Abstract
Objective:
Nearly two-third of the patients with type 2 diabetes have degrees of fatty liver; this may induce some side effects in them. This study aimed to find effect of salsalate on treatment of steatohepatitis and correlation of fatty liver with metabolic syndrome in the setting of impaired glucose metabolism.
Methods:
In a double-blind randomized trial within two distinct groups, i.e., recently diagnosed diabetics and prediabetic cases allocated in two arms of the intervention to receive 3 g salsalate or placebo. All cases underwent glucose and lipid level studies and liver ultrasound study.
Findings:
Out of 46 patients with diabetes, 34 (74%) had fatty liver in ultrasound; this ratio was 75% in 113 prediabetic cases. Relative frequency of fatty liver stages did not differ between diabetics and prediabetics. Within diabetics, mean aspartate aminotransferase (AST) level of fatty liver cases (23 ± 7 IU/dl) was higher than others (18 ± 3 IU/dl) (P < 0.05). Changes in transaminase levels following intervention did not significantly differ, comparing drug and placebo arms in two subgroups.
Conclusion:
According to the findings, if diabetes could be assumed as the logical consequence of prediabetic state, it seems that fatty liver did develop before this preliminary status. In this study, salsalate could not change biochemical markers of fatty liver significantly.
Keywords: Diabetes, fatty liver, salsalate
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a condition which is characterized by abnormal lipid infiltration in liver (steatosis) in the absence of excess alcohol intake; Triglyceride (TG) is the most common abnormal lipid that accumulates in hepatocells. Fatty acid metabolism dysfunction could cause fatty liver, these patients also suffer from insulin resistance.[1] According to the increased prevalence of obesity, fatty liver and metabolic syndrome were also increased.[2] About 66% of type 2 diabetic patients are reported to have fatty liver.[3] On the other hand, inflammation in the liver and nonalcoholic steatohepatitis could cause intra- and extrahepatic symptoms in patients.[4] In addition, it was reported that cirrhosis might be seen in up to 10% of these patients. Blood sugar and lipid metabolic dysfunction and cardiac disease in NAFLD patients are common,[5] but their quality of life is lower than the general population.[6] One of the probable pathways of metabolic syndrome due to fatty liver is excessive activity of immunity and inflammatory systems.[7,8] Efficacy of controlling inflammatory systems as a pathogenesis pathway of metabolic syndrome is being evaluated in different studies. There are recent reports about restraining inflammatory systems and its effect on controlling blood sugar;[9] but we had not find any report about using anti-inflammatory agents in fatty liver treatment.
The aim of this study was to evaluate the efficacy of salsalate as an anti-inflammatory agent in fatty liver treatment and metabolic syndrome symptom reduction.
METHODS
This was a double-blind randomized clinical trial study [Figure 1]. Two groups were evaluated in this study: first group of patients were newly diagnosed diabetic patients who did not have any history of medication. Their blood glucose level was more than 125 mg/dl and HgA1c between 6 and 9. The second group of patients were prediabetic participants, their fasting blood sugar (FBS) level being more than 100 mg/dl or their blood glucose level was more than 140 mg/dl (2 hours after administration of 75 g oral glucose). Classification and diagnostic criteria of American diabetes association were used for including patients.[10] All participants were included from Endocrinology and Metabolism Research Center. All participants had signed our consent form. Salsalate prescription contraindications are as follows: history of reaction to nonsteroidal anti-inflammatory drugs, asthma, heart failure class III and IV, hepatic failure, ongoing steroid usage, leucopenia, and thrombocytopenia. Contraindications were ruled out in the included patients. Patients in both groups were randomly divided into two subgroups (drug or placebo subgroups). Salsalate (Caraco Pharmaceuticals, USA) dosage was 750 mg every 12 hours. Fatty liver and its severity were evaluated by a sonographist before and after our intervention in all patients. Height, body weight, FBS, total cholesterol, TG, high-density lipoprotein (HDL), low-density lipoprotein (LDL), alanine transaminase (ALT), and aspartate transaminase (AST) were evaluated before and after medication too.
Figure 1.
Randomized division of participants
Statistical procedure was done by SPSS software (Version 14, SPSS Inc., Chicago, IL, USA). Salsalate efficacy was determined by ALT and AST levels before and after examination. T-test, Mann–Whitney test, and Spearman tests were used for data analysis. The significance level (P) was set at 0.05.
RESULTS
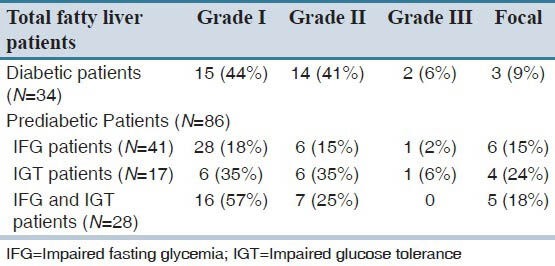
A total of 46 diabetic and 113 prediabetic patients were evaluated in this study; 34 (74%) in diabetic patients (group A1) and 86 (75%) in prediabetic group (group B1) had fatty liver which is diagnosed by sonography. Spearman test showed that there was no significant difference between groups in fatty liver before our intervention (P > 0.05). Sonographic findings are listed in Table 1.
Table 1.
Sonographic findings of fatty liver distribution
In prediabetics group, mean ALT in patients with fatty liver (group B1) was 26 ± 12 IU/dl and in patients without fatty liver (group B2) was 25 ± 12 IU/dl. In this group, mean AST level was 22 ± 8 IU/dl in patients with fatty liver and 22 ± 5 IU/dl in participants without fatty liver. There was no statistical difference in ALT and AST between groups (P > 0.05). Between prediabetic patients with or without fatty liver, there was only a significant difference between groups in metabolic syndrome symptoms. TG in B1 group was higher than group B2 (179 ± 85 vs 120 ± 48 mg/dl) and there was a significant difference between groups (P = 0.02). There was no significant difference between B1 and B2 groups in FBS, cholesterol, LDL, HDL, and body mass index (BMI) (P > 0.05).
In diabetic group, mean AST in patients with fatty liver (group A1) was 23 ± 7 IU/dl and in patients without fatty liver (group A2) was 18 ± 3 IU/dl. There was significant difference between groups (P < 0.05). There was also a significant difference between groups in mean ALT blood level (P < 0.05), but there was no significant difference between A1 and A2 groups in FBS, cholesterol, LDL, and HDL (P > 0.05).
The mean TG in diabetic patients without fatty liver was 131 ± 29 and in diabetic patients with fatty liver was 207 ± 117. Statistical analysis showed a significant difference between groups (P = 0.041). The mean BMI in group A1 was 30 and in group A2 was 28; there was no significant difference between groups in BMI (P > 0.05). There was a significant difference between fatty liver group (A1 and B1) and patients without fatty liver (A2 and B2) in BMI (30.5 ± 4.1 vs 27.8 ± 4.2) (P = 0.034).
There were no significant differences in ALT and AST between groups before and after study (P > 0.05). We also analyzed data for patients who had fatty liver before intervention; there was no significant difference between our results, comparing before and after intervention in both salsalate and placebo groups (P > 0.05). There were no significant differences between salsalate and placebo subgroups in A1 and B1 groups in fatty liver severity, ALT, and AST before and after intervention (P > 0.05).
No significant differences were seen between salsalate and placebo groups (in both diabetic and prediabetic groups) in FBS, total cholesterol, TG, HDL, and LDL (P > 0.05).
DISCUSSION
Most of our participants had fatty liver in both diabetic and prediabetic groups, and fatty liver distribution was almost the same in both groups; if we consider diabetes as a condition which is followed by prediabetic stage, fatty liver likely seems to be appear before this phase.
It was mentioned in many reports that metabolic syndrome symptoms are more frequent in fatty liver patients, but in our study TG was the only marker which had a higher significant level in fatty liver patients. We can assume that the most important symptom in metabolic syndrome is blood glucose adjustment dysfunction and other symptoms have less importance. As metabolic syndrome pathogenesis will increase after fatty liver, all of these symptoms such as cholesterol and blood pressure increase before blood sugar increases.
Among these factors TG is the only factor which could cause fatty liver due to hepatic aggregation.[11] In this study, TG level was higher in fatty liver patients in both groups.
Our other finding was ALT and AST level between fatty liver groups in prediabetic patients. In some other studies, it was reported that there was no significant relation between ALT and inflammatory hepatic conditions,[12] but hepatic inflammation and ALT increase could be a primary event and could be presented before prediabetics stage.
Although salsalate has a proved anti-inflammatory effect,[13] our results did not show a significant change in fatty liver treatment and it does not agree with our primary hypothesis.
In previous studies, salsalate had showed a significant effect on blood sugar reduction.[14] It was reported that salsalate administration had improved glucose metabolism in obese patients.[15]
Lack of salsalate efficacy in fatty liver biochemical markers reduction could be explained by: (1) difference in salsalate dosage (3 vs 4 g/day); (2) medication duration (1 vs 3 months); and (3) we think, it is possible that hepatic inflammatory pathway cannot stop or adjust after its onset.
However, our findings could not show that 3 g/day salsalate administration had a significant effect on diabetic and prediabetic patients with fatty liver.
AUTHORS’ CONTRIBUTION
All authors contributed in conception and design of the study, data analysis and manuscript preparation.
ACKNOWLEDGMENTS
The authors wish to thank all the employees of Endocrinology and Metabolism Research Center of Isfahan University of Medical Sciences where this study was performed and Mr. Majid Abyar for analyzing our data.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.El-Koofy NM, Anwar GM, El-Raziky MS, El-Hennawy AM, El- Mougy FM, El-Karaksy HM, et al. The association of metabolic syndrome, insulin resistance and non-alcoholic fatty liver disease in overweight/obese children. Saudi J Gastroenterol. 2012;18:44–9. doi: 10.4103/1319-3767.91738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moscatiello S, Di Luzio R, Sasdelli AS, Marchesini G. Managing the combination of nonalcoholic fatty liver disease and metabolic syndrome. Expert Opin Pharmacother. 2011;12:2657–72. doi: 10.1517/14656566.2011.629188. [DOI] [PubMed] [Google Scholar]
- 3.Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2009;16:141–9. doi: 10.1097/MED.0b013e3283293015. [DOI] [PubMed] [Google Scholar]
- 4.Bugianesi E, Vanni E, Marchesini G. NASH and the risk of cirrhosis and hepatocellular carcinoma in type 2 diabetes. Curr Diab Rep. 2007;7:175–80. doi: 10.1007/s11892-007-0029-z. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947–53. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 6.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 7.Tataranni PA, Ortega E. A burning question: Does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005;54:917–27. doi: 10.2337/diabetes.54.4.917. [DOI] [PubMed] [Google Scholar]
- 8.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 9.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–7. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–90. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 12.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology. 2008;48:792–8. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 13.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koska J, Ortega E, Bunt JC, Gasser A, Impson J, Hanson RL, et al. The effect of salsalate on insulin action and glucose tolerance in obese nondiabetic patients: Results of a randomised double-blind placebo-controlled study. Diabetologia. 2009;52:385–93. doi: 10.1007/s00125-008-1239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–94. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]


