Abstract
Social stressors such as depressed maternal care and family conflict are robust challenges which can have long-term physiological and behavioral effects on offspring and future generations. The current study investigates the transgenerational effects of an ethologically relevant chronic social stress on the behavior and endocrinology of juvenile and adult rats. Exposure to chronic social stress during lactation impairs maternal care in F0 lactating dams and the maternal care of the F1 offspring of those stressed F0 dams. The overall hypothesis was that the male and female F2 offspring of stressed F1 dams would display decreased social behavior as both juveniles and adults and that these behavioral effects would be accompanied by changes in plasma corticosterone, prolactin, and oxytocin. Both the female and male F2 offspring of dams exposed to chronic social stress displayed decreased social behavior as juveniles and adults, and these behavioral effects were accompanied by decreases in basal concentrations of corticosterone in both sexes, as well as elevated juvenile oxytocin and decreased adult prolactin in the female offspring. The data support the conclusion that social stress has transgenerational effects on the social behavior of the female and male offspring which are mediated by changes in the hypothalamic–pituitary–adrenal axis and hypothalamic–pituitary–gonadal axis. Social stress models are valuable resources in the study of the transgenerational effects of stress on the behavioral endocrinology of disorders such as depression, anxiety, autism, and other disorders involving disrupted social behavior.
Keywords: Social stress, Early life stress, Postpartum depression, Anxiety, Autism, Corticosterone, Oxytocin, Prolactin, Transgenerational, Social behavior
Introduction
Social stressors such as impaired maternal care and family conflict are robust challenges which can have long-term physiological and behavioral effects on the offspring. Disruptions in social behavior are particularly common in children exposed to maternal depression and/or family conflict (Davalos et al., 2012; Goodman, 2007; Hay et al., 2008; Josefsson and Sydsjo, 2007; Moehler et al., 2007; Verbeek et al., 2012). Furthermore, prior exposure to life stress confers susceptibility to stress-related psychopathologies including postpartum depression (Heim and Binder, 2011; Heim and Nemeroff, 2001). The current study investigated the transgenerational effects of an ethologically relevant social stress on the social behavior and endocrinology of juvenile and adult rats.
In the present model of chronic social stress (CSS), lactating dams (F0 generation) are exposed to intruder male rats for 1 hour daily. F0 dams receiving CSS exhibit deficits in the duration of pup grooming and nursing and increased maternal aggression (Nephew and Bridges, 2011), and thus the female offspring (F1 generation) receive both direct (through decreased maternal care) and indirect (through exposure to aggressive conflict between the dam and intruder males) early life stress. Adult F1 dams exposed to early life CSS also display decreases in maternal care (reduced pup grooming and nursing), impaired lactation through decreased milk intake by the pups, decreased saccharine intake (a measure of anhedonia), decreased maternal aggression, and increased restlessness/anxiety through increases in non-maternal care behaviors such as nesting, self grooming, and activity. On day 2 of lactation, F1 dams exposed to early life CSS exhibit a 60% decrease in the duration of maternal care that is driven by a 79% decrease in nursing and a 60% increase in non-maternal care behaviors. These behavioral effects are associated with decreases in hypothalamic oxytocin (OXT), vasopressin (AVP), and prolactin (PRL) gene expression (Murgatroyd and Nephew, 2013) as well as decreases in basal plasma concentrations of estradiol and prolactin and increased corticosterone during lactation (Carini and Nephew, 2013). Additional developmental elements of the model include the effects of potential hormonal changes in the F0 dams on developing F1 offspring and germ line exposure of the F2 generation during early life stress exposure in the F1 animals. Both the F0 and F1 dams represent ethologically relevant models of postpartum depression and anxiety, and the current study investigated the transgenerational effects of CSS on the F2 generation. In addition to assessing ethologically relevant changes in social behavior to compare with previous studies using maternal care as a measure of anhedonia, saccharin preference was included as a standard abstract measure of anhedonia. Based on previous studies on the transgenerational impact of differences in maternal care and related data from the F1 dams, it was hypothesized that the F2 male and female offspring of CSS dams would exhibit decreases in social behavior which would be accompanied by decreased basal concentrations of plasma corticosterone, oxytocin and prolactin.
Several studies have concluded that the hypothalamic–pituitary–adrenal (HPA) axis plays a central role in mediating the behavioral and physiological effects of early life stress in both animals (Bosch et al., 2007; Catalani et al., 2011; Murgatroyd and Spengler, 2011; Veenema, 2009) and humans (Essex et al., 2002, 2011; Francis et al., 1999; Meaney and Szyf, 2005; Tu et al., 2007). Children exposed to the early life stress of maternal depression exhibit disturbances in HPA activity which may include stressor specific changes in the pattern of basal plasma cortisol (Essex et al., 2002, 2011). Previous studies of the CSS model have reported elevated basal corticosterone levels in the CSS F1 females as nulliparous adults and dams (Carini and Nephew, 2013), which parallels data in adolescents exposed to both maternal depression and family anger (Essex et al., 2011), as the F1 females are exposed to decreased maternal care and the conflict between the dam and the novel male intruder. Several studies in rodents have observed decreased basal corticosterone in adult offspring of dams administered exogenous corticosterone in drinking water (see Catalani et al., 2011 for review). It has been suggested that the level of corticosterone exhibited in these lactating dams is similar to corticosterone levels after maternal stressors (Catalani et al., 2011). It was hypothesized that CSS F2 offspring would have lower basal corticosterone, similar to adolescents exposed primarily to maternal depression during early life and the adult offspring of dams treated with corticosterone during lactation (Catalani et al., 2011; Essex et al., 2011).
The importance of OXT as a mediator of social behavior is well known (Feldman, 2012; Nephew, 2012; Veenema, 2012; Young et al., 2008), and previous data indicate that OXT is a potential mediator of the adverse effects of early life CSS on maternal behavior in lactating dams and their adult offspring (Murgatroyd and Nephew, 2013). While there are numerous reports in animals and humans which indicate that impairments in social behavior are associated with a decrease in OXT (Feldman, 2012; Nephew, 2012), recent clinical work suggests that both high and low levels of OXT can indicate a disruption in typical social interaction, depending on gender and social context (Miller et al., 2013; Parker et al., 2010). The effects of OXT on social behavior can be transgenerational (Champagne, 2008; Curley et al., 2012), which is specifically relevant to the current study of the social behavior of the F2 offspring of socially stressed dams. Based on the decreased hypothalamic OXT gene expression in the F1 generation (Murgatroyd and Nephew, 2013), it was hypothesized that the F2 offspring of CSS dams would have decreased plasma concentrations of OXT.
Prolactin is a key mediator of both mammalian lactation (Freeman et al., 2000; McNeilly et al., 1983; Powe et al., 2010) and maternal care (Bridges, 1994; Bridges et al., 1997; Grattan, 2002; Grattan et al., 2001), although less is known about its role in non-maternal social behavior. PRL also has inhibitory actions on HPA activity following stress exposure which may be related to its role in maternal care (Torner and Neumann, 2002). Study of early life CSS exposed females indicates that plasma PRL concentrations are decreased in both juvenile and maternal early life CSS exposed females, and in maternal dams this decrease in peripheral PRL is associated with impaired maternal care and lactation and decreased expression of the long form of the PRL receptor in the PVN (Carini and Nephew, 2013; Murgatroyd and Nephew, 2013). Since many endocrine mediators of maternal care are also known to affect other forms of social behavior, it was hypothesized that the female F2 offspring would exhibit lower levels of social behavior accompanied by decreased plasma PRL.
The current study investigated the social behavior of the male and female F2 offspring of dams exposed to early life stress as both juveniles and adults and whether these behaviors would be accompanied by changes in plasma corticosterone, PRL, and OXT. The current investigation extends the transgenerational value and application of the CSS model of postpartum mood disorders to early life stress associated disorders in both males and females such as depression, anxiety, and autism. The overall hypothesis was that the male and female F2 offspring of social stressed dams would display decreased social behavior as both juveniles and adults and that these behavioral effects would be accompanied by changes in plasma corticosterone, PRL, and OXT.
Methods
Animals
Sprague-Dawley rats (Charles River Inc., Kingston, NY) in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts Institutional Animal Care and Use Committee. “CSS dams” refers to the adult females exposed to chronic social stress during lactation (F0), “early life CCS F1 dams” refers to the adult female offspring of the CSS dams, and their F2 offspring are the focus of the present study.
CSS model: Creation of F0 dams
The CSS dams were subjected to a chronic social stress protocol from days 2 to 16 of lactation as reported (Carini et al., 2013; Nephew and Bridges, 2011). This procedure consisted of placing a similarly sized (220–300 g) novel male intruder into a lactating female’s home cage for 1 hour from days 2 to 16 of lactation. Control dams were not exposed to the CSS protocol; they were only tested for maternal care and maternal aggression on days 2, 9, and 16 of lactation. The pups are left in the cage during the intruder presentation, and the CSS exposure results in reduced maternal care (pup grooming and nursing) and increased anxiety-related behavior and maternal aggression (Nephew and Bridges, 2011).
Early life CSS: Creation of F1 females and their F2 offspring
The control and early life CSS F1 females were the offspring of the F0 control and CSS dams; the differences between the treatments of the control and early life CSS F1 females were limited to the exposure of the early life CSS F1 females to attenuated maternal care and conflict between their F0 mothers and the male intruders during age 2 to 16 days. The F1 control and early life CSS animals were treated identically after the age of 16 days. After weaning all F1 pups on day 23, the female offspring from the twelve control and twelve CSS dams were housed in groups of four until 70 days of age when two from each litter were mated with 6 proven breeder males (24 F1 females for the control and early life CSS groups). Successful mating was determined by body weight gain. Behavioral and endocrine data from those F1 females have been previously reported (Carini and Nephew, 2013). Total F2 pup number and litter weights were recorded on the day of parturition, and litters were then culled to four females and four males. The F2 control and early life CSS animals were treated identically throughout the study; the only difference between the two groups was the attenuated maternal care (including deficits in pup grooming, nursing, and milk intake by pups) and increased restlessness and anxiety-related behavior (nesting, self-grooming, locomotor activity) expressed by the early life CSS F1 dams towards their F2 offspring. In addition, the F2 animals were exposed to the decreased prolactin and increased corticosterone levels of their F1 mothers during lactation (Carini and Nephew, 2013). The early life stress behavioral experience of the F1 generation included decreased maternal care from the F0 dams and the conflict between the dam and male intruders, where the early life stress experience of the F2 generation only included the exposure to attenuated maternal care from the F1 dams. The final F2 sample sizes were 10 for the control groups and 13 for the early life CSS group, and there were no treatment differences in litter size or number or bodyweights at the juvenile or adult stage, (all p’s > 0.2).
Juvenile social behavior
Juvenile social behavior of the F2 generation was assessed on day 35. From the four same-sex littermates, one was chosen at random to be the focal animal and another was the stimulus animal. These two were then separated and housed individually for 30 min. At the end of the separation period, the focal and stimulus animals were then reunited in a clean cage and filmed for 10 min. The behavior of the focal animal was later scored using Odlog software (Macropod Inc. USA). Behaviors scored consisted of rostral and caudal investigation, lateral contact, dorsal contact, self grooming, allogrooming, wrestling, climbing on the wire cage top, feeding, locomotor activity and total social contact (sum of investigation, contact, allogrooming, and wrestling). All experimental animals were sacrificed between 0800 and 1000 the morning following behavioral testing, and trunk blood was collected for the analysis of basal hormone levels. Littermates and the short separation prior to testing were used to focus on the effects of CSS on littermate social interactions and minimize the effects of juvenile behavioral testing on the later adult behavioral testing.
Adult behavioral testing
Saccharin intake
Randomly selected adult (60–70 day old) experimental animals were separated into single housing the day before social behavior testing. At 1600 the day before behavioral testing, 0.02% saccharin bottles were added to the cage (alternating between right and left side of the cage with each animal) and percentage intake of saccharin versus water in a 16 h period, ending at 0800, was measured by weighing the bottles before and after exposure and dividing the weight of saccharin intake by total fluid intake. Adult social behavior testing commenced after this test.
Adult social behavior testing
The experimental rat was removed from the home cage and placed in a clean breeding cage (16 × 20 × 8 in. for 10 min to allow for locomotor acclimation to the novel environment. An empty clear plastic mouse cage cover with a plastic mesh top was then placed in the breeding cage for 10 min, and a randomly selected same gender novel rat from the same treatment group was placed under the cage top to test for social approach for 10 min. At the end of the 10 min of social approach recording, the mouse cage top was removed, and the focal and novel animals were allowed to interact for 10 min. Behaviors scored for social approach consisted of time spent near and distant from the novel rat (area next to the mouse top was divided into two sections), time spent on top of the mouse cage top, olfactory investigation of the novel rat through the mesh of the mouse cage cover, self grooming, and total social approach (the sum of time near novel rat, on top of mouse cage top, and olfactory investigation). Adult social behaviors scored consisted of rostral and caudal investigation, lateral contact, dorsal contact, tail grabbing, allogrooming, self grooming, locomotor activity, aggression, and total social contact (the sum of investigation, contact, tail grabbing, and allogrooming). All experimental animals were sacrificed between 0800 and 1000 the day following testing, and trunk blood was collected for the analysis of basal hormone levels. Estrous cycle was not assessed in the adult females to avoid the potential effects of increased handling.
Endocrine assays
Plasma corticosterone was measured by commercial RIA kits (Siemens), prolactin was measured using the RIA kit from Dr. A.F. Parlow at the National Hormone and Peptide Program, Harbor-UCLA Medical Center, and oxytocin was measured by commercial ELISA (Enzo Life Sciences). All samples were run in duplicate. Assay sensitivities were as follows: corticosterone = 5.7 ng/ml, prolactin = 0.5 ng/ml, oxytocin = 11.7 pg/ml. Intraassay variability was 3–7%, and interassay variability was 5–10%.
Statistics
Behaviors and hormone levels were compared with 1-way ANOVAs for each gender at the juvenile and adult stages. These ANOVAs were followed by independent one-tailed t-tests if justified by a priori data from related studies or an overall effect on social behavior in the present study. To be clear, a priori decreases in maternal care in previous studies of the CSS F0 and F1 dams (Carini and Nephew, 2013; Nephew and Bridges, 2011) were used to justify the use of one-tailed t-tests for decreases in direct social interaction and related work on early life stress and social recognition (Lukas et al., 2011) was used to justify the use of one-tailed t-tests for the increases in investigation. All assumptions of normality and equality of variance between groups were met. Cohen’s d values were calculated to provide a measure of effect size for all significant differences. All graphical results are presented as mean + SEM, and the level of statistical significance was p ≤ 0.05.
Results
Juvenile social behavior
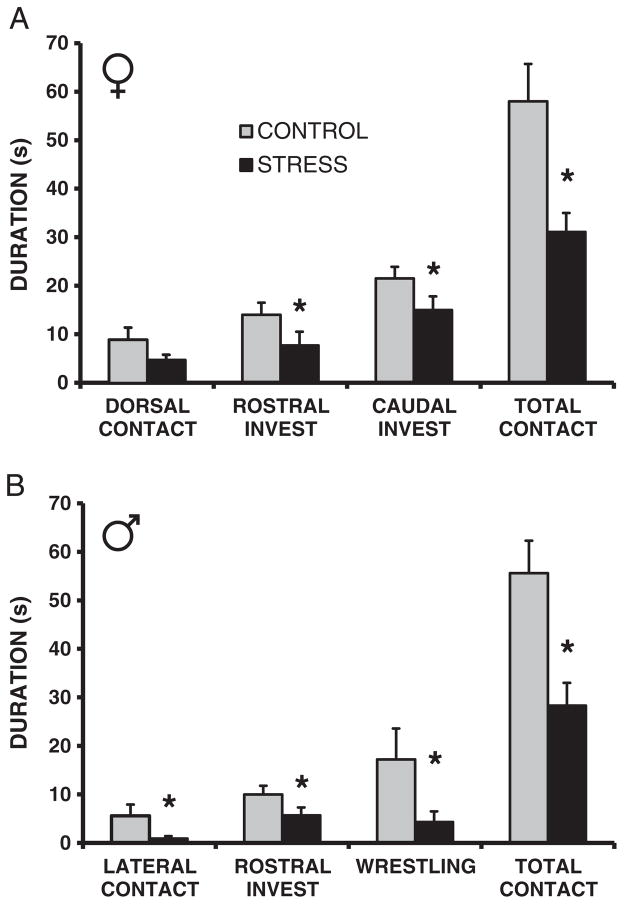
When allowed to interact with a single littermate, the female F2 juvenile offspring of early life CSS treated dams displayed decreased durations of rostral investigation (F1,22 = 6.1, p < 0.05, d = 1.13), caudal investigation (F1,22 = 3.1, p = 0.09, t(21) = 1.8, p < 0.05, d = 0.74), and total social contact (F1,22 = 10.2, p < 0.01, d = 1.38, Fig. 1A). The male CSS F2 juvenile offspring displayed decreased durations of rostral investigation (F1,22 = 3.3, p = 0.09, t(21) = 1.8, p < 0.05, d = 1.16), lateral contact (F1,22 = 4.3, p = 0.05, d = 1.0), wrestling (F1,22 = 3.9, p = 0.06, t(21) = 2.0, p < 0.05, d = 0.9), and total social contact (F1,22 = 11.4, p < 0.01, d = 1.41 Fig. 1B).
Fig. 1.
Transgenerational effect of chronic social stress on juvenile social behavior. Both female (A) and male (B) juvenile offspring of dams exposed to early life stress exhibit reduced social behavior toward a same-sex conspecific compared to offspring of control dams. * = p < 0.05 compared to controls. Invest = investigation.
Adult saccharin preference
There was no effect of CSS on saccharin preference in the adult male CSS F2 rats (control = 82.7 ± 5.2%, CSS = 83.2 ± 4.8%; F1,22 = 0, p = 0.9) or adult female CSS F2 rats (control = 87.7 ± 3.1%, CSS = 79.3 ± 6.9%; F1,22 = 1.1, p = 0.3).
Adult social approach
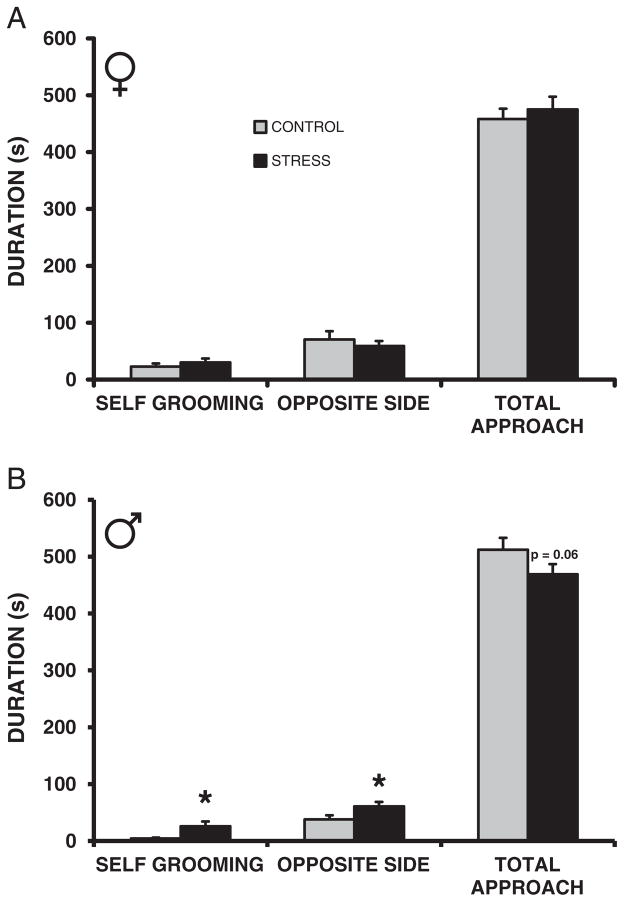
The CSS F2 adult females exhibited a decrease in the duration of rostral investigation (control = 61.5 ± 8.5, css = 34.8 ± 8.0; F1,22 = 5.1, p < 0.05, d = 0.92) during the social approach test, but durations of self grooming, time spent away from the novel animal, and total approach did not differ from controls (Fig. 2A). The CSS F2 adult male offspring spent more time grooming themselves (F1,22 = 5.6, p < 0.05, d = 1.17), away from the novel rat (F1,22 = 4.5, p < 0.05, d = 0.88), and there was a trend for an attenuation in overall social approach (F1,22 = 2.1, p = 0.15, t(21) = 1.7, p = 0.06, d = 0.54) compared to the control F2 offspring (Fig. 2B).
Fig. 2.
Transgenerational effect of chronic social stress on adult social approach behavior. Adult female (A) and male (B) behavior toward a confined same-sex adult conspecific. * = p < 0.05 compared to controls.
Adult social interaction
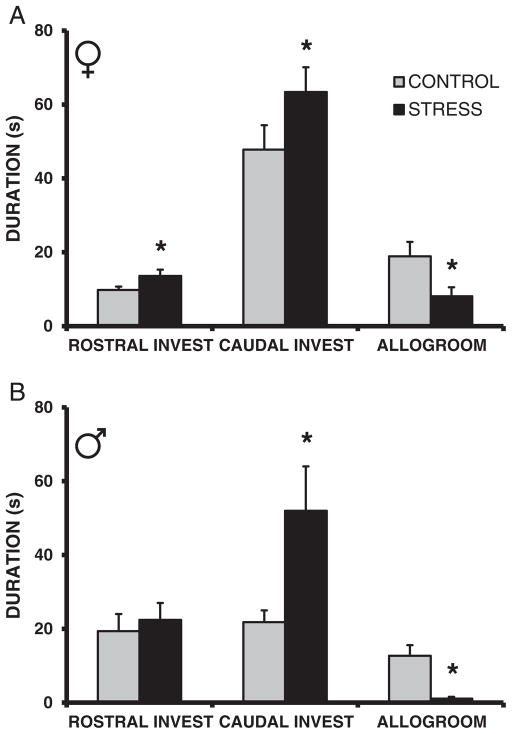
When allowed to directly interact with the novel rat, the CSS F2 adult females had increased durations of rostral (F1,22 = 2.9, p = 0.09, t(21) = 1.8, p < 0.05, d = 0.73) and caudal (F1,22 = 3.0, p = 0.09, t(21) = 1.9, p < 0.05, d = 0.68) investigation but decreased levels of allogrooming (F1,22 = 5.7, p < 0.05, d = 0.96; Fig. 3A). The CSS F2 adult males displayed a similar pattern, with increased caudal investigation (F1,22 = 4.1, p = 0.05, d = 1.13) and attenuated allogrooming (F1,22 = 19.4, p < 0.01, d = 2.04; Fig. 3B).
Fig. 3.
Transgenerational effect of chronic social stress on adult social interaction behavior. Social behavior exhibited by adult female (A) and male (B) offspring of dams exposed to early life stress is altered compared to offspring of control dams. * = p < 0.05 compared to controls. Invest = investigation.
Basal hormones
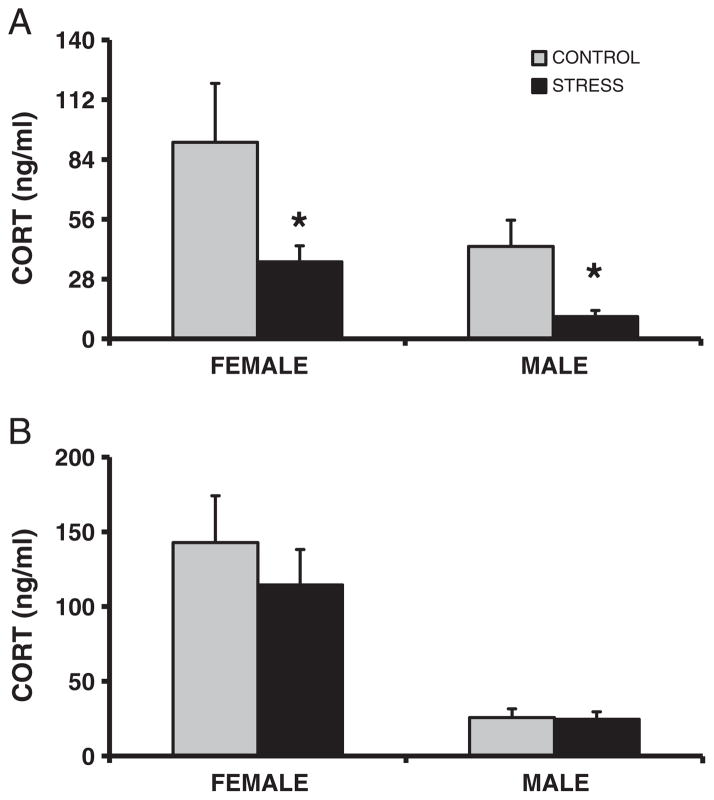
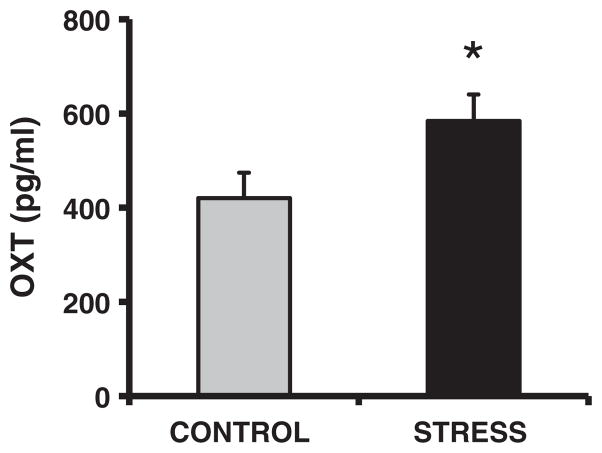
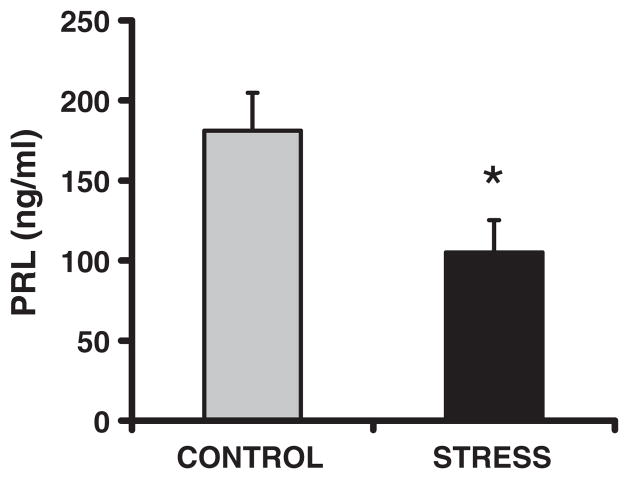
Basal plasma corticosterone concentrations were lower in both the female (F1,22 = 4.4, p < 0.05, d = 0.91) and male (F1,22 = 7.9, p < 0.01, d = 1.19) CSS F2 juveniles (Fig. 4A), however, there was no effect of CSS on this measure in the male or female F2 adults (all p’s > 0.4; Fig. 4B). Basal plasma OXT concentrations were elevated in the female CSS F2 juveniles (F1,22 = 4.4, p < 0.05, d = 1.0 Fig. 5). In the CSS F2 adult females, basal prolactin levels were attenuated (F1,22 = 5.6, p < 0.05, d = 1.12 Fig. 6).
Fig. 4.
Transgenerational effect of chronic social stress on basal corticosterone concentrations. Juvenile (A), but not adult (B), basal plasma corticosterone (CORT; means +1SEM) concentrations are attenuated in the offspring of dams exposed to early life stress compared to offspring of control dams. * = p < 0.05 compared to controls.
Fig. 5.
Basal plasma oxytocin (OXT; means + 1SEM) concentrations are elevated in female juvenile offspring of dams exposed to social stress compared to female juvenile offspring of control dams. * = p < 0.05 compared to controls.
Fig. 6.
Basal plasma prolactin (PRL; means + 1SEM) concentrations are attenuated in adult female offspring of stressed dams, compared to adult females of control dams. * = p < 0.05 compared to controls.
Discussion
Both the female and male F2 offspring of dams exposed to CSS display decreases in social behavior as juveniles and altered social behavior as adults, and these behavioral effects were accompanied by a decrease in basal concentrations of corticosterone in both sexes, as well as elevated juvenile OXT and decreased adult PRL in the female offspring. The data support the hypothesis that CSS has transgenerational effects on the social behavior of female and male offspring and the HPA and hypothalamic pituitary gonadal (HPG) axes. Since these F2 offspring are not directly exposed to the CSS paradigm, it is postulated that changes in the maternal care of the F1 dams (Carini and Nephew, 2013) mediate the effects on F2 behavior and endocrinology.
The observed decreases in F2 juvenile social behavior were consistent across both sexes. Compared to the control juveniles, the socially stressed animals displayed consistently lower levels of social investigation. These data support previous work in rodents (Champagne and Meaney, 2007; Johnson et al., 2011; Meaney, 2001) and humans (Champagne, 2008; Matthews and Phillips, 2012; Pawlby et al., 2008; Plant et al., 2013; Ricks, 1985) on the transgenerational transmission of social behavior. In humans, the offspring of depressed mothers often suffer from mental health disorders that involve deficits in social behavior, such as infant social engagement and depression and anxiety (Burk et al., 2008; Feldman et al., 2009). Emotional distress in mothers is associated with an increased risk of externalizing problems in children which may be mediated by compromised early parenting (Choe et al., 2013; Lovejoy et al., 2000). In addition, attachment behaviors (Ricks, 1985), autistic traits (Constantino and Todd, 2005) and social anxiety (Murray et al., 2008) have been reported to involve transgenerational transmission. Antenatal depression is independently associated with adolescent disruptive behavior disorders and depression (Hay et al., 2010; Pawlby et al., 2009), and can have synergistic effects with childhood maltreatment on adolescent psychopathology (Pawlby et al., 2011; Plant et al., 2013). Pawlby and colleagues (2011) concluded that maternal cumulative stress is a key factor in predicting adolescent antisocial behavior, and it is postulated that interventions which ameliorate the decreased maternal care and/or endocrine changes in the CSS F1 dams may prevent the attenuation of social investigation reported in the current study of the CSS F2 offspring.
As adults, the female CSS F2 offspring spent less time investigating the novel rats during the social approach test, similar to the decrease in the juveniles, and increased self grooming. These effects support previous social and anxiety data from the F0 and F1 dams (Carini and Nephew, 2013; Nephew and Bridges, 2011). Since there were no differences in activity during the social approach test, it is unlikely that the differences in social approach were due to a general effect on locomotor activity. The behavioral patterns changed when the animals were allowed to interact, as the female and male CSS F2 adults displayed increases in rostral and/or caudal investigation. It is possible that this is a compensatory response in the females following the decreased levels of investigation during the social approach test. In contrast to the olfactory investigation data, allogrooming, a more direct form of social interaction, was attenuated in the CSS F2 females and males. While the control animals displayed a typical pattern of rostral and caudal investigation followed by allogrooming, the stress groups spent more time investigating with little allogrooming by the end of the 10 min observation. This increase in olfactory investigation in the CSS F2 animals may indicate impaired social recognition (Lukas et al., 2011). Taken together, the social approach and social interaction data indicate that the F2 CSS offspring were more tentative in engaging in social interaction as adults. In contrast to the changes in adult social behavior, a reward mediated behavior, there were no changes in saccharin preference, a standard measure of anhedonia in animal models of depression. It is suggested that an increased focus on ethologically relevant reward mediated behaviors, such as social behaviors, may generate valuable insights on the development of depression and associated disorders such as social anxiety. The overall decreases in social behavior in the juvenile and adult F2 animals are similar to the decreases in pup grooming reported in the CSS F0 and F1 dams (Carini and Nephew, 2013; Nephew and Bridges, 2011), indicating that CSS has transgenerational effects on female and male social behavior at multiple life history stages. It is hypothesized that these data, along with the previous reports from F0 and F1 dams, represent the transgenerational transmission of social anxiety (Champagne and Meaney, 2007; Curley et al., 2012; Murray et al., 2008).
Changes in the HPA axis are a key mediator of the long-term physiological and psychiatric effects of early life stress (McEwen, 1998). While most rodent studies of early life stress report increases in corticosterone, most of these studies are focused on stress induced corticosterone responses (Meaney and Szyf, 2005). Studies that have investigated basal HPA axis functioning in adult rodents exposed to early life stress have reported mixed results. Attenuated basal corticosterone concentrations have been observed in adult rats exposed to early life stress in the forms of maternal separation/neonatal handling (Faure et al., 2006; Haley et al., 2013; Panagiotaropoulos et al., 2004; Papaioannou et al., 2002; Slotten et al., 2006), postnatal novelty exposure (Tang et al., 2003), and restraint given to pregnant dams (Belay et al., 2011). However, others have observed either no difference or increased basal corticosterone concentrations following early life stress (Kao et al., 2012; Lukkes et al., 2009; Tiba et al., 2008; Veenema and Neumann, 2009). Interestingly, juvenile offspring of dams given corticosterone in the drinking water also display decreased basal corticosterone concentrations (McCormick et al., 2001), similar to the effect observed in the juvenile offspring in the present study. Clearly though, almost all the studies mentioned here represent drastically different forms of early life stress compared to the present study. It is possible that differences in methodology and timing of early life stress protocols could lead to differential outcomes on adult offspring. Due to the observation of attenuated basal corticosterone concentrations in the offspring of dams given drinking water supplemented with corticosterone (Catalani et al., 2011; McCormick et al., 2001) and the elevated basal corticosterone in the F1 dams (Carini and Nephew, 2013), it is postulated that the effects of early life CSS on the F1 mothers mediated the decreased basal corticosterone concentrations observed here in the F2 offspring.
The data presented here may relate to long-term consequences of psychosocial stressors in other species. For example, the decrease in basal corticosterone in the CSS F2 rats parallels studies in nonhuman primates (Capitanio et al., 2005; Feng et al., 2011) and the offspring of depressed mothers (Essex et al., 2011). Nursery reared Rhesus monkeys exposed to suboptimal parental care have lower basal cortisol levels compared to mother raised controls, and an increase in peer exposure during the nursery rearing (increased social experience) increases basal cortisol levels (Capitanio et al., 2005). Study of maternal separation in primates has revealed that peer-reared monkeys have decreased basal hair cortisol levels compared to mother reared monkeys at 1.5 and 3 years old. The peer-reared monkeys display abnormal patterns of social behavior after 3 years of normal social life, indicating that the effects of maternal separation are persistent (Feng et al., 2011). Human offspring exposed to the early life stress of maternal depression have lower basal cortisol concentrations compared to control populations exposed to moderate levels of early life stress (Essex et al., 2011). Furthermore, basal hypoactivity of the HPA axis is associated with several stress-related psychopathologies in humans, including melancholic depression and PTSD (Ehlert et al., 2001). As in humans, impaired maternal care in the early life CSS F1 mothers predicted depressed basal corticosterone levels in the F2 offspring. These data may support the hypothesis that the direction of the effects of stress on the HPA axis is dependent on cumulative severity, or allostatic load (McEwen, 1998). Hypocortisolism may be a marker for allostatic load in children, as offspring with high levels of internalization symptoms have higher stress levels and attenuated average cortisol levels (Badanes et al., 2011). Furthermore, hypocortisolemia may be a marker for susceptibility to developmental stress-related disorders in children (Gunnar and Vazquez, 2001, 2006). Institutional neglect of children is associated with low cortisol (Carlson and Earls, 1997), and a similar observation has been made in children of parents with a diagnosed psychopathology (Fernald et al., 2008). Similar to the clinical findings of Essex et al. (2011), it appears that the CSS model has persistent effects on the HPA activity of young offspring, underscoring the importance of early prevention or intervention measures.
The juvenile F2 rats from stressed dams exhibited substantial attenuations in social behavior which was accompanied by elevated basal OXT in the females. Unlike the substantial attention paid to changes in the HPA axis in rodents exposed to early life stress, very little attention has been paid to long-term changes in either oxytocin or prolactin levels as a result of early life stress, and to our knowledge, none have investigated plasma levels of OXT as a result of early life manipulations. As a result of neonatal handling, adult rats display less OXT neurons in the hypothalamus compared to controls (Todeschin et al., 2009; Winkelmann-Duarte et al., 2007), which is in contrast to the elevated plasma OXT observed here. The direction of the transgenerational effect of early life CSS on F2 plasma OXT was in the opposite direction of our initial hypothesis, but this result supports recent observations in humans (Miller et al., 2013; Parker et al., 2010). Depression has been associated with elevations in plasma OXT, and it has been postulated that this increase is a biomarker for emotional distress and impaired social relationships that are often features of major depression, especially in females (Holt-Lunstad et al., 2011; Parker et al., 2010). Although our initial hypothesis was based on the reported decrease in hypothalamic OXT expression in F1 females (Murgatroyd and Nephew, 2013), maternal rodent studies suggest that peripheral and central OXT may not be correlated (Kojima et al., 2012). It is postulated that general dysregulation of OXT is indicative of disruptions in social behavior, which is supported by studies of couples facing relationship stress. A recent review of the effects of OXT in humans concluded that OXT may promote pro-social behavior in the presence of safe social cues, but it may promote defensive and antisocial behaviors in environments with unsafe or threatening social cues (Olff et al., 2013). Basal plasma OXT is correlated with distressed pair bonds (Taylor et al., 2010), and elevated OXT in females correlates with elevated attachment anxiety (Weisman et al., 2013). Depressed women are also more likely to display greater variability in peripheral OXT release in response to a stressful situation (Cyranowski et al., 2008), and higher OXT is correlated with elevated anxiety in children with high functioning autism as well as healthy controls (Miller et al., 2013). Furthermore, maternal exposure to early life stress is correlated with a threefold increase in the risk of autism in offspring (Roberts et al., 2013). While the mechanism of this relationship is unknown, autism has also been associated with induced or augmented childbirth (which involves OXT treatment) (Gregory et al., 2013) and a single neonatal OXT treatment has long-term effects on developmental and behavioral neural pathways in rodents (Hashemi et al., 2013). Several studies have documented OXT mediated effects of early social environment on social behavior in rodents, and the current study supports this relationship (Veenema, 2012). Central OXT has been shown to mediate the effects of early experience in prairie voles (Bales and Perkeybile, 2012; Carter et al., 2009), and it was concluded that these experiences may play an adaptive role in the development of social behavior. One potential neuroendocrine function of the increased OXT in the female CSS F2 juveniles may be to suppress HPA activity, as long-term elevations of oxytocin decrease corticosterone levels in rats (Petersson et al., 1999). Since the changes in basal plasma OXT were only significant in juveniles, more detailed studies of peripheral and central OXT in adults are needed to determine if OXT mediates adult behavioral patterns as well.
The decrease in basal plasma PRL observed in the adult F2 females supports related data from the F1 dams, where PRL concentrations were attenuated in the maternal females (Carini and Nephew, 2013). Consistent with our current and previous findings, several studies have reported attenuated basal and stress-induced PRL concentrations in adult rats exposed to early life stress in the form of maternal separation (Benetti et al., 2007; Fóscolo et al., 2008; Severino et al., 2004). In conjunction with depressed hypothalamic PRL receptor expression (Murgatroyd and Nephew, 2013), it is concluded that CSS has potent transgenerational suppressive effects on central and peripheral PRL activity. In addition to being a relevant target for the behavioral and physiological symptoms of postpartum depression and anxiety (Powe et al., 2010), PRL may also be involved in disorders that involve changes in social behavior in the juvenile and adult male and female offspring of depressed and anxious mothers.
While it is unknown whether the effects of CSS on F2 behavior and endocrinology are mediated through maternal care, developmental effects, and/or epigenetic mechanisms, it is clear that the social behavior, HPA axis, and reproductive hormones of the F2 juveniles and adults are altered by the ethologically and clinically relevant CSS paradigm. This social stress model and similar paradigms are valuable resources in the study of the transgenerational effects of stress on the behavioral endocrinology of disorders such as depression, anxiety, autism and the many other disorders that are characterized by altered social behavior.
Acknowledgments
We would like to thank the Tufts University Cummings School Laboratory Animals Medicine Service for its outstanding animal care. Gavin Nephew assisted with data collection. This project was funded by NICHD R00 HD HD059943 and a Tufts CTSI Catalyst grant NIH CTSA UL1 TR001064 to BCN.
References
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: associations with family risk and internalizing disorders. Dev Psychopathol. 2011;23:881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM. Developmental experiences and the oxytocin receptor system. Horm Behav. 2012;61:313–319. doi: 10.1016/j.yhbeh.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Belay H, Burton CL, Lovic V, Meaney MJ, Sokolowski M, Fleming AS. Early adversity and serotonin transporter genotype interact with hippocampal glucocorticoid receptor mRNA expression, corticosterone, and behavior in adult male rats. Behav Neurosci. 2011;125:150–160. doi: 10.1037/a0022891. [DOI] [PubMed] [Google Scholar]
- Benetti F, Andrade de Araujo P, Luiz Sanvitto G, Lucion AB. Effects of neonatal novelty exposure on sexual behavior, fear, and stress-response in adult rats. Dev Psychobiol. 2007;49:258–264. doi: 10.1002/dev.20181. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Musch W, Bredewold R, Slattery DA, Neumann ID. Prenatal stress increases HPA axis activity and impairs maternal care in lactating female offspring: implications for postpartum mood disorder. Psychoneuroendocrinology. 2007;32:267–278. doi: 10.1016/j.psyneuen.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Bridges RS. The role of lactogenic hormones in maternal behavior in female rats. Acta Paediatr. 1994;397:33–39. doi: 10.1111/j.1651-2227.1994.tb13263.x. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Robertson MC, Shiu RPC, Sturgis JD, Henriquez BM, Mann PE. Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology. 1997;138:756–763. doi: 10.1210/endo.138.2.4921. [DOI] [PubMed] [Google Scholar]
- Burk LR, Park JH, Armstrong JM, Klein MH, Goldsmith HH, Zahn-Waxler C, Essex MJ. Identification of early child and family risk factors for aggressive victim status in first grade. J Abnorm Child Psychol. 2008;36:513–526. doi: 10.1007/s10802-007-9196-2. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic–pituitary–adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Carini LM, Nephew BC. Effects of early life social stress on endocrinology, maternal behavior, and lactation in rats. Horm Behav. 2013;64:634–641. doi: 10.1016/j.yhbeh.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini LM, Murgatroyd CA, Nephew BC. Using chronic social stress to model postpartum depression in lactating rodents. J Vis Exp. 2013:e50324. doi: 10.3791/50324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann N Y Acad Sci. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pounajafi-Nazarloo H, Bales KL. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci. 2009;31:25–37. doi: 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalani A, Alema GS, Cinque C, Zuena AR, Casolini P. Maternal corticosterone effects on hypothalamus–pituitary–adrenal axis regulation and behavior of the offspring in rodents. Neurosci Biobehav Rev. 2011;35:1502–1517. doi: 10.1016/j.neubiorev.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121:1353. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Choe DE, Olson SL, Sameroff AJ. Effects of early maternal distress and parenting on the development of children’s self-regulation and externalizing behavior. Dev Psychopathol. 2013;25:437–453. doi: 10.1017/S0954579412001162. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57:655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Curley JP, Jensen CL, Franks B, Champagne FA. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm Behav. 2012;61:454–461. doi: 10.1016/j.yhbeh.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Womens Ment Health. 2012;15:1–14. doi: 10.1007/s00737-011-0251-1. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus–pituitary–adrenal axis. Biol Psychol. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Kalin NH, Armstrong JM. Influence of early life stress on later hypothalamic–pituitary–adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23:1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure J, Uys JK, Marais L, Stein D, Daniels WU. Early maternal separation followed by later stressors leads to dysregulation of the HPA-axis and increases in hippocampal NGF and NT-3 levels in a rat model. Metab Brain Dis. 2006;21:172–179. doi: 10.1007/s11011-006-9013-6. [DOI] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Horm Behav. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Feng X, Wang L, Yang S, Qin D, Wang J, Li C, Lv L, Ma Y, Hu X. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proc Natl Acad Sci. 2011;108:14312–14317. doi: 10.1073/pnas.1010943108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LCH, Burke HM, Gunnar MR. Salivary cortisol levels in children of low-income women with high depressive symptomatology. Dev Psychopathol. 2008;20:423–436. doi: 10.1017/S0954579408000205. [DOI] [PubMed] [Google Scholar]
- Fóscolo D, Fóscolo R, Marubayashi U, Reis A, Coimbra C. Neonatal maternal separation affects endocrine and metabolic stress responses to ether exposure but not to restraint exposure in adult rats. Metab Brain Dis. 2008;23:375–385. doi: 10.1007/s11011-008-9102-9. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney M. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annu Rev Clin Psychol. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Grattan DR. Behavioural significance of prolactin signalling in the central nervous system during pregnancy and lactation. Reproduction. 2002;123:497–506. doi: 10.1530/rep.0.1230497. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Pi XJ, Andrews ZB, Augustine RA, Kokay IC, Summerfield MR, Todd B, Bunn SJ. Prolactin receptors in the brain during pregnancy and lactation: implications for behavior. Horm Behav. 2001;40:115–124. doi: 10.1006/hbeh.2001.1698. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda M. Association of autism with induced or augmented childbirth in North Carolina birth record (1990–1998) and education research (1997–2007) databases. JAMA Pediatr. 2013;167:959–966. doi: 10.1001/jamapediatrics.2013.2904. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cohen DCDJ, editor. Developmental psychopathology. 2. Vol. 2. John Wiley & Sons Inc; Hoboken, NJ, US: 2006. pp. 533–577. Developmental Neuroscience. [Google Scholar]
- Haley S, Neff K, Gulliver K, Gough G, Slater H, Lane RH, Moyer-Mileur LJ. Mechanical-tactile stimulation (MTS) intervention in a neonatal stress model alters adult adipose tissue deposition and prevents hyperinsulinemia in male rats. Early Hum Dev. 2013;89:387–392. doi: 10.1016/j.earlhumdev.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Hashemi F, Tekes K, Laufer R, Szegi P, Tóthfalusi L, Csaba G. Effect of a single neonatal oxytocin treatment (hormonal imprinting) on the biogenic amine level of the adult rat brain: could oxytocin-induced labor cause pervasive developmental diseases? Reprod Sci. 2013;20:1255–1263. doi: 10.1177/1933719113483010. [DOI] [PubMed] [Google Scholar]
- Hay DF, Pawlby S, Waters CS, Sharp D. Antepartum and postpartum exposure to maternal depression: different effects on different adolescent outcomes. J Child Psychol Psychiatry. 2008;49:1079–1088. doi: 10.1111/j.1469-7610.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- Hay DF, Pawlby S, Waters CS, Perra O, Sharp D. Mothers’ antenatal depression and their children’s antisocial outcomes. Child Dev. 2010;81:149–165. doi: 10.1111/j.1467-8624.2009.01386.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp Neurol. 2011;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham W, Light KC. The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology. 2011;36:1249–1256. doi: 10.1016/j.psyneuen.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM. Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Front Psychiatry Front Res Found. 2011;2:29. doi: 10.3389/fpsyt.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson A, Sydsjo G. A follow-up study of postpartum depressed women: recurrent maternal depressive symptoms and child behavior after four years. Arch Womens Ment Health. 2007;10:141–145. doi: 10.1007/s00737-007-0185-9. [DOI] [PubMed] [Google Scholar]
- Kao GS, Cheng LY, Chen LH, Tzeng WY, Cherng CG, Su CC, Wang CY, Yu L. Neonatal isolation decreases cued fear conditioning and frontal cortical histone 3 lysine 9 methylation in adult female rats. Eur J Pharmacol. 2012;697:65–72. doi: 10.1016/j.ejphar.2012.09.040. [DOI] [PubMed] [Google Scholar]
- Kojima S, Stewart RA, Demas GE, Alberts JR. Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother–pup interaction that induces a filial huddling preference. J Neuroendocrinol. 2012;24:831–840. doi: 10.1111/j.1365-2826.2012.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–853. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Matthews S, Phillips D. Transgenerational inheritance of stress pathology. Exp Neurol. 2012;233:95–101. doi: 10.1016/j.expneurol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Rioux T, Fisher R, Lang K, MacLaury K, Teillon SM. Effects of neonatal corticosterone treatment on maze performance and HPA axis in juvenile rats. Physiol Behav. 2001;74:371–379. doi: 10.1016/s0031-9384(01)00574-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Robinson IC, Houston MJ, Howie PW. Release of oxytocin and prolactin in response to suckling. Br Med J. 1983;286:257–259. doi: 10.1136/bmj.286.6361.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney M, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Bales KL, Taylor SL, Yoon J, Hostetler CM, Carter CS, Solomon M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: sex differences and associations with symptoms. Autism Res. 2013;6:91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehler E, Kagan J, Parzer P, Brunner R, Reck C, Wiebel A, Poustka L, Resch F. Childhood behavioral inhibition and maternal symptoms of depression. Psychopathology. 2007;40:446–452. doi: 10.1159/000107429. [DOI] [PubMed] [Google Scholar]
- Murgatroyd CA, Nephew BC. Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology. 2013;38:219–228. doi: 10.1016/j.psyneuen.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Spengler D. Epigenetic programming of the HPA axis: early life decides. Stress. 2011;14:581–589. doi: 10.3109/10253890.2011.602146. [DOI] [PubMed] [Google Scholar]
- Murray L, De Rosnay M, Pearson J, Bergeron C, Schofield E, Royal-Lawson M, Cooper PJ. Intergenerational transmission of social anxiety: the role of social referencing processes in infancy. Child Dev. 2008;79:1049–1064. doi: 10.1111/j.1467-8624.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- Nephew BC. Behavioral Roles of Oxytocin and Vasopressin. In: Sumiyoshi T, editor. Neuroendocrinology and Behavior. InTech; 2012. [Google Scholar]
- Nephew BC, Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011;14:677–684. doi: 10.3109/10253890.2011.605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Panagiotaropoulos T, Papaioannou A, Pondiki S, Prokopiou A, Stylianopoulou F, Gerozissis K. Effect of neonatal handling and sex on basal and chronic stress-induced corticosterone and leptin secretion. Neuroendocrinology. 2004;79:109–118. doi: 10.1159/000076633. [DOI] [PubMed] [Google Scholar]
- Papaioannou A, Gerozissis K, Prokopiou A, Bolaris S, Stylianopoulou F. Sex differences in the effects of neonatal handling on the animal’s response to stress and the vulnerability for depressive behaviour. Behav Brain Res. 2002;129:131–139. doi: 10.1016/s0166-4328(01)00334-5. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, Schatzberg AF. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res. 2010;178:359–362. doi: 10.1016/j.psychres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlby S, Sharp D, Hay D, O’Keane V. Postnatal depression and child outcome at 11 years: the importance of accurate diagnosis. J Affect Disord. 2008;107:241–245. doi: 10.1016/j.jad.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Pawlby S, Hay DF, Sharp D, Waters CS, O’Keane V. Antenatal depression predicts depression in adolescent offspring: prospective longitudinal community-based study. J Affect Disord. 2009;113:236–243. doi: 10.1016/j.jad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Pawlby S, Hay D, Sharp D, Waters CS, Pariante CM. Antenatal depression and offspring psychopathology: the influence of childhood maltreatment. Br J Psychiatry. 2011;199:106–112. doi: 10.1192/bjp.bp.110.087734. [DOI] [PubMed] [Google Scholar]
- Petersson M, Hulting AL, Uvnäs-Moberg K. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neurosci Lett. 1999;264:41–44. doi: 10.1016/s0304-3940(99)00159-7. [DOI] [PubMed] [Google Scholar]
- Plant DT, Barker ED, Waters CS, Pawlby S, Pariante CM. Intergenerational transmission of maltreatment and psychopathology: the role of antenatal depression. Psychol Med. 2013;43:519–528. doi: 10.1017/S0033291712001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe CE, Allen M, Puopolo KM, Merewood A, Worden S, Johnson LC, Fleischman A, Welt CK. Recombinant human prolactin for the treatment of lactation insufficiency. Clin Endocrinol. 2010;73:645–653. doi: 10.1111/j.1365-2265.2010.03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricks MH. The social transmission of parental behavior: attachment across generations. Monogr Soc Res Child Dev. 1985;50:211–227. [Google Scholar]
- Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, Weisskopf MG. Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry. 2013;70:508–515. doi: 10.1001/jamapsychiatry.2013.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severino GS, Fossati IAM, Padoin MJ, Gomes CM, Trevizan L, Sanvitto GL, Franci CR, Anselmo-Franci JA, Lucion AB. Effects of neonatal handling on the behavior and prolactin stress response in male and female rats at various ages and estrous cycle phases of females. Physiol Behav. 2004;81:489–498. doi: 10.1016/j.physbeh.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KCF. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: gender-dependent effects. Brain Res. 2006;1097:123–132. doi: 10.1016/j.brainres.2006.04.066. [DOI] [PubMed] [Google Scholar]
- Tang AC, Reeb BC, Romeo RD, McEwen BS. Modification of social memory, hypothalamic–pituitary–adrenal axis, and brain asymmetry by neonatal novelty exposure. J Neurosci. 2003;23:8254–8260. doi: 10.1523/JNEUROSCI.23-23-08254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair–bond relationships? Psychol Sci. 2010;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Tiba PA, Tufik S, Suchecki D. Long lasting alteration in REM sleep of female rats submitted to long maternal separation. Physiol Behav. 2008;93:444–452. doi: 10.1016/j.physbeh.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Todeschin AS, Winkelmann-Duarte EC, Jacob MHV, Aranda BCC, Jacobs S, Fernandes MC, Ribeiro MFM, Sanvitto GL, Lucion AB. Effects of neonatal handling on social memory, social interaction, and number of oxytocin and vasopressin neurons in rats. Horm Behav. 2009;56:93–100. doi: 10.1016/j.yhbeh.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Torner L, Neumann ID. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. 2002;5:249–257. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- Tu MT, Grunau RE, Petrie-Thomas J, Haley DW, Weinberg J, Whitfield MF. Maternal stress and behavior modulate relationships between neonatal stress, attention, and basal cortisol at 8 months in preterm infants. Dev Psychobiol. 2007;49:150–164. doi: 10.1002/dev.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Veenema AH. Toward understanding how early-life social experiences alter oxytocin- and vasopressin-regulated social behaviors. Horm Behav. 2012;61:304–312. doi: 10.1016/j.yhbeh.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology. 2009;34:463–467. doi: 10.1016/j.psyneuen.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Verbeek T, Bockting CL, van Pampus MG, Ormel J, Meijer JL, Hartman CA, Burger H. Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. J Affect Disord. 2012;136:948–954. doi: 10.1016/j.jad.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, Feldman R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology. 2013;38:694–701. doi: 10.1016/j.psyneuen.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Winkelmann-Duarte EC, Todeschin AS, Fernandes MC, Bittencourt LC, Pereira GAM, Samios VN, Schuh AFS, Achaval ME, Xavier LL, Sanvitto GL, Mandarimde-Lacerda CA, Lucion AB. Plastic changes induced by neonatal handling in the hypothalamus of female rats. Brain Res. 2007;1170:20–30. doi: 10.1016/j.brainres.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol Toxicol Pharmacol. 2008;148:401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]