Abstract
Purpose
We examined whether obesity and a history of diabetes, hypertension, and elevated cholesterol, individually and in combination, are associated with breast density, a strong risk factor for breast cancer.
Methods
We measured percent density and dense area using a computer-assisted method (n=191; age range=40-61 years). We used linear regression models to examine the associations of each metabolic condition and the number of metabolic conditions (0, 1, 2, and 3 or 4 conditions) with breast density.
Results
Among individual metabolic conditions, only high blood cholesterol was inversely associated with percent density (β=-5.4, 95% CI: -8.5, -2.2) and dense area (β= -6.7, 95% CI=-11.1, -2.4). Having multiple metabolic conditions was also associated with lower breast density, with 2 conditions and 3 or 4 conditions vs. 0 conditions associated with 6.4% (95% CI:-11.2, -1.6) and 7.4% (95% CI:-12.9, -1.9) reduction in percent density and with 6.5 cm2 (95% CI: -13.1, -0.1) and 9.5 cm2 (95% CI: -17.1, -1.9) smaller dense area.
Conclusions
A history of high blood cholesterol and multiple metabolic conditions were associated with lower relative and absolute measures of breast density. The positive association between metabolic abnormalities and breast cancer risk may be driven by pathways unrelated to mammographic breast density.
Obesity and diabetes have been associated with an increased risk of postmenopausal breast cancer, but the results for other metabolic risk factors, such as lipid abnormalities and elevated blood pressure, are less consistent (reviewed in (1-3)). More recently, studies have also shown modest positive associations between the presence of multiple metabolic conditions, as frequently defined by the metabolic syndrome (MetS), and breast cancer risk in postmenopausal women (reviewed in (2)).
While most risk factors for breast cancer are similarly associated with mammographic breast density, a strong intermediate marker of breast cancer, increasing body size has consistently been associated with reduced breast density in both pre- and postmenopausal women (4, 5). Diabetes and high-density lipoprotein cholesterol (HDL-C) have also respectively been inversely and positively associated with breast density in premenopausal women in some (6-9), but not all studies (9-12).
The majority of research investigating multiple metabolic conditions and breast cancer risk has been conducted among women of European descent (2). There are racial/ethnic (hereafter racial) differences in the prevalence of specific metabolic conditions; for example, hypertension, high blood glucose and dyslipidemia are respectively more common among African American, Hispanic and White populations in the U.S. (13). Research on metabolic abnormalities and breast cancer risk in racial minorities is further warranted given the disproportionately greater prevalence and earlier age of onset of metabolic disorders in many racial minority populations (14, 15), and the potentially adverse impact of metabolic disorders on breast cancer mortality (16). The purpose of this study was to examine whether diagnoses of hypertension, diabetes and high cholesterol, individually and in combination with each other and with having an obese body size, are associated with breast density in a predominantly African American and Caribbean sample of women.
METHODS
Study Population
The New York City Multiethnic Breast Cancer Project includes epidemiologic risk factor and breast density data for 200 women who were recruited as they presented for screening mammography at a hospital in Brooklyn, New York. As described previously (17), we collected in-person interview data between January 2007 and April 2008, and collected mammograms from the same date as the interview for 84% of the participants; the median time between the dates of mammograms and interviews for the remainder of participants was 14 days. We excluded data from five participants with a previous diagnosis of breast cancer and four participants with poor quality mammograms. The final sample of 191 women were 42% African American, 22% African Caribbean, 22% White, 12% Hispanic and 2% other ethnicities; 36% were foreign-born.
Metabolic Risk Factors
Participants reported whether they were ever told by a physician that they had high blood pressure, high blood cholesterol and type II diabetes, and if so reported their age at the first diagnosis for each condition. Data on height and self-reported current weight were used to calculate body mass index (BMI, kg/m2). BMI was categorized into < 30 and ≥ 30, with the latter category signifying obesity. We considered each of the conditions and age at first diagnosis (categorized into ≤ 45 and > 45 years) in relation to breast density. To examine the associations for multiple metabolic conditions, we assessed the number of conditions by summing the presence of each of the three diagnoses and obesity, ranging from 0 to 4. We combined those with 3 or 4 conditions into a single category due to small number of participants with four conditions (n=7), and used this measure as a categorical variable in our analysis.
Breast Density Assessment
We measured mammographic density using left cranio-caudal view mammograms; right cranio-caudal views were used for four participants for whom left views were not available. We digitized all films using a Kodak Lumisys Film Digitizer, and used Cumulus software to measure breast density. A single trained reader, blinded to exposure data, outlined the total areas of the breast and dense tissue, and the computer software measured the number of pixels in these areas. Percent density was calculated as dense area divided by total breast area (in percentage), and the number of pixels for the dense area was converted into cm2 to capture the amount of dense tissue. We assessed density from one film per participant in batches of approximately 50. We re-read a 10% randomly selected sample of films, obtaining Pearson correlation coefficients of 0.99 for breast area and 0.88 for dense area for the repeated readings (17).
Statistical Analyses
We performed linear regression models to examine the associations of each metabolic risk factor, age at first diagnosis and the number of metabolic risk factors with breast density, adjusting for continuous measures of age at mammogram and BMI. We examined whether menstrual and reproductive factors substantially affected these associations. Only age at menarche and menopausal status altered the majority of the estimates of associations for the metabolic conditions by at least 10% and were included in the final multivariable models. We also explored whether the associations between the number of metabolic conditions and breast density differed by obesity and menopausal statuses, through inclusion of cross-product terms between these factors in the multivariable models and through stratification across levels of obesity and menopausal status.
RESULTS
The study participants had an average BMI of 29.8 kg/m2 and a mean age of 50.0 years (range: 40-61); 35% were postmenopausal (Table 1). Over one third of the participants had a history of high blood pressure or cholesterol, and 8% had a history of diabetes (Table 2). The average ages at first diagnosis of these conditions were 42.0 (SD=10.3), 47.8 (SD=6.8) and 46.8 (SD=7.4) years for hypertension, elevated cholesterol and diabetes, respectively. Obesity, hypertension and elevated cholesterol level had a similar prevalence among participants with a single condition. Among those with multiple metabolic risk factors, diabetes always co-occurred with other conditions, and hypertension was reported by all participants with 3 or 4 conditions. African Americans had a higher prevalence of hypertension (48%) than other racial groups (e.g., 26% in African Caribbean, 24% in Whites), but there were no other significant racial differences in the prevalence or age at onset for other conditions (data not shown).
Table 1.
Sample characteristics of New York City Multiethnic Breast Cancer Project (n=191)
| Mean (SD) or Percent (n) | |
|---|---|
| Age at interview (year) | 50.0 (5.7) |
| Educational attainment | |
| ≤ high school | 29.1 (55) |
| Some college/associate degree | 33.9 (64) |
| ≥ college degree | 37.0 (70) |
| Race/ethnicity | |
| Non-Hispanic African American | 42.4 (81) |
| Non-Hispanic African Caribbean | 22.0 (42) |
| Non-Hispanic White | 21.5 (41) |
| Hispanic | 12.5 (24) |
| Non-Hispanic Other | 1.6 (3) |
| Age at menarche (year) | 12.4 (1.8) |
| Body mass index (kg/m2) | 29.8 (6.7) |
| Positive family history of breast cancer | 13.2 (25) |
| Parity | 1.6 (1.5) |
| Age at first full-term pregnancy (in parous women, year) | 23.0 (7.0) |
| Lifetime history of breast feeding | 39.5 (75) |
| Menopausal statusa | |
| Premenopausal | 64.9 (124) |
| Postmenopausal | 35.1 (67) |
Postmenopausal women did not have a menstrual period within the last 1 months or had bilateral oophorectomy. Premenopasual women had a peri within the last 12 month and did not have bilateral oophorectomy. If data last menstrual cycle and gynecological surgery were missing, women youn than 54 (90th percentile of age of natural menopause among postmenopau women in this study population) were considered premenopausal and wom 54 years or older were considered postmenopausal.
Table 2.
Age and body mass index (kg/m2) adjusted regression coefficients for the association between metabolic conditions and breast density (n = 191)
| Percent density | Dense area (cm2) | ||||
|---|---|---|---|---|---|
| N | β | 95% CI | β | 95% CI | |
| Obese body mass index | |||||
| <30 kg/m2 | 111 | Ref | Ref | ||
| ≥ 30 kg/m2 | 76 | −3.53 | −8.21, 1.47 | −5.75 | −12.14, 0.64 |
| High blood pressure | |||||
| No | 122 | Ref | |||
| Yes | 69 | -0.30 | −3.70, −3.10 | −0.55 | −4.10, 5.20 |
| Age at high blood pressure diagnosis | |||||
| No high blood pressure | 122 | Ref | Ref | ||
| ≤ 45 | 42 | 0.11 | −3.71, 3.92 / | 1.47 | −3.75, 6.70 |
| > 45 | 27 | −1.12 | −6.00, 3.76 | −1.30 | −7.97, 5.37 |
| Diabetes | |||||
| No | 175 | Ref | Ref | ||
| Yes | 16 | −2.35 | −7.63, 2.93 | −3.88 | −11.11, 3.34 |
| Age at diabetes diagnosis | |||||
| No diabetes diagnosis | 175 | Ref | Ref | ||
| ≤ 45 | 6 | −4.37 | −12.53, 3.80 | −7.46 | −18.62, 3.70 |
| > 45 | 10 | −1.07 | −7.66, 5.51 | −1.62 | --10.62, 7.38 |
| High Blood Cholesterol | |||||
| No | 127 | Ref | Ref | ||
| Yes | 63 | −5.35 | −8.51, −2.18 | −6.74 | −11.09, −2.38 |
| Age at high blood cholesterol diagnosis | |||||
| No high blood cholesterol | 127 | Ref | Ref | ||
| ≤ 45 | 22 | −6.64 | −11.21, −2.07 | −7.21 | −13.50, −0.92 |
| > 45 | 41 | −4.45 | −8.36, −0.54 | −6.40 | −11.79, −1.02 |
| Number of conditions | |||||
| 0 | 71 | Ref | Ref | ||
| 1 | 46 | −4.15 | −7.93, −0.37 | −0.20 | −5.39, 4.98 |
| 2 | 40 | −6.40 | −11.23, −1.57 | −6.48 | −13.11, 0.14 |
| 3 or 4 | 30 | −7.40 | −12.92, −1.87 | −9.49 | −17.07, −1.91 |
Table 2 displays the regression coefficient estimates and corresponding 95% confidence interval (CI) for age and BMI adjusted associations of each metabolic condition, age at each diagnosis, and the number of multiple conditions with percent density and dense area. Obesity, hypertension and diabetes were not associated with dense area in any of the models. Obesity and hypertension had inverse age-adjusted associations with percent density, but these associations were no longer statistically significant after further adjustment for BMI. High blood cholesterol was associated with significantly lower percent density (β=-5.4, 95% CI: -8.51, -2.18) and dense area (β= -6.74, 95% CI=-11.1, -2.4) in models that adjusted for age and BMI. The associations between high cholesterol diagnosis and breast density were similar for age at diagnosis (e.g., age at diagnosis ≤ 45 and > 45 for percent density were respectively: β=-6.6 95% CI: -11.2, -2.1 and β=-4.45 95% CI: -8.4, -0.5).
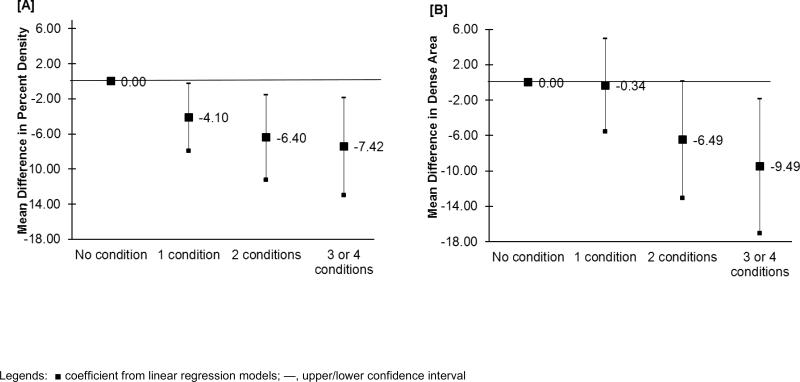
The age and BMI adjusted associations between the number of metabolic conditions and breast density showed a trend toward declining levels of both percent density and dense area with an increasing number of conditions (p-values for trends of <0.01 and =0.01 for percent density and for dense area respectively) (Table 2). Further adjustment for menopausal status and age at menarche did not substantially reduce these associations. For example, having 1, 2, and 3 or 4 conditions were associated with approximately 4.1% (95% CI:-7.9, -0.3), 6.4% (95% CI:-11.2, -1.6), and 7.4% (95% CI: -13.0, -1.9) lower percent density, respectively, as compared with having no metabolic conditions; similar patterns were observed for dense area (Figure 1). These associations were consistent with results from logistic regression models of breast density (categorized into high and low density at the median) (data not shown).
Figure 1.
Associations between the number of metabolic conditions (0 conditions as reference group) and percent breast density (Panel A) and dense area (Panel B), adjusted for age at mammogram, body mass index (kg/m2), age at menarche and menopausal status.
In analysis stratified by obesity, multiple metabolic conditions continued to have inverse associations with percent density and dense area in both obese and nonobese groups (e.g., having 2 or 3 conditions vs. 0 conditions was associated with 5.7% (95% CI: -10.6, -0.7) and 6.3% (95% CI: -14.1, 1.6) reduction in percent density and 9.4 cm2 (95% CI: -17.1, -1.7) and 4.6 cm2 (95% CI: -14.5, 5.4) reduction in dense area among obese and nonobese women, respectively). Similarly, the overall results were similar in pre- and postmenopausal women (data not shown). These results were confirmed by lack of statistical significance of the interaction terms for obesity and menopausal status with the number of metabolic conditions (all p values > 0.11).
Given that diabetes can be a clinical manifestation of other metabolic conditions, we repeated our final multivariable analysis excluding diabetes from the count of number of conditions, and obtained similar overall results (e.g., 5.3% (95% CI: -9.0, -1.6), 9.4% (95% CI:-13.1, -5.7) and 11.2% (95% CI:-16.2, -6.3) reduction in percent density for 1, 2 and 3 conditions, respectively, all relative to no condition).
DISCUSSION
We examined whether obesity, hypertension, high cholesterol and diabetes, individually and in combination, are associated with breast density in a racially diverse sample of women. We observed inverse associations between the presence of each condition and percent density, a relative measure of breast density that incorporates information about dense (mostly fibroglagular) and non-dense (mostly fat) tissues. Accounting for BMI attenuated most of the measures of association between hypertension, high cholesterol and diabetes and percent density. We only observed a statistically significant association between high cholesterol and dense area. Dense area is an absolute measure of breast density that is less susceptible to the confounding influences of body size. We found strong inverse associations between the number of metabolic conditions and both percent density and dense area. These results suggest that the influence of diabetes, hypertension and high cholesterol on breast density are similar to that of body size, and may be mostly explained through their associations with body size. However, our results suggest that the accumulation of metabolic risk factors may confer a greater influence on breast density, beyond the contribution of obesity or any individual conditions.
Most research has considered the contribution of one metabolic risk factor to breast density, and has reported null to modest associations (7, 10, 11, 18). Two studies have examined breast density in relation to the MetS, defined as having three or more the following metabolic conditions: elevated glucose, triglycerides, and blood pressure, low HDL-C and large waist circumference. One study reported modest inverse associations between breast density and the MetS cluster and its individual components in predominantly premenopausal White and Asian women (11), while the other study reported a positive association between breast density and the MetS cluster and low HDL-C among premenopausal women in one of the two cohorts of Mexican women (9). Our study differs from these studies in racial/ethnic composition as well as the measurement of the metabolic conditions. Specifically, These studies measured metabolic conditions through blood biomarkers (HDL-C, triglycerides, glucose) and physical measurements (blood pressure, waist circumference), while we relied on self-reported data on current body size and lifetime diagnosis of metabolic conditions. Other studies of breast density have used self-reports of diabetes, with one study reporting no associations in pre- and postmenopausal women (10), and another study observing strong inverse associations in premenopausal Native American women (7).
While limited data exist for the association between diabetes, hypertension, cholesterol and breast density, body size has been extensively examined and consistently show differential associations with breast cancer and breast density. Specifically, increasing body size is inversely associated with breast density regardless of menopausal status, and though larger body size is inversely associated with premenopausal breast cancer, it is a well-established risk factor for postmenopausal breast cancer risk (4). The MetS is also being increasingly linked with higher risk of poor breast cancer prognostic factors including recurrence, later stage and node positive status, and absence of estrogen, progesterone and/or human epidermal growth factors receptors (19-21). In contrast, breast density has not been associated with these prognostic factors (22-24). It is possible that breast density may not be a mechanism through which metabolic risk factors increase the risk of breast cancer. Given the cross-sectional design of most studies including our own, we cannot rule out the possibility of influences of metabolic conditions on breast density in other life periods and/or on rate of decline in breast density over time.
Self-reports of physician diagnosis of chronic disease conditions have shown acceptable validity in different populations (25, 26). Our measure of cholesterol did not distinguish between HDL and LDL subcomponents, limiting the interpretation of our results. Despite these limitations, our study provides preliminary results suggesting that having multiple metabolic conditions may be associated with lower levels of relative and absolute measures of breast density in a sample primarily composed of premenopausal racial minority women. The reduction in breast density associated with multiple metabolic conditions appears to be greater than the influence of any single condition on breast density. Furthermore, our results suggest that the increased risk of breast cancer conferred by metabolic conditions, as reported in other studies (2), may not involve pathways related to breast density and may be stronger after accounting for the influence of metabolic conditions on breast density.
Acknowledgement
We would like to thank the study participants for contributing data to this analysis and the following research staff for assisting with recruitment activities and data collection/management: Diane Levy, Wendy Lewis, Gladys Rivera, Zoe Quandt, Joy White, Jessica Cabildo, Renata Khanis and Angeline Protacio. This research was funded by grants from the National Cancer Institute (grant numbers: U54 CA101598; K07 CA151777), Susan B. Komen Foundation Career Catalyst award (grant number KG110331) and the National Institute of Environmental Health Sciences Center Support (grant number ES009089).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare there is no conflict of interest.
REFERENCES
- 1.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. The American journal of clinical nutrition. 2007;86:s823–35. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 2.Esposito K, Chiodini P, Capuano A, et al. Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause. 2013 doi: 10.1097/GME.0b013e31828ce95d. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–62. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 4.Boyd NF, Martin LJ, Sun L, et al. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2086–92. doi: 10.1158/1055-9965.EPI-06-0345. [DOI] [PubMed] [Google Scholar]
- 5.Harris HR, Tamimi RM, Willett WC, Hankinson SE, Michels KB. Body size across the life course, mammographic density, and risk of breast cancer. Am J Epidemiol. 2011;174:909–18. doi: 10.1093/aje/kwr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd NF, Connelly P, Byng J, et al. Plasma lipids, lipoproteins, and mammographic densities. Cancer Epidemiol Biomarkers Prev. 1995;4:727–33. [PubMed] [Google Scholar]
- 7.Roubidoux MA, Kaur JS, Griffith KA, et al. Correlates of mammogram density in southwestern Native-American women. Cancer Epidemiol Biomarkers Prev. 2003;12:552–8. [PubMed] [Google Scholar]
- 8.Furberg AS, Jasienska G, Bjurstam N, et al. Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA Study. Cancer Epidemiol Biomarkers Prev. 2005;14:33–40. [PubMed] [Google Scholar]
- 9.Rice MS, Biessy C, Lajous M, et al. Metabolic syndrome and mammographic density in Mexican women. Cancer prevention research. 2013;6:701–10. doi: 10.1158/1940-6207.CAPR-12-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sellers TA, Jensen LE, Vierkant RA, et al. Association of diabetes with mammographic breast density and breast cancer in the Minnesota breast cancer family study. Cancer Causes Control. 2007;18:505–15. doi: 10.1007/s10552-007-0128-9. [DOI] [PubMed] [Google Scholar]
- 11.Conroy SM, Butler LM, Harvey D, et al. Metabolic syndrome and mammographic density: the Study of Women's Health Across the Nation. Int J Cancer. 2011;129:1699–707. doi: 10.1002/ijc.25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamburrini AL, Woolcott CG, Boyd NF, et al. Associations between mammographic density and serum and dietary cholesterol. Breast Cancer Res Treat. 125:181–9. doi: 10.1007/s10549-010-0927-7. [DOI] [PubMed] [Google Scholar]
- 13.Smith SC, Jr., Clark LT, Cooper RS, et al. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: report of the Obesity, Metabolic Syndrome, and Hypertension Writing Group. Circulation 2005. 111:e134–9. doi: 10.1161/01.CIR.0000157743.54710.04. [DOI] [PubMed] [Google Scholar]
- 14.Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004;89:2590–4. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 15.Boden-Albala B. Current understanding of multiple risk factors as the metabolic syndrome: distillation or deconstruction. Semin Neurol. 2006;26:108–16. doi: 10.1055/s-2006-933314. [DOI] [PubMed] [Google Scholar]
- 16.Bjorge T, Lukanova A, Jonsson H, et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev. 2010;19:1737–45. doi: 10.1158/1055-9965.EPI-10-0230. [DOI] [PubMed] [Google Scholar]
- 17.Tehranifar P, Reynolds D, Flom J, et al. Reproductive and menstrual factors and mammographic density in African American, Caribbean, and white women. Cancer Causes Control. 2011;22:599–610. doi: 10.1007/s10552-011-9733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamburrini AL, Woolcott CG, Boyd NF, et al. Associations between mammographic density and serum and dietary cholesterol. Breast Cancer Res Treat. 2011;125:181–9. doi: 10.1007/s10549-010-0927-7. [DOI] [PubMed] [Google Scholar]
- 19.Maiti B, Kundranda MN, Spiro TP, Daw HA. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0591-y. [DOI] [PubMed] [Google Scholar]
- 20.Healy LA, Ryan AM, Carroll P, et al. Metabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clinical oncology. 2010;22:281–8. doi: 10.1016/j.clon.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119:236–8. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson L, Hall P, Czene K, et al. Mammographic density and molecular subtypes of breast cancer. Br J Cancer. 2012;107:18–23. doi: 10.1038/bjc.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma H, Luo J, Press MF, Wang Y, Bernstein L, Ursin G. Is there a difference in the association between percent mammographic density and subtypes of breast cancer? Luminal A and triple-negative breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:479–85. doi: 10.1158/1055-9965.EPI-08-0805. [DOI] [PubMed] [Google Scholar]
- 24.Antoni S, Sasco AJ, dos Santos Silva I, McCormack V. Is mammographic density differentially associated with breast cancer according to receptor status? A meta-analysis. Breast Cancer Res Treat. 2013;137:337–47. doi: 10.1007/s10549-012-2362-4. [DOI] [PubMed] [Google Scholar]
- 25.White K, Avendano M, Capistrant BD, Robin Moon J, Liu SY, Maria Glymour M. Self-reported and measured hypertension among older US- and foreign-born adults. Journal of immigrant and minority health / Center for Minority Public Health. 2012;14:721–6. doi: 10.1007/s10903-011-9549-3. [DOI] [PubMed] [Google Scholar]
- 26.Margolis KL, Lihong Q, Brzyski R, et al. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clinical trials. 2008;5:240–7. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]