Abstract
Neutrophils are multifaceted cells that are often the immune system’s first line of defense. Human and murine cells release extracellular DNA traps (ETs) in response to several pathogens and diseases. Neutrophil extracellular trap (NET) formation is crucial to trapping and killing extracellular pathogens. Aside from neutrophils, macrophages and eosinophils also release ETs. We hypothesized that ETs serve as a mechanism of ensnaring the large and highly motile helminth parasite Strongyloides stercoralis thereby providing a static target for the immune response. We demonstrated that S. stercoralis larvae trigger the release of ETs by human neutrophils and macrophages. Analysis of NETs revealed that NETs trapped but did not kill larvae. Induction of NETs was essential for larval killing by human but not murine neutrophils and macrophages in vitro. In mice, extracellular traps were induced following infection with S. stercoralis larvae and were present in the microenvironment of worms being killed in vivo. These findings demonstrate that NETs ensnare the parasite facilitating larval killing by cells of the immune system.
Keywords: Neutrophils, Extracellular traps, NET, Mice, Human, Strongyloides stercoralis
1. Introduction
Extracellular traps (ETs) are produced by several immune cells in humans and mice including neutrophils [1], eosinophils [2], mast cells [3] and monocytes/macrophages [4]. The release of ETs is a distinct process of cell death termed ‘Etosis’ [5] that results in extrusion of a fibrous network of nuclear [1] or mitochondrial DNA [6], histones and a concentration of granular proteins. Neutrophil extracellular traps (NETs) have been implicated in a growing number of human diseases with beneficial roles in bacterial and fungal infections [7] and deleterious roles in preeclampsia [8], cystic fibrosis [9], septic shock [10] and autoimmunity [11,12]. Human NETs are capable of both trapping and killing pathogenic bacteria such as Staphylococcus species, Salmonella typhimurium [1,13], Escherichia coli [13] and fungi such as Candida albicans [14]. Conversely, mouse NETs are associated with trapping of pathogens such as Streptococcus pneumonia [15], C. albicans and Listeria monocytogenes [16]. The antimicrobial activity of NETs can be attributed to NET-bound histone 2a [1] or concentrated granular proteins such as MPO [17]. NETs also modulate protozoan infections, trapping and killing Leishmania amazonensis promastigotes [18] while the promastigotes of Leishmania donovani are captured but not killed [19]. The protozoan parasite Toxoplasma gondii induces human and mouse neutrophils to release NETs that have microbicidal effects [20]. Yet, a role for NETs during parasitic helminth infections has not been described.
Strongyloides stercoralis, a parasitic nematode of humans, primates and canines, infects an estimated 60–100 million people worldwide [21,22]. The protective innate and adaptive immune response to this parasite has been studied in mice [23]. Neutrophils, eosinophils and macrophages are found in the parasite microenvironment in high numbers in naïve mice [24]. Neutrophils and eosinophils are directly recruited to the parasite microenvironment [25–27], with neutrophils recruited in a CXCR2 [26] and Gαi2 [25] dependent manner. MPO from neutrophils is required for larval killing during innate and adaptive immune responses [24,28]. Alternatively activated macrophages also can kill S. stercoralis larvae in the innate and adaptive responses. In vitro killing by alternatively activated macrophages requires collaboration with neutrophils and complement [29]. These results were confirmed using human neutrophils, macrophages and complement, which as a combination also killed the worms [29]. Although the triad of neutrophils, macrophages and complement from humans and mice can kill the worms, it is unknown if they use the same mechanism.
The goal of this study was to determine the role of ETs from humans and mice in the killing of S. stercoralis larvae. It was hypothesized that ETs serve as a mechanism to trap or impede larval movement allowing immune cells to migrate to the microenvironment of the worm and kill it. In vitro assays demonstrate that ET formation is required for human neutrophils, macrophages and complement to kill the worms but not for the mouse triad to kill the parasite. However, in vivo results demonstrate that mice do produce ETs in response to infection with S. stercoralis and NETs are associated with trapping and killing the parasites in vivo.
2. Materials and methods
2.1. Mice and parasites
Wild type C57BL/6J mice, 6–8 weeks old, were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were housed in ventilated cages in a pathogen-free facility at Thomas Jefferson University (Philadelphia, PA). All experiments were in compliance with the guidelines set forth by the Institutional Animal Care and Use Committee at Thomas Jefferson University. S. stercoralis infective larvae (L3) were obtained from cultures made with the stool of infected laboratory dogs housed at the University of Pennsylvania School of Veterinary Medicine. The larvae were collected as previously described [30] and washed five times in NI medium (1:1 mixture of NCTC-135 and Iscove’s modified Dulbecco’s media, Sigma, St. Louis, MO) supplemented with 100 U/ml penicillin plus 100 μg/ml streptomycin (Corning Cellgro, Manassas, VA), 0.1 mg/ml gentamicin (Life Technologies, Grand Island, NY), and 0.25 mg/ml levofloxacin (Ortho-McNeil, Raritan, NJ).
2.2. Macrophage culture and differentiation
Human macrophages were generated as previously described [29] from CD34− negative stem cell fractions. Briefly, the CD34− fractions were lysed of erythrocytes, using lysis buffer (BD Biosciences, San Jose, CA), prior to monocyte adherence for 2 h at 37 °C on tissue culture treated petri dishes. The plates were then gently washed to remove nonadherent cells and the monocytes were allowed to mature into macrophages in medium containing 5% human serum (Cedarlane, Burlington, NC) and 20 ng/mL of human M-CSF (eBiosciences, San Diego, CA). The macrophages were harvested for in vitro experiments on day 7 using TrypLE (Life Technologies). All human studies were in compliance with the guidelines set forth by the Institutional Review Board at Thomas Jefferson University. Murine macrophages were cultured as previously described [29] by isolating bone marrow from the femurs and tibias of naïve mice. Erythrocytes were eliminated from the single-cell suspension by hypotonic lysis, and cells were cultured in DMEM containing L-glutamine supplemented with 25% heat inactivated fetal bovine serum (Hyclone, Thermo Fisher Scientific, Waltham, MA), 25% L929 cell (ATCC, Manassas, VA) conditioned medium as a source of macrophage colony-stimulating factor [31], sodium pyruvate, 100 U/ml penicillin plus 100 μg/ml streptomycin, and 50 μM 2-ME. Medium was replaced on day 3 and macrophages were harvested for in vitro experiments on day 5.
2.3. Neutrophil isolation
Human neutrophils were isolated from the blood of healthy donors as previously described [29]. In brief, the blood was layered on a Lympholyte-poly column (Cedarlane, Burlington, NC), following centrifugation the neutrophil layer was collected and lysed of contaminating erythrocytes resulting in neutrophil purity of greater than 90%. Murine neutrophils were isolated as previously described [28] by harvesting bone marrow from the femurs, tibias and humeri of C57BL/6J mice. The cells were resuspended in a 45% Percoll solution and layered onto a Percoll gradient column of 80% and 62% Percoll. The cell layer between the 80% and 62% Percoll interface was then incubated with anti-B220 and anti-Thy1.2 MACS beads (Miltenyi Biotec, Auburn, CA) prior to negative selection using MACS LS columns (Miltenyi Biotec). To eliminate monocytes, the cells were plated on untreated dishes for 1 h at 37 °C in RPMI supplemented with 10% (FBS) (Corning Cellgro). The nonadherent cells were removed and the percentage of neutrophils determined. The purity of the neutrophil preparations was typically greater than 95%.
2.4. In vitro killing assay
Human or mouse neutrophils (4 × 106) and macrophages (1 × 106) were plated in 96-well plates with 50 larvae with fresh or frozen autologous serum (25% total volume). In vitro assays were performed for 48 h (for human cells) and 24 h (for mouse cells). DNase I (Worthington, Freehold, NJ) was added to select wells at the start of each experiment at a concentration of 100 U/mL. Larval survival was determined based on morphology and motility of larvae. The viability of neutrophils and macrophages, not found within the fibrous network, from the in vitro killing assays were uniformly greater than 90% [29].
2.5. Visualization of extracellular DNA
Human neutrophils (2 × 106) were plated overnight on glass bottom microwell dishes (MatTek, Ashland, MA) coated with 50 μg/ml poly-L lysine. After 2 h of incubation with 1000 dead larvae, the neutrophils were fixed with 4% paraformaldehyde for 15 min. Dead larvae were used as movement of live larvae disrupted the fine structure of NETs in the assay. The neutrophils were then washed with PBS, and stained for 1hr with rabbit polyclonal anti-histone 3 (Abcam, Cambridge, MA) and mouse monoclonal anti-MPO (Santa Cruz Biotechnology, Dallas, TX) primary antibodies in blocking buffer (20% goat serum and 5 mg/ml BSA in PBS). Following several washes with PBS, the cells were incubated with Hoechst (Life Technologies), goat anti-mouse AlexaFluor594- and goat anti-rabbit AlexaFluor488-conjugated antibodies (Life Technologies) for 1 h. The coverslips were then washed and the presence of NETs was analyzed on a Zeiss LSM 510 Meta Confocal Laser Scanning Microscope (Carl Zeiss, Oberkochen, Germany). Image analysis was performed using ImageJ (NIH, Bethesda, MD).
2.6. Quantification of extracellular DNA
Human and mouse neutrophils (1 × 105) were cultured with normal or heat inactivated (56 °C/30 min) human or mouse serum and stimulated with 100 larvae or 100 nM phorbol myristate acetate (PMA) for 3, 6, 24 and 48 h in vitro. The culture supernatants were collected and the extracellular DNA was quantified using the Quant-iT PicoGreen® dsDNA Assay kit (Life Technologies), following manufacturer instructions. Samples were cultured with the PicoGreen reagent (1:1 dilution) for 5 min. The samples were measured with a spectro-fluorometer at 480 nm excitation and 520 nm emission. A DNA standard curve was used to determine the concentration of Free DNA from in vitro and in vivo samples. In vivo extracellular DNA was measured following injection of NI culture medium or 10,000 live larvae into the peritoneal cavity of naïve C57BL/6J mice. Peritoneal lavages were performed 3 h after the larvae were injected; lavage fluids were centrifuged prior to PicoGreen assay.
2.7. Light and immunofluorescence microscopy
Neutrophils and larvae were cultured in vitro for 24 h and imaged using a light microscope or Zeiss Axiovert 200M inverted microscope with environmental chamber (Carl Zeiss). The movie was imaged during the final 2 min of a 10 min incubation with 100 U/ml DNase I. Immunofluorescent imaging of Sytox Green stained clots were performed by fixing diffusion chamber contents in 2% para-formaldehyde (PFA) followed by staining in 1:1000 fold dilution of Sytox Green according to manufacturer instructions. Diffusion chamber contents were placed in 8-well chamber slides (LabTek, Scotts Valley, CA) and images were acquired using an Andor/Nikon TiE inverted microscope with PFS for image stability control (Nikon, Melville, NY) at room temperature and MetaMorph v7.6.5 software (Molecular Devices, Sunnyvale, CA).
2.8. Diffusion chambers
Diffusion chambers were constructed as previously described [28]. Briefly, cell impermeable diffusion chambers were constructed using 0.1 μm-pore-size membranes glued to 14 mm Lucite rings (Millipore, Billerica, MA). Cell impermeable chambers containing 50 larvae and mouse neutrophils (2 × 106), as indicated, were surgically implanted subcutaneously in the dorsal flank of naïve mice. All surgical procedures were performed on mice anesthetized with isoflurane (Webster Veterinary, Webster, NY).
2.9. Statistics
In vivo experiments consisted of at least 5 mice per group, unless otherwise stated, and independent experiments were performed at least twice, with each experiment having similar outcomes. Data are presented as mean ± standard deviation. Statistical analysis was by multivariate general linear hypothesis ANOVA using the SYSTAT (version 11) software (Systat Software Inc, Chicago, IL). Fisher’s least significant difference test was performed for post hoc analysis, and probability values less than 0.05 were considered significant.
3. Results
3.1. Human neutrophils release NETs following exposure to larvae of S. stercoralis
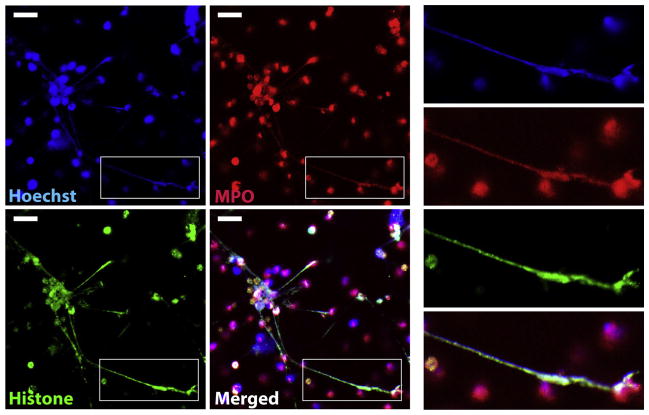
Dead larvae were cocultured with human neutrophils for 2 h and then stained with Hoechst to label DNA to determine if S. stercoralis was capable of inducing NETs. Confocal microscopy revealed the release of DNA from S. stercoralis-stimulated human neutrophils (Fig. 1, Hoechst). To further characterize the NETs, extracellular DNA was labeled for histone and the granular protein MPO. Similar to those induced by bacteria [1], the NETs released from human neutrophils cocultured with S. stercoralis also stained positive for both MPO and histone (Fig. 1, MPO and Histone).
Fig. 1.
Human neutrophils release NETs following exposure to S. stercoralis larvae. Human neutrophils (2 × 106) were exposed to 1000 dead larvae for 2 h at 37 °C. Cells were labeled with Hoechst (Blue) for DNA, anti-MPO (Red) and anti-histone (Green) to visualize NETs. Inserts on the right depict an enlarged NET co-labeled with Hoechst, anti-MPO, and anti-histone. Scale bar = 20 μm.
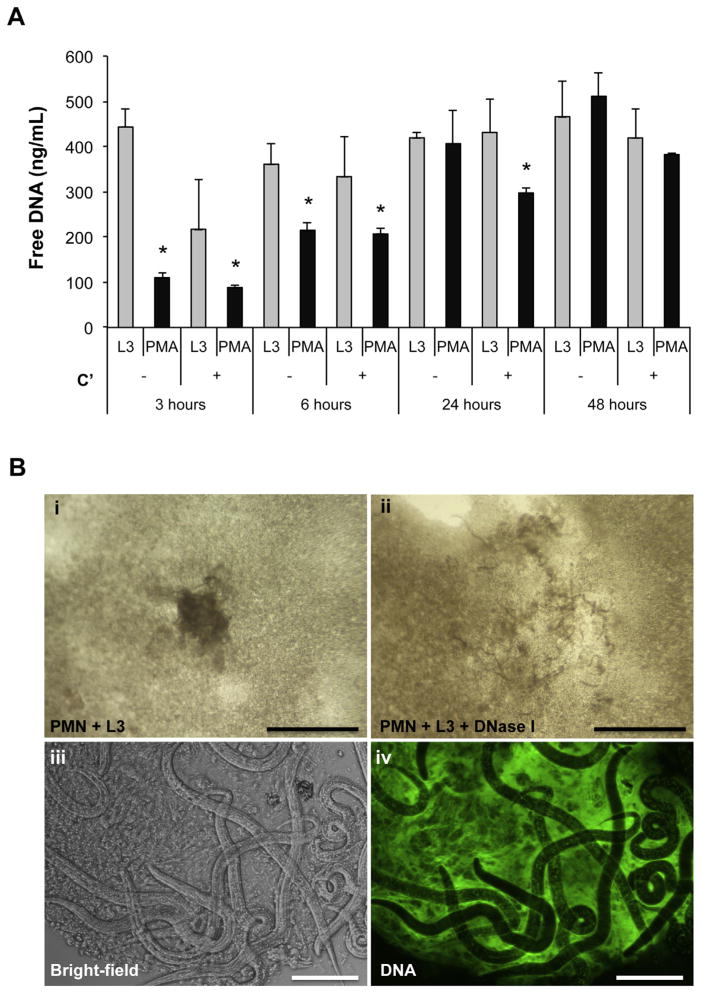
The capacity of human neutrophils to release NETs following exposure to the S. stercoralis larvae was further evaluated by in vitro stimulation of human neutrophils with live larvae or PMA for 3, 6, 24 and 48 h in the presence or absence of active complement components. Neutrophils exposed to live larvae triggered a rapid and significant NET release after only 3 h of stimulation (Fig. 2A). The level of free DNA released into the culture supernatant by neutrophils exposed to larvae remained elevated throughout the course of the experiment. Free DNA release by neutrophils stimulated with PMA increased overtime until 24 h, when the DNA levels in the supernatant were equivalent to levels from larval exposed neutrophils. Furthermore, the release of free DNA following both larvae and PMA stimulation occurred irrespective of complement activity.
Fig. 2.
Human neutrophils release NETs that trap the larvae of S. stercoralis. A) Human neutrophils (1 × 105) were stimulated ex vivo with 100 larvae (L3) or PMA in the presence of autologous human serum as a complement source (C′) or heat inactivated serum for 3, 6, 24 and 48 h. The culture supernatants were analyzed for extracellular DNA using the PicoGreen assay. Data are shown as mean ± SD. B) Human neutrophils, larvae and autologous human serum were cocultured for 24 h in vitro. Cultures revealed live larvae trapped within clots in wells containing only PMN and L3 (i, iii). The resulting clots were then extracted and stained with Sytox Green, a cell impermeable DNA dye, to visualize NET formation using a fluorescent microscope for Bright-field and fluorescent DNA (Green), (iv). Scale bars = 100 μm for (iii–iv). The larvae containing clots completely dissolved within 10 min of treatment with 100 U/ml of DNase I (ii). Scale bars = 6 mm for (i–ii).
Observation of cultures with human neutrophils exposed to live larval S. stercoralis revealed that within 24 h distinct clot-like structures were clearly visible without magnification (Fig. 2B-i). Live larvae could be seen moving within the clots (Supplemental Fig. 1S, Movie). Examination of the neutrophil-derived clots by fluorescent microscopy revealed positive staining with the cell impermeable DNA dye Sytox Green (Fig. 2B-iii–iv), which stains the extracellular DNA backbone on NETs [1]. To confirm that the clots were indeed NETs the clots containing larvae were treated with DNase I to disrupt the DNA [1]. After 10 min of exposure to DNase I, the clots dissolved, releasing the trapped larvae (Fig. 2B-ii and Supplemental Fig. 1S, Movie), demonstrating that human neutrophils extrude DNA, in a manner consistent with NETs, which trap live larvae in vitro. These findings indicate that S. stercoralis larvae induce human neutrophils to release NETs that ensnare larvae in vitro.
Supplementary video related to this article can be found at http://dx.doi.org/10.1016/j.micinf.2014.02.012.
3.2. Human ETs are required for larval killing by neutrophils and macrophages in vitro
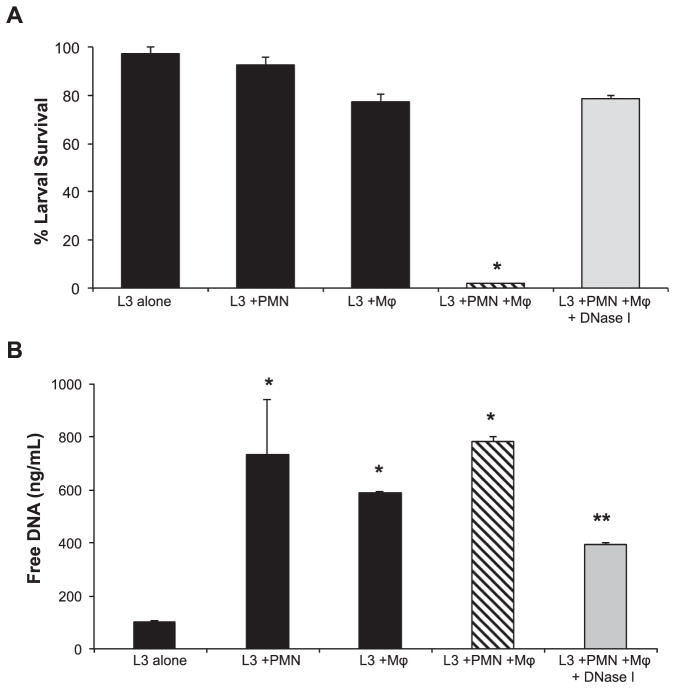
Purified human neutrophils and macrophages collaborate to kill 90% of larvae when cultured in the presence of complement from normal human serum. Culture with only neutrophils or macrophages did not kill the larvae. The role of human ET formation during the in vitro larval killing process was examined by treating cultures with DNase I to dissolve the NETs. Addition of DNase I to human neutrophils and macrophages in vitro resulted in only 20% killing of larvae (Fig. 3A). Culture well supernatants were examined using PicoGreen assays to detect free DNA as an indicator of ET formation. Wells with larvae, where neutrophils and macrophages were cultured individually or in combination, had significant levels of free DNA. The quantity of free DNA was significantly lower in cultures where killing by the combination of macrophages and neutrophils was blocked by DNase I treatment (Fig. 3B). These findings demonstrate that ETs are vital to the in vitro larval killing mechanism used by human neutrophils and macrophages and that both neutrophils and macrophages release DNA. Furthermore, it appears that ETs ensnare the parasites thereby facilitating the larval killing process by human neutrophils, macrophages and complement.
Fig. 3.
NETs are required for larval killing by human neutrophils in vitro. A) Human macrophages (Mϕ) and neutrophils (PMN) cultured in vitro with 50 larvae (L3) for 48 h. The cultures were treated with 100 U/ml of DNase I to block NET formation and assessed for larval killing. Data are shown as mean ± SD. *, p < 0.001 when compared with all other groups. B) Supernatants from the in vitro assay were analyzed for extracellular DNA using the PicoGreen assay. Data are shown as mean ± SD. *, p < 0.001 compared to L3 alone group or **, p < 0.001 compared to the L3 + PMN + Mϕ group. Each group was performed in triplicate for at least two independent experiments.
3.3. Mouse NETs are not required for larval killing by neutrophils and macrophages in vitro
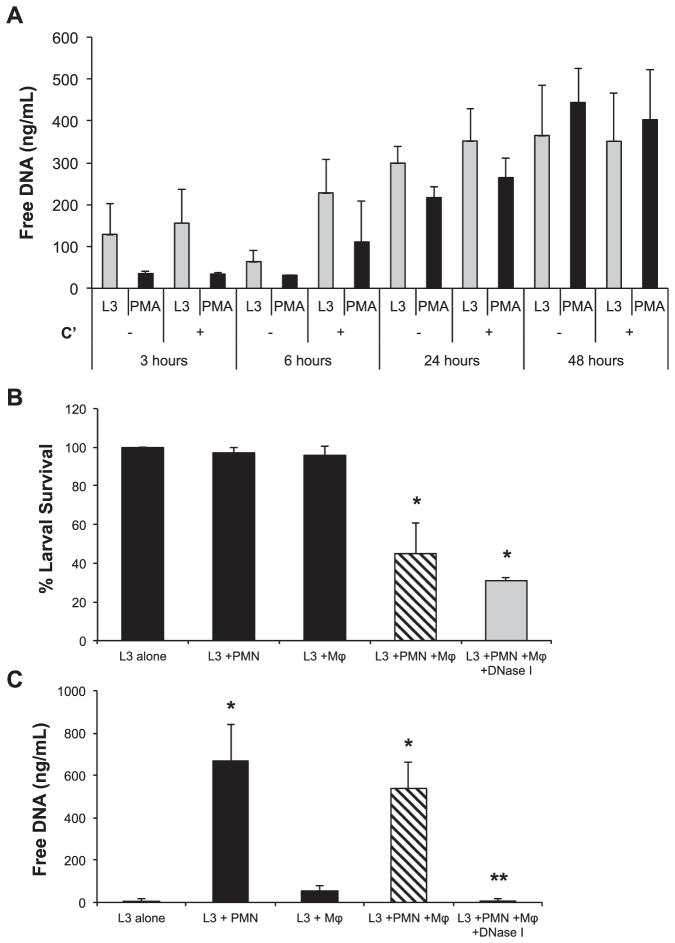
Neutrophils were isolated from the bone marrow of naïve mice and placed in culture with larvae of S. stercoralis. Unlike human neutrophils, mouse neutrophils did not form visible clots in vitro. When mouse neutrophils were cultured with larvae or PMA for 3, 6, 24 and 48 h, the amount of free DNA for both larvae and PMA stimulated cells were equivalent and increased overtime (Fig. 4A). As with human neutrophils, complement activity had no effect on the release of free DNA by mouse neutrophils in vitro. Thus, mouse neutrophils like human neutrophils, are induced by live S. stercoralis larvae to release NETs.
Fig. 4.
Mouse NETs are not required for larval killing in vitro. A) Mouse neutrophils (1 × 105) were stimulated ex vivo with 100 larvae (L3) or PMA in the presence of mouse serum as a complement source (C′) or heat inactivated serum for 3, 6, 24 and 48 h. The culture supernatants were analyzed for extracellular DNA using the PicoGreen assay. Data are shown as mean ± SD. B) Mouse bone marrow-derived macrophages (Mϕ) were cultured with neutrophils (PMN) and 50 larvae (L3) for 22 h. The cultures were treated with 100 U/ml of DNase I to degrade NETs and assessed for larval killing. Data are shown as mean ± SD. *, p < 0.01 when compared with wells containing L3 alone. C) Supernatants from the in vitro assay were analyzed for extracellular DNA using the PicoGreen assay. Data are shown as mean ± SD. *, p < 0.001 compared to L3 alone group or **, p < 0.001 when compared to wells with Mϕ and PMN but not treated with DNase I. Each group was performed in duplicate for at least two independent experiments.
DNase I treatment was used to evaluate the requirement for mouse NETs during the in vitro larval killing process. The combination of mouse neutrophils, macrophages and complement resulted in larval killing in vitro; however, addition of DNase I to the cultures containing both neutrophils and macrophages did not reduce the killing capacity of the cells (Fig. 4B). Examination of the supernatants from the in vitro killing assay revealed that mouse neutrophils cultured with larvae released a significant amount of free DNA, whereas macrophages did not release measurable levels of free DNA. The quantity of free DNA measured from mouse neutrophils cultured with larvae was equivalent to the levels recorded using the same number of human neutrophils. Free DNA in wells containing both cell types was completely ablated following DNase I treatment, yet larval killing was not affected (Fig. 4C). Therefore, based on the presence of visible clots in culture wells with human but not mouse cells, human neutrophils produce ETs that are phenotypically different from NETs produced by murine neutrophils, while having equivalent amounts of DNA released from both cell sources. Human macrophages release ETs in vitro whereas mouse macrophages do not release measurable levels of ETs in vitro. Finally, the larval killing mechanism used by mouse macrophages and neutrophils in vitro occurs independently of NET formation while human cells require ET formation to kill the larvae in vitro.
3.4. Mice develop both ETs and NETs following exposure to S. stercoralis larvae in vivo
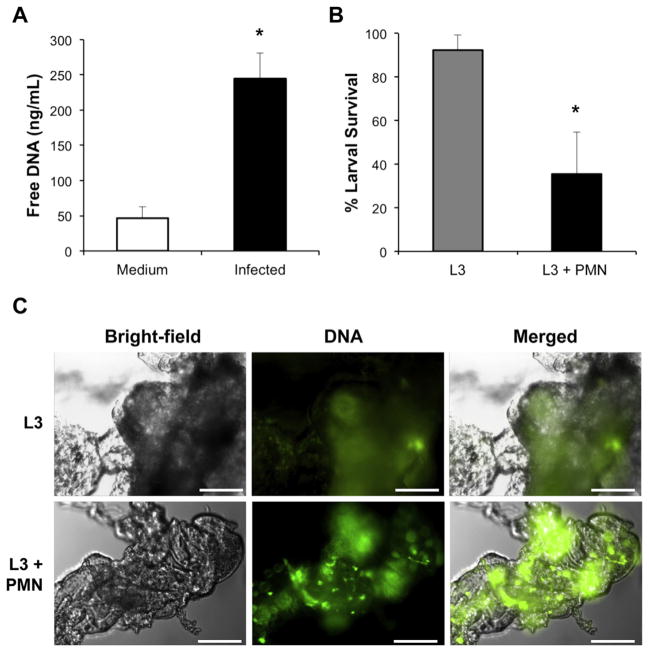
Experiments were performed to determine the role of ETs and NETs in response to larval S. stercoralis in vivo. Naïve mice were infected with live larvae and examined for the release of extracellular DNA to quantify ET release in vivo. Sterile culture medium or larvae were injected into the peritoneal cavity of naive mice and after 3 h the peritoneal lavage fluid was examined for extracellular DNA using a PicoGreen assay. Examination of the peritoneal exudate cells following intraperitoneal injection of live L3 revealed an approximate 10 fold increase in influx of neutrophils into the peritoneal cavity after 4 h. Increased free DNA was detected in the peritoneal cavities of mice infected with larvae, suggesting that infection with S. stercoralis induces ETs in vivo (Fig. 5A).
Fig. 5.
Mice release both ET and NETs following exposure to S. stercoralis larvae in vivo. A) Naïve C57BL/6J mice were injected with medium or 10,000 larvae in the peritoneal cavity (infected) and subjected to peritoneal lavages after 3 h. The peritoneal lavage fluid was measured for extracellular DNA using the PicoGreen assay. Data displayed as mean ± SD, where n = 3 mice per group, representative of at least three independent experiments. Data are shown as mean ± SD. *, p < 0.04 when compared to mice injected with medium. B) Neutrophils (PMN) (2 × 106) were isolated from naïve C57BL/6J mice and 50 larvae (L3) were placed in cell impermeable diffusion chambers, with a 0.1 um pore-size membrane. The chambers were then implanted into naive WT mice for 3 days at which time larval survival was assessed. Data are shown as mean ± SD. *, p ≤ 0.001 when compared to L3 alone control. C) Fluorescent images of Sytox Green stained clots taken from diffusion chambers containing L3 or L3 + PMN. Scale bars = 40 μm.
Neutrophils isolated from naïve mice were utilized to examine NET formation during parasite killing in vivo. Cell impermeable diffusion chambers containing: (1) culture medium, (2) larvae or (3) larvae and bone marrow isolated neutrophils were implanted in naïve mice for 3 days. The diffusion chamber contents were assessed for larval survival and NET formation. Neutrophils within the diffusion chamber killed larvae resulting in a 59% reduction in larval survival (Fig. 5B). Visible clots did not form in implanted diffusion chambers that contained only culture medium (data not shown). However, small clots formed in implanted diffusion chambers containing larvae alone. Examination of the clots using fluorescent microscopy revealed positive staining for Sytox Green indicating release of ETs in vivo that entered through the pores of the diffusion chamber membranes. Finally, clots isolated from diffusion chambers where neutrophils were in contact with the larvae and killed them, stained more intensely for Sytox Green than clots from diffusion chambers that contained only larvae (Fig. 5C). Of note, the majority of the live worms were found trapped within the clots in these diffusion chambers. The Sytox Green staining pattern of clots recovered from in vivo was different from clots that developed in vitro. In vitro derived clots were uniformly stained with Sytox Green whereas in vivo derived clots had patches of Sytox Green staining. DNase I treatment of the in vivo derived clots did not dissolve the clots as it did with the in vitro derived clots. These observations suggest that the in vivo derived clots were composed of ETs in combination with other host-derived factors.
4. Discussion
The objective of this study was to determine if release of ETs by human and mouse cells would serve as a mechanism for ensnaring and killing large and highly motile nematode parasites. The release of extracellular DNA by human neutrophils was confirmed by visualization of cellular clots comprised of extracellular DNA, which disintegrated following DNase I treatment. The colocalization of DNA, histone and MPO following exposure of neutrophils to the larvae of S. stercoralis indicate the presence of NETS which are structurally similar to NET induction by bacteria, fungi and protozoa [1,14,18]. Despite the induction of NETs by human neutrophils the NET based clots trapped but did not kill the parasite. The larval killing process by human cells in vitro requires the presence of multiple immune components including neutrophils, macrophages and complement [29]. This confirms previous reports that culture of S. stercoralis larvae with either human neutrophils or monocytes alone lead to cell adherence but not parasite death [31]. Human neutrophils rapidly release NETs when exposed to worms in vitro, independent of complement activity. Interestingly, ETs were also quantified in culture wells where larvae were cultured with macrophages, yet killing only occurred when neutrophils, macrophages and complement were present. Therefore, both human neutrophils and macrophages are required for killing the larvae of S. stercoralis through a mechanism dependent on ET formation. The relative importance of the contributions of neutrophils and macrophages to ET formation remains unknown.
In vitro experiments were performed to determine whether cells from mice would recapitulate the results obtained with human neutrophils and macrophages. As with human cells, killing of larvae in vitro requires murine neutrophils, macrophages and complement, as previously reported [29]. In vitro exposure of mouse neutrophils to larvae resulted in NET release that was also independent of complement activity. Culture of larvae with neutrophils alone did not result in a visible clot although there was measurable DNA release. Unlike human macrophages, culture of larvae with murine macrophages did not result in measurable DNA release. Treatment with DNase I eliminated the presence of released DNA, but did not block killing of the larvae by mouse neutrophils and macrophages in vitro. This observation suggests that in contrast to human neutrophils and macrophages, mouse cells do not require ET formation in vitro to kill the worms. Thus, although human and mouse neutrophils, macrophages and complement kill the larvae, the mechanism used by this triad appears to be different in mice and humans.
There are several possible reasons for the discrepancy between the results obtained with human and mouse cells. There may be fundamental differences in the biology of human and mouse cells that is manifest in how they respond to pathogens. Alternatively, the data may reflect the ability of neutrophils from different sources to release NETs. Mouse neutrophils were isolated from the bone marrow of naïve mice reflecting a more immature activation state as compared to peripheral human neutrophils which were isolated from the blood of healthy uninfected donors [29]. Likewise, the macrophages used in the human and mouse studies may have been in different activation states, which might explain why human macrophages produced ETs while mouse macrophages did not. Finally, multiple killing mechanisms have been described for the larvae of S. stercoralis in the mouse primary and secondary responses [23]. It is unknown if similar redundancies occur in the human protective immune response, although the fact that humans are susceptible to the infection suggests that humans will have a limited repertoire of killing mechanisms. The redundancy in killing responses found in mice may mask the capacity of ETs to participate in killing the larvae. Thus, ETs may function in killing the larvae but are not required in vitro for killing the worms.
Experiments were performed in mice to determine whether ETs formed in vivo in response to infection with S. stercoralis. It was hypothesized that while ETs were not required for mouse cells to kill the parasite in vitro, the culture conditions may have obscured a role that ETs may play in control of the infection in vivo. Mice infected with S. stercoralis larvae developed a significant increase in free DNA in the peritoneal cavity within 3 h after larval infection. The modest increase in free DNA released by mock infected mice may be the result of nonspecific NET release in response to the needle stick which alone is sufficient to elicit a swarming response by neutrophils [32]. Release of ETs in the peritoneal cavity of infected mice was likely produced by neutrophils, as they are the dominant cell type in the peritoneal cavity at this time point [33]. However, the ability of eosinophils, basophils, monocytes/macrophages and mast cells [3,4,34] to produce extracellular traps following exposure to larvae remains a possibility.
To further test the role of NETs in killing larvae in vivo, mouse neutrophils were implanted with S. stercoralis larvae in diffusion chambers in vivo. The diffusion chambers were cell impermeable; cells on the inside could not leave and cells on the outside of the diffusion chamber could not enter. Diffusion chambers that contained neither larvae nor cells did not develop visible clots, demonstrating that surgical implantation of diffusion chambers did not induce ET formation. Diffusion chambers containing larvae alone had small visible clots that stained positive for extracellular DNA indicating that ETs entered through the pores of the diffusion chamber. Chemotactic studies have demonstrated that a soluble extract of larval S. stercoralis directly recruits neutrophils in a CXCR2 [26] and Gαi2 [25] dependent manner. Neutrophils may have been attracted to soluble parasite material that diffused out of the diffusion chamber and in response underwent ETosis giving rise to ETs that entered the diffusion chamber. Examination of the contents of diffusion chambers containing both neutrophils and larvae showed larval killing, as previously reported [24]. More intense staining of extracellular DNA in clots was associated with diffusion chambers containing neutrophils and larvae where parasite killing occurred, which suggests an association between mouse NETs and parasite killing in vivo. This conclusion was supported by the observation that the majority of live worms recovered from these diffusion chambers, were not in the fluid, but rather were trapped within a fibrous network.
Killing larval S. stercoralis unlike bacteria or fungi presents two critical challenges to the cells of the immune system. The first challenge is that the infective larvae of S. stercoralis migrate through tissue at a rate of 10 cm/h [35,36], 20 times faster than neutrophils, which migrate through skin at 0.5 cm/h [32]. The second challenge is that there is an enormous disparity between the size of S. stercoralis infective larvae, 500–570 μm [37], and that of neutrophils and macrophages which range from 8 to 12 μm [38] to 12–22 μm [39], making phagocytosis impossible. The rapid release of NETs by human neutrophils exposed to live and dead larvae may represent a solution to both challenges. Invading infective larvae induce the release of NETs, which trap free parasites for killing by cells of the innate immune response, independent of phagocytosis [23,29].
Results obtained using human neutrophils in vitro demonstrate that NETs trap the worms, but do not kill them. NETs entrapping pathogens without killing them have been observed in L. donovani [19], Plasmodium falciparum [40], Mycobacterium tuberculosis [41], T. gondii [20] and Streptococcus species [42]. We therefore hypothesize that killing larvae of S. stercoralis by the innate immune response is initiated by neutrophils that are recruited directly to the parasite [26] and induced to release NETs. The live parasites are trapped within the NETs, which completely blocks their ability to migrate away from danger. Cells, including macrophages, neutrophils and eosinophils, undergo chemotaxis to the worms and upon contact or close proximity release toxic molecules such as major basic protein from eosinophils or myeloperoxidase from neutrophils [28] onto the immobilized worms.
In conclusion, the current study demonstrates that human and mouse neutrophils release NETs following exposure to S. stercoralis. Human NETs ensnare the parasite and facilitate killing by human neutrophils and macrophages. Mice produce ETs in response to infection with S. stercoralis and NETs are associated with trapping and killing of the worms. This study indicates that ETs, in particular NETs, play a role in the control of the large extracellular parasite S. stercoralis.
Supplementary Material
Acknowledgments
We thank Jessica Breslow-Deckman for critically reviewing the manuscript and Eric B. Wong for technical assistance. We also thank Volker Brinkmann for expert and enthusiastic guidance. We acknowledge the Bone Marrow Transplant Program and the Bioimaging Shared Resource of the Kimmel Cancer Center (NCI 5 P30 CA-56036) at Thomas Jefferson University Hospital for services provided. This work was supported by grants from the National Institutes of Health, United States, number AI076345 and AI078314 (David Abraham, Thomas Jefferson University), AI82548, AI50668 and AI22662 (James B. Lok, University of Pennsylvania) and RR02512 (Mark Haskins, University of Pennsylvania).
References
- 1.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–53. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 3.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–80. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 4.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–54. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci STKE. 2007;2007:pe11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- 6.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–44. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 7.Logters T, Margraf S, Altrichter J, Cinatl J, Mitzner S, Windolf J, et al. The clinical value of neutrophil extracellular traps. Med Microbiol Immunol. 2009;198:211–9. doi: 10.1007/s00430-009-0121-x. [DOI] [PubMed] [Google Scholar]
- 8.Gupta AK, Hasler P, Holzgreve W, Hahn S. Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia? Semin Immunopathol. 2007;29:163–7. doi: 10.1007/s00281-007-0073-4. [DOI] [PubMed] [Google Scholar]
- 9.Papayannopoulos V, Staab D, Zychlinsky A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One. 2011;6:e28526. doi: 10.1371/journal.pone.0028526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6:415–20. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–5. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yost CC, Cody MJ, Harris ES, Thornton NL, McInturff AM, Martinez ML, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113:6419–27. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida Abicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–76. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 15.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumonia to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–7. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 16.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1:181–93. doi: 10.1159/000205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker H, Albrett AM, Kettle AJ, Winterbourn CC. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol. 2011;3:369–76. doi: 10.1189/jlb.0711387. [DOI] [PubMed] [Google Scholar]
- 18.Guimaraes-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceicao-Silva F, et al. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci USA. 2009;106:6748–53. doi: 10.1073/pnas.0900226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabriel C, McMaster WR, Girard D, Descoteaux A. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J Immunol. 2010;185:4319–27. doi: 10.4049/jimmunol.1000893. [DOI] [PubMed] [Google Scholar]
- 20.Abi Abdallah DS, Lin C, Ball CJ, King MR, Duhamel GE, Denkers EY. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect Immun. 2012;80:768–77. doi: 10.1128/IAI.05730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 22.Croker C, Reporter R, Redelings M, Mascola L. Strongyloidiasis-related deaths in the United States, 1991–2006. Am J Trop Med Hyg. 2010;83:422–6. doi: 10.4269/ajtmh.2010.09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonne-Annee S, Hess JA, Abraham D. Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model. Immunol Res. 2011;51:205–14. doi: 10.1007/s12026-011-8258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galioto AM, Hess JA, Nolan TJ, Schad GA, Lee JJ, Abraham D. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. Infect Immun. 2006;74:5730–8. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padigel UM, Stein L, Redding K, Lee JJ, Nolan TJ, Schad GA, et al. Signaling through Galphai2 protein is required for recruitment of neutrophils for antibody-mediated elimination of larval Strongyloides stercoralis in mice. J Leukoc Biol. 2007;81:1120–6. doi: 10.1189/jlb.1106695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connell AE, Redding KM, Hess JA, Lok JB, Nolan TJ, Abraham D. Soluble extract from the nematode Strongyloides stercoralis induces CXCR2 dependent/IL-17 independent neutrophil recruitment. Microbes Infect. 2011;13:536–44. doi: 10.1016/j.micinf.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein LH, Redding KM, Lee JJ, Nolan TJ, Schad GA, Lok JB, et al. Eosinophils utilize multiple chemokine receptors for chemotaxis to the parasitic nematode Strongyloides stercoralis. J Innate Immun. 2009;1:618–30. doi: 10.1159/000233235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connell AE, Hess JA, Santiago GA, Nolan TJ, Lok JB, Lee JJ, et al. Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect Immun. 2011;79:2770–8. doi: 10.1128/IAI.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonne-Annee S, Kerepesi LA, Hess JA, O’Connell AE, Lok JB, Nolan TJ, et al. Human and mouse macrophages collaborate with neutrophils to kill larval Strongyloides stercoralis. Infect Immun. 2013;9:3346–55. doi: 10.1128/IAI.00625-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerepesi LA, Hess JA, Nolan TJ, Schad GA, Abraham D. Complement component C3 is required for protective innate and adaptive immunity to larval Strongyloides stercoralis in mice. J Immunol. 2006;176:4315–22. doi: 10.4049/jimmunol.176.7.4315. [DOI] [PubMed] [Google Scholar]
- 31.Stanley ER, Heard PM. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977;252:4305–12. [PubMed] [Google Scholar]
- 32.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–4. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerepesi LA, Hess JA, Leon O, Nolan TJ, Schad GA, Abraham D. Toll-like receptor 4 (TLR4) is required for protective immunity to larval Strongyloides stercoralis in mice. Microbes Infect. 2007;9:28–34. doi: 10.1016/j.micinf.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Schorn C, Janko C, Latzko M, Chaurio R, Schett G, Herrmann M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front Immunol. 2012;3:277. doi: 10.3389/fimmu.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKerrow JH, Brindley P, Brown M, Gam AA, Staunton C, Neva FA. Strongyloides stercoralis: identification of a protease that facilitates penetration of skin by the infective larvae. Exp Parasitol. 1990;70:134–43. doi: 10.1016/0014-4894(90)90094-s. [DOI] [PubMed] [Google Scholar]
- 36.Napier LE. Strongyloides stercoralis infection. J Trop Med Hyg. 1949;52:25. [PubMed] [Google Scholar]
- 37.Sing A, Leitritz L, Bogner JR, Heesemann J. First-glance diagnosis of Strongyloides stercoralis autoinfection by stool microscopy. J Clin Microbiol. 1999;37:1610–1. doi: 10.1128/jcm.37.5.1610-1611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worthen GS, Schwab B, 3rd, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989;245:183–6. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- 39.Cannon GJ, Swanson JA. The macrophage capacity for phagocytosis. J Cell Sci. 1992;101(Pt 4):907–13. doi: 10.1242/jcs.101.4.907. [DOI] [PubMed] [Google Scholar]
- 40.Baker VS, Imade GE, Molta NB, Tawde P, Pam SD, Obadofin MO, et al. Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malar J. 2008;7:41. doi: 10.1186/1475-2875-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos-Kichik V, Mondragon-Flores R, Mondragon-Castelan M, Gonzalez-Pozos S, Muniz-Hernandez S, Rojas-Espinosa O, et al. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb) 2009;89:29–37. doi: 10.1016/j.tube.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Lauth X, von Kockritz-Blickwede M, McNamara CW, Myskowski S, Zinkernagel AS, Beall B, et al. M1 protein allows Group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J Innate Immun. 2009;1:202–14. doi: 10.1159/000203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.