Abstract
Biobank Ireland Trust (BIT) was established in 2004 to promote and develop an Irish biobank network to benefit patients, researchers, industry, and the economy. The network commenced in 2008 with two hospital biobanks and currently consists of biobanks in the four main cancer hospitals in Ireland. The St. James's Hospital (SJH) Biobank coordinates the network. Procedures, based on ISBER and NCI guidelines, are standardized across the network. Policies and documents—Patient Consent Policy, Patient Information Sheet, Biobank Consent Form, Sample and Data Access Policy (SAP), and Sample Application Form have been agreed upon (after robust discussion) for use in each hospital. An optimum sequence for document preparation and submission for review is outlined. Once consensus is reached among the participating biobanks, the SJH biobank liaises with the Research and Ethics Committees, the Office of the Data Protection Commissioner, The National Cancer Registry (NCR), patient advocate groups, researchers, and other stakeholders. The NCR provides de-identified data from its database for researchers via unique biobank codes. ELSI issues discussed include the introduction of prospective consent across the network and the return of significant research results to patients. Only 4 of 363 patients opted to be re-contacted and re-consented on each occasion that their samples are included in a new project. It was decided, after multidisciplinary discussion, that results will not be returned to patients. The SAP is modeled on those of several international networks. Biobank Ireland is affiliated with international biobanking groups—Marble Arch International Working Group, ISBER, and ESBB. The Irish government continues to deliberate on how to fund and implement biobanking nationally. Meanwhile BIT uses every opportunity to promote awareness of the benefits of biobanking in events and in the media.
Introduction and Objectives
Biobank Ireland Trust (BIT) was established in 2004 to promote and develop an Irish hospital biobank network, facilitating collaborations among academic and industrial researchers to fast-track individualized medicine for cancer patients. The objectives of BIT are: 1) To develop an Irish hospital biobank network using international guidelines to maximize Ireland's resources and expertise for patient-focused cancer research, 2) To provide fair access to uniformly obtained, processed, and stored samples and data for academic researchers and industry to collaborate on Irish and international projects that are scientifically and ethically approved, 3) To develop technologies that will lead to more precise diagnosis and targeted therapies, while stimulating Ireland's economy, through genuine collaboration among academic researchers, pathologists, and industrial Research and Development, and 4) To produce a biobank network template for the government and other funders to develop and sustain as part of the patient care pathway in Ireland's principal hospitals.
Biobank Network Set-up
BIT requested that Ireland's National Cancer Forum highlight biobanking in 2005. As a result, biobanking for cancer was subsequently included in the National Cancer Strategy in 2006.1 In 2008, an Expert Group report entitled, “Recommendations for the Establishment of a National Cancer Biobank” was published.2 However, this was not followed by implementation or government funding. The turning point for the network's development came from an unexpected source. A Vodafone Ireland Foundation “World of Difference” award enabled authors BM and EG to establish the St James's Hospital (SJH) Cancer Biobank. The SJH Biobank linked with the established Beaumont/RCSI Biobank, and the same honest broker (neutral custodians of the samples) policy and procedures were adopted. The Irish biobank network had effectively begun. BIT's biobank network is modeled on the Spanish National Tumour Bank Network and the Wales Cancer Bank, with additional features incorporated from other international networks. The network also integrates international best practices and principles with emphasis on trust, openness, and sharing, rather than individual achievement.3–6
The network currently consists of four of Ireland's leading cancer hospitals (Beaumont Hospital, Cork University Hospital, St James's Hospital, and University College Hospital Galway). The network focuses primarily on breast and colon cancers, and samples from over 1300 patients have been banked to date. In addition, a small number of the following cancer sites are biobanked at Beaumont Hospital: adrenal gland, bladder, endometrium, kidney, lung, melanoma and non-melanomatous skin cancers, esophagus, ovary, prostate, testis, and thyroid. A total of 13,970 individual snap-frozen, Allprotect-stabilized, FFPE (formalin-fixed, paraffin-embedded) and cryomold samples has been collected across the network up to May 2012.
BIT focuses on patients as the main beneficiaries, and consults regularly with patient advocacy groups. Researchers, industry, and the Irish economy are the other predictable beneficiaries. Since 2009, BIT has been funded by modest unrestricted grants from biopharmaceutical companies, and by business and voluntary donations. It is hoped that the benefits of the biobank network will help to secure funding at the governmental level in the future. Research consortia (breast cancer and leukemia/myeloma/lymphoma) have recently joined the network, expanding our resources. The very active engagement of the Cork and Galway Health Research Board (HRB)-funded Clinical Research Facilities (CRF) in BIT activities has been a very significant accelerant. These multidisciplinary regional sites of broad-spectrum bioscience research provide vital administrative coordination, and expertise vital to our national biobank. A particularly positive prospect is the creation of a National Clinical Research Framework (NCRF), a system of integrated cores sited at Ireland's CRF, which will help optimize the conduct of Ireland's clinical, translational, and population research. Table 1 highlights milestones in the development of the Irish biobank network since BIT was established in 2004. A recent communication provides a more comprehensive overview of the Irish biobank network.7 Importantly, a Biobanking Subgroup has been established by the HRB to examine the funding and implementation of an Irish biobank network. The structure of the biobank network complements the HRB's current strategy for health service research which noted the advantages of “networks, collaborations and linkages”.8,9
Table 1.
Milestones in Development of Irish Biobank Network
| Event | Year |
|---|---|
| Biobank Ireland Trust established to promote biobanking within Ireland | 2004 |
| Biobanking incorporated into the National Cancer Strategy | 2006 |
| First Biobank Conference in Ireland - Biobank Networks, Cancer Research and Better Global Cancer Care | 2008 |
| Expert Group Report - Recommendations for the Establishment of a National Cancer Biobank[2] | 2008 |
| Biobank established at St. James's Hospital | 2008 |
| Development and ethical approval of Biobank Consent Form & Patient Information Sheet | 2009 |
| Development and ethical approval of Patient Consent Policy for Biobanking | 2010 |
| Network received commendation at Irish Healthcare Awards (Best Hospital Project Category) | 2010 |
| Development of Sample Access Policy (SAP) and standardized Biobank Consent Form (BCF) and Patient Information Sheet (PIS) | 2010–2011 |
| Partnership with National Cancer Registry of Ireland and development of mechanism for sharing data nationally | 2011 |
| Hospitals within network approve SAP, BCF and PIS | 2011 |
| SAP, BCF and PIS approved by the Data Protection Commissioner, Europa Donna Ireland and the Research and Ethics committees of each hospital within the biobank network | 2011 |
| Eoin Gaffney, BIT Co-founder, appointed Professor of Biobanking and Biospecimen Science at Trinity College Dublin | 2012 |
Pathway for Standardization
At the outset, SJH employed Beaumont Hospital's standard operating procedures (SOPs) for tissue collection, storage, and processing. The SOPs currently employed across the network are based on these procedures with minor modifications. Harmonization of policies and SOPs is an ongoing activity and standardization is achieved via workshops and training in data protection, database management, data sharing, tissue collection and storage, ethical considerations, sample access, and quality control. Our group previously described quality control in breast cancer biobanking.10 The SJH biobank provides training and guidance to hospitals entering the network.
The physical act of biobanking samples constitutes only one of the activities of a biobank network. Ethics, patient confidentiality and securing fixed-income streams are factors of increasing importance in the evolving landscape of biopreservation and biobanking, which now spans almost all facets of health research.11
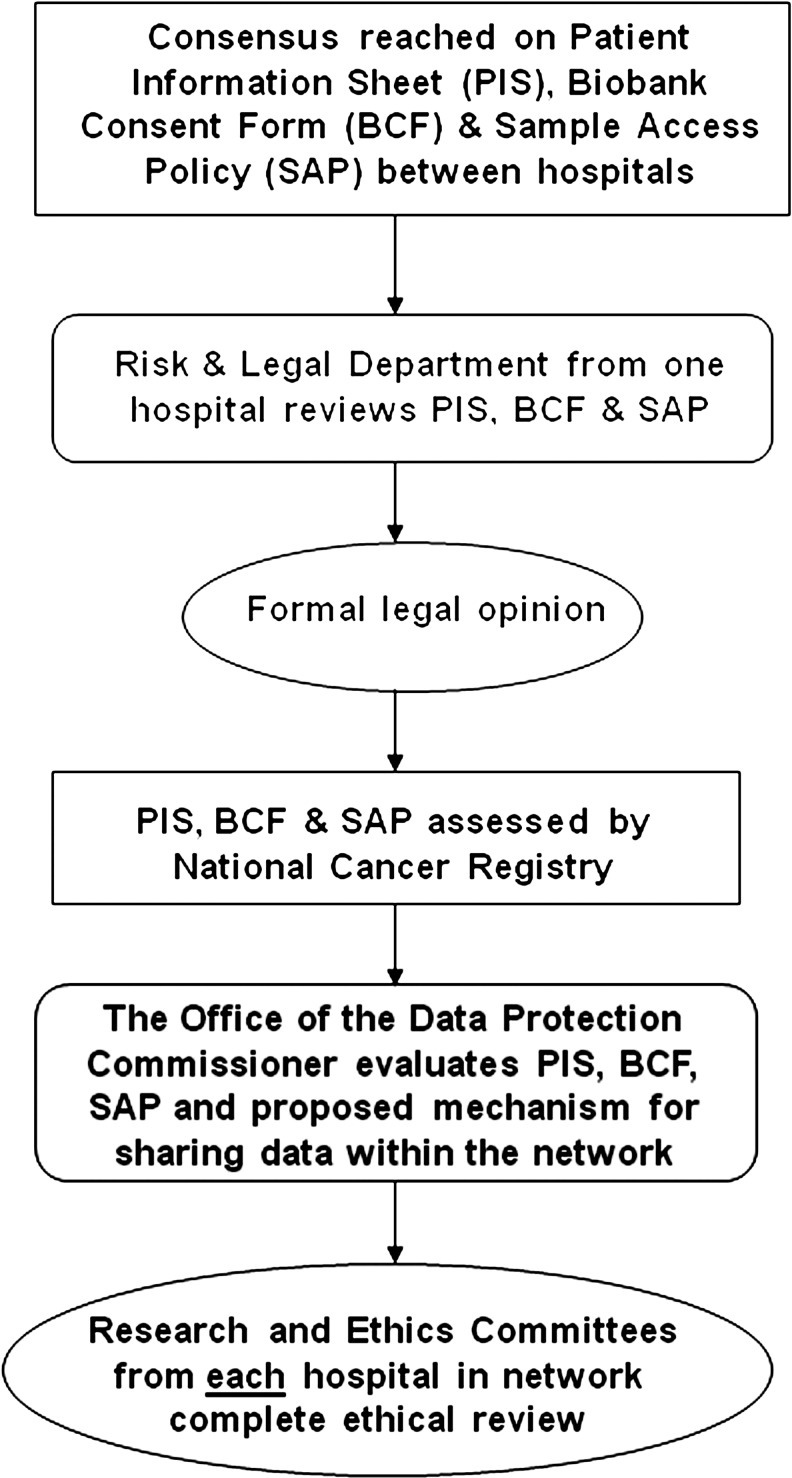
It was important at the outset to develop a sequence for policy development, review, and approval. It was essential that policies were compliant with both national and European guidelines and directives. BIT introduced a standardized Patient Information Sheet (PIS), Biobank Consent Form (BCF), and Sample Access Policy (SAP) in Ireland for the first time (Table 1). It was difficult, in the beginning, to discern the order in which to submit documents for review. The following pathway was identified as the most effective, and others may wish to adopt a similar sequence of document development and submission. First, consensus is reached with each hospital within the network. Pathology as the department most affected by the introduction of biobanking is centrally involved in all discussions from the outset.12 Second, review of documents by the Risk and Legal Department of at least one hospital in the network is undertaken. Third, a formal legal opinion is sought, preferably from an independent medical lawyer. Fourth, the National Cancer Registry assesses all documentation. Fifth, the Data Protection Commissioner evaluates all documentation, focusing particularly on the proposed method of data sharing. Finally, applications are submitted to the Research and Ethics committees of each hospital within the biobank network (Fig. 1).
FIG. 1.
Pathway for standardization.
The recently completed European Health Literacy Study reported that in Ireland “4 out of 10 people (39%) have inadequate or problematic health literacy”.13 This figure was significantly higher than a previous study.14 In light of these findings, BIT availed itself of the plain-English editing and review service provided by NALA (National Adult Literacy Agency). The agency translated the BIT Patient Information Sheet into “plain English”, ensuring the document was simple and easy to follow, in the discussion of generic or broad consent (see below).
Patient Advocate Groups
BIT has invited several Patient Advocate Groups (PAGs) to visit the biobank at SJH in order to gain an improved understanding of the patient's perspective on biobanking. This was crucial in light of the “organ retention controversy” and the distress caused to individuals by doctors' past practices with regard to communication with the next of kin regarding postmortems.15 Visits by PAGs are ongoing and remain an important source of information regarding the views and opinions of patients which are continually monitored in the development of the Irish Biobank Network. Europa Donna Ireland, Fighting Blindness Ireland, the Marie Keating Foundation, and the Irish Cancer Society have visited the SJH cancer biobank. IPPOSI (Irish Platform for Patients' Organisations, Science, and Industry) has also played a complementary role through the organization of conferences and meetings that bring all interested parties together. Recently, E. Gaffney was invited to explain the terminology and significance of breast cancer pathology reports to the members of Europa Donna (Ireland).
The Ethics of Prospective Biobanking
Consent forms and patient information leaflets, designed solely to seek permission for biobanking, are relatively new in Ireland. Historically, patients have been asked to donate blood and/or tissue to specific research groups, and information pertaining to specific project(s) was also provided. Requesting that patients donate samples for prospective and as-yet-undefined research on a national level represents a sizeable shift in the approach to health research in Ireland. To develop the national network, separate ethics submissions were required for each hospital site within the network. The recent introduction of a Standard Ethics Application Form was a welcome development which expedited this procedure.
A Patient Consent Policy (PCP) was developed to harmonize the process of consent across the network. The PCP incorporated existing guidelines pertaining to consent.16,17 Research nurses and members of different clinical teams currently recruit and consent patients for individual research projects. At SJHB, surgeons, surgical registrars and colorectal cancer nurse co-ordinators (CCNCs) have assumed these responsibilities. This is a clear testament to the commitment of hospital staff and underlines the importance placed on biobanking within the hospital. Anecdotal evidence has suggested that patients may be more likely to ask questions of a nurse due to a heightened level of familiarity and trust. Importantly, this may produce a more “informed” consenting process.
The Patient Information Sheet and Biobank Consent Form were modeled on similar documents made available by the international biobanking community (Victorian Cancer Biobank (Australia), Wales Cancer Bank, Oxford Radcliffe Biobank and onCore).18–24 The development of these documents presented BIT with an opportunity to define the ideology and ethos of the network. We consider it important to place patient welfare at the core of decision-making, as all donated tissues are derived from cancer patients. It was therefore important to utilize all available information, including patient feed-back (first hand, anecdotal, and published) in the decision-making process, and to identify solutions which were optimal for patients, while being cautious of paternalism.
The usual norms apply in that the consent must be explicit, informed, and freely given.25–29 If there is a refusal to consent to biobanking, such refusal is valid and would have no impact on the patient's future standard of care. The opportunity to withdraw samples and data from the biobank is also highlighted, though it is made clear that samples and data which have already been released cannot be recalled. It is explained that a pathologist examines each specimen, ensuring that the diagnostic integrity of the patient's specimen is the primary consideration.
The possibility that future research may include genetic influences related to cancer growth, early detection, and treatment is stated clearly. This type of consent could be classified as broad spectrum or generic consent on the grounds that it is designed to maximize the potential information gleaned from each patient sample by allowing samples to be included in multiple projects: however, all research is limited to cancer. Interestingly, a study conducted at St. James's Hospital found that only 4 out of a total of 363 patients opted to be re-contacted and re-consented each time their samples are included in a new research project. However, concerns have been raised regarding the morality of requesting broad consent; particularly in the context of the larger epidemiological biobanks30,31 (see below).
The Return of Significant Research Findings
Do biobanks have a moral duty to inform participants of research results? The duty of care which biobanks owe participants has been hotly debated in the recent past.32–35 The Singapore Tissue Network and UK Biobank have each decided against providing research results to participants.36 Reasonable arguments have been put forward to reinforce this position; for example, that the staff and the administration systems of biobanks are ill-equipped to perform such a role and “to oblige them (biobank researchers) to look beyond the variables under study to findings of potential clinical significance for individual participants would place on them a disproportionate burden”.36 Ethicists have countered that it is reasonable to expect that results which arise from the samples donated by participants should be returned to the originators (i.e., donors).35 The Irish biobank network sought to strike a balance between these views. Does the Irish network have a duty of care to patient donors? Yes. Will patients be made aware of research findings? No.
Initially, we investigated the possibility of returning research results to clinicians and General Practitioners (GPs). Clinicians, it was believed, were best placed to inform biobank donors of clinically relevant research findings, given their role on local Biobank Steering Committees. They would therefore be aware of ongoing studies incorporating blood and/or tissue samples donated by their patients. It was considered important to ensure, as far as possible, that the individuals conveying the research findings to patients could themselves interpret the findings correctly and in turn relay this information faithfully and comprehensively.
Following lengthy discussions that included clinicians, a medical lawyer, pathologists, biobank staff, and ethicists, it became apparent that this was not a workable solution; thus, the specific aims and purposes of the biobank were re-examined. Fundamentally, the biobank cannot be classified as conducting “interventional research”, and there is no certainty as regards research results. The biobank cannot present patients with a defined set of variables. The biobank does not know whether samples will lead to discoveries that are clinically relevant, how many such results will emerge, or whether any such results have the potential to impact on patients themselves or the families of patients who donate samples to the biobank. In short, we cannot ask patients to opt for the return or non-return of results when, at the point of consent, there is no clarification on the degree of specificity, impact, or scope of future research results. The introduction of a system whereby results would be returned to participants has the potential to impose ethical and legal complexities upon clinicians. It also raises questions that must be addressed and sufficiently defined before such a system could be seriously considered. During our discussions, numerous scenarios were debated. What results would be returned? Would all results be returned, or merely results that are peer reviewed and clinically relevant? It appeared reasonable to confine the return of results to those that were clinically relevant. Yet, how is clinical relevance defined? What is the agreed-to definition of clinical relevance within the network? Would it be more advantageous to return only clinically relevant and (independently) validated results? Alternatively, should findings be returned to only those patients whom they specifically affect?
It is simply insufficient to pose the question: Should the biobank return results? Yes/no. The following must first be clarified: To whom should the biobank return results? What action will occur once results are returned? Can these research findings be integrated into the care of specific patients? But what should a clinician do when research results suggest that specific patients may exhibit an enhanced response to an alternative treatment? The clinician cannot act unilaterally to offer such a treatment. Changes in patient treatment can only be introduced within national treatment guidelines, and providing clinicians and/or patients with information they cannot use is a waste of resources. There are many stages in the development of a new diagnostic test or treatment, and many “biomarkers” or “biomolecular targets” that appear promising during early stage in vitro experiments fail to perform in larger (high-powered) in vivo studies. Therefore, there would be real potential to cause confusion among the patients who have donated samples. BIT will publish peer-reviewed publications on the Biobank Ireland website. However to incorporate peer-reviewed, independently validated, and clinically relevant research findings would require substantial infrastructure capable of assessing the power, accuracy, and relevance of each individual project, in addition to recommending accompanying improvements in the clinical care pathway based on the interpretation of these findings. Although the feed-back of results is desirable, it must either be undertaken wholly and after careful consideration of the aforementioned ethical, legal, and resource questions, or not undertaken at all. There is no middle ground, and for small, under-resourced biobanks (the majority) it will probably remain an unobtainable, though desirable objective.
It has previously been suggested that patients may be more receptive to receiving results in a “hypothetical versus actual setting”.33 A number of patients who have donated samples to the Irish biobank network have died and others are currently in palliative care. One would question the morality of obliging biobanks to provide results to patients irrespective of their current state of health, merely to satisfy a moral compunction. Ethicists might with justification be accused of paternalism by determining that the return of research results is in the best interests of patients.34–36 At present, it would be extremely difficult, if not impossible, to return every research result because the time required to comprehend what may be disparate results arising from numerous studies, and the resources required to faithfully relay the information are simply prohibitive. It is clearly unwise to consider the return of nonvalidated results.37 The potential for information overload is also highly likely if patients are constantly re-contacted. The re-contacting of patients who are in recovery, remission, or have relapsed can be extremely upsetting. Former patients often struggle to put distance between themselves and their disease or illness, and “cold calling” patients may cause anxiety, especially in light of recent misdiagnosed cancer cases in Ireland.38 Though this evidence is largely anecdotal, the points raised are no less pertinent. Contacting patients in large epidemiological studies to convey a potential risk of diabetes is very different from re-contacting a patient with a history of a severe illness, and the latter should not even be contemplated unless there is a quantifiable and independently validated risk to the patients themselves and/or family members. Patients are always free to withdraw consent: the BIT Patient Information Sheet clearly states that on receipt of written instructions, all tissue, blood and/or data samples held in the biobank will be destroyed, though it will not be possible to recall samples or data already released to researchers.
Sample Access Policy
Numerous international Sample Access Policies (SAPs) were reviewed in preparing Biobank Ireland's Sample Access Policy.39–52 In Ireland, biobanks have hitherto limited access to specific research groups only. The development of a Sample Access Policy (SAP) required delicate negotiation, and issues surrounding “ownership” were a primary consideration. Some initial concern surrounded the long-term storage location of samples and a general apprehension prevailed that samples might only flow towards the larger institutions within the network. Defining eligibility for sample access and reaching consensus on priority for local research groups was time-consuming, but crucial. A coherent SAP provides researchers with a simple and transparent mechanism for accessing samples. The formation of a multidisciplinary Biobank Steering Committee (BSC) at each hospital within the network was encouraged. Each BSC reviews applications for samples from researchers affiliated with that local hospital. Representatives from each hospital BSC form the Sample Access Committee (SAC) for the national biobank network. Ideally, the SAC is a team of clinicians, research scientists, and biobank personnel who recognize the local, national, and international importance of biobanking. The SAC reviews applications from both national and international investigators conducting large-scale studies that require samples from multiple hospitals within the network. Each BSC has equal voting rights within the SAC (irrespective of the size and composition of the local BSC), thus ensuring that each hospital in the biobank network has equal influence over the distribution of samples, and the power to veto. Therefore, each BSC makes the final decision to include or exclude local samples in larger national or international projects. However, a site which consistently demonstrates a preference for local rather than national/international projects would in all likelihood experience difficulties in obtaining samples from other biobanks within the network. It is therefore mutually beneficial for each site within the network to facilitate collaborations. Members of the network have priority as has been recommended elsewhere.12 Although the SAP was only finalized in 2011, three projects have been approved, and have already led to scientific publications.10,53
Data Protection and the Irish National Cancer Registry
It was equally important to develop a mechanism for sharing data among different hospitals, academic institutions, and biopharmaceutical companies that was compliant with current Irish and European data protection legislation. In the initial phase of the network's development, greater emphasis was placed on the actual samples than on patient data. Potential downstream difficulties swiftly became apparent. For example, while sample release and distribution were relatively straightforward, data could not be disseminated from parent institution(s) without breaching data protection legislation. The solution came in the form of the National Cancer Registry of Ireland (NCR). The NCR collects and distributes data on cancer patients in Ireland and is permitted to do so by specific data protection legislation. It was therefore logical for BIT to partner with the NCR. This allows the network to focus on the biobanking and release of samples, and the NCR to focus on the dissemination of coded (de-identified) patient data, which thereby protects the privacy of individuals. Similar infrastructure exists in Scandinavia and in other countries.54 This signifies a major advance in research infrastructure in Ireland, and constitutes a timely and efficient solution to data sharing. Importantly, researchers can request clinical information from the NCR using a patient-unique biobank code which is generated at the point of collection. Each hospital biobank generates a unique code for every specimen that is biobanked. The code is generated according to the cancer site and hospital of origin and includes a unique biobank number. The code is then maintained on the hospital's biobank database. Thus, patient confidentiality is safeguarded in so far as is possible. The Biobank Consent Form, Patient Information Sheet. and Sample Access Policy illustrate the mechanism for sharing both samples and clinical data.
Biobanks are specifically mentioned in the Working Document on Genetic Data wherein it is recognized that biobanks are an “ongoing study.”55 It is noted that for a “certain period” researchers may need to link data to specific individuals and the possibility of stripping “identifiable characteristics” from databases after a defined period is promoted. The “Huriet Act” (France) is cited to support this position as the act permits the anonymization of clinical trial data 15 years post collection.55 The Irish Data Protection Commissioner's office considers anonymization the “optimal position”56 as anonymized data fall outside data protection legislation. In contrast, the RAND report cites “the ability to effectively collect and store longitudinal data is a best practice”.12 Finally, a new legal framework for the protection of personal data has also been proposed to standardize “General Data Protection Regulation” in Europe, which may influence how European biobanks operate in the future.57
Biobank Ireland considered it unwise to irreversibly anonymize clinical data in the Irish Biobank Network even after a defined period of time, because it may take 10 to 15 years or more for researchers to conclusively identify a new genetic mutation or prognostic marker. There appears to be a degree of discord between ethical and data protection guidelines (Table 2). Ethicists have strongly urged biobanks to return research findings to the patient donors,33–37 an activity which requires the retention of “identifiable patient data”. The Irish Council for Bioethics published a report which noted that while anonymization protected patient confidentiality “it precludes follow-up and feedback to research participants and may be incompatible with, or compromise, the aims of the research study”.16 Conversely, data protection guidelines describe the irreversible anonymization of sensitive patient data as optimal. The BIT solution was to modify the Biobank Consent Form to explicitly request the long-term storage and dissemination of both the samples and de-identified data donated by patients. Thus by its dual approach, BIT in its partnership with the NCR, ensures and satisfies data protection legislation and its permitted public interest mandate, and further protects patient autonomy and privacy by the use of a consent process which requests long-term storage and dissemination (Table 2).
Table 2.
Ethical and Data Protection Guidelines for Biobanking
| Guidelines | Specific documentation |
|---|---|
| Irish Ethical Guidelines for Biobanking | Human Biological Material: Recommendations for Collection, Use and Storage in Research16 |
| Irish and EU Data Protection Guidelines for Biobanking | Working Document on Genetic Data55 |
| Data Protection Guidelines on Research in the Health Sector56 | |
| The Data Protection Acts 1988 and 2003: Some Implications for Public Health and Medical Research: A Discussion Document [58] |
The Future
The prospective biobanking approach adopted by the Irish network has been well received (Table 3). The Irish Biobank Network is continuing to expand, recently partnering with National University of Ireland Galway Prostate Cancer Institute. The next step is to use the current network template to encompass a broader range of illnesses and disease types. To facilitate this expansion, it is important to identify key individuals and research groups who value the network's philosophy of trust, openness, and sharing. The formation of the Health Research Group Biobanking Subgroup to develop a national funding and implementation strategy for biobanking has raised a familiar question. Is biobanking a research or hospital activity? In truth, it is both. Moreover, we are convinced that integrating biobanks within the clinical care pathway allows for the greater committed participation of all hospital staff and the eventual sustainability of patient-focused biobanks. Hence, governance of the Irish network—including management, ethical and legal aspects, and protection of participants—is shared by the participating hospitals and BIT.
Table 3.
Views on Irish Biobank Network
| Europa Donna (Ireland), The Irish Breast Cancer Campaign | EDI and Europa Donna – “The European Breast Cancer Coalition has been very supportive of biobanking for a number of years because of the vital role of translational research in the development of effective cancer treatments. It is to be welcomed that Irish cancer patients can now donate samples through the Irish Biobank Network, thus benefiting future cancer patients”. |
| Researcher #1 | “…A national biobank is a fundamental requirement for excellent and clinically relevant biomedical research. The advantages include availability of optimal quality samples with transparent and timely sample access policies and rich clinical annotation” |
| Researcher #2 | “The Irish Biobank Network allows the researcher to tailor their project, knowing the samples that will be available. This is vital for improving the impact of Irish cancer research and most importantly it reduces the time needed to take research findings from the laboratory to the bedside.” |
Expanding the original (cancer) biobank network to include a wider spectrum of disease types has raised ethical concerns. Ethicists have questioned the legitimacy of “blanket consent”, enabling extremely diverse studies.25–27,31 Clayton noted that “people are far more likely to give permission for research on cancer, for example, than they are for studying mental health problems”.27 Rothstein reported that “a patient/subject may gladly consent to have a blood sample or pathology specimen used for research on the individual's disease, but may strenuously object to having the sample used for research on mental illness, HIV/AIDS, or other conditions”.31 In epidemiological biobanks, the blood of healthy individuals may potentially be included in research projects with a broad remit encompassing the entire spectrum of illnesses ranging from schizophrenia and cancer to high blood pressure.31 Patients may therefore favor research limited to a specific disease but oppose diversification of research fields. Tiered consent or multi-layered consent has been cited as preferable to blanket consent.59 Patients may then determine the scope of future research. This is important given our present understanding that “developments in genetic research indicate that genetic components are involved in a number of psychiatric conditions as well as complex human behaviour—for example, schizophrenia, dyslexia, attention deficit/hyperactivity disorder, and autism”.25 However, tiered/multi-layered consent was considered unsuitable by BIT, as it requires patients to make a “one-time” decision on subjects including: scope of research, feed-back of results, and genetic testing, each of which is likely to have a long-term effect on the patient and his/her family. BIT would prefer to contact and re-consent patients for research unrelated to their primary illness. Although this approach would require additional resources, BIT favors this practice. This is justified as it has been reported that the consensus among patients is that the consent procedure is “a protection of the research institution rather than of themselves”.60
Biobanks evoke strong reactions.34–37 This is not surprising, given the scope of ethical issues raised: respect, informed consent, privacy, risks, return of research results, property rights, benefit sharing, dangers of genetic research, and governance.25–37,59—63 While “nobody has ever died or suffered direct physical harm from having their previously collected biological specimens analyzed by researchers”,31 individuals are nonetheless hesitant regarding “broad consent”, particularly in circumstances where biobanks are not classified as “trustworthy” or in situations where there is “a lack of transparency”.63 It is increasingly important for biobanks to engage with the public in order to foster trust.63 A previous survey reported that only 12% of individuals in Ireland would definitely contribute to a biobank.64 This is in stark contrast to the high uptake observed in the SJH cancer biobank (≈90%) and may reflect a greater willingness to participate in disease-focused rather than in epidemiological biobanks. BIT continues to highlight the progress of the network within Ireland using both established and social media.65–68 In the future, it may be helpful to promote biobanking in a manner similar to organ donation. Ideally, patients should be cognizant of the possibility that consent may be sought for biobanking before they enter a hospital setting, resulting (one would hope) in a more informed decision. The immense potential of biobanks (health, socio-economic, and industry) will not be reached without large scale buy-in from the public.69 In addition, the need for a cohesive European biobanking infrastructure cannot be underestimated if Europe is to compete with the Japanese and US research communities.69 However, a one-size-fits-all approach will never satisfy everyone and although ideological norms can be identified, they can never be rigorously applied without affecting individualism and the rights of individuals to make their own choices rather than making choices within a framework not selected by them.
BIT's short- to medium-term objectives include expanding the current network to include each of the major cancer centers in Ireland. Enlargement of the network could be undertaken with modest capital investment and BIT's small size would facilitate a swift and dynamic approach to network expansion, avoiding a lengthy top-down administrative approach. Ideally, the Irish biobank network should be integrated within a larger pan-European network in the future. BIT is affiliated with the Marble Arch Working Group for international biobanking, ISBER and ESBB. However, the Irish government has not made a decision on membership in Biobanking and the Biomolecular Resources Research Infrastructure (BBMRI).
In conclusion, we have detailed the development of BIT's Irish Biobank Network and outlined the ELSI and other issues that must be considered. It is hoped that this communication will prove useful for those attempting to develop regional or national biobank networks. BIT's network is slowly reaching its goal: to develop infrastructure and foster research on a scale capable of delivering tangible benefits to patients.
The Biobank Ireland Trust Consent Form, Patient Information Sheet and Sample Access Policy are available on request from bmee@stjames.ie
Author Disclosure Statement
Frank Giles received support through NIH grant number R21 CA139476-01.
No competing financial interests exist.
References
- 1.Department of Children and Health. A Strategy for Cancer Control in Ireland. National Cancer Form. 2006. http://www.hse.ie/eng/services/Publications/HealthProtection/Public_Health_/National_Cancer_Control_Strategy.pdf. [Sep 20;2012 ]. http://www.hse.ie/eng/services/Publications/HealthProtection/Public_Health_/National_Cancer_Control_Strategy.pdf
- 2.Department of Children and Health. Recommendations for the Establishment of a National Cancer Biobank—Report of the Expert Group on a National Cancer Biobank. 2008. http://www.hrb.ie/uploads/tx_hrbpublications/Recommendations_for_the_Establishment_of_a_National_Cancer_Biobank.pdf http://www.hrb.ie/uploads/tx_hrbpublications/Recommendations_for_the_Establishment_of_a_National_Cancer_Biobank.pdf
- 3.Organization for Economic Co-operation and Development. OECD Guidelines on Human Biobanks and Genetic Research Databases. 2009.
- 4.Organization for Economic Co-operation and Development. OECD Best Practice Guidelines for Biological Resource Centers. 2007.
- 5.Best Practices for Repositories—Collection, Storage, Retrieval and Distribution of Biological Materials for Research. Cell Preserv Technol. 2008;6:3–58. [Google Scholar]
- 6.National Cancer Institute. Best Practices for Biospecimen Resources. 2010.
- 7.Gaffney EF. Mee B. O'Grady A, et al. An Irish Biobank Network for Patient-focused research. Ir Med J. 2011;104:125. [PubMed] [Google Scholar]
- 8.Health Research Board. Review of Population Health Research and Health Services Research in Ireland. Executive Summary and Main Report. 2011. http://www.hrb.ie/uploads/tx_hrbpublications/Review_of_population_health_research___health_services_research_in_Ireland_Vol1.pdf http://www.hrb.ie/uploads/tx_hrbpublications/Review_of_population_health_research___health_services_research_in_Ireland_Vol1.pdf
- 9.Health Research Board. Review of Population Health Research and Health Services Research in Ireland. Mapping Studies and Appendices. 2011. http://www.hrb.ie/uploads/tx_hrbpublications/Review_of_population_health_research___health_services_research_in_Ireland_Vol2.pdf http://www.hrb.ie/uploads/tx_hrbpublications/Review_of_population_health_research___health_services_research_in_Ireland_Vol2.pdf
- 10.Mee BC. Carroll P. Donatello S, et al. Maintaining breast cancer specimen integrity and individual or simultaneous extraction of quality DNA, RNA, and proteins from Allprotect-stabilized and nonstabilized tissue samples. Biopreserv Biobank. 2011;9:389–398. doi: 10.1089/bio.2011.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meir K. Gaffney EF. Simeon-Dubach D, et al. The human face of biobank networks for translational research. Biopreserv Biobank. 2011;9:279–285. doi: 10.1089/bio.2011.0018. [DOI] [PubMed] [Google Scholar]
- 12.Eiseman E. Bloom G. Brower J, et al. Santa Monica, CA: RAND Corporation; 2003. Case Studies of Existing Human Tissue Repositories: “Best Practices” for a Biospecimen Resource for the Genomic and Proteomic Era. [Google Scholar]
- 13.Health Literacy Initiative. Press release. Nov. 2011. http://healthliteracy.ie/wp-content/uploads/2011/11/FINAL-Survey-results-Crystal-Clear-2011-Launch-Press-Release-NATIONAL-271111.pdf http://healthliteracy.ie/wp-content/uploads/2011/11/FINAL-Survey-results-Crystal-Clear-2011-Launch-Press-Release-NATIONAL-271111.pdf
- 14.Research Report for National Adult Literacy Agency. Health Literacy Policy and Strategy. 2002. http://healthliteracy.ie/wp-content/uploads/2010/11/Health-literacy-policy-and-strategy-2002-research-report.pdf http://healthliteracy.ie/wp-content/uploads/2010/11/Health-literacy-policy-and-strategy-2002-research-report.pdf
- 15.McGuone D. Kay EW. The impact of the organ retention controversy on the practice of hospital necropsy: A four year audit. J Clin Pathol. 2004;57:448. doi: 10.1136/jcp.2003.012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irish Council for Bioethics. Human Biological Material Recommendations for Collection, Use and Storage. 2005. http://www.bioethics.ie/uploads/docs/BiologicalMaterial.pdf http://www.bioethics.ie/uploads/docs/BiologicalMaterial.pdf
- 17.Health Service Executive; . Jun; Guidelines in Relation to Obtaining Consent to Clinical Treatment in an Acute Hospital Setting 2008. http://www.lenus.ie/hse/bitstream/10147/75681/1/Guidelines obtaining consent to clinical treatment.pdf http://www.lenus.ie/hse/bitstream/10147/75681/1/Guidelines obtaining consent to clinical treatment.pdf Reference RM/001/2006.
- 18.The Victorian Cancer Biobank Patient Information Sheet. http://www.viccancerbiobank.org.au/downloads/Intranet_downloads/Patient_Information_Sheet/F4_i2_Patient_Information_Sheet_1_.pdf http://www.viccancerbiobank.org.au/downloads/Intranet_downloads/Patient_Information_Sheet/F4_i2_Patient_Information_Sheet_1_.pdf
- 19.The Victorian Cancer Biobank Donor Consent Form. http://www.viccancerbiobank.org.au/downloads/Forms/Sample_Donor_Consent_form.pdf http://www.viccancerbiobank.org.au/downloads/Forms/Sample_Donor_Consent_form.pdf
- 20.The Wales Cancer Bank Patient Information Sheet. http://www.walescancerbank.com/info%20sheets/patientinformationsheet%20v8%20Grove%20Mews.pdf http://www.walescancerbank.com/info%20sheets/patientinformationsheet%20v8%20Grove%20Mews.pdf
- 21.The Wales Cancer Biobank Consent Form for Patient. http://www.walescancerbank.com/info%20sheets/Patient%20consent%20Grove%20Mews.pdf http://www.walescancerbank.com/info%20sheets/Patient%20consent%20Grove%20Mews.pdf
- 22.The Oxford Radcliffe Biobank Information Sheet: Donating Blood and Tissue Samples for Medical Research. http://wyvern.ndcls.ox.ac.uk/orb/sample_orb_pis.pdf http://wyvern.ndcls.ox.ac.uk/orb/sample_orb_pis.pdf
- 23.The Oxford Radcliffe Biobank Consent Form: Donating Blood and Tissue Samples for Medical Research. http://wyvern.ndcls.ox.ac.uk/orb/sample_orb_consentform.pdf http://wyvern.ndcls.ox.ac.uk/orb/sample_orb_consentform.pdf
- 24.OnCore Patient Information Sheet: Giving Tissue and Blood Samples for Cancer Research. http://www.oncoreuk.org/pages/documents/onCore%20UK%20Donor%20Information%20Sheet%20v2%2008-05-07%20watermarked%202010-01-18.pdf http://www.oncoreuk.org/pages/documents/onCore%20UK%20Donor%20Information%20Sheet%20v2%2008-05-07%20watermarked%202010-01-18.pdf
- 25.Hansson MG. Building on relationships of trust in biobank research. J Med Ethics. 2005;31:415–418. doi: 10.1136/jme.2004.009456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansson SO. The ethics of biobanks. Cambridge Quart Healthcare Ethics. 2004;13:319–326. doi: 10.1017/s0963180104134038. [DOI] [PubMed] [Google Scholar]
- 27.Clayton EW. Informed consent and biobanks. J L Med Ethics. 2005;33:15–21. doi: 10.1111/j.1748-720x.2005.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 28.Knoppers BM. Biobanking: International norms. J L Med Ethics. 2005;33:7–14. doi: 10.1111/j.1748-720x.2005.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 29.Cambon-Thomsen A. Rial-Sebbag E. Knoppers BM. Trends in ethical and legal frameworks for the use of human biobanks. Eur Respir J. 2007;30:373–382. doi: 10.1183/09031936.00165006. [DOI] [PubMed] [Google Scholar]
- 30.Andrews LB. Harnessing the benefits of biobanks. J L Med Ethics. 2005;33:22–30. doi: 10.1111/j.1748-720X.2005.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 31.Rothstein MA. Expanding the ethical analysis of biobanks. J L Med Ethics. 2005;33:89–101. doi: 10.1111/j.1748-720x.2005.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 32.Johnson C. Kaye J. Does the UK biobank have a legal obligation to feedback individual findings to participants? Med Law Rev. 2004;12:239–267. [Google Scholar]
- 33.Ormond KE. Disclosing genetic research results: Examples from practice. Am J Bioeth. 2006;6:30–32. doi: 10.1080/15265160600935944. [DOI] [PubMed] [Google Scholar]
- 34.Harmon SH. Semantic, pedantic or paradigm shift? Recruitment, retention and property in modern population biobanking. Eur J Health Law. 2009;16:27–43. doi: 10.1163/157180909x400213. [DOI] [PubMed] [Google Scholar]
- 35.Bovenberg J. Meulenkamp T. Smets E, et al. Biobank research: Reporting results to individual participants. Eur J Health Law. 2009;16:229–247. doi: 10.1163/157180909x453062. [DOI] [PubMed] [Google Scholar]
- 36.Bovenberg J. Meulenkamp T. Smets E, et al. Your biobank, your doctor? The right to full disclosure of population biobank findings. GSP J. 2009;5:55–79. [Google Scholar]
- 37.Knoppers BM. Kharaboyan L. Deconstructing biobank communication of results. Scripted. 2009;6:677–684. [Google Scholar]
- 38.Independent team to probe alleged cancer misdiagnosis. Irish Independent. 2007. Dec 10, http://www.independent.ie/health/health-news/independent-team-to-probe-alleged-cancer-misdiagnosis-1242163.html http://www.independent.ie/health/health-news/independent-team-to-probe-alleged-cancer-misdiagnosis-1242163.html
- 39.Australian Biospecimen Network. Biorepository Protocols. http://www.abrn.net/pdf/ABN_SOPs_Review_Mar07_final.pdf http://www.abrn.net/pdf/ABN_SOPs_Review_Mar07_final.pdf
- 40.Australian Prostate Cancer BioResource. Tissue Access Policy. http://www.apccbioresource.org.au/downloads/FinalAccessPolicy%200409.pdf http://www.apccbioresource.org.au/downloads/FinalAccessPolicy%200409.pdf
- 41.The CNIO Tumour Bank Network. How to Obtain Tissue Through the BTN. http://www.cnio.es/ing/programas/progTumor05.asp http://www.cnio.es/ing/programas/progTumor05.asp
- 42.The Chernobyl Tissue Bank (CTB) Fact Sheet. http://www.chernobyltissuebank.com/docs/Fact_sheet_April_2011.doc http://www.chernobyltissuebank.com/docs/Fact_sheet_April_2011.doc
- 43.OnCoreBiosample Access Policy. http://www.oncoreuk.org/pages/documents/onCoreUK-AccessPolicyv102009-03-26o.pdf http://www.oncoreuk.org/pages/documents/onCoreUK-AccessPolicyv102009-03-26o.pdf
- 44.Cooperative Human Tissue Network. Application Form. http://www.chtn.nci.nih.gov/docs/chtn_app.508.20110104.doc http://www.chtn.nci.nih.gov/docs/chtn_app.508.20110104.doc
- 45.Kathleen Cunningham Foundation. Information and Policies for Access to Biospecimens. http://www.kconfab.org/Documents/Application%20for%20Biospecimens%20Aug%202006.pdf http://www.kconfab.org/Documents/Application%20for%20Biospecimens%20Aug%202006.pdf
- 46.The Singapore Bio-Bank. Samples Withdrawal Application Form. www.stn.org.sg www.stn.org.sg
- 47.European Human Tumor Frozen Tissue Bank. http://tubafrost.org/research/index.php http://tubafrost.org/research/index.php
- 48.Victorian Cancer Biobank. Information on How to Apply and Conditions for Use. http://www.viccancerbiobank.org.au/downloads/Researchers/F1_i2_Information_and_Conditions.pdf http://www.viccancerbiobank.org.au/downloads/Researchers/F1_i2_Information_and_Conditions.pdf
- 49.Wales Cancer Bank. Mechanism of approval for access to materials from the WCB. http://www.walescancerbank.com/info%20sheets/Mechofapprovaldraft3.doc http://www.walescancerbank.com/info%20sheets/Mechofapprovaldraft3.doc
- 50.AIDS and Cancer Specimen Resource. Standard Application Form. http://acsr.ucsf.edu/Application/tabid/59/Default.aspx http://acsr.ucsf.edu/Application/tabid/59/Default.aspx
- 51.Lemrow SM. Colditz GA. Vaught JM, et al. Key elements of access policies for biorepositories associated with population based science research. Cancer Epidemiol Biomarkers Prev. 2007;16:1533–1535. doi: 10.1158/1055-9965.EPI-07-0101. [DOI] [PubMed] [Google Scholar]
- 52.Vaught J. Kelly A. Hewitt R. A review of international biobanks and networks: Success factors and key benchmarks. Biopreserv Biobank. 2009;7:143–150. doi: 10.1089/bio.2010.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carroll PA. Healy L. Lysaght J, et al. Influence of the Metabolic Syndrome on Leptin and Leptin Receptor in Breast Cancer. Molecular Carcinogenesis. 2011;50:643–651. doi: 10.1002/mc.20764. [DOI] [PubMed] [Google Scholar]
- 54.Anderson K. Bray F. Arbyn M, et al. The interface of population-based cancer registries and biobanks in etiological and clinical research-current and future perspectives. Acta Oncol. 2010;49:1227–1234. doi: 10.3109/0284186X.2010.496792. [DOI] [PubMed] [Google Scholar]
- 55.Working document on genetic data. Article 29 Data Protection Working Party (12178/03/EN WP 91) http://ec.europa.eu/justice/policies/privacy/docs/wpdocs/2004/wp91_en.pdf http://ec.europa.eu/justice/policies/privacy/docs/wpdocs/2004/wp91_en.pdf
- 56.Data Protection Guidelines on Research in the Health Sector. Data Protection Commissioner. http://www.dataprotection.ie/documents/guidance/Health_research.pdf http://www.dataprotection.ie/documents/guidance/Health_research.pdf
- 57.(General Data Protection Regulation) COM/2012/011 final—2012/0011 (COD) Proposal for a regulation of the European parliament and of the council on the protection of individuals with regard to the processing of personal data and on the free movement of such data. http://ec.europa.eu/justice/data-protection/document/review2012/com_2012_11_en.pdf http://ec.europa.eu/justice/data-protection/document/review2012/com_2012_11_en.pdf
- 58.Sheikh AA. The Data Protection Acts 1988 and 2003: Some Implications for Public Health and Medical Research: HRB Discussion Document. 2008. http://www.hrb.ie/uploads/tx_hrbpublications/Data_Protection_Opinion.pdf http://www.hrb.ie/uploads/tx_hrbpublications/Data_Protection_Opinion.pdf
- 59.Elgar BS. Caplan AL. Consent and anonymisation in research involving biobanks. EMBO Rep. 2006;7:661–666. doi: 10.1038/sj.embor.7400740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoeyer K. The ethics of research biobanking: A critical review of the literature. Biotechnol Genet Eng Rev. 2008;25:429–452. doi: 10.5661/bger-25-429. [DOI] [PubMed] [Google Scholar]
- 61.Björkman B. Hansson SO. Bodily rights and property rights. J Med Ethics. 2006;32:209–214. doi: 10.1136/jme.2004.011270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karlsen JR. de Faria PL. Solbakk JH. To know the value of everything—A critical commentary on B Björkman and SO Hansson's “bodily rights and property rights”. J Med Ethics. 2006;32:215–219. doi: 10.1136/jme.2005.012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gottweis H. Gaskell G. Starkbaum J. Connecting the public with biobank research: reciprocity matters. Nat Rev Genet. 2011;12:738–739. doi: 10.1038/nrg3083. [DOI] [PubMed] [Google Scholar]
- 64.Gottweis H. Gaskel G. Starkbaum J, et al. University of Vienna 2011/2. Biobanks in public perception, explaining European heterogeneity. http://www.univie.ac.at/LSG/papers2011/LSG%20Working%20Paper%202011%202.pdf http://www.univie.ac.at/LSG/papers2011/LSG%20Working%20Paper%202011%202.pdf
- 65.Irish Independent (Jobs and Careers) Real Life and Work: Making a Difference. 2008. Jun 5, http://www.independent.ie/lifestyle/jobs-careers/real-life-and-work-making-a-difference-1398744.html http://www.independent.ie/lifestyle/jobs-careers/real-life-and-work-making-a-difference-1398744.html
- 66.A Sample Network. Irish Times. 2009. May 9, http://www.irishtimes.com/newspaper/innovation/2009/0511/1224246164735.html http://www.irishtimes.com/newspaper/innovation/2009/0511/1224246164735.html
- 67.Taking Stock of our Pooled rResearch. Irish Times. 2011. Nov 10, http://www.irishtimes.com/newspaper/sciencetoday/2011/1110/1224307305267.html http://www.irishtimes.com/newspaper/sciencetoday/2011/1110/1224307305267.html
- 68.Gaskell G. Gottweis H. Biobanks need publicity. Nature. 2011;471:159–160. doi: 10.1038/471159a. [DOI] [PubMed] [Google Scholar]
- 69.European Science Foundation. Population Surveys and Biobanking. European Science Foundation. 2008. http://www.esf.org/fileadmin/links/EMRC/SPB32Biobanking%5B1%5D.pdf http://www.esf.org/fileadmin/links/EMRC/SPB32Biobanking%5B1%5D.pdf