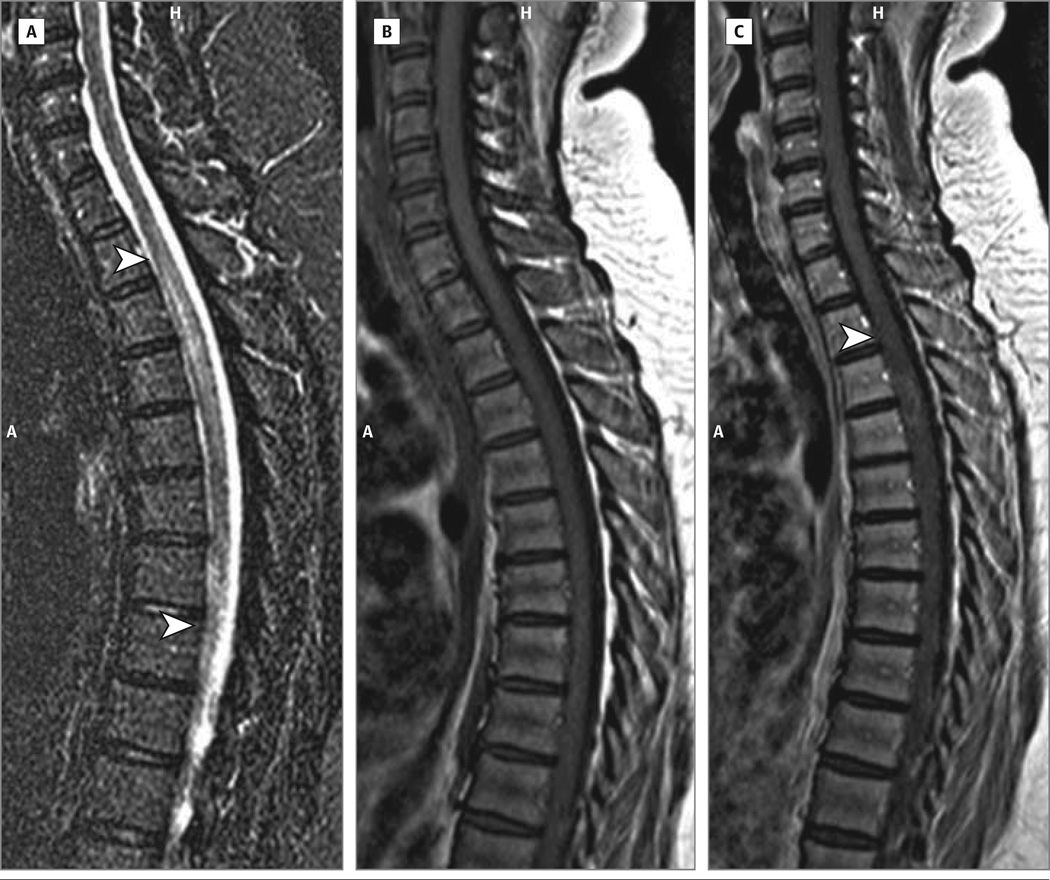
Spine MRIs showed continuous T2 hyperintensity (C6 to T4 at the time of maximum illness), with focal patchy gadolinium enhancement and cord swelling at T3-T4 (Figure 1). Brain MRI findings were normal. Cerebrospinal fluid was a cellular with normal total protein, IgG index was 0.60 (normal), and there were no supernumerary oligoclonal bands (5 noted in serum). Positive serology results included antinuclear antibody (1:160, mixed speckled and homogeneous pattern), double-stranded DNA (1:80), and Smith antibody (25.1; normal, <25.0), as well as persistent lupus anticoagulant and AQP4 IgG (enzyme-linked immunosorbent assay, >160 units; normal, <5 units/mL; positive AQP4-transfected cell-binding assay). The results of a comprehensive paraneoplastic antibody panel were positive for muscle acetylcholine receptor antibodies (2.3 nmol/L, normal <0.02 nmol/L) but were negative for other paraneoplastic antibodies including antineuronal nuclear antibody–1, amphiphysin IgG, and collapsin response-mediator protein 5 IgG, which are antibodies associated with myelopathies. Antimuscle acetylcholine receptor is encountered in 11% of AQP4-IgG–positive NMO serum samples and 2% of patients with NMO have myasthenia gravis.10 High-dose intravenous prednisolone therapy was followed by plasma exchange.
Figure 1. 1.5-T Magnetic Resonance Imaging Scan of Spine.
A, Sagittal T2 turbo inversion recovery magnitude image of the spine. The arrowheads indicate the caudal and rostral limits of T2 increased signal. B, Sagittal T1 image of the spine. C, Sagittal T1 image of the spine postinfusion of gadolinium. The arrowhead indicates the area of gadolinium enhancement. A indicates anterior; H, head.
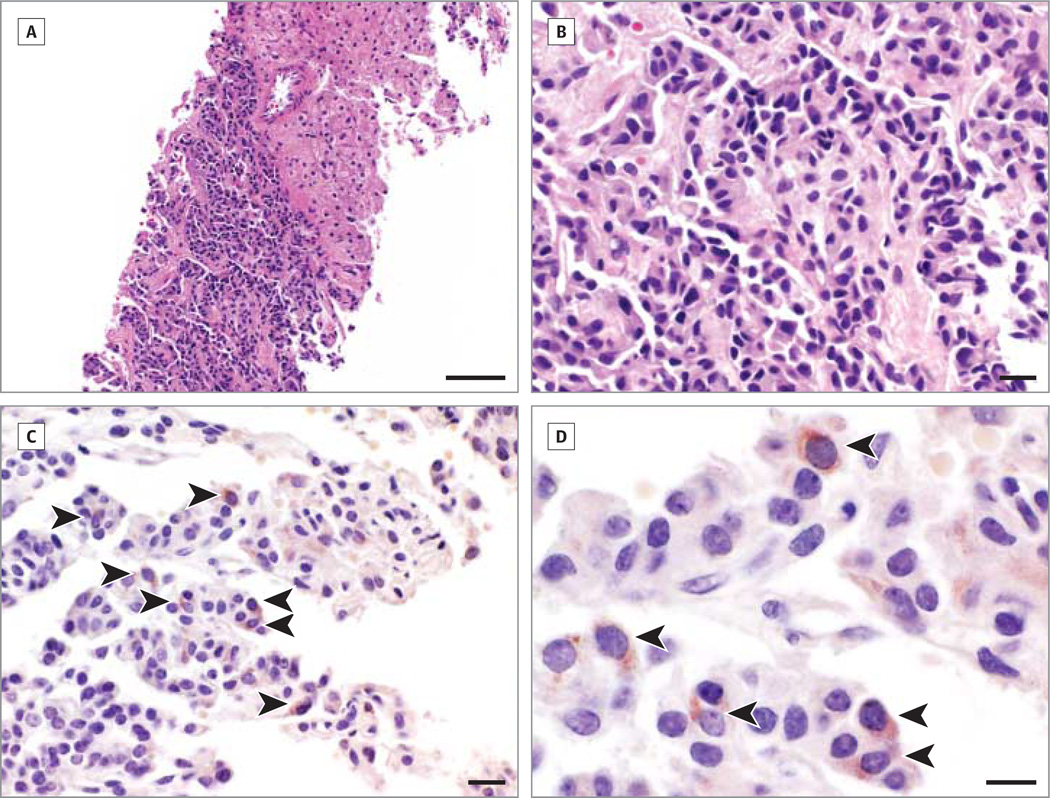
Anemia was noted at hospital admission and abdominal pain, hematemesis, and diarrhea developed. Her history included resection of a small-bowel neuroendocrine tumor 6 years earlier (no follow-up). An abdominal computed tomographic scan revealed several hepatic hypervascular masses. Biopsy confirmed metastatic carcinoid. Hematoxylin and eosin and immunohistochemical (anti-AQP4 antibody incubated at 4°C overnight, Sigma-Aldrich, A5971, dilution 1:250; no antigen retrieval) stainings of formalin-fixed paraffin embedded sections (5 µm) revealed AQP4-positive tumor cells (reddish cytoplasm) scattered within nests of nonimmunoreactive tumor cells (Figure 2). Octreotide acetate therapy was started, right hepatic artery radioembolization was performed, and rituximab was infused every 6 months. The patient had no further episodes of leg weakness, is able to ambulate without assistance (25-foot walk), and has no progression of her liver metastasis 30 months after her initial presentation.
Figure 2. Aquaporin-4 Immunoreactivity in Carcinoid Tumor Cells.
A, There is a sharp border between the hypercellular tumor (lower left) and normal liver parenchyma (upper right; hematoxylin and eosin). B, Tumor cells are shaped irregularly with hyperchromatic nuclei and pink cytoplasm (hematoxylin and eosin). C, Aquaporin-4–positive tumor cells (reddish cytoplasm) are relatively sparse and scattered within nests of nonimmunoreactive tumor cells (arrowheads). D, Aquaporin-4 immunoreactivity appears to be predominately cytoplasmic (arrowheads). Scale bars indicate 100 µm (A), 20 µm (B and C), and 10 µm (D).
Wayne State University institutional review board approval was waived and written informed consent was obtained from the patient.
Discussion
The demonstration of AQP4 immunoreactivity in this patient’s tumor further supports a paraneoplastic etiology for some NMOSD cases. Breast carcinoma is the most common neoplasm reported with paraneoplastic NMOSD, but a previous case of carcinoid has been reported.6 Carcinomas of the lung, uterus, thymus, cervix, bladder, and thyroid; seminoma; ovarian teratoma; pituitary adenoma; lymphoma; and monoclonal gammopathy also have been reported.6,9 Aquaporin-4 is expressed normally in a variety of noncentral nervous system tissues11,12 and has been reported in the breast carcinoma tissues of patients with and without clinical evidence of NMOSD8 and in an ovarian teratoma from a patient with NMOSD.9
Nonorgan-specific autoantibodies commonly coexist with AQP4 IgG in patients with NMO, as found in this patient. Detection of AQP4-IgG seropositivity in a patient presenting with myelopathy or optic neuritis in the setting of a nonorgan-specific autoimmunity supports NMOSD diagnosis rather than a vasculopathic or other complication of Sjögren syndrome or lupus.13
It is noteworthy that 2 other paraneoplastic autoimmune mimics of multiple sclerosis exhibit symmetric, longitudinally extensive tract changes resembling signal abnormalities characteristic of spinal cord MRIs typical of NMOSD lesions14: relapsing optic neuropathy and myelopathy associated with collapsin response-mediator protein 5 IgG(usually associated with small-cell carcinoma)15,16 and myelopathy associated with amphiphysin IgG (with breast or lung carcinoma).17
The median onset age for idiopathic NMO is 39 years,4,6–9 but patients with paraneoplastic NMO generally present at older than age 55 years. Recent reports indicate that 12% to 16% of patients with NMOSD are older than age 50 years at initial diagnosis.18,19 Generally, one considers a paraneoplastic disorder in those older than 55 years of age but, with respect to NMO recommendations, await more investigations.
Acknowledgments
Funding/Support: This work was supported by the Parker Webber Chair in Neurology Endowment, Detroit Medical Center Foundation/Wayne State University (Dr Lisak) and grants from the National Institutes of Health (RO-1-NS065829, Drs Pittock and Lennon) and the Guthy-Jackson Charitable Foundation (Drs Lucchinetti and Pittock).
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Lucchinetti and Lisak had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pittock, Lennon, Lucchinetti, Lisak.
Acquisition of data: Figueroa, Guo, Tselis, Lennon, Lucchinetti.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Figueroa, Guo, Tselis, Lisak.
Critical revision of the manuscript for important intellectual content: All authors.
Obtained funding: Pittock, Lennon, Lucchinetti, Lisak.
Conflict of Interest Disclosures: Dr Tselis has received research funding from Acorda, Biogen Idec, Genzyme, Novartis, Roche, and Teva. Dr Pittock is a named inventor on patents (12/678,350 filed 2010 and 12/573,942 filed 2008) that relate to functional aquaporin-4 (AQP4)/neuromyelitis optica (NMO) IgG assays and NMO IgG as a cancer marker. He has provided consultation to Alexion Pharmaceuticals, Chugai Pharmaceutical, and MedImmune Inc but has received no personal fees or personal compensation for these consulting activities. All compensation for consulting activities is paid directly to the Mayo Clinic. Dr Pittock receives research support from Alexion Pharmaceuticals Inc. Dr Lennon is a named inventor on a patent (7101679, issued 2006) relating to AQP4 antibodies for diagnosis of NMO and receives royalties for this technology. Dr Lennon is a named inventor on patents (12/678,350 filed 2010 and 12/573,942 filed 2008) that relate to functional AQP4/NMO IgG assays and NMO IgG as a cancer marker. Serological testing for neural autoantibodies is offered on a service basis by Mayo Collaborative Service Inc, an agency of the Mayo Foundation. Neither Dr Lennon nor her laboratory benefit financially from this testing. Dr Lucchinetti is a named inventor on patents (12/678,350 filed 2010 and 12/573,942 filed 2008) that relate to the function of AQP4/NMO-IgG assays and NMO IgG as a cancer marker. Dr Lisak has consulted for Teva Pharmaceuticals, Novartis, Geon, and Questcor. He has served as an expert witness and consultant in patent cases for Teva and is on a speakers list for nonpromotional talks for Teva. Dr Lisak has received research funding from Acorda, Avanir, Biogen Idec, Genzyme, Novartis, Questcor, Roche, and Teva. No other disclosures were reported.
REFERENCES
- 1.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7(4):327–340. doi: 10.1016/S1474-4422(08)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol. 2004;56(5):715–719. doi: 10.1002/ana.20269. [DOI] [PubMed] [Google Scholar]
- 3.Iorio R, Lennon VA. Neural antigen-specific autoimmune disorders. Immunol Rev. 2012;248(1):104–121. doi: 10.1111/j.1600-065X.2012.01144.x. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 5.Hinson SR, McKeon A, Lennon VA. Neurological autoimmunity targeting aquaporin-4. Neuroscience. 2010;168(4):1009–1018. doi: 10.1016/j.neuroscience.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Pittock SJ, Lennon VA. Aquaporin-4 autoantibodies in a paraneoplastic context. Arch Neurol. 2008;65(5):629–632. doi: 10.1001/archneur.65.5.629. [DOI] [PubMed] [Google Scholar]
- 7.De Santis G, Caniatti L, De Vito A, De Gennaro R, Granieri E, Tola MR. A possible paraneoplastic neuromyelitis optica associated with lung cancer. Neurol Sci. 2009;30(5):397–400. doi: 10.1007/s10072-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 8.Armağan H, Tüzün E, Içöz S, et al. Long extensive transverse myelitis associated with aquaporin-4 antibody and breast cancer: favorable response to cancer treatment. J Spinal Cord Med. 2012;35(4):267–269. doi: 10.1179/2045772312Y.0000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frasquet M, Bataller L, Torres-Vega E, et al. Longitudinally extensive transverse myelitis with AQP4 antibodies revealing ovarian teratoma. J Neuroimmunol. 2013;263(1–2):145–147. doi: 10.1016/j.jneuroim.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 10.McKeon A, Lennon VA, Jacob A, et al. Coexistence of myasthenia gravis and serological markers of neurological autoimmunity in neuromyelitis optica. Muscle Nerve. 2009;39(1):87–90. doi: 10.1002/mus.21197. [DOI] [PubMed] [Google Scholar]
- 11.Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci. 1995;108(pt 9):2993–3002. doi: 10.1242/jcs.108.9.2993. [DOI] [PubMed] [Google Scholar]
- 12.Frigeri A, Gropper MA, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci U S A. 1995;92(10):4328–4331. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittock SJ, Lennon VA, de Seze J, et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008;65(1):78–83. doi: 10.1001/archneurol.2007.17. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan EP, McKeon A, Lennon VA, et al. Paraneoplastic isolated myelopathy: clinical course and neuroimaging clues. Neurology. 2011;76(24):2089–2095. doi: 10.1212/WNL.0b013e31821f468f. [DOI] [PubMed] [Google Scholar]
- 15.Cross SA, Salomao DR, Parisi JE, et al. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann Neurol. 2003;54(1):38–50. doi: 10.1002/ana.10587. [DOI] [PubMed] [Google Scholar]
- 16.Keegan BM, Pittock SJ, Lennon VA. Autoimmune myelopathy associated with collapsin response-mediator protein-5 immunoglobulin G. Ann Neurol. 2008;63(4):531–534. doi: 10.1002/ana.21324. [DOI] [PubMed] [Google Scholar]
- 17.Pittock SJ, Lucchinetti CF, Parisi JE, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. 2005;58(1):96–107. doi: 10.1002/ana.20529. [DOI] [PubMed] [Google Scholar]
- 18.Quek AM, McKeon A, Lennon VA, et al. Effects of age and sex on aquaporin-4 autoimmunity. Arch Neurol. 2012;69(8):1039–1043. doi: 10.1001/archneurol.2012.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collongues N, Marignier R, Zéphir H, et al. Neuromyelitis optica in France: a multicenter study of 125 patients. Neurology. 2010;74(9):736–742. doi: 10.1212/WNL.0b013e3181d31e35. [DOI] [PubMed] [Google Scholar]