Abstract
Objective:
Ibuprofen – a non-steroidal anti-inflammatory drug (NSAID)- and glucosamine sulfate – a natural compound and a food supplement- are two therapeutic agents which have been widely used for treatment of patients with temporomandibular joint (TMJ) disorders. This study was aimed to compare the effectiveness and safety of these two medications in the treatment of patients suffering from TMJ disorders.
Methods:
After obtaining informed consent, 60 patients were randomly allocated to two groups. Patients with painful TMJ, TMJ crepitation or limitation of mouth opening entered the study. Exclusion criteria were history of depressive disorders, cardiovascular disease, musculoskeletal disorders, asthma, gastrointestinal problems, kidney or liver dysfunction or diabetes mellitus, dental diseases needing ongoing treatment; taking aspirin or warfarin, or concomitant treatment of TMJ disorder with other agents or methods. Thirty patients were treated with ibuprofen 400 mg twice a day, (mean age 27.12 ± 10.83 years) and 30 patients (mean age 26.60 ± 10) were treated with glucosamine sulfate 1500 mg daily. Patients were visited 30, 60 and 90 days after starting the treatment, pain and mandibular opening were checked and compared within and between two groups.
Findings:
Comparing with baseline measures, both groups had significantly improved post-treatment pain (P < 0.0001 for both groups) and mandibular opening (P value: 0.001 for glucosamine sulfate and 0.03 for ibuprofen). Post treatment pain and mandibular opening showed significantly more improvement in the glucosamine treated patients (P < 0.0001 and 0.01 respectively). Rate of adverse events was significantly lower in the P value glucosamine sulfate group (P < 0.0001).
Conclusion:
This investigation demonstrated that comparing with a commonly prescribed NSAID – ibuprofen-, glucosamine sulfate is a more effective and safer therapeutic agent for treatment of patients with TMJ degenerative join disorder.
Keywords: Glucosamine sulfate, ibuprofen, temporomandibular joint disorder
INTRODUCTION
Degenerative joint disease (DJD) is a common disorder which progresses slowly over a period of years, and results in the destruction of different joints including temporomandibular joint (TMJ).[1]
In addition to pain which is the main feature of TMJ disorder and the main cause of seeking medical help, stiffness, joint clicking, crepitation and movement limitation are other signs and symptoms of degenerative disease of the TMJ which may affect approximately from 60% to 70% of the general population.[2,3,4] Severe TMJ disorders may cause difficulties in everyday activities such as eating, talking, yawning and laughing. Therefore, it can reduce quality of life of patients, and affect both personal and professional life.[4]
Depending on the severity of disease and patients’ condition, both surgical and non-surgical methods could be used for treatment of TMJ disorders.[5]
Many of the surgical and dental therapies suggested for TMJ disorders have no scientifically proven effect;[6,7] therefore, pharmacotherapy is the primary intervention in patients with TMJ disorder.[6] Although pharmacotherapy is not curative for these patients it helps them to manage dysfunction and discomfort caused by such a chronic disorder.[7] Despite the wide range of pharmacotherapeutic agents used for TMJ disorder, there is still controversy regarding the treatment of choice for these patients.[6,7]
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most frequently prescribed medications for TMJ disorders worldwide; however, long term administration of NSAIDs is associated with a variety of adverse effects.[8,9]
Glucosamine is an essential component of glycosaminoglycans which are found in connective tissue, skin, tendons, ligaments, and cartilage. Recently, this compound has been more commonly prescribed for the treatment of TMJ disorders as a drug and nutritional supplement, which has minor side effects.[8,10] Although some investigations suggest glucosamine as an effective treatment for TMJ disorders some other clinical trials did not show any advantage for this treatment over placebo.[11,12,13,14,15]
Given the above, this study was aimed to compare effects of glucosamine sulfate with ibuprofen in the treatment of patients with TMJ disorder.
METHODS
After approval of the study by the ethic committee of Isfahan University of Medical Sciences and obtaining informed consent, this open-label clinical trial was performed on patients who were referred to Clinical Center of Isfahan Dentistry School, Isfahan, Iran, between September 2011 and March 2012.
After clinical diagnosis of TMJ disorder, 60 patients with painful TMJ, TMJ crepitation or limitation of mouth opening entered the study (CONSORT diagram). The diagnosis of TMJ disorder was made according to the research diagnostic criteria for TMJ disorders including joint pain at rest, evoked pain on TMJ palpation or TMJ clicking or noise with mandibular movement examination.[16]
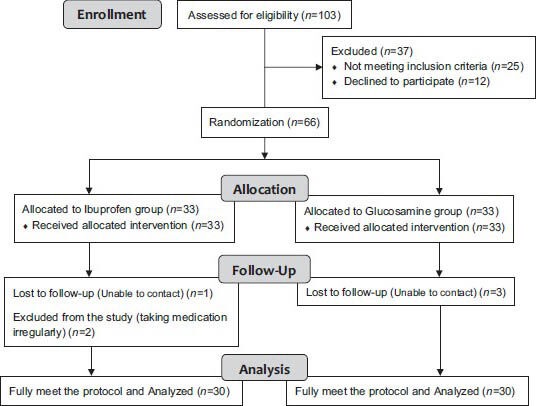
CONSORT diagram of the study
Exclusion criteria were positive history of depressive disorders, musculoskeletal disorders, cardiovascular diseases, asthma, gastrointestinal problems, kidney or liver dysfunction or diabetes mellitus; taking aspirin or warfarin, or concomitant treatment of TMJ disorder with other agents or methods.[16,17,18,19,20,21,22,23,24] Subjects with dental diseases needing ongoing treatment were also excluded.[16]
A single dentist evaluated all subjects regarding medical and dental history. Moreover, a head and neck examination, TMJ function assessment, palpation of the TMJ and masticatory muscles and a dental examination were performed at the beginning of the study.
Using ordinary randomization, patients were randomly allocated to two groups (30 patients in the ibuprofen group and 30 patients in glucosamine sulfate group).
In the first group, patients were commenced on ibuprofen 400 mg twice a day while in the second group, glucosamine sulfate 1500 mg daily was prescribed.
In addition to demographic data, baseline characteristics including severity of pain and maximal comfortable mandibular opening were recorded for all subjects prior to study drug administration.
Visual analogue scale of pain intensity was used to assess pain severity. Maximal comfortable mandibular opening was defined as the maximal inter-incisal distance a patient can open without pain, and was measured at the subject's maximum incisor to incisor mouth opening using a precise caliper.
Patients were re-evaluated regarding aforementioned characteristics at 30, 60 and 90 days after the start of treatment.
Patients’ vital signs were monitored at each follow up session. In addition, appropriate laboratory tests were checked to monitor the cases for renal, hepatic, or hematologic adverse events at the beginning and at 60 days after the start of the treatment. Moreover, a history was taken about any possible adverse effect.
Data were imported and analyzed by SPSS 16.5. According to the type of variables, independent t-test and Chi-square test were used to analyze data. P values less than 0.05 were considered statistically significant (confidence interval 95%).
RESULTS
The study sample consisted of 46 (77%) women and 14 (23%) men. There was no significant difference between two groups in the mean of age (27.12 ± 10.83 in the ibuprofen group versus. 26.60 ± 10.32 in the glucosamine sulfate group, P value: 0.90) and in sex distribution (female/male: 21/9 in the ibuprofen group versus. 25/5 in the glucosamine sulfate group, P value: 0.63).
Although there was no statistically significant difference between groups regarding pre-treatment pain intensity (4.66 ± 2.43 in the ibuprofen group vs. 4.71 ± 2.11in the glucosamine sulfate group, P value: 0.83), pain severity was significantly lower in the glucosamine sulfate treated group on all follow-up visits [Table 1 and Figure 1].
Table 1.
Comparison of pain intensity between two groups before and after treatment
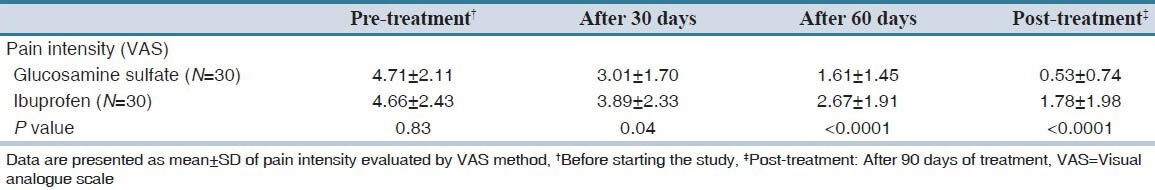
Figure 1.
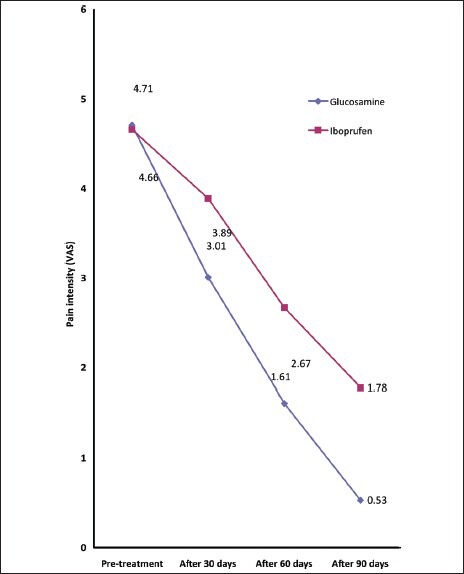
Changes in mean of pain intensity during the study
In addition, post treatment pain intensity was significantly lower than pre-treatment pain intensity in both glucosamine sulfate and ibuprofen treated patients (P < 0.0001 for both groups).
No significant difference was found between two groups regarding mandibular opening before and 30 days after starting treatment; however, mandibular opening of glucosamine sulfate treated subjects were significantly more than ibuprofen group 60 days and 90 days after treatment [Table 2 and Figure 2].
Table 2.
Comparison of mandibular opening between two groups before and after treatment
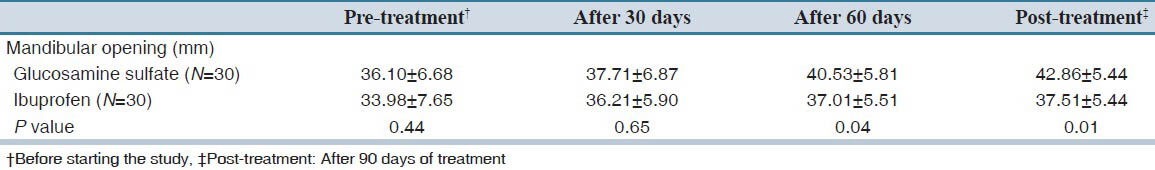
Figure 2.
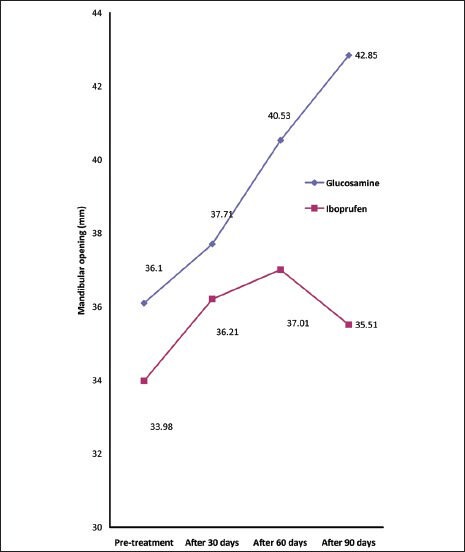
Changes in mean of mandibular opening during the study
Post-treatment mandibular opening was significantly more than pre-treatment values in both glucosamine sulfate and ibuprofen groups (P value: 0.001 and 0.03 respectively).
All patients were asked about adverse effects on every visit. No case of severe adverse effect was observed during the study, and all reported adverse effects were mild to moderate. Gastrointestinal adverse events including dyspepsia, abdominal pain and nausea were reported by 16 (53%) of ibuprofen treated and 5 (16%) of glucosamine sulfate treated patients (P < 0.0001). No case of adverse renal events was found.
DISCUSSION
This study showed that both ibuprofen and glucosamine sulfate improved TMJ pain and mandibular opening in patients suffering from TMJ degenerative joint disorders; however, glucosamine sulfate was found to be more effective, and had a lower rate of adverse effect.
Glucosamine was first reported as a potential therapeutic agent for degenerative joint disorders in 1969 by a German physician. Then, three other German investigators reported that glucosamine can decrease joint pain, and improve joint motility. However, because glucosamine is a natural product, many researchers and pharmaceutical companies have not paid enough attention to it until the previous decade. More recently, several studies have reported glucosamine sulfate superior to ibuprofen for treatment of different types of joint problems such as knee or TMJ degenerative joint disorders. Although there are fundamental differences between the TMJ and other synovial joints, studies performed on patients suffering from DJD of joints other than TMJ can be helpful.[15] Vas et al., performed a double blind study on 40 patients with knee osteoarthritis over 8 weeks. Their results showed that glucosamine sulfate was more effective than ibuprofen in relieving symptoms. Muller-Fassbender et al., published results of another double blind randomized clinical trial on knee osteoarthritis of 200 patients and found glucosamine sulfate as effective as ibuprofen with fewer adverse effects.[25] Another study on 178 patients by Qiu et al., reported similar results, and showed more effectiveness and fewer side effects of treatment with glucosamine sulfate compared with ibuprofen.[26] Similar to what we found, Thie et al., reported that glucosamine sulfate has greater influence in reducing TMJ pain and improvement of jaw movement than ibuprofen.[15] Comparing with ibuprofen, we found that glucosamine is associated with significantly lower rate of adverse events, which is confirmed by most of the previous studies.[10,15]
Now, the question is how glucosamine sulfate can compete with ibuprofen, and what its mechanism of action is. Glucosamine sulfate is an aminomonosaccharide and is the crucial part of O-linked and N-linked glycosaminoglycans. Matrix of all connective tissues such as cartilage are formed by glycosaminoglycans.[27] Over the previous decades, it has been believed that glucosamine compounds could be effective in ameliorating pain caused by degenerative joint disorders. Glucosamine sulfate acts as a substrate and stimulant of glycosaminoglycan production within articular cartilage, but the exact biochemical effects of glucosamine sulfate - which lead to symptom relief in patients with degenerative joint disorders-are not well known.[15] In contrast to glucosamine sulfate, which is not dependent on cyclooxygenase (COX) and can induce cartilage metabolism, traditional NSAIDs act by inhibition of COX and interfere with cartilage metabolism.[15]
In addition to beneficial effects of glucosamine sulfate on cartilage metabolism, this supplement may have some secondary effects including inhibition of catabolic mechanisms of degenerative joint disorders. Proinflammatory cytokines such as interleukin 1 and tumor necrosis factor alpha induce these destructive events, and it is believed that glucosamine sulfate can inhibit their activities. However, further studies are required to confirm the results of these hypotheses.[15]
In summary, this investigation demonstrated that comparing with a commonly prescribed NSAID – ibuprofen-, glucosamine sulfate may be used as a more effective and safer therapeutic agent for treatment of patients with TMJ degenerative joint disorder.
AUTHORS’ CONTRIBUTION
All authors contributed the idea or research, design of study, data analysis and manuscript preparation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Guarda-Nardini L, Ferronato G, Favero L, Manfredini D. Predictive factors of hyaluronic acid injections short-term effectiveness for TMJ degenerative joint disease. J Oral Rehabil. 2011;38:315–20. doi: 10.1111/j.1365-2842.2010.02164.x. [DOI] [PubMed] [Google Scholar]
- 2.Milam SB. Pathophysiology and epidemiology of TMJ. J Musculoskelet Neuronal Interact. 2003;3:382–90. [PubMed] [Google Scholar]
- 3.Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 4.Bessa-Nogueira RV, Vasconcelos BC, Niederman R. The methodological quality of systematic reviews comparing temporomandibular joint disorder surgical and non-surgical treatment. BMC Oral Health. 2008;8:27. doi: 10.1186/1472-6831-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fricton J. Current evidence providing clarity in management of temporomandibular disorders: Summary of a systematic review of randomized clinical trials for intra-oral appliances and occlusal therapies. J Evid Based Dent Pract. 2006;6:48–52. doi: 10.1016/j.jebdp.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Hersh EV, Balasubramaniam R, Pinto A. Pharmacologic management of temporomandibular disorders. Oral Maxillofac Surg Clin North Am. 2008;20:197–210. doi: 10.1016/j.coms.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Mujakperuo HR, Watson M, Morrison R, Macfarlane TV. Pharmacological interventions for pain in patients with temporomandibular disorders. Cochrane Database Syst Rev. 2010;10:CD004715. doi: 10.1002/14651858.CD004715.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Steinmeyer J, Konttinen YT. Oral treatment options for degenerative joint disease: Presence and future. Adv Drug Deliv Rev. 2006;58:168–211. doi: 10.1016/j.addr.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Steinmeyer J. Osteoarthritis-Fundamentals and Strategies for Joint-Preserving Treatment. Berlin: Springer; 2000. Pharmacological basis for the therapy of osteoarthritis; pp. 54–65. [Google Scholar]
- 10.Washington DC: Institute of Medicine and National Research Council; 2005. Institute of Medicine and National Research Council. Prototype monograph on glucosamine. Dietary Supplements: A framework for evaluating safety; pp. 363–6. [Google Scholar]
- 11.Cibere J, Kopec JA, Thorne A, Singer J, Canvin J, Robinson DB, et al. Randomized, double-blind, placebo-controlled glucosamine discontinuation trial in knee osteoarthritis. Arthritis Rheum. 2004;51:738–45. doi: 10.1002/art.20697. [DOI] [PubMed] [Google Scholar]
- 12.Cordoba F, Nimni ME. Chondroitin sulfate and other sulfate containing chondroprotective agents may exhibit their effects by overcoming a deficiency of sulfur amino acids. Osteoarthritis Cartilage. 2003;11:228–30. doi: 10.1016/s1063-4584(02)00351-5. [DOI] [PubMed] [Google Scholar]
- 13.Hughes R, Carr A. A randomized, double-blind, placebo-controlled trial of glucosamine sulphate as an analgesic in osteoarthritis of the knee. Rheumatology (Oxford) 2002;41:279–84. doi: 10.1093/rheumatology/41.3.279. [DOI] [PubMed] [Google Scholar]
- 14.Rindone JP, Hiller D, Collacott E, Nordhaugen N, Arriola G. Randomized, controlled trial of glucosamine for treating osteoarthritis of the knee. West J Med. 2000;172:91–4. doi: 10.1136/ewjm.172.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thie NM, Prasad NG, Major PW. Evaluation of glucosamine sulfate compared to ibuprofen for the treatment of temporomandibular joint osteoarthritis: A randomized double blind controlled 3 month clinical trial. J Rheumatol. 2001;28:1347–55. [PubMed] [Google Scholar]
- 16.Ta LE, Dionne RA. Treatment of painful temporomandibular joints with a cyclooxygenase-2 inhibitor: A randomized placebo-controlled comparison of celecoxib to naproxen. Pain. 2004;111:13–21. doi: 10.1016/j.pain.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Bruyere O, Pavelka K, Rovati LC, Gatterová J, Giacovelli G, Olejarová M, et al. Total joint replacement after glucosamine sulphate treatment in knee osteoarthritis: Results of a mean 8-year observation of patients from two previous 3-year, randomised, placebo-controlled trials. Osteoarthritis Cartilage. 2008;16:254–60. doi: 10.1016/j.joca.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Davis S, Papalia MA, Norman RJ, O’Neill S, Redelman M, Williamson M, et al. Safety and efficacy of a testosterone metered-dose transdermal spray for treating decreased sexual satisfaction in premenopausal women: A randomized trial. Ann Intern Med. 2008;148:569–77. doi: 10.7326/0003-4819-148-8-200804150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Muniyappa R, Karne RJ, Hall G, Crandon SK, Bronstein JA, Ver MR, et al. Oral glucosamine for 6 weeks at standard doses does not cause or worsen insulin resistance or endothelial dysfunction in lean or obese subjects. Diabetes. 2006;55:3142–50. doi: 10.2337/db06-0714. [DOI] [PubMed] [Google Scholar]
- 20.Persiani S, Rotini R, Trisolino G, Rovati LC, Locatelli M, Paganini D, et al. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthritis Cartilage. 2007;15:764–72. doi: 10.1016/j.joca.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Pham T, Cornea A, Blick KE, Jenkins A, Scofield RH. Oral glucosamine in doses used to treat osteoarthritis worsens insulin resistance. Am J Med Sci. 2007;333:333–9. doi: 10.1097/MAJ.0b013e318065bdbe. [DOI] [PubMed] [Google Scholar]
- 22.Rozendaal RM, Koes BW, van Osch GJ, Uitterlinden EJ, Garling EH, Willemsen SP, et al. Effect of glucosamine sulfate on hip osteoarthritis: A randomized trial. Ann Intern Med. 2008;148:268–77. doi: 10.7326/0003-4819-148-4-200802190-00005. [DOI] [PubMed] [Google Scholar]
- 23.Stumpf JL, Lin SW. Effect of glucosamine on glucose control. Ann Pharmacother. 2006;40:694–8. doi: 10.1345/aph.1E658. [DOI] [PubMed] [Google Scholar]
- 24.Tannock LR, Kirk EA, King VL, LeBoeuf R, Wight TN, Chait A. Glucosamine supplementation accelerates early but not late atherosclerosis in LDL receptor-deficient mice. J Nutr. 2006;136:2856–61. doi: 10.1093/jn/136.11.2856. [DOI] [PubMed] [Google Scholar]
- 25.Muller-Fassbender H, Bach GL, Haase W, Rovati LC, Setnikar I. Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee. Osteoarthritis Cartilage. 1994;2:61–9. doi: 10.1016/s1063-4584(05)80007-x. [DOI] [PubMed] [Google Scholar]
- 26.Qiu GX, Gao SN, Giacovelli G, Rovati L, Setnikar I. Efficacy and safety of glucosamine sulfate versus ibuprofen in patients with knee osteoarthritis. Arzneimittelforschung. 1998;48:469–74. [PubMed] [Google Scholar]
- 27.Harris ED, Budd RC, Firestein GS, Genovese MC, Sergent JS, Ruddy S, et al. 7th ed. Philadelphia: Elsevier; 2005. Kelley's Textbook of Rheumatology. [Google Scholar]


