Abstract
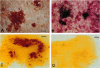
The brain amyloid of Alzheimer disease (AD) may potentially be imaged in patients with AD by using neuroimaging technology and a radiolabeled form of the 40-residue beta-amyloid peptide A beta 1-40 that is enabled to undergo transport through the brain capillary endothelial wall, which makes up the blood-brain barrier (BBB) in vivo. Transport of 125I-labeled A beta 1-40 (125I-A beta 1-40) through the BBB was found to be negligible by experiments with both an intravenous injection technique and an internal carotid artery perfusion method in anesthetized rats. In addition, 125I-A beta 1-40 was rapidly metabolized after either intravenous injection or internal carotid artery perfusion. BBB transport was increased and peripheral metabolism was decreased by conjugation of monobiotinylated 125I-A beta 1-40 to a vector-mediated drug delivery system, which consisted of a conjugate of streptavidin (SA) and the OX26 monoclonal antibody to the rat transferrin receptor, which undergoes receptor-mediated transcytosis through the BBB. The brain uptake, expressed as percent of injected dose delivered per gram of brain, of the 125I,bio-A beta 1-40/SA-OX26 conjugate was 0.15 +/- 0.01, a level that is 2-fold greater than the brain uptake of morphine. The binding of the 125I,bio-A beta 1-40/SA-OX26 conjugate to the amyloid of AD brain was demonstrated by both film and emulsion autoradiography performed on frozen sections of AD brain. Binding of the 125I,bio-A beta 1-40/SA-OX26 conjugate to the amyloid of AD brain was completely inhibited by high concentrations of unlabeled A beta 1-40. In conclusion, these studies show that BBB transport and access to amyloid within brain may be achieved by conjugation of A beta 1-40 to a vector-mediated BBB drug delivery system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bickel U., Kang Y. S., Pardridge W. M. In vivo cleavability of a disulfide-based chimeric opioid peptide in rat brain. Bioconjug Chem. 1995 Mar-Apr;6(2):211–218. doi: 10.1021/bc00032a009. [DOI] [PubMed] [Google Scholar]
- Dewan M. J., Gupta S. Toward a definite diagnosis of Alzheimer's disease. Compr Psychiatry. 1992 Jul-Aug;33(4):282–290. doi: 10.1016/0010-440x(92)90054-t. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Brandon M. R., Williams A. F., Hunt S. V. Analysis of lymphopoietic stem cells with a monoclonal antibody to the rat transferrin receptor. Immunology. 1985 Feb;54(2):333–341. [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kang Y. S., Pardridge W. M. Use of neutral avidin improves pharmacokinetics and brain delivery of biotin bound to an avidin-monoclonal antibody conjugate. J Pharmacol Exp Ther. 1994 Apr;269(1):344–350. [PubMed] [Google Scholar]
- Maggio J. E., Stimson E. R., Ghilardi J. R., Allen C. J., Dahl C. E., Whitcomb D. C., Vigna S. R., Vinters H. V., Labenski M. E., Mantyh P. W. Reversible in vitro growth of Alzheimer disease beta-amyloid plaques by deposition of labeled amyloid peptide. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5462–5466. doi: 10.1073/pnas.89.12.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. J., Sisodia S. S., Koo E. H., Cork L. C., Dellovade T. L., Weidemann A., Beyreuther K., Masters C., Price D. L. Amyloid precursor protein in aged nonhuman primates. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1461–1465. doi: 10.1073/pnas.88.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson G., Conklin E., Desai S., Nielander G., Savage M. D., Morgensen S. A practical approach to crosslinking. Mol Biol Rep. 1993 Apr;17(3):167–183. doi: 10.1007/BF00986726. [DOI] [PubMed] [Google Scholar]
- Nordstedt C., Näslund J., Tjernberg L. O., Karlström A. R., Thyberg J., Terenius L. The Alzheimer A beta peptide develops protease resistance in association with its polymerization into fibrils. J Biol Chem. 1994 Dec 9;269(49):30773–30776. [PubMed] [Google Scholar]
- Oldendorf W. H., Hyman S., Braun L., Oldendorf S. Z. Blood-brain barrier: penetration of morphine, codeine, heroin, and methadone after carotid injection. Science. 1972 Dec 1;178(4064):984–986. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Boado R. J., Kang Y. S. Vector-mediated delivery of a polyamide ("peptide") nucleic acid analogue through the blood-brain barrier in vivo. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5592–5596. doi: 10.1073/pnas.92.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Kang Y. S., Buciak J. L., Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res. 1995 Jun;12(6):807–816. doi: 10.1023/a:1016244500596. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Vinters H. V., Yang J., Eisenberg J., Choi T. B., Tourtellotte W. W., Huebner V., Shively J. E. Amyloid angiopathy of Alzheimer's disease: amino acid composition and partial sequence of a 4,200-dalton peptide isolated from cortical microvessels. J Neurochem. 1987 Nov;49(5):1394–1401. doi: 10.1111/j.1471-4159.1987.tb01005.x. [DOI] [PubMed] [Google Scholar]
- Roher A. E., Lowenson J. D., Clarke S., Woods A. S., Cotter R. J., Gowing E., Ball M. J. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samii A., Bickel U., Stroth U., Pardridge W. M. Blood-brain barrier transport of neuropeptides: analysis with a metabolically stable dermorphin analogue. Am J Physiol. 1994 Jul;267(1 Pt 1):E124–E131. doi: 10.1152/ajpendo.1994.267.1.E124. [DOI] [PubMed] [Google Scholar]
- Schlageter N. L., Carson R. E., Rapoport S. I. Examination of blood-brain barrier permeability in dementia of the Alzheimer type with [68Ga]EDTA and positron emission tomography. J Cereb Blood Flow Metab. 1987 Feb;7(1):1–8. doi: 10.1038/jcbfm.1987.1. [DOI] [PubMed] [Google Scholar]
- Tomlinson B. E., Blessed G., Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970 Sep;11(3):205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Triguero D., Buciak J., Pardridge W. M. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990 Jun;54(6):1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Vinters H. V., Pardridge W. M., Secor D. L., Ishii N. Immunohistochemical study of cerebral amyloid angiopathy. II. Enhancement of immunostaining using formic acid pretreatment of tissue sections. Am J Pathol. 1988 Oct;133(1):150–162. [PMC free article] [PubMed] [Google Scholar]
- Walker L. C., Masters C., Beyreuther K., Price D. L. Amyloid in the brains of aged squirrel monkeys. Acta Neuropathol. 1990;80(4):381–387. doi: 10.1007/BF00307691. [DOI] [PubMed] [Google Scholar]
- Walker L. C., Price D. L., Voytko M. L., Schenk D. B. Labeling of cerebral amyloid in vivo with a monoclonal antibody. J Neuropathol Exp Neurol. 1994 Jul;53(4):377–383. doi: 10.1097/00005072-199407000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E. T., Inman C. B., Weller R. O. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990 Jun;170:111–123. [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B. V., Ghiso J., Mackic J. B., McComb J. G., Weiss M. H., Frangione B. Blood-brain barrier transport of circulating Alzheimer's amyloid beta. Biochem Biophys Res Commun. 1993 Dec 30;197(3):1034–1040. doi: 10.1006/bbrc.1993.2582. [DOI] [PubMed] [Google Scholar]