Abstract
An elderly man with long-standing, non-resectable pheochromocytoma rapidly developed rectal adenocarcinoma despite close endoscopic surveillance. We determined that the patient’s colorectal cancer over-expressed M3 subtype muscarinic receptors, whereas his pheochromocytoma expressed choline acetyltransferase, an enzyme required to produce acetylcholine, a muscarinic receptor agonist. These findings suggested that acetylcholine release from the pheochromocytoma stimulated rapid growth of the rectal neoplasm. As proof-of-principle, we demonstrate here that culture media conditioned by pheochromocytoma cells stimulates proliferation of a human colon cancer cell line, an effect attenuated by atropine, a muscarinic receptor inhibitor. Our observations provide both clinical and laboratory evidence that muscarinic receptor agonists promote the growth of colorectal neoplasia.
The development of colorectal cancer, the second most common cause of cancer death in the United States, is associated with the accumulation of somatic gene mutations that promote cell dysplasia.1,2 Environmental factors augment and accelerate transition of normal colon epithelial cells to adenomas and adenocarcinomas. For example, diets high in fat and meat content increase fecal bile acids and colon cancer risk.3 Muscarinic receptors, primarily subtype three (M3R), are expressed widely in the gastrointestinal tract and over-expressed in colon cancer.4 Conjugated bile acids may interact functionally with M3R, thereby stimulating colon cancer cell proliferation.5–7 Moreover, human colon cancer cells commonly express choline acetyltransferase (ChAT) and release acetylcholine, which can result in autocrine stimulation of colon cancer cell proliferation.8 Collectively, these findings provide evidence that muscarinic receptor agonists may be important growth factors in colon cancer. Indeed, in mouse models of intestinal neoplasia, deficiency of CHRM3, the gene encoding M3R, robustly attenuates intestinal tumor formation,9,10 whereas treatment with a muscarinic receptor agonist promotes tumorigenesis.11
Here we describe a man with a long-standing unresectable pheochromocytoma who, despite close endoscopic surveillance, developed invasive rectal adenocarcinoma in an exceedingly short time after removal of a rectal adenoma. The rapidity of rectal cancer development in this patient is distinctly unusual. Studies of the natural history of colorectal polyps indicate that normally little or no growth is observed over several years of surveillance.12,13 Prior work demonstrating that pheochromocytoma cells can synthesize acetylcholine,14 suggested to us that acetylcholine production and release from our patient’s pheochromocytoma was likely to have played a role in stimulating rapid growth of the rectal cancer.
CASE
In the late 1970’s, an African-American man born in 1932 was diagnosed with a right adrenal pheochromocytoma when he presented with palpitations, headache and diaphoresis. In 1982 he underwent right adrenalectomy and nephrectomy, and was well until 1993 when these symptoms returned. In 1995, he was hospitalized at the Johns Hopkins University Medical Center in hypertensive crisis with blood pressures as high as 300/180 mmHg. He was diagnosed with recurrent pheochromocytoma but declined surgery. Symptoms were initially controlled with adrenergic blockade, but recurred. In 2003, he was evaluated at the Baltimore VA Medical Center; CT and PET scans demonstrated a 5 × 4 cm mass in the right renal fossa and aortocaval region, consistent with recurrent pheochromocytoma. In 2004, he was referred to the National Institutes of Health where resection of the pheochromocytoma was unsuccessful due to tumor infiltration into the inferior vena cava – only an incisional biopsy was performed.
Also in 2004, the patient underwent colonoscopy at the Baltimore VA Medical Center for evaluation of a positive fecal occult blood test. A 1-cm rectal polyp (Figure 1, A) was removed by snare electrocautery. Histological examination revealed a tubular adenoma with a focus of high-grade dysplasia. Six months later, sigmoidoscopy revealed residual rectal polyp tissue versus scar (Figure 1, B), which was removed and identified histologically as tubular adenoma without high-grade dysplasia. Three months later, repeat sigmoidoscopy with biopsies of the prior polypectomy site (Figure 1, C) revealed no residual adenomatous tissue. In 2007, 28 months after the unremarkable sigmoidoscopy and biopsies, he developed rectal bleeding. Colonoscopy revealed a 3- to 4-cm moderately well-differentiated rectal adenocarcinoma (Figure 1, D), stage T2N0. He was treated with neoadjuvent chemotherapy and radiation followed by a low-anterior resection, and 6 years later has no evidence of recurrence.
Figure 1. Endoscopic Findings.
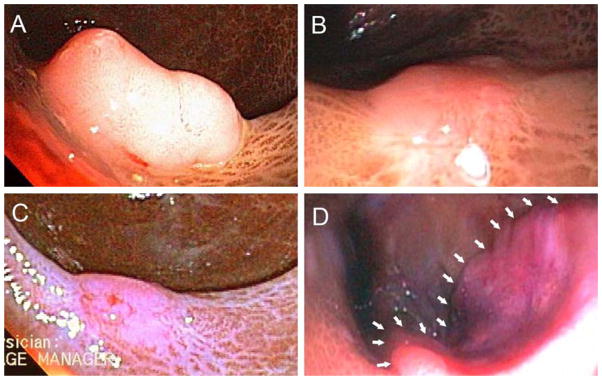
Panel A shows our patient’s rectal polyp prior to initial polypectomy. Panel B displays the site six-months later when additional polypectomy revealed residual adenoma. Panel C shows a polypectomy scar after an additional three months. Multiple biopsies obtained at this time showed no adenomatous tissue. Panel D demonstrates the rectal adenocarcinoma found 28 months after the unremarkable sigmoidoscopy with biopsy (white arrows outline the proximal tumor margin).
METHODS
Immunohistochemistry and Real-Time PCR
Immunohistochemistry
Five-micrometer sections were obtained from formalin-fixed, paraffin-embedded sections of pheochromocytoma, tubular adenoma prior to development of cancer, colorectal cancer prior to anti-cancer therapy, and normal colorectal tissue removed at the time of surgical resection. Additional sections were obtained from five archival pheochromocytoma pathology specimens. Pheochromocytoma sections were stained for ChAT using goat anti-ChAT affinity purified polyclonal antibody (Millipore) according to the manufacturer’s protocol. Sections derived from the colorectal polyp, colorectal cancer, and normal colon were stained for M3R using purified rabbit anti-M3R polyclonal antibody (Alomone Labs) according to the manufacturer’s protocol. Sections were examined under light microscopy and photographed using a Nikon 80i photo-microscope.
Quantitative real-time PCR
RNA was extracted from formalin-fixed, paraffin-embedded samples of colorectal cancer obtained by endoscopic biopsy prior to anti-cancer treatment and non-cancerous colorectal tissue obtained from uninvolved areas of the surgical resection specimen. Total RNA was extracted using the RNeasy FFPE kit (Qiagen). RNA purity and integrity were determined by the A260/280 ratio and the RNA integrity number (RIN) from an Agilent 2100 Bioanalyzer. Equal amounts of RNA were converted to cDNA with M-MLV reverse transcriptase (Promega). Specific primers for all five muscarinic subtypes and the housekeeping gene GAPDH were used for SYBR green qRT-PCR, measuring cycle thresholds on an Applied Biosystems 7500 real-time PCR system. Results were analyzed using the 2−ΔΔCt comparative method with GAPDH as control.15
Conditioned Media Experiments
Cell culture
T-75 culture flasks coated with type IV collagen (BD Biosciences) were seeded with ~5×106 PC-12 rat pheochromocytoma cells (ATCC # CRL-1721) and grown in RPMI-1640 media (GIBCO BRL) supplemented with 10% heat-inactivated horse serum (ATCC), 5% fetal bovine serum (ATCC) and 100 ng/mL nerve growth factor (Sigma-Aldrich) to promote a neuronal phenotype. Growth media were renewed every two to three days. H508 human colon cancer cells (ATCC # CCL-253) were grown in RPMI-1640 supplemented with 10% FBS. Media were renewed every two to three days, and cells were passaged weekly at sub-confluence after trypsinization. Cell cultures were maintained in incubators at 37°C in an atmosphere of 5% CO2 and 95% air.
Conditioned Media
Serum-free RPMI-1640 with potassium concentration adjusted to 55 mM and 10 μM eserine hemisulfate added served as the basis of all test media. After 8 days growth, adherent PC-12 cells were washed with 10 mL serum-free media and incubated for 10 min with 10 mL of test media. To test its effect on H508 cell proliferation, the resulting ‘conditioned media’ was used immediately.
Cell Proliferation Assay
H508 cells were seeded in 96-well plates (Corning Glass Works, Corning, NY) at ~10% confluence and allowed to attach for 24 h. Cells were washed with serum-free media and serum starved for 2 h before incubation with 200 μL of conditioned or control media (15 wells per test condition). In parallel experiments, cells were pre-incubated with 5 μM atropine for 30 min before incubation with unconditioned or conditioned media containing 5 μM atropine. After adding test media, cells were incubated for 5 days at 37°C in an atmosphere of 5% CO2-95% air without further additions. After 5-day incubation, cell proliferation was measured by adding 20 μL CellTiter 96 Aqueous One solution (Promega) to each well. After three to four h incubation at 37°C, absorbance was measured at 490 nm using a 96-well microtiter plate reader (SpectraMax384). Data were analyzed using the two-tailed Student’s t-test (GraphPad Prism, version 5).
RESULTS
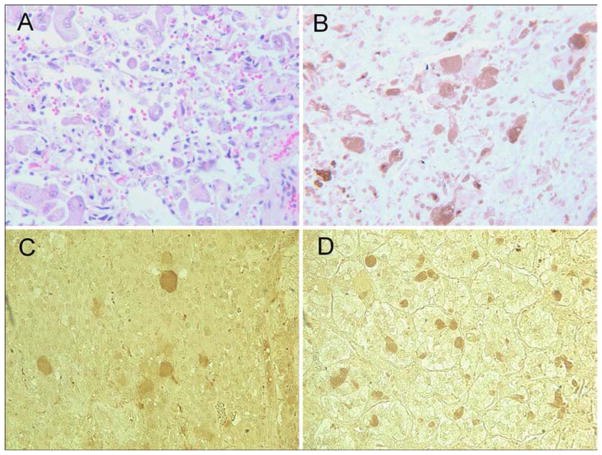
Immunohistochemical staining of our patient’s pheochromocytoma tissue revealed strong staining for ChAT (Figure 2, B), the principal enzyme required for acetylcholine production. ChAT staining was also detected in two of five additional archival pheochromocytomas from other patients (Figure 2, C and D).
Figure 2. Histopathological and Immunohistochemical Evaluation of Pheochromocytoma.
Histopathological evaluation of the pheochromocytoma showed connective tissue with clusters and isolated pleomorphic tumor cells with abundant and granular cytoplasm (Panel A, H&E stain). Immunohistochemical staining for choline acetyltransferase (ChAT) shows strong cytoplasmic staining of most tumor cells in this field (Panel B, anti-ChAT stain). Two of five additional archival specimens stained positively for ChAT (Panels C & D, anti-ChAT stain). ChAT = choline acetyltransferase.
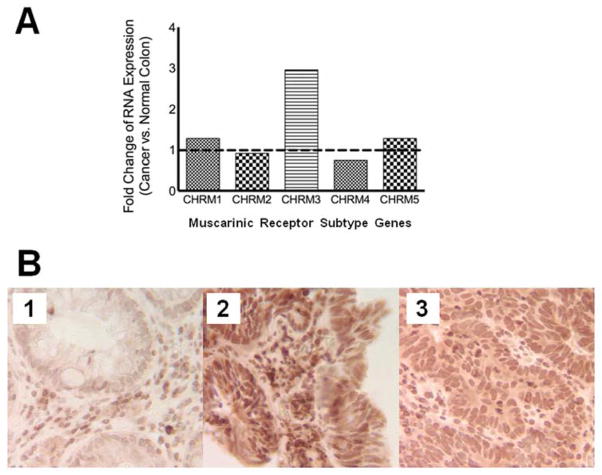
Expression of all five muscarinic receptor subtypes was identified by qRT-PCR in our patient’s colorectal cancer as well as in adjacent normal colon. However, for only CHRM3, the gene encoding M3R, expression was greater (2.95-fold) in the cancer compared to normal colon (Figure 3, A). Because there were no meaningful differences in expression of the other four muscarinic receptor genes when comparing cancer to normal colon (fold-change range 0.74 – 1.28), we focused on expression of M3R.
Figure 3. M3 muscarinic receptor (M3R) gene and protein expression.
Panel A shows muscarinic receptor subtype gene expression in our patient’s colorectal cancer compared to adjacent normal colon tissue. The dashed line represents equivalence. Results were obtained by real-time PCR using the 2−ΔΔCt comparative method using GAPDH as the relative control. Panel B shows immunohistochemical staining for M3R (see Methods) and reveals weak staining in normal colon mucosa (Panel B1) whereas robust staining is observed in a fragment of the tubular adenoma removed by endoscopic biopsy prior to progression to cancer (Panel B2). Staining for M3R in an endoscopic biopsy of the moderately well-differentiated colorectal cancer (Panel B3) was less intense than that observed in the polyp (Panel B2) but substantially greater than that observed in normal colon (Panel B1). M3R = muscarinic receptor subtype 3.
Immunohistochemical staining for M3R in tissue samples from our patient revealed weak staining in normal colorectal mucosa (Figure 3, B1), strong staining in endoscopic biopsies of his tubular adenoma obtained prior to progression to cancer (Figure 3, B2), and intermediate staining in endoscopic biopsies of his moderately well-differentiated colorectal cancer (Figure 3, C). Notably, M3R staining in the cancer was less intense than that observed in the polyp, but substantially greater than that observed in normal colon (Figure 3, B2 and B3).
To provide additional evidence that acetycholine release from pheochromocytoma cells could stimulate human colon cancer cell proliferation, we used pheochromocytoma cells that were previously shown to synthesize and release acetylcholine.14 We found that adding PC-12 pheochromocytoma cell ‘conditioned media’ to H508 human colon cancer cells stimulated a 130% increase in cell proliferation compared to otherwise identical, but unconditioned, media (P<0.001). Moreover, adding atropine significantly attenuated the proliferative effects of ‘conditioned media’ (P<0.001) (Figure 4). In control experiments, addition of atropine to unconditioned media resulted in a small, but significant (P<0.01) reduction in H508 cell proliferation; however, this reduction was substantially less than that observed when atropine was added to conditioned media (P<0.001) (data not shown). We concluded that H508 cell proliferation was driven, in part, by acetylcholine release from the pheochromocytoma cells.
Figure 4. Results of Conditioned Media Experiments.
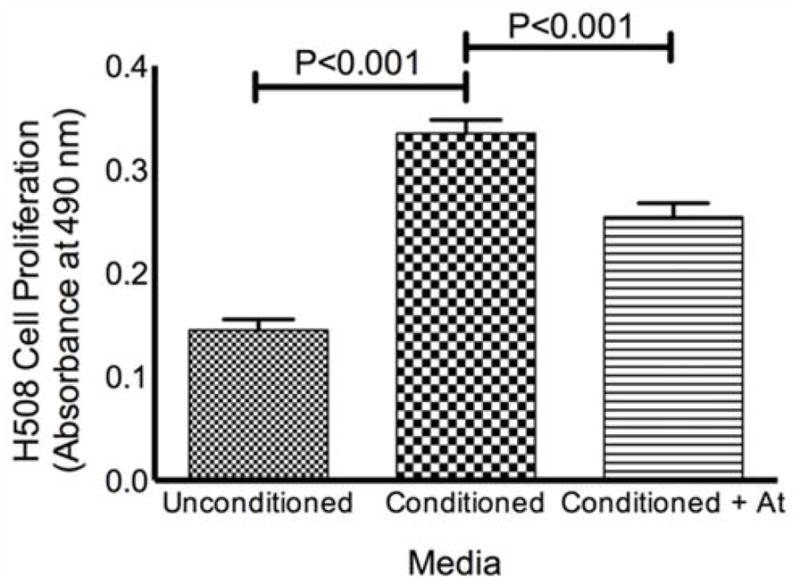
Media exposed to PC-12 pheochromocytoma cells (‘conditioned media’) stimulates proliferation of H508 human colon cancer cells. H508 cell proliferation was determined by absorbance at 490 nm as described in Methods. The proliferative effects of ‘conditioned media’ were significantly attenuated in the presence of 5 μM atropine. Data represent means and standard errors from three independent experiments. At = atropine.
DISCUSSION
Genetic and environmental factors modulate the development and rate of progression of colorectal cancer. Whereas previous in vitro and in vivo animal studies identified a role for muscarinic receptor signaling in modulating progression of colorectal neoplasia,9,16–18 this unusual case and the experimental data presented herein provide compelling evidence that acetylcholine is an important growth factor in human colorectal cancer. We have demonstrated that our patient’s pheochromocytoma expressed the principal enzyme required for acetylcholine production; that his colorectal cancer over-expressed CHRM3, the gene encoding M3 muscarinic receptors; and that, compared to his normal colon epithelium, both his colorectal polyp and cancer over- expressed M3R protein. It is of interest that M3R expression was greater in the polyp than the cancer; this finding suggests that muscarinic receptor activation may also play a role in progression of colon dysplasia to cancer, a hypothesis that we will pursue in future studies.
Collectively, these findings provide a plausible explanation for ultrarapid growth of colorectal cancer in our patient; acetylcholine, and possibly other growth factors, produced and released into the blood stream from the pheochromocytoma stimulated rapid growth of the colon cancer. Our in vitro experiments provide additional support for this hypothesis; we demonstrated that bioactive agents released by pheochromocytoma cells stimulate colon cancer cell proliferation. Inhibition of this effect by atropine identifies acetylcholine as one of these bioactive agents.
We acknowledge that the present work has limitations. PC12 cells, used to condition media, derive from a rat pheochromocytoma cell line (human pheochromocytoma cell lines are unavailable). Moreover, other bioactive products released from the pheochromocytoma (e.g. catecholamines such as epinephrine and norepinephrine) may have contributed to rapid progression of colorectal cancer. Others have shown in vitro that catecholamines stimulate migration and proliferation of human colon cancer cell lines.19,20 On-going treatment of our patient with alpha- and beta-adrenergic blockers may have minimized the effects of these agents. Additionally, we were unable to measure acetylcholine levels in our patient’s serum. Hydrolysis by serum cholinesterases of acetylcholine released by the pheochromocytoma may have attenuated the impact on colorectal neoplasia. Nonetheless, release of bioactive agents by other hormone-secreting tumors has been shown to exert potent pro-neoplastic effects at distant sites despite degradation by serum enzymes. For example, despite rapid degradation of gastrin by serum endopeptidases, continuous release of gastrin from gastrinomas maintains increased steady-state serum gastrin concentrations that drive transformation of gastric enterochromaffin-like cells into gastric carcinoid tumors.
This unique case highlights the important role of muscarinic receptor activation in colorectal cancer. In view of increasing efforts to target multiple pro-neoplastic pathways simultaneously and to personalize cancer treatment, our findings suggest that in conjunction with standard therapies, a subset of colorectal cancer patients might benefit from addition of anti-cholinergic agents, primarily those directed selectively at blocking M3 receptor activation.
CONCLUSION
Inspired by the exceptional case of a patient with an unresectable pheochromocytoma who rapidly developed colorectal cancer, our observations and experimental findings implicate muscarinic receptor agonists in the progression of human colorectal neoplasia.
Acknowledgments
Financial Support: This work was supported by grants from the National Cancer Institute (CA120407, to Dr. Raufman) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK080843, to Dr. Xie and DK093406, to Dr. Raufman).
ABREVIATIONS
- ChAT
choline acetyltransferase
- M3R
muscarinic receptor subtype 3
- At
atropine
Footnotes
Disclosures: The authors have no disclosures relevant to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erik C. von Rosenvinge, Division of Gastroenterology & Hepatology, University of Maryland School of Medicine & VA Maryland Health Care System, Baltimore, Maryland, USA
Kunrong Cheng, Division of Gastroenterology & Hepatology, University of Maryland School of Medicine, Baltimore, Maryland, USA
Cinthia B. Drachenberg, Department of Pathology, University of Maryland School of Medicine, Baltimore, Maryland, USA
Carol B. Fowler, Office of Research and Development, Veterans Health Administration, Department of Veterans Affairs, Washington, D.C., USA
David L. Evers, Office of Research and Development, Veterans Health Administration, Department of Veterans Affairs, Washington, D.C., USA
Guofeng Xie, Division of Gastroenterology & Hepatology, University of Maryland School of Medicine & VA Maryland Health Care System, Baltimore, Maryland, USA
Jean-Pierre Raufman, Division of Gastroenterology & Hepatology, University of Maryland School of Medicine & VA Maryland Health Care System, Baltimore, Maryland, USA
References
- 1.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361(25):2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn C, Montrose DC, Swank DL, Nakanishi M, Ilsley JN, Rosenberg DW. Deoxycholic acid promotes the growth of colonic aberrant crypt foci. Mol Carcinog. 2007;46(1):60–70. doi: 10.1002/mc.20253. [DOI] [PubMed] [Google Scholar]
- 4.Frucht H, Jensen RT, Dexter D, Yang WL, Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin Cancer Res. 1999;5(9):2532–2539. [PubMed] [Google Scholar]
- 5.Cheng K, Chen Y, Zimniak P, Raufman J, Xiao Y, Frucht H. Functional interaction of lithocholic acid conjugates with M3 muscarinic receptors on a human colon cancer cell line. Biochim Biophys Acta. 2002;1588(1):48–55. doi: 10.1016/s0925-4439(02)00115-1. [DOI] [PubMed] [Google Scholar]
- 6.Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol. 2005;70(7):1035–1047. doi: 10.1016/j.bcp.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Raufman JP, Cheng K, Zimniak P. Activation of muscarinic receptor signaling by bile acids: physiological and medical implications. Digestive Diseases and Sciences. 2003;48(8):1431–1444. doi: 10.1023/a:1024733500950. [DOI] [PubMed] [Google Scholar]
- 8.Cheng K, Samimi R, Xie G, et al. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2008;295(3):G591–597. doi: 10.1152/ajpgi.00055.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raufman JP, Samimi R, Shah N, et al. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res. 2008;68(10):3573–3578. doi: 10.1158/0008-5472.CAN-07-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raufman JP, Shant J, Xie G, et al. Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin/+ mice. Carcinogenesis. 2011;32(9):1396–1402. doi: 10.1093/carcin/bgr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng Z, Heath J, Drachenberg C, Raufman JP, Xie G. Cholinergic muscarinic receptor activation augments murine intestinal epithelial cell proliferation and tumorigenesis. BMC Cancer. 2013;13:204. doi: 10.1186/1471-2407-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welin S, Youker J, Spratt JS. The rates and patterns of growth of 375 tumors of the large intestine and rectum observed serially by double contras enema study (Malmö Technique) Am J Roentgenol Radium Ther Nucl Med. 1963;90:673–87. [PubMed] [Google Scholar]
- 13.Hofstad B, Vatn MH, Andersen SN, et al. Growth of colorectal polyps: redetection and evaluation of unresected polyps for a period of three years. Gut. 1996;39(3):449–56. doi: 10.1136/gut.39.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene LA, Rein G. Synthesis, storage and release of acetylcholine by a noradrenergic pheochromocytoma cell line. Nature. 1977;268(5618):349–51. doi: 10.1038/268349a0. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Raufman JP, Cheng K, Saxena N, et al. Muscarinic receptor agonists stimulate matrix metalloproteinase 1-dependent invasion of human colon cancer cells. Biochem Biophys Res Commun. 2011;415(2):319–24. doi: 10.1016/j.bbrc.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belo A, Cheng K, Chahdi A, et al. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G749–60. doi: 10.1152/ajpgi.00306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Rosenvinge EC, Raufman J-P. Muscarinic Receptor Signaling in Colon Cancer. Cancers. 2011;3(1):971–81. doi: 10.3390/cancers3010971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61(7):2866–9. [PubMed] [Google Scholar]
- 20.Wong HP, Ho JW, Koo MW, et al. Effects of adrenaline in human colon adenocarcinoma HT-29 cells. Life Sci. 2011;88(25–26):1108–12. doi: 10.1016/j.lfs.2011.04.007. [DOI] [PubMed] [Google Scholar]