Abstract
Objective:
Various non-hormonal agents have been used for the treatment of hot flashes in women with menopause. Some studies have reported that gabapentin appears to be an effective and well-tolerated treatment modality. The aim of this study was to evaluate whether the treatment with gabapentin is effective in reducing hot flash frequency and severity and also to compare gabapentin 100 mg/day, 300 mg/day and conjugated estrogen in this regards.
Methods:
In this comparative clinical trial, 100 post-menopausal women attending outpatient clinics of Isfahan University hospitals were included from April 2008 to February 2009. Participants randomly received gabapentin 300 mg/day, gabapentin 100 mg/day, or conjugated estrogen 0.625 mg/day for 12 weeks. Frequency and severity of hot flashes and adverse effects were compared among the three groups.
Findings:
From all, 16 participants dropped out. There were no significant differences among the groups before intervention in terms of age, body mass index and baseline hot flash frequency and severity. Hot flash diaries were used to record the frequency and severity of hot flashes. After the treatment period, there was a significant decrease in both severity and frequency of hot flashes in all three groups. Post-hoc analyses showed that the frequency and severity of hot flashes were significantly lower in those who received gabapentin 300 mg/day or estrogen 0.625 mg/day compared to those who received gabapentin 100 mg/day. There was not statistically significant difference between those who received gabapentin 300 mg/day and those who received estrogen. Very few adverse effects, mostly gastrointestinal discomfort were observed in both gabapentin groups (8%).
Conclusion:
Gabapentin 300 mg/day could be useful to relieve hot flashes in women for whom hormone therapy is not suitable or when hot flashes do not respond to other therapies. Further researches are needed to determine the efficacy of gabapentin use for longer periods or at higher doses.
Keywords: Estrogen, gabapentin, hot flash, menopause
INTRODUCTION
A woman is considered to be menopausal after 12 consecutive months of amenorrhea. The symptoms of menopause can range from mild to severe. The most common and often most troubling symptoms are vasomotor - including hot flashes and night sweats. Other symptoms associated with menopause are vaginal dryness, dyspareunia, mood changes, fatigue, sleep disturbances, and sexual dysfunction.[1]
Since hot flashes are the most common adverse effect of menopause, occurring in 30-80% of women and possibly diminishing their quality of life, they typically are the reason women seek medical treatment for menopause. The highest prevalence of hot flashes is seen within the first two post-menopause years, although 10% of women will experience hot flashes for more than 10 years.[2,3] Hot flashes occur spontaneously and may last for only seconds or up to minutes. Although the mechanism of a hot flash is not fully understood, it is likely due to changes in the thermoregulatory center in the hypothalamus.[4] Hot flashes are increased in severity and frequency by hot weather, caffeine, spicy foods ingestion, and alcohol. Current non-pharmacologic treatments for hot flashes include life-style changes such as weight loss, smoking cessation, and wearing light clothing in layers.[5]
The most effective treatment option for hot flashes is estrogen therapy, which was the standard therapy for more than 60 years. A report from the women's Health Initiative published in 2002 raised concerns about the long-term safety of estrogen therapy. This study indicated an increase in cardiovascular and thromboembolic events and breast cancer among healthy patients receiving estrogen or estrogen-progestin therapy compared with placebo. As a result, concern about the safety of hormonal therapy for symptoms of menopausal has lead practitioners and patients to seek non-hormonal treatment options.[6,7,8]
Treatment of hot flashes include life-style modification, such as regular exercise, use of over the counter remedies such as soy, black cohosh, vitamin E and red clover, and use of prescription drugs, such as selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, clonidine, gabapentin, and progestin. These therapies have had variable effects on hot flashes and each has its own adverse effects.[9]
Gabapentin is an anticonvulsant, which the United States food and Drug Administration approved as an adjunct therapy for partial seizures and post-herpetic neuralgia. Its mechanism of action for alleviating hot flashes is unknown. The drug possibly affects the thermoregulatory centers in the hypothalamus and it may possess nociceptive properties due to its high-affinity binding sites on calcium channels. The North American Menopause society and the American College of Obstetricians and Gynecologists recommend the use of gabapentin as an option for managing hot flashes in women who are unwilling to take estrogen-containing supplements. Previous studies showed gabapentin to be safe and effective in the treatment of hot flashes.[10]
There are some controlled trials on the efficacy of gabapentin in the treatment of hot flashes in post-menopausal women. However, very few of them compared gabapentin with estrogen and also few of them compared the efficacy and safety of different dosages simultaneously.[11] Therefore, much more data are still needed for a clear conclusion on the efficacy, safety, and dosage of gabapentin in the treatment of hot flashes in postmenopausal women. In the present study, we aimed to evaluate and compare the efficacy and safety of gabapentin 100 mg/day or 300 mg/day versus estrogen for the treatment of hot flashes in women with menopause.
METHODS
This prospective single-blinded, randomized controlled Clinical trial was carried out in which 100 healthy post-menopausal women who had hot flashes, attending out-patient clinics of Isfahan University hospitals were included from April 2008 to February 2009.
All subjects were informed about the study and its objectives and gave written consent to participate.
Inclusion criteria were post-menopausal women aged 45-65 years defined as those who had experienced natural cessation of menses for at least 1 year, having hot flashes for at least 2 moderate to severe episodes per day for more than 2 months, and volunteer to participate.
Exclusion criteria were current or previous history of cardiovascular, neurologic, liver, gallbladder, and/or chronic renal disease, focal neurological signs and/or other systemic disorders like hypothyroidism that confound the results regarding drug side-effects, being on estrogen or gabapentin for the treatment of hot flashes or receiving such drugs and also SSRIs or other antiepileptic medicines in the past 3 months.
We recruited 100 post-menopausal women who fulfilled the criteria of inclusion from those who referred to out-patient clinics of obstetrics and gynecology. Using random table list, they were randomly assigned into three groups: Group 1 received gabapentin 100 mg/day; group two received gabapentin 300 mg/day; and group three received conjugated estrogen 0.625 mg/day all for 12 weeks. Compliance was checked by patient report and also by observing the box of the drugs. The study was conducted in Al-Zahra and Shahid Beheshti University Hospital outpatient clinics, Isfahan from April 2008 to February 2009.
Participant eligibility was assessed at an initial visit according to the inclusion and exclusion criteria. Patient characteristics were assessed by participant self-report. Hot flash frequency was assessed with a numeric visual scale,[1,2,3,4,5,6,7,8,9,10] within the study period. Hot flash diaries were used to record the severity (1 = mild, 4 = severe) of the hot flashes. Women registered hot flash diaries for one week before to therapy initiation and at the planned interval (4 and 12 weeks after) then mean value was calculated. The researcher visited women 4 weeks and 12 weeks after starting the intervention to complete their hot flash diaries and to collect reports of side-effects or other perceived adverse events other than hypersensitivity to the study medication.
The primary outcome was the percentage of declining in hot flashes frequency from baseline to after treatment that its mean were compared among the three groups using analysis of variance (ANOVA) and Tukey as post-hoc analyses. For comparison of the severity among the three groups, Kruskal-Wallis test with Mann-Whitney as post-hoc analyzes were used. To compare the severity of hot flash before and after treatment in each group, Wilcoxon Test was used.
RESULTS
From April 2008 to February 2009, 100 post-menopausal women were randomized to receive gabapentin 100 mg/day, gabapentin 300 mg/day or conjugated estrogen. Of the 16 women who did not continue the study protocol, 5 dropped out from the gabapentin 100 mg/day arm, 5 dropped out from the gabapentin 300 mg/day arm, and 6 dropped out from the estrogen arm. Dropouts were excluded in the baseline data and in the analysis of primary outcomes.
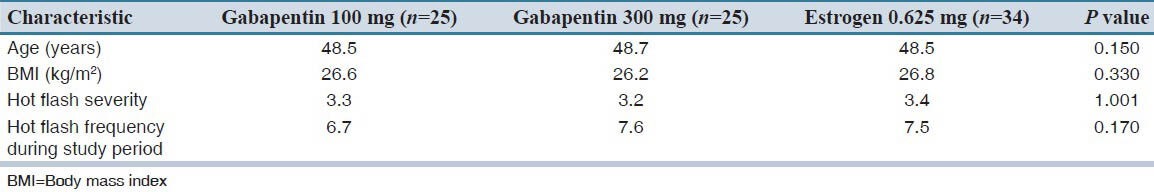
The participants in each group did not differ in baseline characteristics of age, body mass index and baseline hot flash frequency and severity [Table 1]. All women were white, married and some of them were educated beyond high school. All participants were non-smoker and were not taking other non-hormonal treatment at baseline. During the baseline week, women in the three study groups did not differ significantly in hot flash frequency according to ANOVA test (P = 0.170) or severity according to Kruskal-Wallis test (P = 1.001).
Table 1.
Baseline of demographic and clinical characteristics of the studied patients
Changes in frequency and severity of hot flashes from baseline to the end of treatment in each group are presented in Table 2. There was a significant decrease in frequency and severity after treatment in all groups after 4 and 12 weeks as compared to baseline, but, this decrease was statistically more evident for those who received gabapentin 300 mg/day and estrogens.
Table 2.
Patients’ characteristics in frequency and severity of hot flashes post-treatment
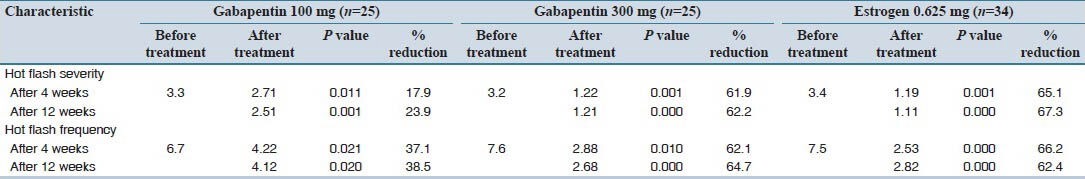
Post-hoc analyzes showed that the frequency and severity of hot flashes were significantly lower in those who received gabapentin 300 mg/day and estrogen. According to Table 2, after 12 weeks, gabapentin 300 mg/day reduced 62.2% in severity and 64.7% in frequency of hot flashes, which is similar to estrogen results with 67.3% reduction in severity and 62.4% in frequency; but those who received gabapentin 100 mg/day had only 23.9% reduction in severity and 38.5% reduction in frequency of hot flashes.
The pattern of adverse events was similar among the two groups. Indeed, no patients had headache, dizziness or disorientation symptoms. The only side-effect was gastrointestinal (GI) tract disturbance in two (8%) patients who received gabapentin 100 mg/day and two (8%) who received gabapentin 300 mg/day.
DISCUSSION
Gabapentin is currently approved as an anticonvulsant and a treatment for post-herpetic neuralgias. Although the precise mechanism of action of gabapentin on hot flashes is unknown, it is thought to act as a gamma-aminobutyric acid (GABA) agonist and affect the temperature regulation by binding to calcium channels. It was designed to cross the blood-brain barrier and mimic the physiologic effects of the neurotransmitter GABA, but it binds to a previously unknown receptor rather than to any of the known GABA receptors.[12,13]
In the present study, gabapentin 300 mg/day significantly decreased hot flash frequency and severity similar to that observed for estrogen. But gabapentin 100 mg/day had mildly decreases on frequency and intensity of hot flash.
Gabapentin approved for the management of seizure disorders and post-herpetic neuralgia, but has been used off-label for other indications as well. Gabapentin appears to be effective in reducing the frequency of hot flashes.[11,14]
Several studies have been conducted on the efficacy and safety of gabapentin in treatment of hot flashes of post-menopausal and other (e.g., breast cancer) origins.[15] The results of this study confirm previous findings that, gabapentin is effective for the reduction of hot flash in post-menopausal women. New findings were that low dose of gabapentin (300 mg/day) appears to be comparable with estrogen, in reducing the frequency and severity of hot flashes with very few adverse effects, GI disturbances.
During the past time, multiple randomized and open-label studies have been performed to evaluate the use of gabapentin in treating menopausal hot flashes. Aguirre et al.,[16] compared gabapentin versus low-dose transdermal estradiol for treating post-menopausal women with moderate to very sever hot flashes. A total of 45 women were prospectively and single blinded randomized to receive oral gabapentin 600 mg/night or transdermal 25 μg/day estradiol/week. Hot flash intensity and frequency significantly decreased for both groups at 1, 4 and 8 weeks of treatment as compared to baseline. Compliance to treatment was 95.6% (gabapentin group) as compared to 90.9% for the estradiol group. In placebo-controlled trial, patients were randomly selected to receive gabapentin 300 mg or placebo 3 times/day for 4 weeks. At week 4, hot-flash composite scores were significantly reduced in the gabapentin group. Regarding individual domains for the menopause-specific quality-of-life score, vasomotor measures significantly improved (P < 0.001), but psychosocial (P = 0.12), physical (P = 0.03), and sexual (P = 0.02) measures did not. Compared with placebo, gabapentin was associated with worsening dizziness (18%), unsteadiness (14%), and drowsiness (12%) at week 1. However, none of the parameters significantly differed on statistical analysis at week 4.[17] In another study, patients were randomly chosen to receive gabapentin as an addition or as a replacement after weaning from the antidepressant.[18] Patients received gabapentin 300 mg once/day for 3 days, twice/day for 3 days, then to a target dose of 3 times/day for 22 days. After 4 weeks, hot-flash composite scores were significantly reduced from baseline scores for gabapentin and gabapentin plus antidepressant. Adverse reactions did not significantly differ between treatment groups, although a trend for increased dizziness, nervousness and negative mood was identified in the gabapentin group. In another small study, Reddy et al.,[19] examined 60 post-menopausal women to assess the efficacy of estrogen and gabapentin in the treatment of moderate-to-severe hot flashes. Participants were randomly assigned to receive either 0.625 mg/day of conjugated estrogens (n = 20), placebo (n = 20), or gabapentin titrated to 2,400 mg/d (n = 20) for 12 weeks. Participants recorded frequency and severity of baseline hot flashes on a hot flash diary for 2 weeks before randomization and for 12 weeks after randomization. At 12 weeks, both estrogen and gabapentin significantly decreased the hot flash composite score compared to placebo. No significant differences in composite scores were observed when estrogen and gabapentin were compared with each other (P = 0.63). Headache, dizziness and disorientation occurred more frequently with gabapentin (50%) than with placebo (20%), but the difference was not statistically significant. Comparing these results with our study, it was interesting in our study that with a lower dose of gabapentin (300 mg/day vs. 2400 mg/day), the efficacy is still comparable to estrogens 0.625 mg/day while very few adverse effects have been occurred.
Doses in the previous studies ranged from 300 to 2400 mg/day for 4-12 weeks. These doses are within the range indicated for the control of epilepsy and post-herpetic neuralgia. Given the schedules for beginning treatment in the study protocols described earlier, it appears reasonable to start gabapentin with 300 mg/day on first day, 300 mg twice/day on day 2, and 300 mg 3 times/day on day 3.[20,21,22,23] This stepwise titration was well-tolerated in clinical studies. It also has the advantage of being simple, yet the dosage can rapidly be escalated to 900 mg/day. These features may contribute to patient adherence and may decrease the risk of dose related adverse effects. In general, 900 mg has been effective in minimizing hot flashes; dosage increases up to 2400 mg/day have demonstrated further decreases in symptoms.[23] To our knowledge, no study has demonstrated the true dose-ranging response with gabapentin. If an increase is indicated, the dose of gabapentin should be increased to achieve the target dose in stepwise fashion or until intolerable adverse effects occur.[24] Anyway, the results of the present study showed that a lower dose of gabapentin can be as effective as estrogen with few side effects and larger doses are not required, though further trials with larger sample sizes in a double-blind fashion should confirm our results. There are some limitation to our study; importantly the study was not double-blind and we did not use placebo control group. The sample size was also small and we only measured frequency and severity as outcome measures. Moreover, this study didn’t have follow-up period.
CONCLUSION
In conclusion, hormone therapy remains the treatment of choice for menopausal women with moderate to severe hot flashes unless appreciable risk of venous thromboembolism, cardiovascular disease or breast cancer exists. Gabapentin 300 mg/day could be useful; however, to relieve hot flashes in women for whom hormone therapy is not suitable or when hot flashes do not respond to other therapies. Nevertheless, side-effects such as drowsiness, dizziness, ataxia, and withdrawal syndrome are of concern with larger doses of Gabapentin but with lower doses (300 mg/day) only GI discomfort may appear. Further, research is needed to determine the effectiveness of Gabapentin use for longer periods or at high doses.
AUTHORS’ CONTRIBUTION
Zahra Allameh carried out the design and coordinated the study. Zahra Allameh, Safoura Rouholamin and Sonia Valaie carried out the interviews with patients and performed clinical follow up. Safoura Rouholamin prepared the first draft of the manuscript and conducted the statistical analysis. All authors have read and approved the content of the manuscript.
Footnotes
Source of Support: Isfahan University of Medical Sciences.
Conflict of Interest: None declared.
REFERENCES
- 1.Shifren Jan L, Schiff I. Menopause. In: Berek JS, Berek DL, Hengst TC, editors. Berek and Novakxs Gynecology. 15th ed. New York: Lippincott Williams and Wilkins; 2012. p. 1234. [Google Scholar]
- 2.Schiff I, Regestein Q, Tulchinsky D, Ryan KJ. Effects of estrogens on sleep and psychological state of hypogonadal women. JAMA. 1979;242:2405–4. [PubMed] [Google Scholar]
- 3.Siddle N, Sarrel P, Whitehead M. The effect of hysterectomy on the age at ovarian failure: Identification of a subgroup of women with premature loss of ovarian function and literature review. Fertil Steril. 1987;47:94–100. doi: 10.1016/s0015-0282(16)49942-5. [DOI] [PubMed] [Google Scholar]
- 4.Freedman RR, Subramanian M. Effects of symptomatic status and the menstrual cycle on hot flash-related thermoregulatory parameters. Menopause. 2005;12:156–9. doi: 10.1097/00042192-200512020-00009. [DOI] [PubMed] [Google Scholar]
- 5.Kronenberg F, Barnard RM. Modulation of menopausal hot flashes by ambient temperature. J of Therm Biol. 1992;17:43–9. [Google Scholar]
- 6.Bachmann GA, Schaefers M, Uddin A, Utian WH. Lowest effective transdermal 17beta-estradiol dose for relief of hot flushes in postmenopausal women: A randomized controlled trial. Obstet Gynecol. 2007;110:771–9. doi: 10.1097/01.AOG.0000284450.51264.31. [DOI] [PubMed] [Google Scholar]
- 7.Notelovitz M, Lenihan JP, McDermott M, Kerber IJ, Nanavati N, Arce J. Initial 17beta-estradiol dose for treating vasomotor symptoms. Obstet Gynecol. 2000;95:726–31. doi: 10.1016/s0029-7844(99)00643-2. [DOI] [PubMed] [Google Scholar]
- 8.Sturdee DW. The menopausal hot flush: Anything new? Maturitas. 2008;60:42–9. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Health. National Institutes of Health State-of-the-Science Conference statement: Management of menopause-related symptoms. Ann Intern Med. 2005;142:1003–13. [PubMed] [Google Scholar]
- 10.Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin's effects on hot flashes in postmenopausal women: A randomized controlled trial. Obstet Gynecol. 2003;101:337–45. doi: 10.1016/s0029-7844(02)02712-6. [DOI] [PubMed] [Google Scholar]
- 11.Brown JN, Wright BR. Use of gabapentin in patients experiencing hot flashes. Pharmacotherapy. 2009;29:74–81. doi: 10.1592/phco.29.1.74. [DOI] [PubMed] [Google Scholar]
- 12.Stearns V. Clinical update: New treatments for hot flushes. Lancet. 2007;369:2062–4. doi: 10.1016/S0140-6736(07)60959-3. [DOI] [PubMed] [Google Scholar]
- 13.Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, et al. Nonhormonal therapies for menopausal hot flashes: Systematic review and meta-analysis. JAMA. 2006;295:2057–71. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 14.Guttuso TJ., Jr Gabapentin's effects on hot flashes and hypothermia. Neurology. 2000;54:2161–3. doi: 10.1212/wnl.54.11.2161. [DOI] [PubMed] [Google Scholar]
- 15.Collaborative Group on Hormonal factors in Breast cancer. Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–59. [PubMed] [Google Scholar]
- 16.Aguirre W, Chedraui P, Mendoza J, Ruilova I. Gabapentin vs. low-dose transdermal estradiol for treating post-menopausal women with moderate to very severe hot flushes. Gynecol Endocrinol. 2010;26:333–7. doi: 10.3109/09513590903511539. [DOI] [PubMed] [Google Scholar]
- 17.Butt DA, Lock M, Lewis JE, Ross S, Moineddin R. Gabapentin for the treatment of menopausal hot flashes: A randomized controlled trial. Menopause. 2008;15:310–8. doi: 10.1097/gme.0b013e3180dca175. [DOI] [PubMed] [Google Scholar]
- 18.Loprinzi CL, Kugler JW, Barton DL, Dueck AC, Tschetter LK, Nelimark RA, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol. 2007;25:308–12. doi: 10.1200/JCO.2006.07.5390. [DOI] [PubMed] [Google Scholar]
- 19.Reddy SY, Warner H, Guttuso T, Jr, Messing S, DiGrazio W, Thornburg L, et al. Gabapentin, estrogen, and placebo for treating hot flushes: A randomized controlled trial. Obstet Gynecol. 2006;108:41–8. doi: 10.1097/01.AOG.0000222383.43913.ed. [DOI] [PubMed] [Google Scholar]
- 20.Pandya KJ, Morrow GR, Roscoe JA, Zhao H, Hickok JT, Pajon E, et al. Gabapentin for hot flashes in 420 women with breast cancer: A randomised double-blind placebo-controlled trial. Lancet. 2005;366:818–24. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albertazzi P, Bottazzi M, Purdie DW. Gabapentin for the management of hot flushes: A case series. Menopause. 2003;10:214–7. doi: 10.1097/00042192-200310030-00007. [DOI] [PubMed] [Google Scholar]
- 22.Bunyarataveij N, Songpatanaslip T. Application of Gabapentin in Thia women with menopausal syndrome. J Med Assoc Thia. 2005;88:521–3. [PubMed] [Google Scholar]
- 23.Toulis KA, Tzellos T, Kouvelas D, Goulis DG. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: A systematic review and meta-analysis. Clin Ther. 2009;31:221–35. doi: 10.1016/j.clinthera.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Hayes LP, Carroll DG, Kelley KW. Use of gabapentin for the management of natural or surgical menopausal hot flashes. Ann Pharmacother. 2011;45:388–94. doi: 10.1345/aph.1P366. [DOI] [PubMed] [Google Scholar]


