Abstract
Dendritic cells (DC) are essential for the first-line innate defense against influenza infection. The greater susceptibility to severe influenza infection in young infants and neonates may be attributed in part to their defective DC function. We sought to investigate the effect of influenza A virus (IAV) infection on the maturation, apoptosis, and function of monocyte-derived dendritic cells (MoDCs) from umbilical cord blood (UCB) and compared this with responses from adult peripheral blood (APB). Our findings were as follows. First, MoDCs derived from UCB showed deficient CD40, CD80, CD86, and HLA-DR upregulation following IAV infection compared to APB MoDCs. Second, IAV induced a multiplicity of infection (MOI)-dependent increase of apoptosis in UCB MoDCs, similar to that observed with APB. Third, the ability of UCB MoDCs to uptake dextran is decreased following IAV infection. Fourth, deficient TNF-α, but not IL-6, IFN-α response was induced by IAV infection of UCB MoDCs. Fifth, the ability of UCB MoDCs to promote allogeneic CD3 T-cell proliferation is inhibited by IAV infection. Taken together, we demonstrated a differential response of UCB and APB MoDCs following IAV infection, which may contribute in part to the increased susceptibility to severe influenza infection observed in young infants and neonates.
Introduction
Influenza A virus (IAV), a single-stranded RNA virus, can evoke seasonal epidemics or widespread pandemic disease (26). The 2009 H1N1 pandemic highlights the importance of developing more effective antiviral therapies (29). Severe IAV infection is characterized by the production of numerous proinflammatory cytokines known as hypercytokinemia (2). Although adaptive immune response plays a pivotal role in protective immunity against IAV infection (5,8), innate immunity players such as dendritic cells (DCs) serve as an important first-line antiviral immune defense. They are distributed throughout the airway, and can sense and capture the invading IAV. They then migrate to lymph nodes and present the processed antigen in association with major histocompatibility complex (MHC) to initiate T-cell response (10,27).
Young infants are particularly vulnerable to severe influenza infection (3,4), which may be attributed in part to the immaturity of their DC function. Kollman et al. showed that neonatal DCs were less efficient in producing TNF-α and IL-12 compared to adults (9). Zhang et al., however, reported that umbilical cord blood (UCB) plasmacytoid DCs can elicit potent antiviral innate responses (31). Few studies, however, have compared the differential DC response against influenza infection between adults and neonates.
The present study aims to investigate the effect of IAV infection on maturation and function of MoDCs from UCB compared with responses from adult peripheral blood (APB).
Materials and Methods
Samples
Mononuclear cells (MNCs) were isolated using Ficoll-Hypaque density gradient centrifugation from heparinized APB and UCB samples with informed consent obtained from each subject, and with the pre-approval for the study by the Medical Ethics and Human Clinical Trial Committee of the Chang Gung Memorial Hospital, Taiwan. UCB was collected in sterile tubes and was processed within 24 h of birth. MNCs were then resuspended in RPMI with 10% fetal calf serum (FCS) at a concentration of 1×106/mL.
Preparation of IAV
Influenza A/William Smith Neurotropic (WSN) virus/33 strain virus was grown in 10-day-old embryonated specific pathogen-free hen eggs (Animal Health Research Institute, Council of Agriculture, Executive Yuan, Taiwan). Allantoic fluid was harvested 48 h after infection and virus concentration was determined. Virus-containing allantoic fluid was pooled and centrifuged to pellet IAV particles. The virus pellet was resuspended in phosphate buffered saline (PBS) and further purified by a continuous 15–60% sucrose gradient centrifugation. The purified virus was reconstituted in PBS, stabilized with sucrose-phosphate-glutamate buffer (Sigma, St. Louis, MO), dispensed into single-use aliquots, and stored at −70°C. The virus titer was determined with Madin-Darby canine kidney cells by standard procedures (12).
Dendritic cell preparation
CD14+ monocytes were isolated from APB and UCB MNCs by depleting T-, B-, and NK-cells using a Monocyte Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of isolated CD14+ monocytes was around 90–95%, as determined by flow cytometry. To generate immature DC, the monocytes (1×106/mL) were cultured with GM-CSF (50 ng/mL; Peprotech, Rocky Hill, NJ) and IL-4 (50 ng/mL; Peprotech) in complete medium for 5 days (21).
Maturation of dendritic cells by IAV
Immature DCs (1×106/mL) were exposed or sham-exposed to IAV at a multiplicity of infection (MOI) of 0.1 and 1 for 1 h at 37°C in serum-free RPMI1640 supplemented with 2 mM glutamine, 100 U of penicillin G, and 100 μg of streptomycin/mL. For sham exposures, cells were exposed to a volume of Dulbecco's modified Eagle's medium (DMEM) cultured fluid equal to that used for virus infections. After 1 h of exposure or sham exposure to virus, infected DC cells (1×106/mL) were washed in warm medium, centrifuged, and reincubated at 37°C in medium supplemented with 10% heat-inactivated FCS for 18 h.
Flow cytometric analysis
DCs were washed in cold PBS with 2% FCS and 0.1% sodium azide and then stained with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated mouse anti-human monoclonal antibodies including CD80, CD83, major histocompatibility complex class II (MHC-II), CD40 (Becton-Dickinson/BD Pharmingen, Franklin Lakes, NJ) for flow cytometric analysis. DC apoptosis was assessed using an annexin-V (FITC)/propidium iodie (PI) apoptosis detection kits (BD Pharmingen). The fluorescent staining was analyzed on a FACS Calibur (BD Biosciences) flow cytometer. The percentage of cells stained with each monoclonal antibody was determined by comparing each histogram with one from control cells stained with FITC- or PE-labeled isotype control monoclonal antibodies.
FITC-labeled dextran uptake
Infected DC endocytosis was measured by the cellular uptake of FITC-dextran. Cultured DC (1×106/mL) were washed twice and resuspended in 1 mL RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 100 U/mL penicillin, 100 U/mL streptomycin, and 25 mM HEPES. The cells were then incubated with FITC-labeled dextran (0.1 and 0.2 mg/mL) (mol. Wt 40,000; Sigma) at 4°C or 37°C for 1 h. After incubation, cells were washed twice with cold PBS and fixed in 4% polyfluoroalkoxy. The quantitative uptake of FITC-dextran by the cells was determined using FACS.
Measurement of IFN-γ and TNF-α protein in culture supernatant
Secreted interleukin (IL)-6, IFN-α, and TNF-α was quantitated in cell-free supernatants using a human IL-6, human IFN-α, and human TNF-α enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Inc. Minneapolis, MN) as recommended by the manufacturer.
Mixed lymphocyte reaction
Allogeneic peripheral CD3+ T-cells were isolated from adult PBMC with a CD3+ T-cell isolation kit (Miltenyi Biotec). The purity of the isolated CD3+ T-cells was around 90–95%, as determined by flow cytometry. The CD3+ T-cells were stained with carboxyfluorescein succinimidyl ester (CFSE; Sigma) for 15 min in serum-free RPMI-1640. Then, CFSE-labeled CD3+ T-cells were washed by complete RPMI-1640. The CFSE-labeled CD3+ T-cells were cultured with LPS or Flu-A-treated DC at a ratio 1:10 or 1:100 for 5 days. After 5 days, CD3+ T-cell division was determined by flow cytometric measurement of CFSE dye dilution.
Statistics
The Wilcoxon signed rank test was used to analyze the difference in responses before and after treatment (calculated with SPSS v9.0; SPSS, Inc., Chicago, IL). The Mann–Whitney U-test was used to compare CB and APB responses. The data are presented as means±standard error of the mean (SEM). Groups being compared were considered significantly different if p<0.05.
Results
Deficient CD80, CD86, and HLA-DR expression on UCB MoDCs compared to APB MoDCs
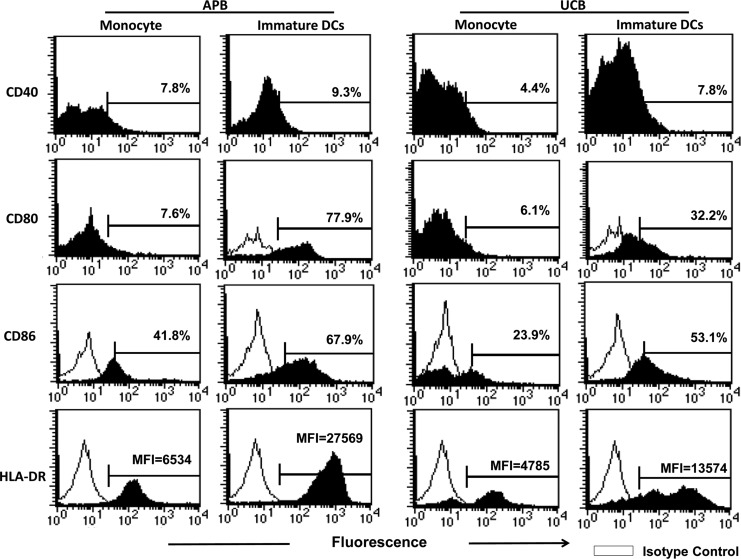
UCB and APB CD14+ monocytes were cultured with GM-CSF and IL-4 for 5 days to generate immature MoDCs. At the end of the culture, they lost the CD14 expression and showed typical cytoplasmic projections under light microscopy (data not shown). Figure 1 shows a representative profile of various maturation marker expression during the transition of monocytes into the immature MoDCs. The immature MoDCs showed markedly enhanced CD40, CD80, CD86, and HLA-DR compared to monocytes. However, the CD83 expression was only slightly enhanced. MoDC derived from UCB monocytes, when compared to APB MoDCs, showed deficient CD80 (39.7±8.3% vs. 72.1±6.9%; p=0.032), CD86 (45.3±7.8% vs. 74.0±5.1%; p=0.01), and HLA-DR (MFI=18905±4531 vs. MFI=21331±3080; p=0.015; as almost all cells expressed HLA-DR, MFI was used) expression. The expression of CD40 remained low and were comparable to adults (9.4±3.5% vs. 10.7±2.9%; p=0.772)
FIG. 1.
A representative profile showing the changes of the CD40, CD80, CD86, and HLA-DR expression of maturation markers from monocytes to immature dendritic cells (DCs). Purified human monocytes were cultured with GM-CSF and IL-4 for 5 days. Then cells were analyzed for surface markers by flow cytometry.
IAV infection induced maturation of UCB MoDCs
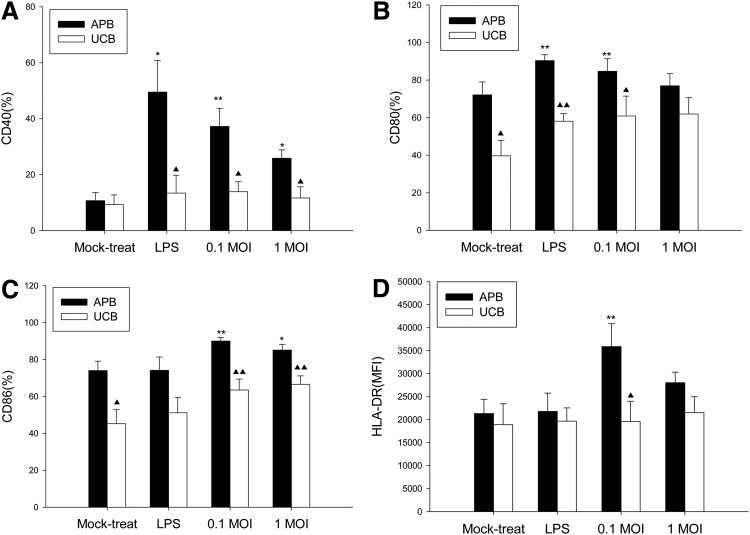
The immature MoDCs from UCB and APB were exposed to IAV for 1 h, washed, and then cultured for 18 h. Figure 2 shows the effect of IAV exposure on the maturation marker expression of UCB MoDCs, using LPS as a positive control, and compared with adults. For APB MoDCs, IAV infection at 0.1 MOI resulted an increased expression of CD40 (37.2±6.5% vs. 10.7±2.9%; p=0.007), CD86 (90.0±1.9% vs. 74.0±5.1%; p=0.002), and HLA-DR (MFI=35,884±4,973 vs. MFI=21,331±3,080, p=0.008) compared to mock-treated controls respectively. A similar yet lower level of maturation effect was observed at 1 MOI. However, we observed that IAV infection at 0.1 MOI did not enhance the expression of CD40 (13.9±3.6% vs. 9.4±3.4%; p=0.893), CD80 (60.9±10.6% vs. 39.7±8.3%; p=0.208), CD86 (63.5±5.9% vs. 45.3±7.8%; p=0.128), and HLA-DR (MFI=19,607±4,382 vs. MFI=18,905±4,531; p=0.401) on UCB MoDCs compared to mock-treated controls respectively. Thus, MoDC derived from UCB showed deficient CD40, CD80, CD86, and HLA-DR upregulation following IAV infection compared to APB MoDCs
FIG. 2.
Effect of LPS and influenza A virus (IAV) infection on (A) CD40, (B) CD80, (C) CD86, and (D) HLA-DR expression on monocyte-derived dendritic cells (MoDCs) derived from adult peripheral blood (APB) and umbilical cord blood (UCB). Data are expressed as percent expression for CD40, CD86, and mean fluorescence intensity (MFI) for HLA-DR, obtained from 13 adults and 8 cord blood samples. Values were expressed as mean percentage±standard error of the mean (SEM). *p<0.05 and **p<0.01 between mock-treated cells and IAV-infected cells. ▲, p<0.05 and ▲▲, p<0.01 between APB and UCB.
IAV induces apoptosis and impaired endocytosis in UCB and APB MoDCs
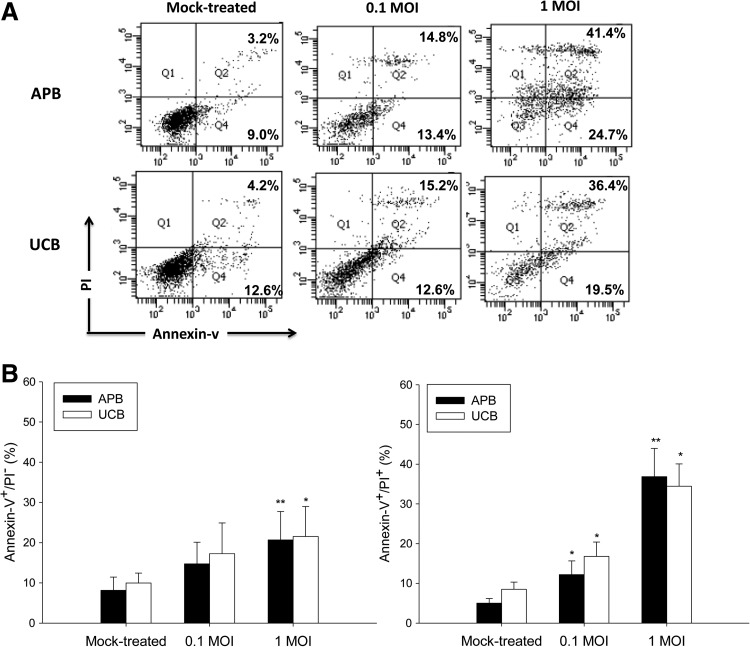
We next determined the effect of IAV on the survival and function of UCB and APB MoDC. As shown in Figure 3, MoDC exposed to IAV resulted in a dose-dependent increase of the percentages of annexin V+/PI− (early apoptotic) UCB MoDCs from 9.9±2.5% in mock-treated controls to 17.3±7.6% at 0.1 MOI (p=0.083), and further to 21.5±7.5% at 1 MOI (p=0.008), comparable to that observed with APB MoDCs. The percentages of annexin V+/PI+ (late apoptotic) UCB MoDCs also increased by IAV infection in a dose-dependent fashion (for 0.1 MOI: 16.8±3.6% vs. 8.5±1.8%, p=0.043; for 1 MOI: 34.5±5.6% vs. 8.5±1.8%, p=0.043), UCB MoDCs were not more susceptible to IAV-induced apoptosis than were APB MoDCs.
FIG. 3.
(A) A representative profile showing the IAV infection-induced apoptosis of MoDCs derived from APB and UCB. (B) The percentages of cells bearing annexin-V+/PI− (early apoptosis) and annexin-V+/PI+ (late apoptosis) were shown. Values were expressed as mean percentage±SEM obtained from 13 adults and 8 cord blood samples.*p<0.05; **p<0.01.
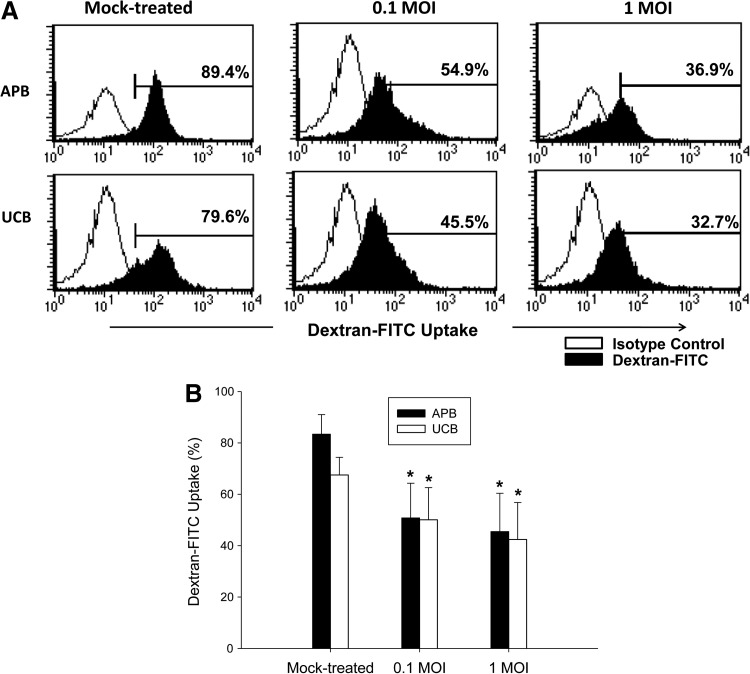
We also determined the IAV induction of MoDCs endocytosis by detecting dextran-FITC uptake by MoDCs (14). Similar to that observed with APB MoDCs, the ability to uptake dextran-FITC decreased from 67.5±6.9% for mock-treated UCB MoDC to 50.8±13.5% following IAV infection at 0.1 MOI (p=0.043), and further to 42.4±14.4% (p=0.043) at 1 MOI, suggesting that IAV infection promotes maturation of UCB MoDCs (Fig. 4).
FIG. 4.
(A) A representative profile showing the effect of IAV on dextran-uptake of MoDCs derived from APB and UCB. Cells were cultured with Dextran-FITC (2 mg/mL) for 1 h. (B) The percentage of dextran-FITC uptake was analyzed by flow cytometry. Values were expressed as mean percentage±SEM obtained from six adults and six cord blood samples.*p<0.05.
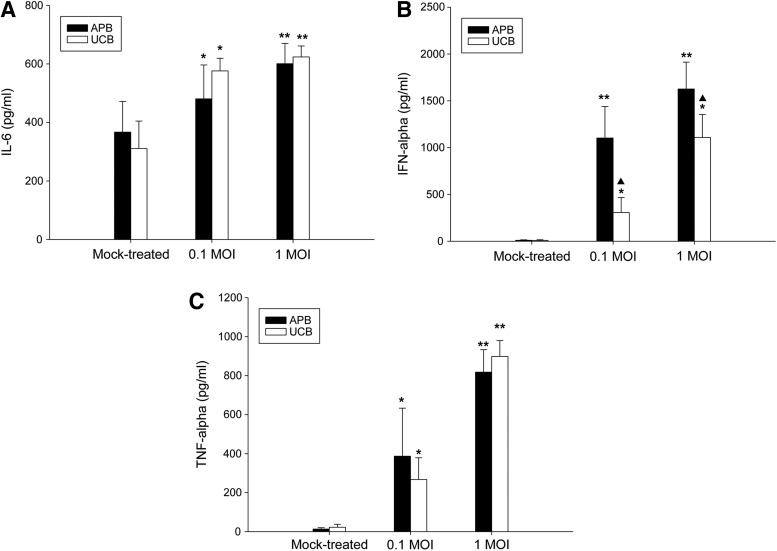
Effect of IAV on IL-6, IFN-α, and TNF-α production from UCB MoDCs
We next compared the effects of IAV exposure on IL-6, IFN-α, and TNF-α production of UCB and APB MoDCs (Fig. 5). IAV induced a dose-dependent increase of IL-6 production of UCB MoDCs from 311±94 pg/mL for mock-treated controls to 576±43 pg/mL at 0.1 MOI (p=0.043), and further to 625±37 pg/mL at 1 MOI (p=0.008). Mock-treated UCB and APB MoDCs barely produced IFN-α and TNF-α. Similar to that observed with IL-6, IAV infection resulted in a dose-dependent increase of IFN-α and TNF-α production in UCB MoDCs. In general, UCB MoDCs produced comparable amounts of cytokines compared to their adult counterparts, except for a decreased production of IFN-α production at 0.1 MOI (306±162 pg/mL for UCB vs. 1,102±337 pg/mL for APB; p=0.013) and at 1 MOI (1,109±245 pg/mL for UCB vs. 1,626±288 pg/mL for APB; p=0.017).
FIG. 5.
Effect of IAV infection on (A) IL-6, (B) IFN-α, (C) TNF-α production of MoDcs from APB and UCB. Data were obtained from 13 adults and 8 cord blood samples. Values were expressed as mean percentage±SEM. *p<0.05 and **p<0.01 between mock-treated cells and IAV-infected cells. ▲, p<0.05 between APB and UCB.
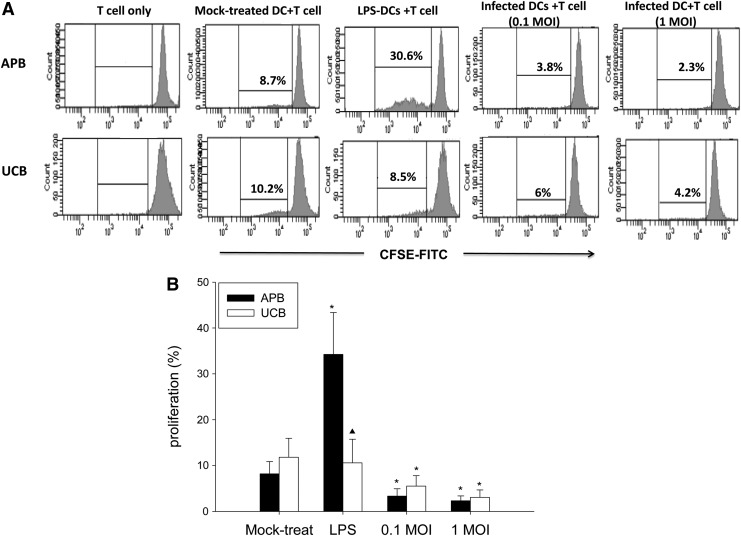
Deficient MLR response of UCB MoDCs compared to APB MoDCs
We next tested the T-cell stimulatory ability of UCB and APB MoDCs following IAV infection by co-culturing with carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled allogeneic CD3+ T-cells as responders. As shown in Figure 6, LPS-treated APB MoDCs showed enhanced induction of CD3+ T-cell proliferation compared to controls (34.2±9.1% vs. 8.2±2.7%; p=0.02), while UCB MoDCs showed much less response compared to APB (10.6±5.2% vs. 34.2±9.1%; p=0.043). In contrast, IAV-infected APB MoDCs inhibited allogeneic CD3+ T-proliferation to a greater extent compared to mock-treated controls (at 0.1 MOI: 3.4±1.6% vs. 8.2±2.7%, p=0.043; at 1 MOI: 2.3±1.0% vs. 8.2±2.7%, p=0.018), which was similarly observed in UCB MoDCs (for 0.1 MOI: 5.5±2.3% vs. 11.8±4.2%, p=0.018; for 1 MOI: 3.1±1.6% vs. 11.8±4.2%, p=0.028).
FIG. 6.
(A) A representative profile showing the ability of MoDCs derived from APB and UCB to promote the proliferation of allogeneic CD3+ T-cells. (B) The percentage of proliferating allogeneic CD3+ cells analyzed by flow cytometry. MoDCs were infected with IAV or stimulated with LPS, and were co-cultured with allogeneic purified carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD3+ T-cells at a DC:T ratio of 1:100 for 5 days. Data were obtained from 11 adults and 7 cord blood samples. Values were expressed as mean percentage±SEM. *p<0.05 between mock-treated cells and IAV-infected cells. ▲, p<0.05 between APB and UCB.
Discussion
The biological process of DC maturation represents a crucial step in the initiation of adaptive immune responses. MoDCs accumulate in the lung and play an important pathogenic role during influenza infection (1,15). Experimental studies regarding the fate of human respiratory DCs following exposure to IAV have been hampered by low cell yield and purity. Immature functional DCs generated from peripheral blood monocytes by culturing with GM-CSF and IL-4 may provide a reproducible alternative to study the role of DC in IAV infections. Similar to our study, Thitihanyanont et al. used MoDCs to demonstrate the high susceptibility of human DCs to H5N1 (24).
We and others have demonstrated the deficient neonatal immune function by studying T-cells and NK-cells isolated from UCB, an enriched source of hematopoietic precursors and immune cells (16,17,23). The present study is the first to compare the effect of IAV infection on maturation of APB and UCB MoDCs. We observed that IAV infection induced a lesser degree of maturation marker expression in UCB MoDCs compared to their adult counterparts. The effect of IAV infection on apoptosis, endocytosis, and cytokine production in UCB MoDCs was similar to that observed in adults. The ability of UCB MoDCs to stimulate allogeneic T-cell proliferation was deficient compared to adults, and was further compromised by IAV infection.
CD40 and CD40L interaction is important in the regulation of dendritic T-cell and dendritic B-cell crosstalk (19). CD80 and CD86 are co-stimulatory molecules belonging to the B7 family, capable of promoting a full T-cell activation (20). HLA-DR expression on DCs were found to be correlated with immune activation (6). We observed a deficient expression of CD40, CD80, CD86, and HLA-DR on immature DC of UCB and following LPS-induced maturation compared to their adult counterpart, in agreement with previous studies (7,28). We found that UCB MoDCs also showed deficient maturation following IAV infection, contributing in part to the increased susceptibility to severe IAV infection in the young infants and neonates.
IAV infection resulted in a dose-dependent increase of apoptosis of MoDCs, consistent with Wu et al. (30). The degree of IAV induced apoptosis, either early or late in UCB MoDCs, was comparable to their adult counterparts. Therefore, the impaired maturation marker expression and function in UCB MoDCs compared to adults is not due to the cytotoxicity of IAV infections. The uptake of FITC-dextran is known to be maximal in the immature MoDCs and gradually decreased during their maturation process (13,14). We demonstrated a reduction in FITC-dextran uptake when UCB and APB MoDCs were exposed to IAV, suggesting its maturation-inducing effect. The decrease in endocytotic ability did not differ between UCB and APB MoDCs.
Previous reports have shown the decreased cytokine production of neonatal MoDCs compared to adults (7,18), Krumbiegel et al. showed that multiple proinflammatory mediators were required to induce cytokine synthesis of DCs derived from hematopoietic stem cells (11), suggesting a higher threshold of activation for neonatal DCs. We, however, observed a robust IL-6 and TNF-α secretion of neonatal MoDCs in response to IAV infection, comparable to that observed in adults, though the IFN-α secretion of UCB MoDCs of neonatal MoDCs in response to IAV infection was still deficient. It may be that the neonatal immune system is Th2-biased, while IFN-α is involved in the development of Th1 immunity by promoting IFN-γ production (22).
When the capacity of MoDCs to stimulate allogeneic CD3+ T-cells was analyzed, UCB MoDCs showed impaired proliferation-promoting capacity following LPS stimulation compared to their adult counterparts. Our finding is consistent with Velilla et al. who proposed that UCB MoDCs, in contrast to mature adult MoDCs that can efficiently prime T-cells, are poor inducers of proliferation or production of IFN-γ by T-cells (25). We found that IAV adversely affected the MLR response in both UCB and APB, which may be a possible mechanism for IAV to evade antiviral immune defense.
Taken together, we demonstrated a differential response of UCB and APB MoDCs following IAV infection. Our findings suggest that the defective DC function in neonates and young infants may contribute to their greater susceptibility to severe influenza infection.
Acknowledgments
This work was supported by the National Science Council of Republic of China (grant NSC101-2314-B-182-033) and Chang Gung Memorial Hospital (grants CMRPG 4A0051-CMRPG4A0053).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aldridge JR, Jr, Moseley CE, Boltz DA, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A 2009;106:5306–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care 2009;13:R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003–2004. New Engl J Med 2005;353:2559–2567 [DOI] [PubMed] [Google Scholar]
- 4.Coffin SE, Zaoutis TE, Rosenquist AB, et al. Incidence, complications, and risk factors for prolonged stay in children hospitalized with community-acquired influenza. Pediatrics 2007;119:740–748 [DOI] [PubMed] [Google Scholar]
- 5.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, and Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev 1997;159:105–117 [DOI] [PubMed] [Google Scholar]
- 6.Drénou B, Amiot L, Setterblad N, et al. MHC class II signaling function is regulated during maturation of plasmacytoid dendritic cells. J Leukoc Biol 2005;77:560–567 [DOI] [PubMed] [Google Scholar]
- 7.Goriely S, Vincart B, Stordeur P, et al. Deficient IL-12 (p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol 2001;166:2141–2146 [DOI] [PubMed] [Google Scholar]
- 8.Graham MB, and Braciale TJ: Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med 1997;186:2063–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009;183:7150–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreijtz JH, Fouchier RA, and Rimmelzwaan GF. Immune responses to influenza virus infection. Virus Res 2011;162:19–30 [DOI] [PubMed] [Google Scholar]
- 11.Krumbiegel D, Rohr J, Schmidtke P, Knuf M, Zepp F, and Meyer CU. Efficient maturation and cytokine production of neonatal DCs requires combined proinflammatory signals. Clin Dev Immunol 2005;12:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lennette DA. General principles for laboratory diagnosis of viral, rickettsial, and chlamydial infections. In: Lennette EH, Lennette DA, and Lennette ET, eds. Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections. Washington, DC: American Public Health Association, 1995: 3–25 [Google Scholar]
- 13.Leslie DS, Vincent MS, Spada FM, et al. CD1-mediated gamma/delta T cell maturation of dendritic cells. J Exp Med 2002;196:1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine TP, and Chain BM. Endocytosis by antigen presenting cells: dendritic cells are as endocytically active as other antigen presenting cells. Proc Natl Acad Sci U S A 1992;89:8342–8346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol 2008;180:2562–2572 [DOI] [PubMed] [Google Scholar]
- 16.Lin SJ, and Kuo ML. Effect of cyclosporin-A on interleukin-15 activated umbilical cord blood natural killer cell function. Cytotherapy 2008;10:397–405 [DOI] [PubMed] [Google Scholar]
- 17.Lin SJ, Li JJ, Cheng PJ, and Kuo ML. Susceptibility to Fas and tumor necrosis factor-( receptor mediated apoptosis of anti-CD3/anti-CD28-activated umbilical cord blood T cells. Pediatr Allergy Immunol 2009:20:392–398 [DOI] [PubMed] [Google Scholar]
- 18.Liu E, Tu W, Law HK, and Lau YL. Decreased yield, phenotypic expression and function of immature monocyte-derived dendritic cells in cord blood. Br J Haematol 2001;113:240–246 [DOI] [PubMed] [Google Scholar]
- 19.Ma DY, and Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol 2009;1:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzotti CN, Liu MK, Burke F, Dussably L, Zheng Y, and Sansom DM. Integration of CD28 and CTLA-4 function results in differential responses of T cells to CD80 and CD86. Eur J Immunol 2006;36:1413–1422 [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994;179:1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sareneva T, Matikainen S, Kurimoto M, and Julkunen I. Influenza A virus-induced IFN-alpha/beta and IL-18 synergistically enhance IFN-gamma gene expression in human T cells. J Immunol 1998;160:6032–6038 [PubMed] [Google Scholar]
- 23.Satwani P, Morris E, van de Ven C, and Cairo MS. Dysregulation of expression of immunoregulatory and cytokine genes and its association with the immaturity in neonatal phagocytic and cellular immunity. Biol Neonate 2005;88:214–227 [DOI] [PubMed] [Google Scholar]
- 24.Thitithanyanont A, Engering A, Ekchariyawat P, et al. High susceptibility of human dendritic cells to avian influenza H5N1 virus infection and protection by IFN-alpha and TLR ligands. J Immunol 2007;179:5220–5227 [DOI] [PubMed] [Google Scholar]
- 25.Velilla PA, Rugeles MT, and Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin Immunol 2006;121:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viboud C, Miller M, Olson D, Osterholm M, and Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLOS Curr 2010;2:RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waithman J, and Mintern JD. Dendritic cells and influenza A virus infection. Virulence 2012;3:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willems F, Vollstedt S, and Suter M. Phenotype and function of neonatal DC. Eur J Immunol 2009;39:26–35 [DOI] [PubMed] [Google Scholar]
- 29.Writing Committee of the WHOCoCAoPI, Bautista E, Chotpitayasunondh T, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010;362:1708–1719 [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Mao H, Ling MT, et al. Successive influenza virus infection and Streptococcus pneumoniae stimulation alter human dendritic cell function. BMC Infect Dis 2011;11:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Lepelley A, Azria E, et al. Neonatal plasmacytoid dendritic cells (pDCs) display subset variation but can elicit potent anti-viral innate responses. PloS One 2013;8:e52003. [DOI] [PMC free article] [PubMed] [Google Scholar]