Abstract
Objective:
The purpose of this study is to analyze the antibiotic sensitivity pattern of microorganisms, to study the antibiotic usage pattern, and to conduct a cost-effectiveness analysis (CEA) for the antibiotics prescribed in a tertiary care teaching hospital in south India.
Methods:
This prospective study was carried out in the General Medicine and Pulmonology departments of the hospital for a period of 6 months. The study was carried out in three phases: A prospective analysis to check the sensitivity pattern of microorganisms to various antibiotics, data extraction and determining the cost of antibiotics and finally evaluation of the sensitivity pattern of microorganisms and the antibiotic usage. A total of 796 documented records were analyzed.
Findings:
It was found that Escherichia coli was the major organism identified in 36.4% of the isolated specimens, followed by Klebsiella sp. (18.9%), Streptococcus pneumoniae (15.8%), Staphylococcus aureus (12.4%), and Pseudomonas (9.3%). The sensitivity pattern data of the prospective study revealed that E. coli was highly sensitive to Amikacin (99.3%), Klebsiella to Amikacin (93.8%), Pseudomonas to Meropenem (97.6%), and S. pneumoniae to Ofloxacin (93.8%). In the prescribing pattern study, it was found that the most common disease (21.2%) was found to be lower respiratory tract infection in 51 patients. Cephalosporins (73%), in particular Ceftriaxone (63.5%) was highly prescribed, followed by fluoroquinolones (53.9%). In the CEA, it was revealed that Ceftriaxone was the cost-effective antibiotic with a cost-effectiveness ratio (CER) of 78.27 compared to Levofloxacin, which had a CER of 95.13.
Conclusion:
Continuous surveillance of susceptibility testing is necessary for cost-effective customization of empiric antibiotic therapy. Furthermore, reliable statistics on antibiotic resistance and policies should be made available.
Keywords: Antibiotics, cost-effectiveness analysis, prescribing pattern, sensitivity
INTRODUCTION
Anti-microbial resistance patterns can vary regionally and even among different hospitals within the same community. Infections are the most common reasons for patients to seek medical advice and for antibiotics to be prescribed.[1] Inappropriate or indiscriminate use of antibiotics can increase the cost of care by increasing drug cost, increasing toxicity, increasing resistance, and increasing laboratory costs. Prophylactic antibiotic use in some hospitals remains a problem.[2] Antibiotics are prescribed unnecessarily and empirically for complaints where no antibiotic is required or where culture and sensitivity results could be safely awaited.[3] The key action by the clinician should be the provision of a specimen for accurate identification of the offending pathogen by means of culture and sensitivity method.[4] The pharmacist can present information at the point of care regarding antibiotic susceptibility and individual patient factors to improve antibiotic prescribing. The pharmacist can play a significant role in recommending the prescriber about the necessary changes to be made in the patient regimen, dose, and duration of antibiotic therapy. The costs of drug therapy are increasing dramatically, especially as new products, derived from biotechnology, are introduced. Cost is one among the various factors to be taken into account in antibiotic prescribing.[5] Cost-effectiveness analysis (CEA) evaluates the relative costs and benefits of different medical technologies, procedures or clinical strategies as measured in physical units, for example, lives saved or reduced morbidity.[6] CEA is considered to be the most appropriate method for the evaluation of health economics when at least two alternatives are being compared and when outcomes can be expressed in a common unit, such as cost per life years saved.[7] Thus, the purpose of this study is to conduct a detailed study on the sensitivity pattern of microorganisms, to analyze the antibiotic usage pattern, and to conduct a CEA for the antibiotics prescribed.
METHODS
This prospective cross-sectional study was carried out in a 700-bedded multi-specialty private corporate hospital in South India during 6 months. All patients hospitalized in the General Medicine and Pulmonology departments for whom at least one antibiotic was prescribed were included in the study. The study protocol was submitted to the Dean of the study hospital, Coimbatore. The authorization from the Dean was procured. The author was permitted to utilize the hospital facilities to make a follow-up of the prescriptions in the selected department. A specially designed format was used for entering the prevalence and sensitivity pattern of microorganisms among the patients during the study period. A separate data entry format was designed for noting the pattern of antibiotic use.
The study was carried out in three phases. The first phase involves a prospective analysis to check the sensitivity pattern of microorganisms to various antibiotics for a 6 month period. The documented data were reviewed and necessary information such as specimen collected, organism isolated, and their sensitivity pattern were noted down. During the 2nd phase, information regarding the pattern of antibiotics prescribed in the Pulmonology and General Medicine departments and also the cost of the antibiotics were obtained. During the final phase, the sensitivity pattern of microorganisms and the antibiotic usage pattern were analyzed in detail.
A CEA was conducted by calculating the cost per failure avoided to find out the most cost-effective antibiotic in the Pulmonology and General Medicine departments. A decision tree was created on the basis of the data collected and this tree was used to determine the expected value (anticipated therapeutic cost per patient) for each antibiotic prescribed. Using the therapeutic effect of the antibiotic against infection and the anticipated therapeutic cost per patient, the cost-effectiveness ratio (CER) was calculated. The antibiotic with the lower CER was found to be the most cost-effective antibiotic.
RESULTS
During the first phase, a total of 796 documented records were analyzed. Escherichia coli was the major organism isolated in 36.4% of the specimens, followed by Klebsiella sp. (18.9%), Streptococcus pneumonia (15.8%), Staphylococcus aureus (12.4%), and Pseudomonas (9.3%) [Table 1]. Urine, sputum, and pus cells were the major specimen samples collected. E. coli was more common in urine (78.6%), Streptococcus pneumoniae was found extensively in sputum (76.2%), and Proteus sp. was more common in urine (82.3%) samples [Figure 1].
Table 1.
Sensitivity pattern studies of antibiotics
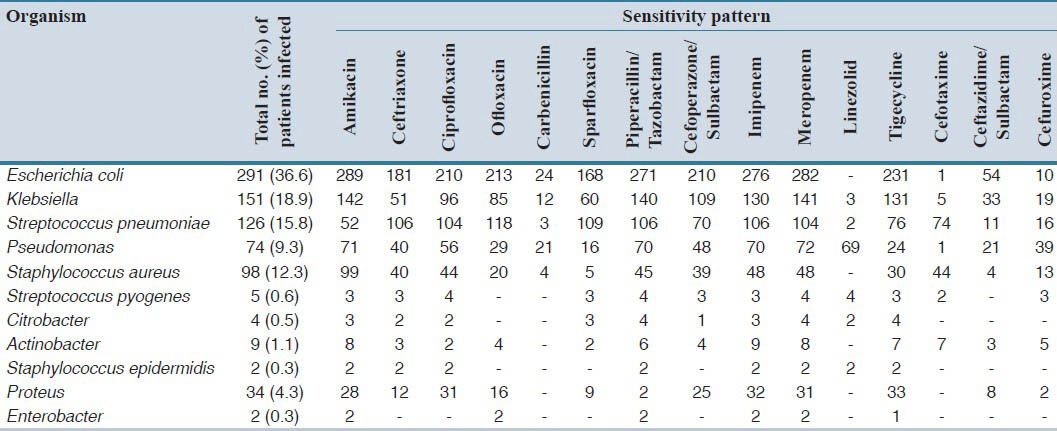
Figure 1.

Percentage of microorganisms found in different patients’ specimens (n = 796)
The prospective data revealed that almost all the organisms isolated were highly sensitive to Amikacin. It was found that Amikacin showed the best sensitivity in S. aureus (100%), E. coli (99.3) Klebsiella species (93.8%), and Pseudomonas (96.3%) [Figure 2]. Proteus showed high sensitivity toward Tigecycline (95.8%) and Actinobacter showed high sensitivity toward Meropenem (91.9%) [Table 1].
Figure 2.

Percentage of microorganisms’ sensitivity to different antibiotics (n = 796)
Phase II of the study was to collect information on the antibiotic prescribing pattern along with the cost of antibiotics from the General Medicine and Pulmonology wards for 6 months period. Lower respiratory tract infections were the major diseases for which antibiotics were prescribed (21.2%). Cephalosporins were the major category of antibiotics prescribed (73%), followed by fluoroquinolones (53.9%) [Figure 3].
Figure 3.

Major antibiotics prescribed for treating infections in general medicine and pulmonology departments (n = 241)
The third phase involved the CEA of the antibiotics prescribed. For CEA, decision tree was created on the basis of the data collected [Figure 4]. The decision tree was used to determine the expected value. One hundred fifty three patients received Ceftriaxone of which 112 treatments (73.2%) were successful. Using the drug cost only, the average cost per patient in this path was 0.92 United States dollars (USD). Forty one patients in the Ceftriaxone arm failed therapy and were switched over to either Levofloxacin or Amikacin. Ninety eight patients received Levofloxacin. The total anticipated therapeutic cost per patient is calculated on the basis of the decision-tree model, which was found out to be 1.06 USD for the Ceftriaxone group and 1.77 USD for the Levofloxacin group. The CER was calculated to be 1.45 for Ceftriaxone group and 1.77 for the Levofloxacin group. CER suggests that Ceftriaxone is the most cost-effective antibiotic at our institution. The results were limited to the drug acquisition cost only and revealed that Ceftriaxone is a cost-effective alternative to Levofloxacin in so far as the only drug cost was considered.
Figure 4.

Decision tree for cost-effectiveness analysis (USD: United States Dollar)
DISCUSSION
Hospital anti-biograms can be a useful means for guiding empiric therapy and tracking the emergence of resistance among bacterial isolates, as it is shown in the present study. Similar study was conducted by Gayathri et al.,[8] on antibiotic susceptibility pattern of rapidly growing mycobacterium. Out of the 148 rapidly growing mycobacterium isolates, 146 (98%) were susceptible to Amikacin, 138 (91%) to Gatifloxacin, 132 (87%) to Moxifloxacin, 122 (76%) to ciprofloxacin, and 116 (74%) to Norfloxacin. In the other study conducted by Perveen et al.,[9] on the prevalence and antimicrobial susceptibility pattern of Methicillin-Resistant Staphylococcus aureus (MRSA) and Methicillin-Resistant Coagulase-Negative Staphylococci (MRCoNS), out of the total 350 staphylococcal isolates from different clinical specimens, 148 isolates (60.40%) were identified as MRSA and 46 isolates (43.80%) were screened as MRCoNS. All isolates of MRSA and MRCoNS were multi-drug resistant. Antibiotic resistance pattern of these isolates was high against penicillin, whereas all the MRSA strains were resistant to penicillin and oxacillin (100%). The MRCoNS strains also showed closely similar drug resistance pattern with 97.82% isolates being resistant to penicillin. However, all the MRSA and MRCoNS isolates were uniformly susceptible to vancomycin. Chloramphenicol and rifampicin also showed excellent activity against methicillin-resistant isolates. This study indicated a high level prevalence of MRSA and MRCoNS strains resistance against widely used antimicrobial agents. Hoogendoorn et al.,[10] performed a study on Prevalence of Antibiotic Resistance of the Commensal Flora in Dutch Nursing Homes. A total of 125 patients were included in the study. The resistance and intermediate susceptibility of E. coli varied from 4% (ceftriaxone) to 43% (amoxicillin). Extended spectrum β-lactamase-producing Enterobacteriaceae were found in 6% of the patients. Amoxicillin and/or co-amoxiclav users were significantly more resistant to these antibiotics (69%) than non-users (38%). Antibiotic use was associated with antibiotic resistance of E. coli.
The present study also analyzed the data obtained for any changes in the sensitivity pattern of microorganisms and the pattern of antibiotic use in the study department. This phase includes the CEA on the antibiotics prescribed. Ceftriaxone was the cost-effective alternative to levofloxacin with a CER of 78.27. Similar study was conducted by Lavoie et al,[11] on the cost-effectiveness of antibiotics used for community acquired pneumonia and acute exacerbation of chronic bronchitis. The study was conducted on 3,610 patients and it revealed that Azithromycin, which is widely prescribed antibiotic, appears to be the most cost-effective treatment strategy for lower respiratory tract infections.
Continuous surveillance of susceptibility testing is necessary for cost-effective customization of empiric antibiotic therapy. Furthermore, reliable statistics on antibiotic resistance and policies that are mandatory to control spread of resistant pathogens should be made available. Clinical pharmacists play a significant role in promoting optimal antibiotic prescribing practice among physicians, during their routine visit toward.
AUTHORS’ CONTRIBUTION
All authors of this article made substantial contributions to conception and design, and/or acquisition of data. S. Sriram, V. Aiswaria, A. Cijo and T. Mohankumar analyzed and interpreted data. Also participated in drafting the article, revising it critically for important intellectual content; and all authors gave final approval of the version to be submitted and any revised version.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Little P, Watson L, Morgan S, Williamson I. Antibiotic prescribing and admissions with major suppurative complications of respiratory tract infections: A data linkage study. Br J Gen Pract. 2002;52:187–90. 193. [PMC free article] [PubMed] [Google Scholar]
- 2.Braden RL. Surgical antibiotic prophylaxis. In: Herfindale ET, Gourley DR, editors. Textbook of Therapeutics: Drug and Disease Management. 6th ed. Maryland, USA: Williams and Wilkins; 1996. [Google Scholar]
- 3.Abate BJ, Barriere SL. Antimicrobial regimen selection. In: Dipiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: A Pathophysiologic Approach. 5th ed. New York: Mc Graw-Hill; 1993. [Google Scholar]
- 4.Barclay L. Resistance to antibiotics prescribed in primary care may last up to 12 months. Medscape. [Last accessed 2013 May 02; 2010 May 20]. Available from: http://www.medscape.com/viewarticle/722032 .
- 5.Kerr JR, Barr JG, Smyth ET, O’Hare J, Bell PM, Callender ME. Antibiotic pharmacoeconomics: An attempt to find the real cost of hospital antibiotic prescribing. Ulster Med J. 1993;62:50–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke J. Pharmacoeconomics. In: Roger W, Clive E, editors. A Textbook of Clinical Pharmacy and Therapeutics. 3rd ed. Spain: Churchill Livingstone; 2003. [Google Scholar]
- 7.Chisin R. Cost-effectiveness analysis. J Nucl Med. 2009;50:338–9. doi: 10.2967/jnumed.108.057489. [DOI] [PubMed] [Google Scholar]
- 8.Gayathri R, Therese KL, Deepa P, Mangai S, Madhavan HN. Antibiotic susceptibility pattern of rapidly growing mycobacteria. J Postgrad Med. 2010;56:76–8. doi: 10.4103/0022-3859.65278. [DOI] [PubMed] [Google Scholar]
- 9.Perveen I, Majid A, Knawal S, Naz I, Sehar S, Ahmad S, et al. Prevalence and antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococci in Rawalpindi, Pakistan. Br J Med Med Res. 2013;3:198–209. [Google Scholar]
- 10.Hoogendoorn M, Smalbrugge M, Stobberingh EE, van Rossum SV, Vlaminckx BJ, Thijsen SF. Prevalence of antibiotic resistance of the commensal flora in dutch nursing homes. J Am Med Dir Assoc. 2013;14:336–9. doi: 10.1016/j.jamda.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Lavoie F, Blais L, Castilloux AM, Scalera A, LeLorier J. Effectiveness and cost-effectiveness of antibiotic treatments for community acquired pneumonia (CAP) and acute exacerbations of chronic bronchitis (AECB) Can J Clin Pharmacol. 2005;12:e212–7. [PubMed] [Google Scholar]


