Abstract
The role of macrophage activation, traffic, and accumulation on cardiac pathology was examined in 23 animals. Seventeen animals were simian immunodeficiency virus (SIV) infected, 12 were CD8 lymphocyte depleted, and the remaining six were uninfected controls (two CD8 lymphocyte depleted, four nondepleted). None of the uninfected controls had cardiac pathology. One of five (20%) SIV-infected, non-CD8 lymphocyte-depleted animals had minor cardiac pathology with increased numbers of macrophages in ventricular tissue compared to controls. Seven of the 12 (58%) SIV-infected, CD8 lymphocyte-depleted animals had cardiac pathology in ventricular tissues, including macrophage infiltration and myocardial degeneration. The extent of fibrosis (measured as the percentage of collagen per tissue area) was increased 41% in SIV-infected, CD8 lymphocyte-depleted animals with cardiac pathology compared to animals without pathological abnormalities. The number of CD163+ macrophages increased significantly in SIV-infected, CD8 lymphocyte-depleted animals with cardiac pathology compared to ones without pathology (1.66-fold) and controls (5.42-fold). The percent of collagen (percentage of collagen per total tissue area) positively correlated with macrophage numbers in ventricular tissue in SIV-infected animals. There was an increase of BrdU+ monocytes in the heart during late SIV infection, regardless of pathology. These data implicate monocyte/macrophage activation and accumulation in the development of cardiac pathology with SIV infection.
Introduction
Effective antiretroviral treatment (ART) has decreased mortality due to HIV infection resulting in an increase in the survival rate of HIV-infected patients.1,2 This increased survival has resulted in new, tissue-specific complications due to HIV infection and chronic immune activation.3,4 Previous studies have shown a link between cardiovascular disease (CVD) and HIV infection, with HIV-infected individuals having approximately a 2-fold increase in the incidence of myocarditis, ventricular dilation, and myocardial infarction compared to age-matched uninfected individuals.5–7 Factors correlating with HIV-associated CVD are likely multifactoral and include antiretroviral drugs,8–10 microbial translocation resulting in chronic immune activation,11,12 increased levels of cardiac myosin-specific autoantibodies,13 opportunistic infections,14,15 and increased inflammation due to immune activation.16,17 Consistent observations in HIV-infected individuals with CVD include mononuclear cellular infiltrates observed in cardiac parenchymal tissue and coronary vessels.12,18
Monocytes/macrophages have been shown to play a role in simian immunodeficiency virus (SIV)-associated and HIV-associated CVD, in particular atherosclerosis and myocarditis.5,19 Developing in the bone marrow, monocytes are released into the blood where they function in immunosurveillance and traffic to specific tissues, normally and in response to infection. Monocytes develop into macrophages in tissues5,20; some of them play roles in inflammation while others, which are alternatively activated, may have antiinflammatory roles.21 Monocytes are prime sources of cytokines and chemokines that can alter cardiac function, and22,23 monocyte/macrophage-associated proinflammatory cytokines including interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α) are elevated during HIV infection and CVD.24,25 The number of CD14+CD16+ proinflammatory blood monocytes is increased with CVD and HIV infection, suggesting that chronic immune activation with HIV infection may increase the risk of CVD.26,27
CD163, a hemoglobin/haptoglobin receptor involved in the clearance of hemoglobin, is expressed on the surface of monocytes/macrophages28,29 and proteolytically cleaved from the surface of activated CD163+ monocytes/macrophages in response to proinflammatory signaling.30,31 Levels of soluble CD163 (sCD163) in plasma inversely correlate with the levels of the membrane bound form of CD163 on monocytes/macrophages32 and function as a biomarker for diseases where macrophage activation and inflammation play a central role.31
We have shown that sCD163 levels in plasma correlate with the rate of AIDS progression in SIV-infected rhesus macaques.33 Levels of sCD163 in acute (<1 year) and chronic (>1 year) HIV infection have been shown to function as a novel marker of HIV activity,34 and correlate with the percentage of noncalcified, vulnerable coronary plaques.35 Lastly, there are increased percentages of noncalcified coronary plaques in HIV-infected elite controllers not on ART, further underscoring the role of monocytes/macrophages in lesion formation.36 While causes of CVD in HIV-infected individuals are likely multifactoral, we sought to examine the role of macrophage activation and accumulation with SIV infection. We examined macrophage accumulation in the hearts of SIV-infected non-CD8 lymphocyte-depleted rhesus macaques and in SIV-infected, CD8 lymphocyte-depleted animals. We found similar pathology in both groups, but SIV-infected CD8 lymphocyte-depleted animals had a higher incidence of inflammation and damage in the heart. Because SIV-infected, CD8 lymphocyte-depleted animals progress to AIDS rapidly (3–4 months), and have a high rate of macrophage-mediated disease,37 we used this model to examine the role of macrophage activation in cardiac inflammation and damage with AIDS.
Materials and Methods
Ethics statement
All animals were either housed at Harvard University's New England Regional Primate Research Center (NERPC) or Tulane University's National Primate Center and handled in accordance with Harvard University's or Tulane University's National Primate Research Center Institutional Animal Care and Use Committee (IACUC). IACUC approval from the NERPC and Tulane National Primate Research Center was granted for all procedures including 5-bromo-2′-deoxyuridine (BrdU) injections.
Animals, viral infection, and CD8+ T lymphocyte depletion
Twenty-three rhesus macaques were used in this study. Seventeen were infected with SIVmac251 (SIV p27) by iv injection (kindly provided by Ronald Desrosiers, Harvard University). To achieve rapid progression to AIDS, 12 of the 17 infected animals were CD8 lymphocyte depleted using a human anti-CD8 antibody, cM-T807, administered sc (10 mg/kg) at 6 days postinfection (dpi) and iv (5 mg/kg) at 8 and 12 dpi, as previously described.4 The cM-T807 antibody was provided by the NIH Non-human Primate Reagent Resource (RR016001, A1040101). Six of the 23 rhesus macaques served as uninfected controls, two of which were CD8 lymphocyte depleted (50 mg/kg single bolus iv injection). Animals were anesthetized with ketamine-HCl, euthanized with a pentobarbital overdose intravenously, and exsanguinated. Following exsanguinations, standard necropsy was performed with a standard set of major organs collected in 10% neutral buffered formalin. Following fixation, all tissues were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Sections of left ventricular myocardium were blindly analyzed by a board certified veterinary pathologist (Andrew D. Miller, D.V.M, D.A.C.V.P). Cardiac tissues were scored based on 10 randomly chosen 400× fields. Pathology was assessed based on the degree of change in the tissue and graded as mild, moderate, or severe inflammation and degeneration.
Plasma viral load determination
SIV-RNA in plasma was quantified using real time PCR as previously described.38 Virions were pelleted from 500 μl EDTA plasma from infected animals by centrifugation at 20,000 × g for 1 h. The threshold sensitivity was 100 copy Eq/ml. Viral loads peaked at day 8 postinfection, and no differences in viral load were observed between SIV-infected, CD8 lymphocyte-depleted animals.
Immunohistochemistry and in situ hybridization for SIV-p28 and -RNA
The presence of SIV viral proteins in heart tissues was identified using SIV-gp41 (KK41 1:500, NIH AIDS Research and Reference Reagent Program) and SIV-p28 (SIVmac251 1:2500, Fitzgerald Industries), along with in situ hybridization with digoxigenin-labeled antisense riboprobes to detect SIV-RNA (Lofstrand Labs, Gaithersburg, MD), as previously described.39 Extensive immunohistochemistry and in situ hybridization in tissue revealed that no SIV-p28 or -RNA was found in the hearts of SIV-infected animals.
5-bromo-2′-deoxyuridine (BrdU) administration
To study monocyte/macrophage traffic to and accumulation in the heart with SIV infection, BrdU was administered to SIV-infected, CD8 lymphocyte-depleted animals as previously described.33 Briefly, a 30 mg/ml BrdU stock solution was prepared with 1× phosphate-buffered saline (PBS) and was heated to 60°C in a water bath. BrdU was administered as a slow bolus iv injection at a dose of 60 mg BrdU/kg body weight. Animals were administered BrdU either early (6 and 20 dpi) or late (48 dpi and 24 h prior to necropsy).
Immunohistochemistry
Formalin-fixed, paraffin-embedded cardiac tissue was cut in 5-μm sections, air dried overnight, then deparaffinized with xylenes, and rehydrated in graded ethanols and finally with deionized water. Following rehydration, antigen-binding sites were unmasked using Antigen Unmasking Solution (Vector Laboratories) for antibodies recognizing CD3, CD68, and BrdU with 20 min heat exposure followed by 20 min cooling at room temperature. Antigen unmasking for HAM56 was accomplished by incubating slides at 37°C for 5 min with Proteinase K (Dako). Following antigen unmasking cardiac tissues were blocked with a dual endogenous enzyme block (Dako). Sections were then incubated with biotin solution (Avidin/Biotin block, Vector Laboratories) for 15 min to block endogenous biotin, followed by protein block using serum-free protein block (Dako) and incubated for 10 min for monoclonal antibodies and 30 min for polyclonal antibodies. Antibodies used to identify macrophages were the pan macrophage marker mouse monoclonal antibody CD68 (1:400, Dako, clone KP1). The CD163 scavenger receptor was detected using mouse monoclonal CD163 (1:250, Serotec). Recently infiltrated macrophages were identified by staining with a mouse monoclonal antibody against MAC387, which recognizes MRP14 and to a lesser extent the MRP14/MRP8 heterocomplex (1:100, Dako). A rabbit polyclonal CD3 antibody (1:300, Dako) was used to identify CD3 T-lymphocytes. To assess the timing of inflammatory cell accumulation in hearts of CD8 lymphocyte-depleted animals that received BrdU, tissues were stained with a mouse monoclonal BrdU antibody (1:100, Dako).
Slides were incubated for 1 h at room temperature. Sections were incubated with the corresponding antimouse or antrabbit horseradish peroxidase-conjugated secondary antibody for 30 min at room temperature. The color reaction product was developed using 3,3′-diaminobenzidine tetrahydrochloride (DAB, Dako). Slides were counterstained in hematoxylin, dehydrated in washes of increasing concentration of ethanol, followed by xylene, and mounted. To determine if BrdU-labeled cells in the heart of SIV-infected, CD8 lymphocyte-depleted animals were myeloid cells or CD3+ T-lymphocytes, double label immunohistochemistry was performed using antibodies against MAC387, CD68, CD163 (for macrophages), or CD3 (for T-lymphocytes) and BrdU. Double label immunohistochemistry was performed using the Dako Double Stain System, according to the manufacturer's protocol. Isotype-specific immunoglobins that correspond with the specific test antibodies were used as controls.
The color reaction was developed using DAB and Vector Blue (Vector Labs). All slides were air dried overnight before mounting. The numbers of BrdU+ MAC387+, BrdU+ CD68+, BrdU+ CD163+, and BrdU+ CD3+ double-positive cells were counted. Slides were imaged using a Zeiss Axio Imager M1 microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY) using Plan-Apochromat ×20/0.8 Korr objectives. Positive cells were counted manually for 20 random, nonoverlapping fields of view to determine the number of positive cells/mm2 with a field area of 0.147 mm2 corresponding to a 20× field
Massons trichrome stain
Cardiac ventricular tissues were stained using a Massons Trichrome Stain kit (Newcomer Supply, Middleton, WI) according to the manufacturer's protocol and imaged using a Zeiss Axio Imager M1 microscope using Plan-Apochromat ×20/0.8 Korr objectives. Twenty random, nonoverlapping fields of view were imaged for each slide and the percent of collagen relative to total cardiac tissue area examined was calculated. The percent collagen of total tissue area was quantified using ImageJ analysis software (v1.46, National Institute of Health) and color deconvolution vectors developed for Massons trichrome stain that separate histological dyes into individual red, blue, and green colors.40 We quantified the area of red and blue dyes corresponding to cytoplasm and collagen, respectively, to determine the percentage of total tissue area for each. The percentage of collagen used here serves as a qualitative measurement of fibrosis and cardiac tissue damage as previously described.41
Statistical analysis
Statistical analyses were performed using Prism version 5.0d (GraphPad Software, Inc., San Diego, CA) software. A Mann–Whitney nonparametric U test was used to compare all groups. Spearman's rank correlation was used for all correlations. Significance was accepted at p<0.05.
Results
Increased numbers of macrophages in the heart with SIV infection in non-CD8 lymphocyte-depleted animals
To investigate the role of monocytes/macrophage activation and traffic on cardiac pathology with SIV infection, 23 rhesus macaques were used in this study. Six animals (two CD8 lymphocyte depleted, four nondepleted) were uninfected controls. Seventeen animals were SIV infected, and of these 12 were CD8 lymphocyte-depleted (Table 1). Immunohistochemistry revealed a significant increase in the number of CD163+, CD68+, and MAC387+ macrophages in SIV-infected non-CD8 lymphocyte–depleted animals compared to uninfected controls. There was a 1.32-fold increase in the number of activated CD163+ macrophages, a 1.91-fold increase in the number of resident CD68+ macrophages, and a 1.45-fold increase in the number of newly infiltrating MAC387+ macrophages in cardiac tissue of SIV-infected non-CD8 lymphocyte-depleted animals compared to uninfected controls. In CD8 lymphocyte-depleted animals we found a 4.52-, 9.44-, and 2.36-fold increase (p<0.05) in the number of CD163+, CD68+, and MAC387+ macrophages, respectively. CD3+ T-lymphocytes in SIV-infected, CD8 lymphocyte-depleted animals were also significantly increased 7.91-fold (p<0.05) compared to uninfected controls (Table 2).
Table 1.
Rhesus Macaques Used in the Study
| Animal type | SIV status | N | Heart pathology |
|---|---|---|---|
| Controls | − | 6 | 6 NSF |
| Non-CD8 depleted | + | 5 | 4 NSF |
| 1 Mild, myocardial fibrosis and immune cell infiltration | |||
| CD8 depleted | + | 12 | 5 NSF |
| 3 Mild | |||
| 3 Moderate | |||
| 1 Severe, myocardium degeneration, inflammation, and fibrosis |
Pathology was determined by a veterinary pathologist. Cardiac tissue sections were scored based on 10 randomly chosen 400× fields. Inflammation was graded according to mild, moderate, and severe as it related to immunoreactive cells present within the section.
NSF, no significant findings.
Table 2.
Comparison of the Numbers of Macrophages and T-Lymphocytes in Uninfected, SIV+, and SIV+ CD8 Lymphocyte-Depleted Rhesus Macaques
| SIV+ | p value | Fold change | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Immune markers | Uninfected (UN) (n=6) | Nondepleted (ND) (n=5) | CD8 Depleted (CD8) (n=12) | UN vs. ND | UN vs. CD8 | ND vs. CD8 | NDUN | CD8UN | CD8ND |
| CD163 | 56.33 (±5.30) | 74.70 (±3.74) | 254.83 (±21.37) | * | ** | ** | 1.32 | 4.52 | 3.41 |
| CD68 | 10.85 (±2.03) | 20.76 (±1.63) | 102.52 (±23.3) | * | ** | ** | 1.92 | 9.44 | 4.93 |
| HAM56 | 92.50 (±23.20) | 77.12 (±10.19) | 163.50 (±34.9) | ns | ns | * | — | — | 2.12 |
| MAC387 | 10.92 (±3.20) | 15.83 (±1.96) | 25.83 (±2.25) | * | * | * | 1.45 | 2.36 | 1.63 |
| CD3 | 3.47 (±0.75) | 5.74 (±1.07) | 27.45 (±7.85) | ns | * | ** | — | 7.91 | 4.78 |
Uninfected controls comprised of two uninfected, CD8 T-lymphocyte-depleted animals and four uninfected, nondepleted animals. No differences were observed in the number of macrophages and T-lymphocytes between these two groups. Numbers represent the mean number of positive cells (cells/mm2)±standard error of the mean (parentheses) based on counts from 20 nonoverlapping fields of view using a 20× objective. p values were calculated by comparing the mean number of positive cells for controls and SIV+ animals using a Mann–Whitney nonparametric U test (*p<0.05, **p<0.01). Fold change was calculated based on the ratio of macrophages or T-lymphocytes present in the heart for the indicated groups.
Increased numbers of macrophages in the heart of CD8 lymphocyte-depleted versus non-CD8 lymphocyte-depleted animals
SIV-infected, CD8 lymphocyte-depleted animals had significantly elevated numbers of macrophages and T-lymphocytes in cardiac tissue compared to SIV-infected, non-CD8 lymphocyte-depleted rhesus macaques (Table 2). CD163+ macrophages were increased 3.41-fold (p<0.01), CD68+ macrophages were increased 4.93-fold (p<0.01), and MAC387+ cells were increased 1.63-fold (p<0.05) in SIV-infected, CD8-depleted animals compared to SIV-infected, non-CD8 lymphocyte-depleted animals. The number of T-lymphocytes in SIV-infected, CD8-depleted animals was elevated 4.78-fold over SIV-infected non-CD8 lymphocyte-depleted animals. Overall, when compared to controls, SIV-infected, CD8 lymphocyte-depleted animals show even greater numbers of macrophages and T-lymphocytes in cardiac tissue (Table 2).
Increased numbers of macrophages in the hearts of animals with cardiac pathology
SIV-infected, CD8 lymphocyte-depleted animals were divided into two groups based on the presence or absence of cardiac pathology as determined by a veterinary pathologist. Five of 12 animals had normal histology, while the remaining seven had mild, moderate, or severe inflammation and degeneration. Animals with cardiac pathology had a 1.66-fold increase of CD163+ macrophages (p<0.01), and CD68+and MAC387+ macrophages were increased by 2.46-fold (p<0.01) and 1.70-fold (p<0.05), respectively, when compared to SIV-infected, CD8-depleted animals without cardiac pathology (p<0.05). In addition, SIV-infected, CD8 lymphocyte-depleted animals with pathology had a 2.51-fold increase in the number of CD3 T-lymphocytes compared to animals without cardiac pathology (Table 3).
Table 3.
Increased Number of Macrophages and T-Lymphocytes in SIV-Infected CD8-Depleted Rhesus Macaques with Cardiac Pathology Compared to SIV-Infected CD8-Depleted Rhesus Macaques Without Cardiac Pathology
| SIV+, CD8 depleted (n=12) | ||||
|---|---|---|---|---|
| Immune markers | w/o pathology (n=5) | w/pathology (n=7) | p value | Fold change |
| CD163 | 183.40 (±34.20) | 305.85 (±14.06) | ** | 1.66 |
| CD68 | 55.98 (±12.06) | 137.75 (±6.20) | ** | 2.46 |
| HAM56 | 196.50 (±14.90) | 131.90 (±12.40) | ns | — |
| MAC387 | 17.34 (±1.99) | 29.48 (±3.01) | * | 1.70 |
| CD3 | 14.60 (±4.72) | 36.62 (±12.07) | * | 2.51 |
Numbers represent the mean number of positive cells (cells/mm2)±standard error of the mean (parentheses) based on counts from 20 nonoverlapping fields of view using a 20×objective. p values were calculated by comparing the mean number of positive cells for SIV+, CD8-depleted animals with and without cardiac pathology using a Mann–Whitney nonparametric U test (*p<0.05, **p<0.01). Fold change was calculated based on the ratio of cells in SIV+, CD8-depleted animals with cardiac pathology compared to animals without pathology.
Correlations between fibrosis and increasing numbers of macrophages in the heart
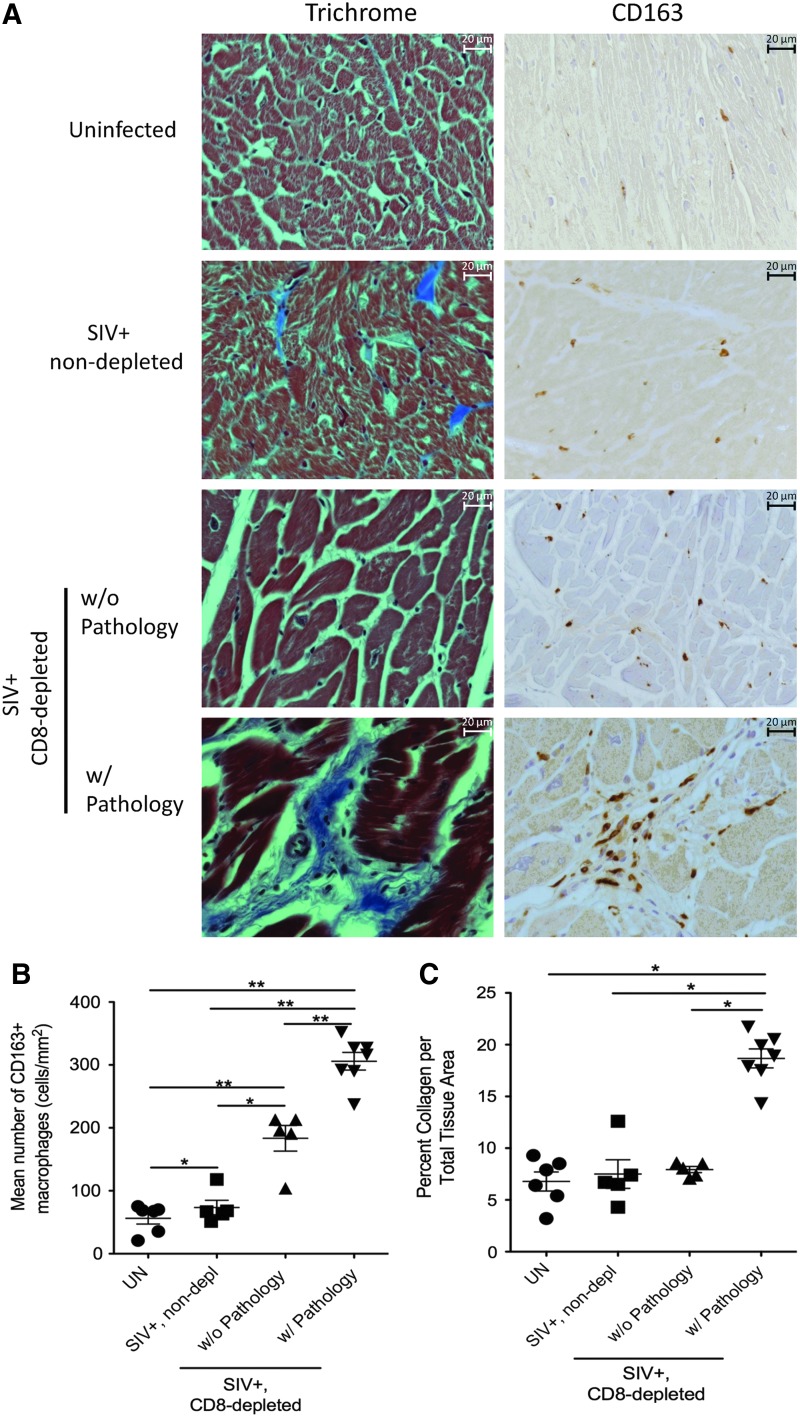
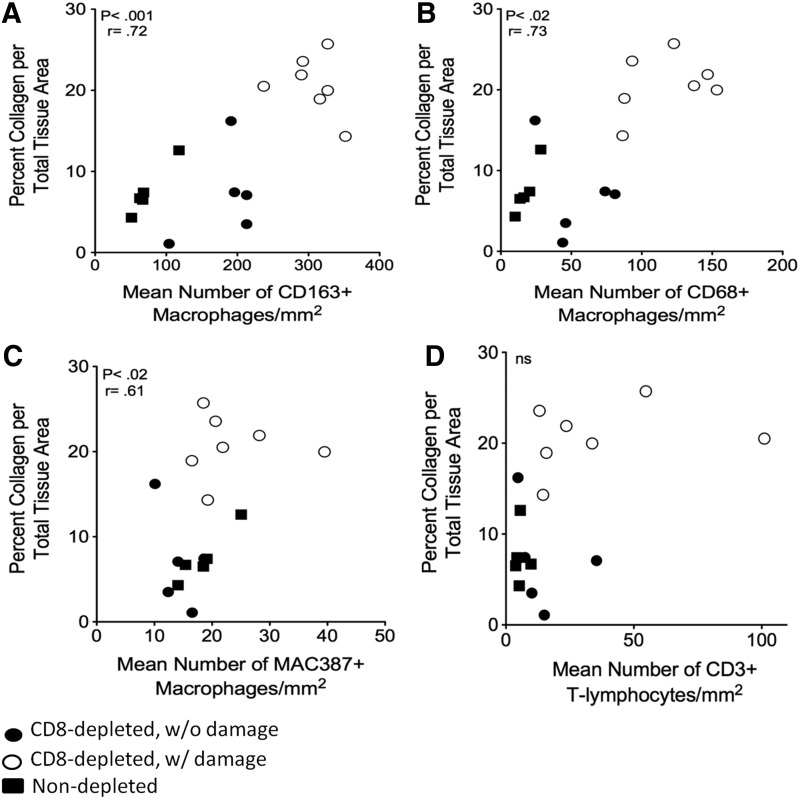
We quantified the percent of collagen per total tissue area using Massons trichrome stain, as a measurement of fibrosis. In SIV-infected non-CD8 lymphocyte-depleted animals one animal had minor pathology and mild fibrosis with 12.6±5.6% collagen per total tissue area, as compared to the remaining four animals with normal histology with an average of 6.2±3.8% collagen. The percent collagen in hearts of SIV-infected non-CD8 lymphocyte-depleted animals was not significantly different from uninfected controls (p>0.05) (Fig. 1A and C). Overall, SIV-infected, CD8 lymphocyte-depleted animals had increased levels of collagen compared to SIV-infected, non-CD8-depleted animals (14.5±1.81 compared to 7.5±1.39) (Fig. 1A and C). SIV-infected, CD8 lymphocyte-depleted animals with cardiac pathology had increased amounts of collagen and numbers of CD163+ macrophages (Fig. 1B and C). The percentage of collagen per total tissue area in SIV-infected, CD8 lymphocyte-depleted rhesus macaques with cardiac pathology was significantly increased when compared to those without pathology (Fig. 1C) (19.2±3.4% vs. 7.93±4.7%, p<0.05). All SIV-infected animals had positive correlations between the percentage of collagen per total tissue area as a marker for fibrosis and the numbers of CD163+ (Fig. 2A), CD68+ (Fig. 2B), and MAC387+ (Fig. 2C) macrophages. Animals with severe cardiac pathology had the highest numbers of macrophages in the heart. There was no correlation between the number of CD3+ T-lymphocytes and the amount of fibrosis in SIV-infected animals (Fig. 2D).
FIG. 1.
Increased numbers of CD163+ macrophages and amount of collagen in heart tissue in simian immunodeficiency virus (SIV)-infected rhesus macaques with cardiac damage. Massons trichrome stain and immunohistochemistry were used to compare the amount of collagen and numbers of CD163+ macrophages present in the heart of uninfected, SIV-infected non-CD8-depleted, and SIV-infected CD8 lymphocyte-depleted rhesus macaques [(A) 400× magnification]. SIV-infected, CD8-depleted animals with cardiac pathology show significantly increased levels of collagen (19.2±3.4%) and increased numbers of CD163+ macrophages are present (305.85±14.06) (B, C). Statistical analysis was done between the indicated groups using the Mann–Whitney nonparametric U test (*p<0.05, **p<0.01).
FIG. 2.
Correlations between the percentage of collagen per total tissue area and numbers of CD163+, CD68+, and MAC387+ macrophages in SIV-infected rhesus macaques. Spearman's rank test was used to determine if there were correlations between the amount of cardiac damage based on the percentage of collagen and the numbers of macrophages in the heart of SIV-infected rhesus macaques (closed circle, SIV+ CD8 depleted w/o damage; open circle, SIV+ CD8 depleted w/damage; closed square, SIV+ nondepleted). Positive correlations were found between the amount of damage in each section and the numbers of CD163+ (A), CD68+ (B), and MAC387 (C) macrophages in SIV-infected rhesus macaques. No correlations were found between the amount of damage and the number of CD3+ T-lymphocytes (D). r=Spearman's coefficient. p<0.05.
Absence of SIV viral protein and RNA in the heart of SIV-infected animals
Extensive immunohistochemistry and in situ hybridization was done on cardiac tissues to determine if SIV-infected cells were present. SIV-p28 and SIV-RNA were found in matched brain and lymph node tissues, but not in cardiac tissues from the same animals (data not shown). There was no difference in plasma viral load between SIV-infected, CD8 lymphocyte-depleted animals with and without cardiac pathology. Plasma viral load peaked at 8 dpi, was virtually indistinguishable between animals, and remained elevated throughout infection. Thus, these levels did not correlate with differences in cardiac pathology between animals (data not shown).
Increased traffic to the heart during late SIV infection
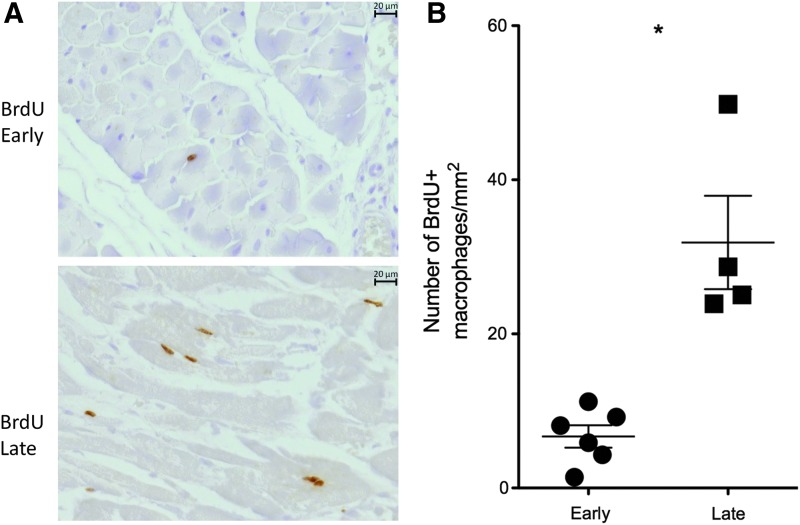
We used BrdU to examine the traffic of recently released macrophages to the heart in SIV-infected, CD8-depleted animals. Animals were grouped based on when they received BrdU, early (6 and 20 dpi) or late (48 dpi and 24 h prior to necropsy). In animals that received BrdU late we found a 4.72-fold increase in the number of BrdU+ macrophages present compared to animals that received BrdU early (Fig. 3A and B).
FIG. 3.
Increased traffic of cells to the heart during late SIV infection. Bromodeoxyuridine (BrdU) experiments were used to determine when inflammatory cells traffic to the heart in SIV-infected, CD8 lymphocyte-depleted rhesus macaques. Animals were grouped based on whether they received BrdU early (6 and 20 dpi) or late (48 dpi and 24 h before necropsy). Immunohistochemistry shows increased traffic of BrdU+ cells in the heart of SIV-infected, CD8 lymphocyte-depleted rhesus macaques that received BrdU late [(A) 400× magnification]. Animals that received BrdU late during infection had 31.85±14.8 BrdU+ cells compared to 6.68±2.9 BrdU+ cells in animals receiving BrdU early (B).
BrdU+ cells in the heart are of myeloid lineage
The majority of BrdU+ cells in the heart were MAC387+ (75.60±3.60% for uninfected controls, 84.48±5.83% for SIV-infected, CD8 lymphocyte-depleted animals without cardiac pathology, and 79.25±4.98% for SIV-infected, CD8 lymphocyte-depleted animals with cardiac pathology). Minor populations of BrdU+ CD68+ (26.75±7.97% for uninfected controls, 14.51±3.92% for SIV-infected, CD8 lymphocyte-depleted animals without cardiac pathology, and 19.31±4.89% for SIV-infected, CD8 lymphocyte-depleted animals with cardiac pathology) and BrdU+ CD163+ (9.90±5.71% for uninfected controls, 1.24±0.53% for SIV-infected, CD8 lymphocyte-depleted animals without cardiac pathology, and 5.87±1.80% for SIV-infected, CD8 lymphocyte-depleted animals with cardiac pathology) macrophages were found. No BrdU+ CD3+ T-lymphocytes were found in the hearts of all animals studied (Table 4).
Table 4.
Bromodeoxyuridine-Positive Cells in the Heart of SIV-Infected, CD8 Lymphocyte-Depleted Rhesus Macaques Are of Myeloid Lineage
| SIV+, CD8 depleted (n=12) | |||
|---|---|---|---|
| Cell phenotype | SIV−, CD8 depleted (n=2) | w/o pathology (n=5) | w/pathology (n=7) |
| BrdU+ MAC387+ | 75.60 (±3.71) | 84.48 (±5.83) | 79.25 (±4.98) |
| BrdU+ CD68+ | 26.75 (±7.97) | 14.51 (±3.92) | 19.31 (±4.89) |
| BrdU+ CD163+ | 9.90 (±5.71) | 1.24 (±0.53) | 5.87 (±1.80) |
| BrdU+ CD3+ | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) |
BrdU+ cells were counted for SIV-infected, CD8 lymphocyte-depleted animals and the average percentage of double positive cells for each indicated group was calculated±standard error of the mean (in parentheses).
BrdU, bromodeoxyuridine.
Discussion
We examined, in a retrospective study, the role of macrophage activation and traffic to the heart with SIV infection leading to cardiac inflammation and pathology. Examining macrophage populations in SIV-infected, non-CD8 lymphocyte-depleted rhesus macaques we found increased numbers of macrophages in cardiac tissues compared to uninfected controls. Twenty percent of SIV-infected, non-CD8 lymphocyte-depleted animals had minor interstitial fibrosis while the remaining had no significant signs of fibrosis when compared to controls. A single animal that had increased levels of fibrosis also had increased numbers of macrophages in the heart when compared to other SIV-infected, non-CD8 lymphocyte-depleted animals without fibrosis. This suggests that macrophage accumulation plays a role in the observed cardiac pathology in a subset of SIV-infected animals.
Compared to SIV-infected, non-CD8 lymphocyte-depleted rhesus macaques SIV-infected, CD8 lymphocyte-depleted animals had significantly increased numbers of macrophages in the heart and a higher incidence of cardiac pathology. While viral load in plasma has been implicated in the progression of cardiac pathology in SIV-infected animals,42 we observed no differences in plasma viral load between SIV-infected, CD8 lymphocyte-depleted animals with and without cardiac pathology. Additionally, we did not find SIV-infected macrophages in the hearts of our animals.
Kelly et al. found scattered SIV-infected macrophages in hearts of a subset of infected animals (12 of 22) using an SIV-gp41 antibody that recognizes productively infected cells.42 But the number of infected cells did not correlate with functional decline.42 Yearley et al. found 1–4 SIV-infected macrophages/mm2 in hearts of 7 of 21 animals.43 These authors used an antibody against SIV-nef that detects latent and productively infected macrophages. We found SIV-p28 and SIV-RNA-positive macrophages in brains and lymph nodes of the animals in our study. Results from our study suggest plasma virus, protein, or RNA alone does not drive cardiac pathology, and other factors, including macrophages, likely do. We found positive correlations between the number of macrophages and the amount of fibrosis as a maker of damage to the heart, where animals with the most severe fibrosis had the highest numbers of macrophages.
While we have reported increased numbers of CD163+ macrophages in SIV-infected animals with cardiac pathology and fibrosis, others have shown decreased numbers of CD163+ macrophages in the heart of SIV-infected, non-CD8 lymphocyte-depleted animals with active and borderline myocarditis44 and an increase in CD3+ T-lymphocytes.43 In these studies, there were increased numbers of CD163+ macrophages in SIV-infected animals with normal, uninflammed heart tissue, and fewer CD163+ macrophages in SIV-infected animals with active or borderline myocarditis.
Because CD163+ macrophages can be considered to be antiinflammatory, and CD163 is upregulated by the antiinflammatory cytokines IL-6 and IL-10,45,46 these authors concluded that CD163+ macrophages are protective. Their observations are in contrast to ours and others, where increased numbers of CD163+ macrophages are present with active inflammation and lesion formation in cardiac and central nervous system tissues. It is interesting that we find more CD163+ macrophages in the heart than CD68+ macrophages because CD68 is considered to be a pan macrophage marker. We note that CD68 expression by macrophages is regulated by activation, which can vary from tissue to tissue and be dictated not only by activation but also by microenvironments. Whether there is differential CD163 and CD68 regulation and expression in the heart, and/or different macrophage subsets based on CD68 and CD163, warrants further study. CD163 is also expressed on plasmacytoid DCs, which also are CD68 negative. Recent reports are just now characterizing resident and inflammatory macrophage populations in cardiac tissue.47
Previous studies have shown that chronic inflammation and T cell activation, without HIV infection, play a role in the formation of atherosclerotic plaques.7 In HIV-infected individuals, atherosclerosis is associated with T cell activation and markers of inflammation predict cardiac events.48,49 In SIV-infected animals CD8+ T-lymphocytes are present and correlate with myocarditis.18,43 Animals in our study were persistently CD8+ T-lymphocyte depleted, and had no CD8+ T cells at necropsy. We found few CD3+ T-lymphocytes in cardiac tissues, which are likely CD4+ T cells or NK cells. Double label immunohistochemistry indicated that a majority of BrdU+ cells are MAC387+ macrophages with no BrdU+ CD3+ cells. Minor populations of CD68+ BrdU+ and CD163+ BrdU+ macrophages were also found. These results indicate that the newly infiltrating cells are myeloid lineage monocyte/macrophages. Thus, our results show there is cardiac pathology and fibrosis without CD8+ T-lymphocytes and few CD3+ T-lymphocytes, further underscoring the role of monocytes/macrophages in pathology and fibrosis.
Using FDG-PET imaging, we have shown that increased monocyte/macrophage accumulation in the ascending aorta with HIV infection correlates with the number of noncalcified, vulnerable plaques.50 In addition, this accumulation of macrophages is associated with sCD163 in plasma, a marker of macrophage activation.50 We have also shown that sCD163 in plasma correlates with the presence of noncalcified, vulnerable coronary plaques in HIV-infected patients.35 Here, we demonstrate that actual macrophage accumulation within ventricular tissue correlates with fibrosis, suggesting that macrophage accumulation drives cardiac pathology with SIV infection. While some studies have associated the toxic effects of ART on coronary artery disease progression in humans and monkeys,8–10 studies examining HIV-1 elite controllers have shown that these individuals have increased rates of atherosclerosis compared to uninfected and chronically HIV-infected individuals.36 HIV-1 elite controllers also had elevated levels of sCD163 in plasma.36 These observations, taken together with data from this study, suggest that immune activation and macrophages play a role in HIV- and SIV-associated cardiac pathology.
Using BrdU, we were able to examine the accumulation of monocytes newly released from the bone marrow. Regardless of cardiac pathology, we show an increase of monocyte/macrophage traffic to the heart during late versus early SIV infection. In the hearts of SIV-infected, CD8 lymphocyte-depleted animals that received BrdU late (48 dpi and 24 h before necropsy) we found a 4.6-fold increase in the number of BrdU+ cells compared to animals that received BrdU early (6 and 20 dpi). This underscores the notion that the majority of inflammatory monocyte/macrophages traffic later in infection and are likely triggered by the development of AIDS. Regardless, these data point to the need to target monocyte/macrophage activation and traffic, in addition to antiretroviral therapy, when considering HIV-associated CVD.
Our studies demonstrated an increase in macrophage numbers in cardiac tissues of SIV-infected animals with cardiac pathology. In particular, the numbers of CD163+ macrophages are significantly increased compared to animals without pathology and uninfected controls. We found a strong correlation between the numbers of macrophages and the degree of fibrosis determined by increasing levels of collagen in animals with cardiac pathology. The correlation between fibrosis and increased numbers of CD163+ macrophages demonstrates that the SIV-infected, CD8 lymphocyte-depleted animals can be a beneficial model to study the effects of macrophage activation and inflammation on cardiac pathology associated with HIV and SIV infection. While tissue sections of coronary arteries were not available for animals in this study, future studies examining whether there is a correlation between macrophage accumulation in cardiac muscle and coronary arteries are needed. Our preliminary studies on HIV-infected cardiac tissues also show an increase in the number of CD163+ macrophages in the heart during HIV infection that correlates with fibrosis (data not shown).
While the causes of HIV- and SIV-associated CVD are likely multifactoral, our data suggest that macrophage accumulation and traffic to the heart play a role in the developing cardiac pathology. Data from this study suggest that therapeutic treatments targeting macrophage activation and traffic, along with effective ART, could potentially reduce inflammation and the resulting damage seen in the heart during infection.
Acknowledgments
We thank the staff at the NEPRC and Tulane University's National Primate Center for animal care, as well as pathology residents and staff for assisting with necropsies and tissue collection. We thank Michael Piatek and Jeffrey Lifson for plasma viral load determination, and Ronald Desrosiers for providing the SIVmac25. This work was supported by the following NIH grants: R01 NS040237 (K.C.W.), R01 NS082116 (T.H.B.), a pilot grant from the Tulane National Primate Research Center NIH P51-RR00164 (T.H.B), and an NERPC base grant NIH OD011103.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Collaboration H-C, Ray M, Logan R, et al. : The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 2010;24:123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coquet I, Pavie J, Palmer P, et al. : Survival trends in critically ill HIV-infected patients in the highly active antiretroviral therapy era. Crit Care 2010;14:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeks SG: HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011;62:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams K, Westmoreland S, Greco J, et al. : Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest 2005;115:2534–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowe SM, Westhorpe CL, Mukhamedova N, Jaworowski A, Sviridov D, and Bukrinsky M: The macrophage: The intersection between HIV infection and atherosclerosis. J Leukoc Biol 2010;87:589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Currier JS, Lundgren JD, Carr A, et al. : Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation 2008;118:e29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho JE. and Hsue PY: Cardiovascular manifestations of HIV infection. Heart 2009;95:1193–1202 [DOI] [PubMed] [Google Scholar]
- 8.Annamalai L, Westmoreland SV, Domingues HG, Walsh DG, Gonzalez RG, and O'Neil SP: Myocarditis in CD8-depleted SIV-infected rhesus macaques after short-term dual therapy with nucleoside and nucleotide reverse transcriptase inhibitors. PLoS One 2010;5:e14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friis-Møller N, Reiss P, Sabin CA, et al. : Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007;356:1723–1735 [DOI] [PubMed] [Google Scholar]
- 10.Friis-Møller N, Sabin CA, Weber R, et al. : Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993–2003 [DOI] [PubMed] [Google Scholar]
- 11.Brenchley JM, Price DA, Schacker TW, et al. : Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 12.Pandrea I, Cornell E, Wilson C, et al. : Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood 2012;120:1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currie PF, Goldman JH, Caforio ALP, et al. : Cardiac autoimmunity in HIV related heart muscle disease. Heart 1998;79:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanai T, Lackner AA, Sakai H, Masegi T, and Simon MA: Systemic arteriopathy in SIV-infected rhesus macaques (Macaca mulatta). J Med Primatol 2006;35:106–112 [DOI] [PubMed] [Google Scholar]
- 15.Liu R, Moroi M, Yamamoto M, et al. : Presence and severity of Chlamydia and pneumoniae and cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. Int Heart J 2006;47:511–519 [DOI] [PubMed] [Google Scholar]
- 16.Roivainen M, Viik-Kajander M, Palosuo T, et al. : Infections, inflammation, and the risk of coronary heart disease. Circulation 2000;101:252–257 [DOI] [PubMed] [Google Scholar]
- 17.Stein JH. and Hsue PY: Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA 2012;308:405–406 [DOI] [PubMed] [Google Scholar]
- 18.Shannon RP, Simon MA, Mathier MA, Geng YJ, Mankad S, and Lackner AA: Dilated cardiomyopathy associated with simian AIDS in nonhuman primates. Circulation 2000;101:185–193 [DOI] [PubMed] [Google Scholar]
- 19.Crowe SM. and Hoy JF: Are monocytes the canary in the coal mine for HIV-related atherosclerosis? J Infect Dis 2012;206:1491–1493 [DOI] [PubMed] [Google Scholar]
- 20.Auffray C, Sieweke MH, and Geissmann F: Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 2009;27:669–692 [DOI] [PubMed] [Google Scholar]
- 21.Martinez FO, Helming L, and Gordon S: Alternative activation of macrophages: An immunologic functional perspective. Annu Rev Immunol 2009;27:451–483 [DOI] [PubMed] [Google Scholar]
- 22.Prabhu SD: Cytokine-induced modulation of cardiac function. Circ Res 2004;95:1140–1153 [DOI] [PubMed] [Google Scholar]
- 23.Monsuez JJ, Escaut L, Teicher E, Charniot JC, and Vittecoq D: Cytokines in HIV-associated cardiomyopathy. Int J Cardiol 2007;120:150–157 [DOI] [PubMed] [Google Scholar]
- 24.Barbaro G, Di Lorenzo G, Soldini M, et al. : Clinical course of cardiomyopathy in HIV-infected patients with or without encephalopathy related to the myocardial expression of tumor necrosis factor-a and nitric oxide synthase. AIDS 2000;14:827–838 [DOI] [PubMed] [Google Scholar]
- 25.Fisher SD, Bowles NE, Towbin JA, and Lipshultz SE: Mediators in HIV-associated cardiovascular disease: A focus on cytokines and genes. AIDS 2003;17:S29–35 [DOI] [PubMed] [Google Scholar]
- 26.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, and Haeffner-Cavaillon N: CD14lowCD16high A cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol 1995;25:3418–3424 [DOI] [PubMed] [Google Scholar]
- 27.Barisione C, Garibaldi S, Ghigliotti G, et al. : CD14CD16 monocyte subset levels in heart failure patients. Dis Markers 2010;28:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristiansen M, Graversen JH, Jacobsen C, Hoffman È, Lawk SKA, and Moestrup SK: Identification of the haemoglobin scavenger receptor. Nature 2001;409:1999–2002 [DOI] [PubMed] [Google Scholar]
- 29.Onofre G, Koláčková M, Jankovičová K, and Krejsek J: Scavenger receptor CD163 and its biological function. Acta Med 2009;52:57–61 [PubMed] [Google Scholar]
- 30.Buechler C, Ritter M, Orsó E, Langmann T, Klucken J, and Schmitz G: Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol 2000;67:97–103 [PubMed] [Google Scholar]
- 31.Etzerodt A. and Moestrup SK: CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal 2013;18:2352–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis BH. and Zarev PV: Human monocyte CD163 expression inversely correlates with soluble CD163 plasma levels. Cytometry B Clin Cytom 2005;63:16–22 [DOI] [PubMed] [Google Scholar]
- 33.Burdo TH, Soulas C, Orzechowski K, et al. : Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 2010;6:e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burdo TH, Lentz MR, Autissier P, et al. : Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011;204:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burdo TH, Lo J, Abbara S, et al. : Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011;204:1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereyra F, Lo J, Triant V, et al. : Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012;26:2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz JE, Simon MA, Kuroda MJ, et al. : A nonhuman primate model for the selective elimination of CD8+lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Pathol 1999;154:1923–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lifson JD, Rossio JL, Piatak M Jr, et al. : Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol 2001;75:10187–10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams K, Schwartz A, Corey S, et al. : Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am J Pathol 2002;161:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruifrok AC. and Johnston DA: Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Hist 2001;23:291–299 [PubMed] [Google Scholar]
- 41.Brower GL, Gardner JD, Forman MF, et al. : The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg 2006;30:604–610 [DOI] [PubMed] [Google Scholar]
- 42.Kelly KM, Tarwater PM, Karper JM, et al. : Diastolic dysfunction is associated with myocardial viral load in simian immunodeficiency virus-infected macaques. AIDS 2012;26:815–823 [DOI] [PubMed] [Google Scholar]
- 43.Yearley JH, Pearson C, Carville A, Shannon RP, and Mansfield K: SIV-associated myocarditis: Viral and cellular correlates of inflammation severity. AIDS Res Hum Retroviruses 2006;22:529–540 [DOI] [PubMed] [Google Scholar]
- 44.Yearley JH, Pearson C, Carville A, Shannon RP, and Mansfield K: Phenotypic variation in myocardial macrophage populations suggests a role for macrophage activation in SIV-associated cardiac disease. AIDS Res Hum Retroviruses 2007;23:512–524 [DOI] [PubMed] [Google Scholar]
- 45.Moestrup SK. and Møller HJ: CD163: A regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med 2004;36:347–354 [DOI] [PubMed] [Google Scholar]
- 46.Moller HJ: Soluble CD163. Scand J Clin Lab Invest 2012;72:1–13 [DOI] [PubMed] [Google Scholar]
- 47.Epelman S, Lavine KJ, Beaudin AE, et al. : Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014;40:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker JV, Henry WK, Patel P, et al. : Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clin Infect Dis 2011;53:826–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplan RC, Sinclair E, Landay AL, et al. : T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 2011;203:452–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramanian S, Tawakol A, Abbara S, et al. : Arterial inflammation in patients with HIV. JAMA 2012;308:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]