Abstract
Objective:
Chronic myeloid leukemia (CML) is a clonal hematopoietic disorder caused by acquired genetic defect in pluripotent stem cells characterized by acquisition of the philadelphia chromosome. The aim of this study was to compare the efficacy, safety and quality of life (QoL) in CML patients treated with imatinib or hydroxyurea.
Methods:
A prospective observational study was conducted on 40 patients with pathologically confirmed CML in an in-patient department of Mahavir Cancer Sansthan and Research Centre (tertiary care cancer hospital) in India. Patients were divided into two groups (group A: Imatinib consuming patients and group B: Hydroxyurea consuming patients). Complete blood count was done every month to assess the efficacy and safety/toxicity profile of these drugs. The results were analyzed 12 months after completion of treatment. QoL was assessed by The European Organization for Research and Treatment of Cancer QoL Questionnaire core 30. Hematological response was analyzed using kaplan-meier survival analysis. Chi-square test was applied to assess the association of two regimens with complete hematological response, hematological and non-hematological toxicity. White blood cell (WBC) was noted each month in every patient of each group and analyzed by generalized linear mode (repeated measures) analysis of variance (ANOVA). Independent t-test was used to compare changes in QoL between treatment groups.
Findings:
At the end of treatment, significant improvement (P = 0.001) in hematological response was observed in the group A (95%) compared to group B (30%). WBC count analyzed at each month of treatment by ANOVA achieved better results for patients treated with imatinib (P = 0.0001). The hematological toxicity was higher in imatinib group while non-hematological toxicity was higher in the hydroxyurea group; however only little toxicities such as nausea and constipation were statistically significant. QoL assessment of patients related to functional scale showed significantly better results in group A (P = 0.046).
Conclusion:
The study showed that imatinib has better profile compared to hydroxyurea, with siginificant statistical differences in terms of efficacy, non-hematological toxicity and QoL in CML patients. Even with such better efficacy and safety profile, pharmacoeconomic evaluation needs to be done to justify and support the use of imatinib for CML patients in India.
Keywords: Chronic myeloid leukemia, Hydroxyurea, Imatinib, quality of life
INTRODUCTION
Chronic myeloid leukemia (CML) is a form of chronic myeloproliferative disease caused by an acquired genetic defect in pluripotent stem cells characterized by acquisition of the philadelphia (Ph) chromosome in leukemic stem cells and their progeny. The abnormal Ph chromosome is a manifestation of a reciprocal translocation between chromosomes 9 and 22. The major consequence of this translocation is the fusion of the (ABL) gene to the (BCR) gene on chromosome 22. The BCR-ABL fusion gene, which is transcribed into an 8.6 kb chimeric messenger ribonucleic acid, encodes a 210 kd hybrid protein that is possibly responsible for the chronic phase of the disease.[1,2] CML represents 14% of all leukemias and 20% of adult leukemias. The annual incidence is 1.6 cases per 100,000 adults with a slight male preponderance. The median age at diagnosis is 65 years; it occurs rare in children and its incidence increases with age. There are no known hereditary, familial, geographic, ethnic, or economical associations with CML.[3] Therapy was palliative during the first century of CML treatment, which included splenic irradiation, various cytostatic agents, of which busulfan and hydroxyurea were standards for almost three decades. Treatment intention became curative with the introduction of stem cell transplantation in the 1970 s. A prolongation of survival could be achieved by interferon alpha (IFN-α) in combination with hydroxyurea or low-dose cytarabine, particularly in low-risk patients and in patients who achieve a cytogenetic remission.[4]
The treatment of CML has experienced a great degree of refinement during the past few years. However, the explosion of the field of molecularly targeted tyrosine kinase inhibitors (TKIs) in recent years led by imatinib mesylate has brought about a change in the natural history of the disease and in the therapeutic approach to the treatment of patient. After imatinib, many new TKIs came with improved efficacy such as Dasatinib, Nilotinib and Bosutinib proved to be one of the recommended treatments for the patients with CML.[3]
We surveyed the drugs used for the treatment of CML in our study site. Imatinib, being the recommended treatment, is very expensive and hence unaffordable to many patients. Hydroxyurea is used as another treatment option as it offers a number of advantages such as economical, easy administration, rapid action, efficacious also in advanced phase and prolongation of survival over busulfan/IFN with less toxicity. No study has yet been reported comparison results between imatinib and hydroxyurea. This study was carried out with aim to compare the efficacy, toxicity and quality of life (QoL) in the patients treated with imatinib or hydroxyurea. As imatinib is comparatively new drug and introduced only a few years ago, so toxicity profile of this regimen is yet to be established; hence, this study also aimed to assess the safety profile of the imatinib in our community.
METHODS
This was a prospective observational study in an inpatient Department of Mahavir Cancer Sansthan and Research Centre (tertiary care cancer hospital) in Patna, India in 2008-2009. A total of 20 patients were included in each group after satisfying the inclusion criteria, consisted of pathologically confirmed cases of CML, age between 18 and 75 years, with no severe organ dysfunction and life expectancy more than 6 months.
This study was approved by the Research and Ethical Committee of Mahavir Cancer Sansthan. Patients satisfying the inclusion criteria were enrolled in the study. A written informed consent was obtained from every patient. A detailed elucidation of the history and clinical examination record of patients was performed prior to enrollment in the study. The patients were followed-up for 12 months of treatment.
Patients who were prescribed imatinib were enrolled in group A while patient prescribed hydroxyurea were kept in group B: All patients were assessed every month of the treatment and dose of the drug was adjusted every month based on the clinical judgment and lab reports. The recommended dose of imatinib for the chronic phase is 400 mg daily, increased to 600 mg daily or 400 mg bid. Recommended dose for the hydroxyurea is 20-30 mg/kg/day in single doses.
Efficacy was evaluated in each group based on the hematological response.[5,6,7] Complete blood count was done after every month of treatment. Efficacy of the treatment was evaluated by complete hematological response rate, hematological response survival curve analysis and white blood cell (WBC) count after each month of treatment.
A complete hematological response defined as normal WBC count with normal differential (WBC count less than 10 × 109/L), bands and metamyelocyte ≤5%, normal platelet count, no CML related symptoms and normal spleen size (11-12 cm max. diameter).
A partial hematological response is defined as a more than 50% decrease of WBC count or a WBC count less than 20 × 109/L.
A failure was defined as WBC count greater than 20 × 109/L.
Hematologic failure was defined as either hematologic resistance (failure to achieve a complete hematologic response after at least 6 months of treatment) or relapse after a complete hematologic response had been achieved, with white-cell counts increased to at least 20,000 mm3 during therapy.
The hematological toxicity was graded according to the National Cancer Institute-Common Toxicity Criteria (NCI-CTC) Version 3.0. QoL was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 Version 03 (EORTC module QLQ-C30).[8,9] This is a 30 item questionnaire composed of five multi-item functional subscales: Physical function, role function, emotional function, cognitive function and social function; three multi-item symptom scales measuring fatigue, pain and emesis; a global health subscale and six items to assess the financial impact and general symptoms. All scales ranged from 0 to 100, with a high score representing better functioning and global health and a higher level of symptomatology. Scoring of the QLQ-C30 was done according to the procedures described in the EORTC manual.
Data analysis was performed using Excel 2007 and Statistical Package for the Social Sciences (SPSS) (version 16.0) software (SPSS; Chicago, Illinois, USA). Complete hematological responses were compared using Chi-square test. Kaplan-Meier survival analysis was used to compare hematological response between two groups. hematological response was evaluated after every month of treatment and if complete response was not achieved within 12 months than data was censored. The mean and median of survival time was evaluated for imatinib and hydroxyurea treatment groups; with this regard, significant difference between two groups was compared with long-rank (Mantel-Cox) test method. WBC count was analyzed in both groups by generalized linear mode (repeated measures), analysis of variance (ANOVA). The hematological and non-hematological toxicities were analyzed using Chi-square test and Fisher's exact test. Independent t-test was used to compare changes in QoL between treatment groups. (P < 0.05) was considered to be significant for all statistical analysis.
RESULTS
A total of 40 enrolled patients were taken for the analysis. The mean age was 44 years (range: 25-75) in the imatinib group as compared to 42.5 years (range: 24-65) in the hydroxyurea group. The maximum number of patients was in the age group of 40-50 years in both groups. There were 13 male and 7 female patients in group A while 11 male and 9 female patients in group B.
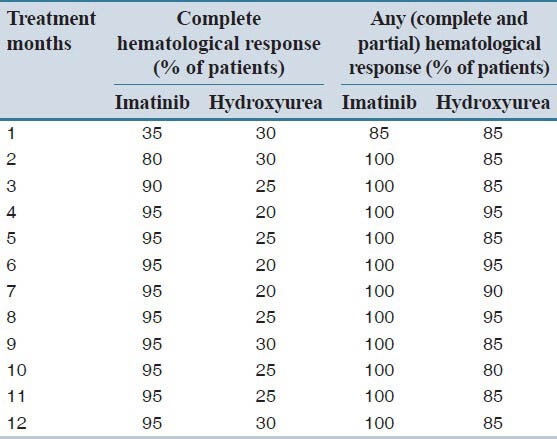
Comparison of complete hematological response rate (%) is presented in Table 1, indicating a significant difference (P = 0.001) between two groups of treatment. The mean response time for imatinib and hydroxyurea groups was noted to be 2.5 and 6.3 months respectively, whereas the median response time for imatinib and hydroxyurea group was noted to be 2 and 4 months respectively. Group A patients achieved significantly better response (P = 0.031) using Long Rank (Mantel-Cox) test.
Table 1.
Hematological response of patients receiving imatinib and hydroxyurea treatment
Hematological response survival curve analysis using Kaplan-Meier method is presented in Figure 1, taking the time to event (month) on horizontal and the probability of survival on the vertical axis. Thus, any point on the survival curve showed the probability that a patient on a given treatment would not have experienced relief by that time. Kaplan-Meier survival curve for the imatinib group was below that of the hydroxyurea group for most of the study period duration.
Figure 1.
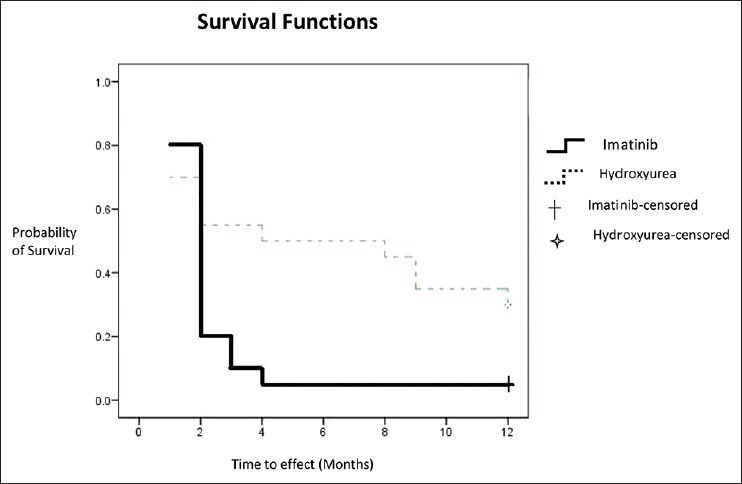
Kaplan-Meier survival curve for hematological response
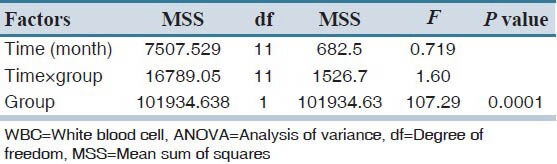
WBC count was analyzed every month of treatment and patient's with normal/subnormal WBC was calculated as shown in Table 2. WBC was monitored regularly after each month in each group of treatment and analyzed by generalized linear mode (repeated measures), ANOVA as presented in Table 3. The values show significantly better therapeutic effect in the imatinib group, with this regard (P = 0.0001).
Table 2.
Average WBC count and patients with normal/subnormal WBC count
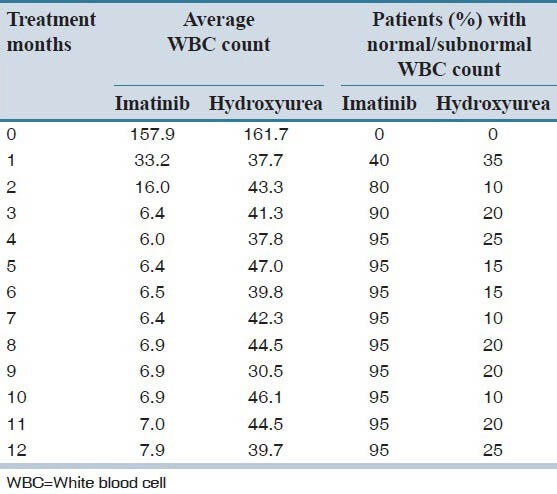
Table 3.
Results of repeated measure ANOVA for WBC counts taken every month for 12 months of treatment in each group
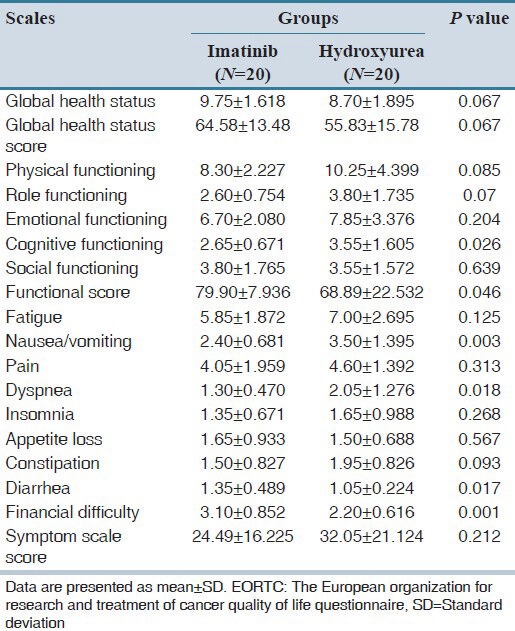
The toxicity profile was calculated for both treatment groups according to NCI-CTC grade, version 3. The grades of hematological toxicity (anemia, leucopenia, thrombocytopenia and neutropenia) as well as non-hematological toxicity (fatigue, nausea, vomiting, dyspnea, insomnia, appetite loss, constipation and diarrhea) are presented in Table 4. Thrombocytopenia was noted to be significantly less prevalent (P < 0.0001) in hydroxyurea group as compare to the imatinib group. Leucopenia and Neutropenia were noted to be less in hydroxyurea group but did not reach statistically significance while anemia was similar in both treatment groups. Fatigue, nausea, vomiting, dyspnea, insomnia and diarrhea were less prevalent in the imatinib group, while appetite loss and constipation were less prevalent in the hydroxyurea group. The EORTC QLQ-C30 questionnaire was used for the assessment of the QoL. Cognitive functioning, nausea, vomiting, and dyspnea were significantly less prevalent in the imatinib group while diarrhea and financial difficulties were significantly less prevalent in the hydroxyurea group. All other functional scales such as physical, role, emotional and social functioning were better, whereas symptom scales such as fatigue, pain and insomnia were less in the imatinib group, although not statistically significant. Appetite loss was also noted to be less in hydroxyurea group, but not statistically significant [Table 5]. Functional score was significantly better in the imatinib group (P = 0.046), while global health status was better (P = 0.067) and symptom scales had more favorable results (P = 0.212) in imatinib group, however, did not reach statistically significance.
Table 4.
Overall toxicity for both treatment groups according to NCI-CTC grade
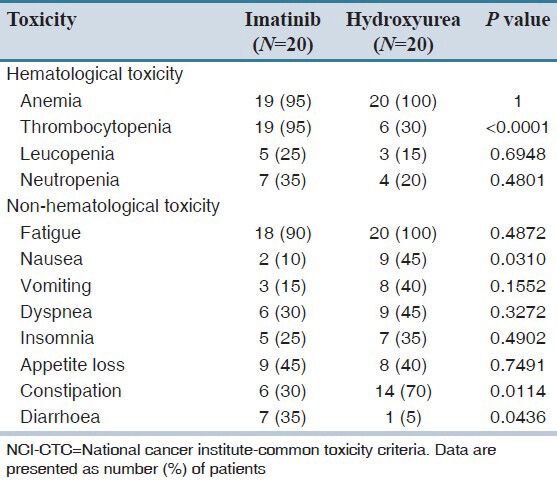
Table 5.
Quality of life assessment in the patients treated with imatinib and hydroxyurea according to EORTC reference manual
DISCUSSION
Complete evaluation of treatment benefits or disadvantages involves not only the assessment of efficacy, but also the safety and QoL In this study after 12 months of treatment, 95% of patients achieved complete hematological response in imatinib group when compared to 30% in hydroxyurea group (P = 0.04). In the study done by Kantarjian et al.,[7] O’Brien et al.[10] and Druker et al.,[11] around 95% of the patients had achieved complete hematological response while in the study conducted by Benelux CML study group, Hehlmann et al. showed that around 35% patients had achieved complete hematological response with hydroxyurea treatment.[12] In our study, hematological response survival analysis showed significantly better results (P = 0.031) in imatinib group when compared to hydroxyurea group. Similarly, WBC count analysis showed significantly better profile (P < 0.0001) in imatinib group.
There were several studies[4,7,11,13] conducted to analyze the hematological and non-hematological toxicity of imatinib and these toxicities found to be very minimal; the most common were nausea, myalgia, edema and diarrhea.[11] In another study conducted by Kantarjian et al.,[7] severe grades of non-hematologic toxic effects were infrequent and hematologic toxic effects were manageable. Only 2% of patients discontinued treatment because of drug-related adverse events and no treatment-related deaths occurred.[7] Study conducted by Talpaz et al.,[13] non-hematologic toxicity showed to be usually mild or moderate and hematologic toxicity was manageable. One study conducted for hydroxyurea by Hehlmann et al.[12] reveals that flu-like (12.3%), gastrointestinal (19.5%), neurological/psychiatric 6.2%) and dermatological symptoms (9.4%) have occurred in patients after treatment with this drug.
Only few studies were conducted to assess the QoL in CML patients. One study was conducted by Hahn et al.[14] to show the difference between QoL in the patients treated with imatinib and IFN-α and concluded that imatinib offers more QoL advantages compared with IFN. In the present study, functional scale was significantly better in imatinib group when compared to hydroxyurea group, while global health status was better and symptom scales had more favorable results in the imatinib group, however, did not reach statistically significance. Financial constraint appeared to be higher in imatinib group.
As a practical limitation, the sample size of this study was small, so in further studies by increasing the sample size, the comparison of efficacy, toxicity profile and QoL of patients could be assessed with a better possible outcome. In developing countries like India, pharmacoeconomic evaluations need to be extensively performed to assess the affordability of imatinib in comparison to hydroxyurea.
AUTHORS’ CONTRIBUTION
All authors contributed actively at all stages of the study and in preparation of the manuscript. All authors participated in the concept design and preparation of the manuscript. AR has done the data and Statistical analysis and also participated in editing and reviewing the manuscript. All authors read and approved the final version submitted for publication.
ACKNOWLEDGMENTS
We would like to thank Dr. Pradeep Das (Director, NIPER and RMRI (ICMR Institute) for valuable support and advice for this project.
Footnotes
Source of Support: National Institute of Pharmaceutical Education and Research-Hajipur, Bihar, India
Conflict of Interest: None declared.
REFERENCES
- 1.Deininger MW, Druker BJ. Specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol Rev. 2003;55:401–23. doi: 10.1124/pr.55.3.4. [DOI] [PubMed] [Google Scholar]
- 2.Cortes J, Deininger M. BCR-ABL as a molecular target. In: Deininger M, editor. Chronic Myeloid Leukemia. 1st ed. New York: Informa Healthcare; 2006. pp. 27–45. [Google Scholar]
- 3.Quintás-Cardama A, Cortes JE. Chronic myeloid leukemia: Diagnosis and treatment. Mayo Clin Proc. 2006;81:973–88. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- 4.Hochhaus A. Advances in the treatment of haematological malignancies: Optimal sequence of CML treatment. (58-63).Ann Oncol. 2007;18(Suppl 9):ix. doi: 10.1093/annonc/mdm295. [DOI] [PubMed] [Google Scholar]
- 5.Hehlmann R, Heimpel H, Hasford J, Kolb HJ, Pralle H, Hossfeld DK, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064–77. [PubMed] [Google Scholar]
- 6.Berger U, Engelich G, Maywald O, Pfirrmann M, Hochhaus A, Reiter A, et al. Chronic myeloid leukemia in the elderly: Long-term results from randomized trials with interferon alpha. Leukemia. 2003;17:1820–6. doi: 10.1038/sj.leu.2403042. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–52. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 8.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 9.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, et al. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer; 2001. The EORTC QLQ-C30 Scoring Manual. [Google Scholar]
- 10.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 11.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 12.Hehlmann R, Berger U, Pfirrmann M, Hochhaus A, Metzgeroth G, Maywald O, et al. Randomized comparison of interferon alpha and hydroxyurea with hydroxyurea monotherapy in chronic myeloid leukemia (CML-study II): Prolongation of survival by the combination of interferon alpha and hydroxyurea. Leukemia. 2003;17:1529–37. doi: 10.1038/sj.leu.2403006. [DOI] [PubMed] [Google Scholar]
- 13.Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: Results of a phase 2 study. Blood. 2002;99:1928–37. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 14.Hahn EA, Glendenning GA, Sorensen MV, Hudgens SA, Druker BJ, Guilhot F, et al. Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon alfa plus low-dose cytarabine: Results from the IRIS Study. J Clin Oncol. 2003;21:2138–46. doi: 10.1200/JCO.2003.12.154. [DOI] [PubMed] [Google Scholar]


