Abstract
Background:
Warfarin use and associated outcomes in patients with heart failure and atrial fibrillation and a cardiovascular implantable electronic device have not been described previously.
Hypothesis:
We hypothesized that warfarin is underused and is associated with lower risks of mortality, thromboembolic events, and myocardial infarction.
Methods:
Using data from a clinical registry linked with Medicare claims, we examined warfarin use at discharge and 30‐day and 1‐year Kaplan‐Meier estimates of all‐cause mortality and cumulative incidence rates of mortality, thromboembolic events, myocardial infarction, and bleeding events in patients 65 years or older, with a history of atrial fibrillation and a cardiovascular implantable electronic device admitted with heart failure between 2001 and 2006, who were naïve to anticoagulation therapy at admission. We compared outcomes between patients who were or were not prescribed warfarin at discharge and tested associations between treatment and outcomes.
Results:
Of 2586 eligible patients in 252 hospitals, 2049 were discharged without a prescription for warfarin. At 1 year, the group discharged without warfarin had a higher mortality rate after discharge (37.4% vs 28.8%; P < 0.001) but similar rates of thromboembolism, myocardial infarction, and bleeding events. After adjustment, treatment with warfarin was associated with lower risk of all‐cause death 1 year after discharge (hazard ratio: 0.76, 95% confidence interval: 0.63–0.92).
Conclusions:
Among older patients with heart failure and atrial fibrillation and a cardiovascular implantable electronic device, 4 of 5 were discharged without a prescription for warfarin. Warfarin nonuse was associated with a higher risk of death 1 year after discharge. Clin. Cardiol. 2011 DOI: 10.1002/clc.22064
Damon M. Seils, MA, Duke University, assisted with manuscript preparation. Mr. Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
This study was supported by a research agreement between Duke University and Janssen Pharmaceuticals.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Atrial fibrillation is the most common cardiac arrhythmia in patients with heart failure and is associated with increased risk of thromboembolism and mortality.1., 2., 3. As the US population ages, the prevalence of atrial fibrillation concomitant with heart failure increases.4., 5. Simultaneously, in light of technological advances, broadening indications, and expanding coverage by Medicare, a growing number of older patients with heart failure are receiving cardiovascular implantable electronic devices, including pacemakers, cardioverter‐defibrillators, and cardiac resynchronization therapy devices.6 Warfarin has been shown to reduce the risk of thromboembolism7., 8., 9. in patients with atrial fibrillation and may have a mortality benefit in patients with ischemic heart disease.10., 11. However, despite professional guideline recommendations supporting the use of anticoagulation therapy for patients with heart failure and atrial fibrillation,12 and the launch of quality‐improvement initiatives,13., 14. rates of warfarin use remain suboptimal.15
Warfarin use and associated outcomes in older patients with concurrent heart failure and atrial fibrillation and a cardiovascular implantable device have not been described previously. We hypothesized that warfarin is underused in this high‐risk population and that the use of warfarin is associated with a lower risk of mortality, thromboembolic events, and myocardial infarction. Using data from the Acute Decompensated Heart Failure National Registry (ADHERE) linked with Medicare claims, we examined relationships between warfarin use at discharge in previously warfarin‐naïve patients and 30‐day and 1‐year outcomes, including mortality, thromboembolic events, myocardial infarction, and bleeding.
Methods
Data Sources
We obtained hospitalization data from the ADHERE registry, which was established to study the characteristics, treatments, and inpatient outcomes of patients hospitalized with acute decompensated heart failure.16 More than 300 community and academic centers in the United States participated, and more than 185,000 patients were enrolled between January 2001 and March 2006. Demographic characteristics, comorbid conditions, medications, hospital course, laboratory values, procedures, and discharge disposition were collected by chart review and entered using a Web‐enabled report form.
To obtain long‐term follow‐up data on these hospitalizations and to analyze outcomes in unique patients, we linked the ADHERE data to the 100% Medicare inpatient and denominator files.17 The inpatient files contain hospital claims generated for reimbursement under Medicare Part A. For beneficiaries in fee‐for‐service Medicare, these files include service dates and diagnosis and procedure codes. The denominator files contain beneficiary demographic characteristics, enrollment information, and death dates. The files contain an encrypted identifier unique to each beneficiary to allow for longitudinal follow‐up. We linked to Medicare Part B claims to identify dates of billing for international normalized ratio laboratory testing. The latest date of Medicare data availability for this study was December 31, 2007.
We linked the ADHERE hospitalizations to the Medicare claims using several indirect identifiers—hospital identifier, admission date, discharge date, patient sex, and either birth date or month and year of birth, as available. Combinations of these identifiers are almost completely unique, enabling identification of registry hospitals and registry hospitalizations in the Medicare claims data. ADHERE records used for linking included hospitalizations of patients 65 years or older with complete data on the identifiers listed above. Medicare inpatient records used for linking included all hospitalizations of patients 65 years or older with an associated diagnosis of heart failure (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] diagnosis code 402.x1, 404.x1, 404.x3, or 428.x) in any position on the inpatient claim.
Study Population
We included patients 65 years or older living in the United States who had an ADHERE hospitalization linked to fee‐for‐service Medicare claims data. Eligible patients had atrial fibrillation indicated in their medical history, had a cardiovascular implantable electronic device in place at admission, were discharged alive to home, were naïve to anticoagulation therapy at admission, and had no contraindications for warfarin. Documentation of a warfarin contraindication was obtained by chart review and transmitted as a binary variable via a Web‐enabled case report form. For patients with multiple registry hospitalizations, we used the earliest registry hospitalization as the index hospitalization. We also defined a comparison population of patients who were discharged without a device in place at admission but met the other inclusion criteria above.
Drug Exposure
The exposure of interest was anticoagulation therapy at discharge. We defined anticoagulation therapy as documentation of a warfarin prescription at the time of discharge in the ADHERE registry.
Outcomes
We followed patients for up to 1 year after discharge from the ADHERE hospitalization. The outcomes of interest were all‐cause mortality, thromboembolic events, myocardial infarction, and bleeding events, including hemorrhagic stroke. We determined all‐cause mortality on the basis of death dates recorded in the Medicare denominator files. We identified new myocardial infarction (ICD‐9‐CM code 410.x1) on the basis of a primary diagnosis listed on an inpatient claim after discharge from the ADHERE hospitalization. Likewise, we identified thromboembolic events on the basis of a primary diagnosis listed on a subsequent Medicare claim. These events included cerebral occlusion, nonhemorrhagic stroke, or transient ischemic attack (433.x–437.x)18; arterial embolism or thrombosis (444.x and 445.x); and deep vein thrombosis, pulmonary embolism, or other venous thrombosis (415.1x, 451.1x, 451.2, 451.81, 451.9, 452.x, and 453.x).19 We defined bleeding events to include gastrointestinal bleeding (ICD‐9‐CM procedure code 44.4x [control of hemorrhage and suture of ulcer of stomach of duodenum]18; or primary diagnosis code 530.82 [esophageal]; 531.0x, 531.2x, 531.4x, 531.6x, 532.0x, 532.2x, 532.4x, 532.6x, 533.0x, 533.2x, 533.4x, 533.6x, 534.0x, 534.2x, 534.4x, 534.6x [ulcer]; 535.x1 [gastritis and duodenitis with hemorrhage]; 537.83, 537.84 [bleeding of stomach or duodenum due to vascular abnormalities]; 569.85, 569.86 [bleeding of intestine due to vascular abnormalities]; 569.3x [rectum]; or 578.x [unspecified]) or cerebrovascular hemorrhage (primary diagnosis code 430.x [subarachnoid hemorrhage]; 431.x [intracerebral hemorrhage]; or 432.x [intracranial hemorrhage]). We censored data for patients who enrolled in a Medicare managed care plan during the follow‐up period from the date of managed care enrollment. As a sensitivity analysis to test for potential unmeasured confounding, we repeated the treatment comparison using an outcome unrelated to treatment (ie, hip fracture) but related to overall health status. We identified hip fracture events (ICD‐9‐CM code 820.xx20) on the basis of a primary diagnosis on a subsequent inpatient claim.
Patient Characteristics
Baseline characteristics from the ADHERE registry included demographic characteristics (age, sex, race), medical history (chronic obstructive pulmonary disease, chronic renal insufficiency, coronary artery disease, diabetes mellitus, hyperlipidemia, hypertension, myocardial infarction, peripheral vascular disease, stroke or transient ischemic attack), findings from the initial clinical evaluation (dyspnea, edema, ejection fraction, fatigue, rales), initial vital signs (heart rate, systolic blood pressure), laboratory test results (creatinine, hemoglobin, sodium), and discharge medications (angiotensin‐converting enzyme [ACE] inhibitor or angiotensin receptor blocker [ARB], aspirin, β‐blocker, diuretic, clopidogrel, lipid‐lowering agent). For variables with low rates of missingness (ie, <5% of records), we handled missing values by imputing continuous variables to the overall median value and dichotomous variables to “no.” For evaluation of left ventricular function (15.6% missing), we created a categorical variable that included a category for missing. We derived CHADS2 and CHA2DS2‐VASc scores using the algorithms described by Gage et al21 and Lip et al,22 respectively. Using the comorbid conditions from the ADHERE registry, the CHADS2 score was created by adding 1 point each for the presence of congestive heart failure, hypertension, age 75 years or older, and diabetes mellitus, and adding 2 points for stroke or transient ischemic attack. The CHA2DS2‐VASc score was created by adding 1 point each for the presence of congestive heart failure, hypertension, age 65 or older, age 75 years or older, diabetes mellitus, prior myocardial infarction or peripheral vascular disease, and female sex, and adding 2 points for stroke or transient ischemic attack. All patients in the study had a CHADS2 score of at least 1 and a CHA2DS2‐VASc score of at least 2, because all were 65 years or older and were admitted to the hospital with heart failure.
Statistical Analysis
We describe the baseline characteristics of the study population, comparing each of the treatment groups. We present categorical variables as frequencies and continuous variables as means with standard deviations. We tested for differences in baseline variables between treatment groups using χ 2 tests for categorical variables and Kruskal‐Wallis tests for continuous variables.
We report unadjusted outcome rates for each treatment group. We used Kaplan‐Meier methods to estimate mortality within 30 days and 1 year after discharge and used log‐rank tests to assess differences in mortality between treatment groups. For the other outcomes, we used the cumulative incidence function, which accounts for the competing risk of death, to calculate cumulative incidence estimates at 30 days and 1 year after discharge. We used Gray tests to assess differences in these outcomes between groups.
To assess differences in outcomes among treatment groups, we used inverse probability‐weighted estimates based on the probability of patients receiving the treatment they received conditional on observed covariates.23 We estimated these probabilities with a propensity model fit as a logistic regression model with treatment as the dependent variable. Independent variables included age, sex, race, medical history, findings from the initial clinical evaluation, initial vital signs, laboratory test results, and length of stay greater than 7 days for the index hospitalization.24 Each patient was then weighted by the inverse of the estimated probability of the treatment received. To assess the effectiveness of the propensity model to balance the treatment groups, we used standardized differences to compare baseline characteristics between treatment groups after weighting.25 In addition, we used weighted χ 2 tests for categorical variables and weighted analysis of variance for continuous variables to compare differences by treatment groups. We estimated the unadjusted relationship between treatment and each outcome of interest using Cox proportional hazards models, in which the treatment indicator was the sole independent variable. Next, we estimated the adjusted relationship between treatment and each outcome using weighted proportional hazards regression models. Finally, we controlled for discharge medications in addition to the treatment indicator using weighted proportional hazards models. We used robust standard errors to account for clustering of patients within hospitals in all Cox models.
We used a significance level of 0.05 and 2‐sided tests for all hypotheses. We used SAS version 9.2 (SAS Institute Inc., Cary, NC) for all analyses. The institutional review board of the Duke University Health System approved the study.
Results
Of 2586 eligible patients from 252 hospitals, 2049 (79.2%) were discharged without warfarin (Table 1). Of 6335 otherwise similar patients without a cardiovascular implantable electronic device, 4811 (75.9%) were discharged without warfarin. Compared with patients discharged with warfarin, patients without warfarin were slightly older; had a higher prevalence of hypertension; had higher rates of CHADS2 score ≥2, rales, pulmonary edema, and tachycardia; and had lower rates of systolic dysfunction on presentation. Patients discharged without warfarin were more likely to be discharged on aspirin and clopidogrel and equally likely to receive ACE inhibitors or ARBs and β‐blockers. Compared with patients discharged with warfarin, patients without warfarin were hospitalized an average of 1.5 fewer days.
Table 1.
Characteristics of the Study Population
| Characteristic | No Warfarin at Discharge (n = 2049) | Warfarin at Discharge (n = 537) | Standardized Difference, % | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD), y | 80.0 (7.4) | 78.1 (6.9) | 26.7 | <0.001 |
| Male, No. (%) | 1100 (53.7) | 295 (54.9) | 2.5 | 0.61 |
| Race, No. (%) | 7.4 | 0.33 | ||
| Black | 168 (8.2) | 36 (6.7) | ||
| White | 1754 (85.6) | 473 (88.1) | ||
| Other/unknown | 127 (6.2) | 28 (5.2) | ||
| Medical history, No. (%) | ||||
| Chronic obstructive pulmonary disease | 656 (32.0) | 164 (30.5) | 3.2 | 0.51 |
| Chronic renal insufficiency | 756 (36.9) | 185 (34.5) | 5.1 | 0.29 |
| Coronary artery disease | 1526 (74.5) | 392 (73.0) | 3.4 | 0.49 |
| Diabetes mellitus | 785 (38.3) | 192 (35.8) | 5.3 | 0.28 |
| Hyperlipidemia/dyslipidemia | 881 (43.0) | 227 (42.3) | 1.5 | 0.76 |
| Hypertension | 1465 (71.5) | 356 (66.3) | 11.3 | 0.02 |
| Peripheral vascular disease | 391 (19.1) | 88 (16.4) | 7.1 | 0.15 |
| Prior myocardial infarction | 816 (39.8) | 193 (35.9) | 8.0 | 0.10 |
| Stroke or transient ischemic attack | 385 (18.8) | 83 (15.5) | 8.9 | 0.07 |
| CHADS2 score, No. (%) | ||||
| 1 | 70 (3.4) | 36 (6.7) | 15.0 | <0.001 |
| 2 | 447 (21.8) | 154 (28.7) | 15.8 | <0.001 |
| 3 | 862 (42.1) | 199 (37.1) | 10.3 | 0.04 |
| 4 | 373 (18.2) | 82 (15.3) | 7.9 | 0.11 |
| 5 | 212 (10.3) | 45 (8.4) | 6.8 | 0.18 |
| 6 | 85 (4.1) | 21 (3.9) | 1.2 | 0.81 |
| ≥2 | 1979 (96.6) | 501 (93.3) | 15.0 | <0.001 |
| CHA2DS2‐VASc score, No. (%) | ||||
| 2 | 28 (1.4) | 12 (2.2) | 6.5 | 0.15 |
| 3 | 162 (7.9) | 71 (13.2) | 17.4 | <0.001 |
| 4 | 460 (22.4) | 142 (26.4) | 9.3 | 0.05 |
| 5 | 618 (30.2) | 148 (27.6) | 5.7 | 0.24 |
| 6 | 438 (21.4) | 91 (16.9) | 11.3 | 0.02 |
| 7 | 219 (10.7) | 43 (8.0) | 9.2 | 0.07 |
| 8 | 108 (5.3) | 24 (4.5) | 3.7 | 0.45 |
| 9 | 16 (0.8) | —a | 3.5 | 0.45 |
| ≥3 | 2021 (98.6) | 525 (97.8) | 6.5 | 0.15 |
| Initial evaluation, No. (%) | ||||
| Dyspnea | 1827 (89.2) | 471 (87.7) | 4.6 | 0.34 |
| Ejection fraction | 25.5 | <0.001 | ||
| Normal or slightly impaired (>40%) | 622 (30.4) | 140 (26.1) | ||
| Moderately impaired (26%–40%) | 481 (23.5) | 140 (26.1) | ||
| Severely impaired (≤25%) | 598 (29.2) | 202 (37.6) | ||
| Missing | 348 (17.0) | 55 (10.2) | ||
| Fatigue | 654 (31.9) | 180 (33.5) | 3.4 | 0.48 |
| Pulmonary edema | 1708 (83.4) | 420 (78.2) | 13.1 | 0.005 |
| Rales | 1375 (67.1) | 312 (58.1) | 18.7 | <0.001 |
| Initial vital signs, No. (%) | ||||
| Pulse | 19.4 | <0.001 | ||
| <80 bpm | 1165 (56.9) | 266 (49.5) | ||
| 80–100 bpm | 644 (31.4) | 174 (32.4) | ||
| >100 bpm | 240 (11.7) | 97 (18.1) | ||
| Systolic blood pressure | 10.9 | 0.08 | ||
| <110 mm Hg | 355 (17.3) | 113 (21.0) | ||
| 110–150 mm Hg | 1122 (54.8) | 293 (54.6) | ||
| >150 mm Hg | 572 (27.9) | 131 (24.4) | ||
| Laboratory test results | ||||
| Serum creatinine | 6.5 | 0.42 | ||
| <1.5 mg/dL | 1031 (50.3) | 282 (52.5) | ||
| 1.5–2.0 mg/dL | 617 (30.1) | 146 (27.2) | ||
| >2.0 mg/dL | 401 (19.6) | 109 (20.3) | ||
| Serum sodium | 10.0 | 0.11 | ||
| <135 mEq/L | 369 (18.0) | 118 (22.0) | ||
| 135–145 mEq/L | 1631 (79.6) | 406 (75.6) | ||
| >145 mEq/L | 49 (2.4) | 13 (2.4) | ||
| Hemoglobin | 12.2 | 0.05 | ||
| <9 g/dL | 74 (3.6) | 18 (3.4) | ||
| 9–11 g/dL | 489 (23.9) | 102 (19.0) | ||
| >11 g/dL | 1486 (72.5) | 417 (77.7) | ||
| Discharge medications, No. (%) | ||||
| ACE inhibitor or ARB | 1247 (60.9) | 349 (65.0) | 8.6 | 0.08 |
| Aspirin | 1215 (59.3) | 216 (40.2) | 38.9 | < 0.001 |
| β‐Blocker | 1336 (65.2) | 361 (67.2) | 4.3 | 0.38 |
| Clopidogrel | 449 (21.9) | 37 (6.9) | 43.8 | <0.001 |
| Diuretic | 1610 (78.6) | 420 (78.2) | 0.9 | 0.86 |
| Lipid‐lowering agent | 741 (36.2) | 213 (39.7) | 7.2 | 0.13 |
| Length of stay | ||||
| Length of stay, mean (SD), d | 4.8 (3.6) | 6.3 (4.7) | 35.9 | <0.001 |
| Length of stay >7 days, No. (%) | 295 (14.4) | 153 (28.5) | 34.9 | <0.001 |
| Device type, No. (%) | ||||
| Pacemaker | 1360 (66.4) | 336 (62.6) | 8.0 | 0.10 |
| CRT‐D | 110 (5.4) | 45 (8.4) | 11.9 | 0.009 |
| CRT‐P | 169 (8.2) | 46 (8.6) | 1.1 | 0.81 |
| ICD | 410 (20.0) | 110 (20.5) | 1.2 | 0.81 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker, CRT‐D, cardiac resynchronization therapy defibrillator; CRT‐P, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter‐defibrillator; SD, standard deviation.
Cells with ≤10 observations are not shown.
As shown in Table 2, 1‐year postdischarge mortality was higher among patients discharged without warfarin (37.4% vs 28.8% for patients discharged with warfarin). Mortality at 30 days after discharge was similar between groups. Figure 1 shows the Kaplan‐Meier curves of all‐cause mortality by treatment. Thromboembolism, myocardial infarction, and bleeding rates at 30 days and 1 year after discharge were similar between groups.
Table 2.
Outcomes of Patients With Heart Failure and Atrial Fibrillation and a Cardiovascular Implantable Electronic Device
| Outcome | No Warfarin at Discharge (n = 2049) | Warfarin at Discharge (n = 537) | P Value |
|---|---|---|---|
| Mortality, No. (%) | <0.001 | ||
| 30 days | 112 (5.5) | 29 (5.4) | |
| 1 year | 761 (37.4) | 153 (28.8) | |
| Thromboembolic events, No. (%) | 0.77 | ||
| 30 days | 24 (1.2) | —a | |
| 1 year | 114 (5.6) | 28 (5.3) | |
| New myocardial infarction, No. (%) | 0.87 | ||
| 30 days | 14 (0.7) | —a | |
| 1 year | 60 (2.9) | 15 (2.8) | |
| Bleeding events, No. (%) | 0.86 | ||
| 30 days | 12 (0.6) | —a | |
| 1 year | 88 (4.3) | 22 (4.1) |
Cells with ≤10 observations are not shown.
Figure 1.
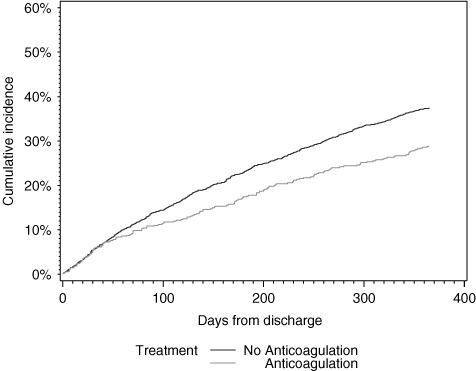
Cumulative incidence of mortality at 1 year by discharge anticoagulation status.
After weighting by the inverse probability of treatment, the treatment groups were well balanced (Supplemental Tables 1, 2). Table 3 shows the unadjusted and inverse probability‐weighted hazard ratios (HRs) for the associations between warfarin use and 30‐day and 1‐year outcomes. Warfarin at discharge was associated with a lower hazard of 1‐year mortality in both the unadjusted (HR: 0.73, 95% confidence interval [CI]: 0.61–0.86) and inverse probability‐weighted analyses (HR: 0.76, 95% CI: 0.63–0.92). Results were similar after adjustment for other discharge medications (HR: 0.77, 95% CI: 0.64–0.92). There was no significant association between receiving warfarin at discharge and the hazard of 30‐day or 1‐year thromboembolism, myocardial infarction, or bleeding. In a sensitivity analysis, warfarin was not associated with a lower hazard of hip fracture events at 1 year after discharge (HR: 1.51, 95% CI: 0.77–2.94).
Table 3.
Associations Between Warfarin Use and Outcomesa
| Outcome | Unadjusted HR (95% CI) | Weighted HR (95% CI) | Weighted and Adjusted HR (95% CI)b |
|---|---|---|---|
| Mortality | |||
| 30 days | 0.99 (0.66–1.48) | 0.96 (0.60–1.53) | 0.96 (0.61–1.52) |
| 1 year | 0.73 (0.61–0.86) | 0.76 (0.63–0.92) | 0.77 (0.64–0.92) |
| Thromboembolic events | |||
| 30 days | —c | —c | —c |
| 1 year | 0.89 (0.58–1.39) | 0.92 (0.60–1.41) | 0.93 (0.60–1.43) |
| New myocardial infarction | |||
| 30 days | —c | —c | —c |
| 1 year | 0.91 (0.51–1.59) | 1.18 (0.62–2.25) | 1.40 (0.72–2.72) |
| Bleeding events | |||
| 30 days | —c | —c | —c |
| 1 year | 0.90 (0.58–1.40) | 1.13 (0.69–1.85) | 1.18 (0.71–1.96) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Warfarin use defined as documentation of a warfarin prescription at the time of discharge in the Acute Decompensated Heart Failure National Registry.
Cox proportional hazards model includes adjustment for prescription of angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, aspirin, β‐blocker, diuretic, clopidogrel, and lipid‐lowering medication at discharge.
Data suppressed because of insufficient observations.
Discussion
Linking the ADHERE registry with Medicare claims data, we examined associations between new warfarin use at discharge among patients 65 years or older with concurrent heart failure and atrial fibrillation and a cardiovascular implantable electronic device. The principal findings are twofold. First, 79.2% of warfarin‐eligible patients were discharged without a prescription, despite being at high risk for stroke. Second, not receiving warfarin was independently associated with a higher risk of death 1 year after discharge.
Limitations of warfarin, which may partly explain its underuse, include the narrow therapeutic window, variable amount of time spent in treatment range, numerous drug‐drug interactions, variable dose requirements, unpredictable anticoagulant response resulting in the need for frequent monitoring, need for a stable diet, slow onset of action, and a bleeding risk most pronounced in older patients. However, according to a meta‐analysis of 29 clinical trials spanning 18 years, warfarin reduces stroke risk in patients with atrial fibrillation by approximately 64%.7 Guidelines from the American College of Cardiology (ACC)/American Heart Association (AHA) reflected this high‐level evidence by establishing warfarin as a class I recommendation in all patients with heart failure and atrial fibrillation in the absence of contraindications.12 Warfarin nonuse ranged from 26%26 to 78.7%27 in various populations with atrial fibrillation over the past decade.15 The magnitude of warfarin nonuse in our study is therefore quite striking, particularly because patients not initiated on warfarin were more likely to have a CHADS2 score ≥2.
Limitations of stroke risk indices merit mention. Patients with a CHADS2 score of 0 have traditionally been classified as having low risk. However, 1‐year stroke rates among these patients range from 0.84% to 3.2%,28 reflecting the considerable heterogeneity in risk prediction accuracy.29 The CHA2DS2‐VASc score, which accounts for ages 65 to 74 years, vascular disease, and female sex, in addition to the risk factors constituting the CHADS2 score, offers greater granularity of risk prediction among patients with CHADS2 scores of 0 to 1.28 However, the c statistics for the CHA2DS2‐VASc score (0.602) and the CHADS2 score (0.586) are similar, suggesting that risk prediction by this index is also heterogeneous.22., 30. The ACC/AHA guidelines state that even patients with a CHADS2 score of 1 in the current analysis should receive anticoagulation therapy in the absence of contraindications.12
Despite the ability of many cardiovascular implantable electronic devices to detect atrial fibrillation with its attendant risk of stroke and systemic embolism,31 the proportion of patients with a device who received warfarin was slightly lower than the proportion of those without a device. The receipt of other evidence‐based prescriptions, such as ACE inhibitors or ARBs and β‐blockers at discharge, and the reasonable safety profile given similar bleeding rates between groups set the low rate of warfarin use in high relief. In spite of the efficacy of warfarin and the professional guidelines, 4 of 5 eligible patients in this real‐world cohort were discharged without a prescription for warfarin.
The observed association between warfarin use and lower mortality risk merits careful consideration. Warfarin may have been withheld in the setting of worse illness, a possibility supported by the higher prevalence of advanced heart failure on admission in patients who did not receive warfarin at discharge. The better systolic function seen in this group may simply reflect a higher prevalence of diastolic heart failure. However, it is these high‐risk patients who are more likely to benefit from warfarin. Counter to the notion of deliberate postponement of warfarin initiation among sicker patients, the prevalence of baseline comorbid conditions was otherwise similar. Our finding of a warfarin‐associated survival benefit is consistent with the results of a previous clinical trial7 and an observational study.32 The reduction in mortality associated with warfarin may be explained in part by the more regular exposure to health care providers required for anticoagulation monitoring. More frequent provider contact may result in higher quality of care among patients receiving warfarin compared with those not receiving warfarin.
We hypothesized that warfarin would also reduce the risk of thromboembolic events and myocardial infarction. Our hypothesis was grounded in a previously demonstrated reduction in the incidence of thromboembolism7., 8., 9., 32. and a suggestion of a mortality benefit in ischemic heart disease with warfarin use.10., 11. However, the rates of nonfatal outcomes coded in claims data were low, and this may partially account for the insignificant associations with warfarin. Reductions in stroke or myocardial infarction may nonetheless underlie the survival benefit associated with warfarin.
Since active enrollment in ADHERE ended in 2006, randomized clinical trials have demonstrated the efficacy of 3 alternative anticoagulants for the prevention of stroke and systemic embolism: dabigatran, rivaroxaban, and apixaban.33., 34., 35. None of these anticoagulants requires laboratory monitoring. Bleeding profiles are at least similar33., 34. if not superior35 to warfarin, with the exception of gastrointestinal bleeding at higher doses of dabigatran. In this changing landscape of anticoagulation alternatives, populations at risk for underuse of anticoagulation should be identified. In view of the substantial underuse of warfarin and the associated increase in mortality observed in our study, older patients with concurrent heart failure and atrial fibrillation and a cardiovascular implantable electronic device merit close attention. Physician, patient, and health system factors associated with warfarin nonuse should be explored in this and other patient populations to inform current and future quality‐improvement initiatives.
Limitations
Our study is strengthened by a new‐user design,36 rich clinical detail, and longitudinal outcomes in a large, national sample. Nonetheless, the retrospective, observational nature of the study does not allow one to discern whether our findings reflect treatment effects or are related to unmeasured or residual confounders, such as preadmission New York Heart Association class, major clinical events during hospitalization such as shock or intracranial hemorrhage, or patient frailty. However, the absence of an association between warfarin use and hip fracture events 1 year after discharge provides evidence that the overall health of patients discharged with warfarin was not substantially dissimilar to that of patients discharged without warfarin. Although clinical data were available from the registry to assess baseline clinical status, all outcomes except death were ascertained on the basis of inpatient claims data, and thus sensitivity is modest. This reduced granularity is reflected in the low rates of nonfatal outcomes. Detection of stroke and myocardial infarction requires that the patient survive to hospitalization. The study's power to detect associations between warfarin use and these outcomes was therefore limited. Death rates, by contrast, are reliably coded in the Medicare denominator file. Absence of data regarding warfarin prescription after discharge is a significant limitation. However, crossover to either warfarin initiation among patients not receiving a prescription,37., 38. or warfarin discontinuation among patients who received a prescription,9., 39. is not expected to occur frequently enough to change the directionality or significance of our findings. Absence of data regarding time in therapeutic range among warfarin recipients and cause of death also limit the explanatory power of our analysis. Potential reasons to defer warfarin initiation, such as fall risk, medication compliance, likelihood of outpatient follow‐up, and a high likelihood of bleeding as indicated by the HAS‐BLED risk score40., 41. were not captured in our data set. Because our study was limited to patients 65 years or older in ADHERE and Medicare, our results may not be generalizable to other populations. However, previous analyses suggest that older patients enrolled in ADHERE are similar to Medicare beneficiaries.17
Conclusion
Among older patients with heart failure and atrial fibrillation, a cardiovascular implantable electronic device, and no contraindications, 4 of 5 were discharged without a prescription for warfarin. Warfarin nonuse was associated with a higher risk of death 1 year after discharge. Future efforts should focus on improving anticoagulant use in this patient population.
References
- 1. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 2. Maisel W. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91: 2–8. [DOI] [PubMed] [Google Scholar]
- 3. Olsson LG, Swedberg K, Ducharme A, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: Results from the candesartan in heart failure‐assessment of reduction in mortality and morbidity (charm) program. J Am Coll Cardiol. 2006;47:1997–2004. [DOI] [PubMed] [Google Scholar]
- 4. Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 5. Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168:418–424. [DOI] [PubMed] [Google Scholar]
- 6. Goldberger Z, Lampert R. Implantable cardioverter‐defibrillators: expanding indications and technologies. JAMA. 2006;295: 809–818. [DOI] [PubMed] [Google Scholar]
- 7. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 8. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 9. Birman‐Deych E, Radford MJ, Nilasena DS, et al. Use and effectiveness of warfarin in medicare beneficiaries with atrial fibrillation. Stroke. 2006;37:1070–1074. [DOI] [PubMed] [Google Scholar]
- 10. Smith P, Arnesen H, Holme I. The effect of warfarin on mortality and reinfarction after myocardial infarction. N Engl J Med. 1990;323:147–152. [DOI] [PubMed] [Google Scholar]
- 11. Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969–974. [DOI] [PubMed] [Google Scholar]
- 12. Hunt SA; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:e1–e82. [DOI] [PubMed] [Google Scholar]
- 13. Shah B, Hernandez AF, Liang L, et al. Hospital variation and characteristics of implantable cardioverter‐defibrillator use in patients with heart failure: data from the GWTG‐HF (Get With The Guidelines‐Heart Failure) registry. J Am Coll Cardiol. 2009;53:416–422. [DOI] [PubMed] [Google Scholar]
- 14. Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence‐based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence‐Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585–596. [DOI] [PubMed] [Google Scholar]
- 15. Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4. [DOI] [PubMed] [Google Scholar]
- 16. Adams KF Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 17. Kociol RD, Hammill BG, Fonarow GC, et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non‐ADHERE Medicare beneficiaries. Am Heart J. 2010;160:885–892. [DOI] [PubMed] [Google Scholar]
- 18. Wahl PM, Rodgers K, Schneeweiss S, et al. Validation of claims‐based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially‐insured population. Pharmacoepidemiol Drug Saf. 2010;19: 596–603. [DOI] [PubMed] [Google Scholar]
- 19. Birman‐Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD‐9‐CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. [DOI] [PubMed] [Google Scholar]
- 20. Rigler SK, Ellerbeck E, Whittle J, et al. Comparing methods to identify hip fracture in a nursing home population using Medicare claims. Osteoporos Int. 2011;22:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: Results from the national registry of atrial fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 22. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 23. Curtis LH, Hammill BG, Eisenstein EL, et al. Using inverse probability‐weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45:S103–S107. [DOI] [PubMed] [Google Scholar]
- 24. Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 25. Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–753. [DOI] [PubMed] [Google Scholar]
- 26. Leckey R, Aguilar EG, Phillips SJ. Atrial fibrillation and the use of warfarin in patients admitted to an acute stroke unit. Can J Cardiol. 2000;16:481–485. [PubMed] [Google Scholar]
- 27. Ageno W, Ambrosini F, Nardo B, et al. Atrial fibrillation and antithrombotic treatment in Italian hospitalized patients: a prospective, observational study. J Thromb Thrombolysis. 2001;12:225–230. [DOI] [PubMed] [Google Scholar]
- 28. Olesen JB, Torp‐Pedersen C, Hansen ML, et al. The value of the CHA2DS2‐VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost. 2012;107:1172–1179. [DOI] [PubMed] [Google Scholar]
- 29. Keogh C, Wallace E, Dillon C, et al. Validation of the CHADS2 clinical prediction rule to predict ischaemic stroke. A systematic review and meta‐analysis. Thromb Haemost. 2011;106:528–538. [DOI] [PubMed] [Google Scholar]
- 30. Karthikeyan G, Eikelboom JW. The CHADS2 score for stroke risk stratification in atrial fibrillation—friend or foe? Thromb Haemost. 2010;104:45–48. [DOI] [PubMed] [Google Scholar]
- 31. Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 32. Gorin L, Fauchier L, Nonin E, et al. Antithrombotic treatment and the risk of death and stroke in patients with atrial fibrillation and a CHADS2 score=1. Thromb Haemost. 2010;103:833–840. [DOI] [PubMed] [Google Scholar]
- 33. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 34. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 35. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 36. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 37. Butler J, Arbogast PG, BeLue R, et al. Outpatient adherence to beta‐blocker therapy after acute myocardial infarction. J Am Coll Cardiol. 2002;40:1589–1595. [DOI] [PubMed] [Google Scholar]
- 38. Butler J, Arbogast PG, Daugherty J, et al. Outpatient utilization of angiotensin‐converting enzyme inhibitors among heart failure patients after hospital discharge. J Am Coll Cardiol. 2004;43:2036–2043. [DOI] [PubMed] [Google Scholar]
- 39. Fang MC, Go AS, Chang Y, et al. Warfarin discontinuation after starting warfarin for atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2010;3:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. European Heart Rhythm Association; European Association for Cardio‐Thoracic Surgery; Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 41. Skanes AC, Healey JS, Cairns JA, et al; Canadian Cardiovascular Society Atrial Fibrillation Guidelines Committee. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 2012;28:125–136. [DOI] [PubMed] [Google Scholar]


