Abstract
Significance: The endoplasmic reticulum (ER) is a specialized organelle for the folding and trafficking of proteins, which is highly sensitive to changes in intracellular homeostasis and extracellular stimuli. Alterations in the protein-folding environment cause accumulation of misfolded proteins in the ER that profoundly affect a variety of cellular signaling processes, including reduction–oxidation (redox) homeostasis, energy production, inflammation, differentiation, and apoptosis. The unfolded protein response (UPR) is a collection of adaptive signaling pathways that evolved to resolve protein misfolding and restore an efficient protein-folding environment. Recent Advances: Production of reactive oxygen species (ROS) has been linked to ER stress and the UPR. ROS play a critical role in many cellular processes and can be produced in the cytosol and several organelles, including the ER and mitochondria. Studies suggest that altered redox homeostasis in the ER is sufficient to cause ER stress, which could, in turn, induce the production of ROS in the ER and mitochondria. Critical Issues: Although ER stress and oxidative stress coexist in many pathologic states, whether and how these stresses interact is unknown. It is also unclear how changes in the protein-folding environment in the ER cause oxidative stress. In addition, how ROS production and protein misfolding commit the cell to an apoptotic death and contribute to various degenerative diseases is unknown. Future Directions: A greater fundamental understanding of the mechanisms that preserve protein folding homeostasis and redox status will provide new information toward the development of novel therapeutics for many human diseases. Antioxid. Redox Signal. 21, 396–413.
Introduction
Life cannot exist without proteins, the macromolecules that need to acquire specific three-dimensional structures for function. The most error-prone step in gene expression is protein folding. In eukaryotic cells, the endoplasmic reticulum (ER) is a membrane-bound organelle that is specialized for the folding and post-translational maturation of almost all membrane proteins and most secreted proteins. In addition, the ER plays important roles in lipid biosynthesis, detoxification, energy metabolism, as well as homeostasis of intracellular Ca2+ and reduction–oxidation (redox) balance. Protein folding and maturation in the ER are subject to “quality control,” an essential surveillance mechanism that ensures only properly folded and modified proteins exit the ER and traffic to other intracellular organelles/vesicles and the plasma membrane. Protein folding in the ER is highly sensitive to extracellular stimuli and changes in intracellular homeostasis, including ER Ca2+, glycosylation, energy stores, redox state, metabolic and inflammatory challenges, increased ER-associated mRNA translation, and expression of proteins that are prone to misfolding. The accumulation of unfolded and misfolded proteins in the ER lumen, a condition called ER stress, activates the unfolded protein response (UPR) to resolve this protein-folding defect. The UPR enhances the ER capacity for protein folding and modification, attenuates global mRNA translation, and disposes terminally misfolded proteins by ER-associated protein degradation (ERAD) and autophagy. However, when ER stress is too severe or chronic, or the UPR is chemically or genetically impaired and unable to mitigate the protein-folding defects, pro-apoptotic signaling pathways are activated in the cell (20, 75, 161).
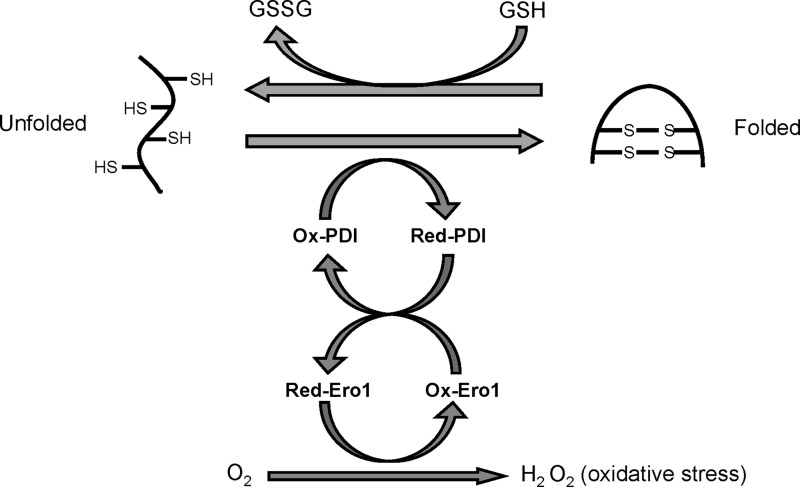
The ER redox state is closely linked to ER protein-folding homeostasis. Disulfide bond formation in the ER lumen is highly sensitive to altered redox balance, where both reducing and oxidizing reagents disrupt protein folding and cause ER stress (104). During oxidative protein folding in the ER, the thiol groups on cysteines of substrate peptides are oxidized and form disulfide bonds with hydrogen peroxide (H2O2) generated as a byproduct (Fig. 1). In a stressed ER, dysregulated disulfide bond formation and breakage may result in reactive oxygen species (ROS) accumulation and cause oxidative stress. In addition, some UPR components such as the C/EBP homologous protein CHOP can contribute to oxidative stress. Meanwhile, ER stress can cause mitochondrial dysfunction and increase mitochondrial ROS production. In many ER stress-related in vitro and in vivo models, ER stress and oxidative stress accentuate each other in a positive feed-forward loop, which interferes with cell function and activates pro-apoptotic signaling (104). Basic and clinical studies in the last decade suggest that ROS crucially impacts the pathogenesis of many human diseases, including metabolic disease, neurodegenerative disease, inflammatory disease, neoplasms, as well as pathologies in the heart, kidney, and lung (6, 20, 178). In this review, we summarize our knowledge regarding the generation of ER stress and oxidative stress in the cell and the signaling pathways activated in response to these two cellular stresses. We also highlight how cross-talk between ER stress and oxidative stress causes multiple human pathologies, which suggest and encourage the development of novel therapeutic applications in the future.
FIG. 1.
Oxidative protein folding in the ER. Oxidative protein folding of eukaryotic cells occurs in the ER, which is mediated by ER protein PDI and ERO1. ROS are generated as a byproduct of oxidative protein folding. Improperly paired disulfide bonds formed during protein folding can be reduced at the expense of glutathione, an essential antioxidant in the ER. See Introduction section for details. ER, endoplasmic reticulum; ROS, reactive oxygen species.
ER Stress and the UPR
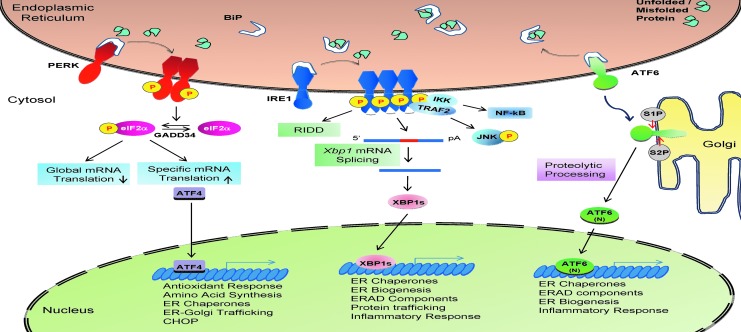
In metazoans, three protein sensors on the ER membrane initiate the UPR: inositol-requiring kinase 1 (IRE1), pancreatic ER eukaryotic translation initiation factor 2 (eIF2α) kinase (PERK), and activating transcription factor 6 (ATF6). Binding of the ER luminal protein chaperone BiP/GRP78 to the UPR sensors prevents their signaling (Fig. 2). Based on the competition-binding model of UPR initiation, unfolded/misfolded proteins in the ER lumen compete with the ER stress sensors for binding to the most abundant protein chaperone in the ER, BiP/GRP78. Accumulation of unfolded/misfolded proteins in the ER activates the three UPR transducers as a consequence of BiP from their luminal domains (20, 75).
FIG. 2.
The mammalian UPR. In most mammalian cells, three UPR branches were identified: the PERK-eIF2α-ATF4-CHOP pathway, the IRE1α-XBP1 pathway, and the ATF6 pathway. The functions of the three pathways overlap and are redundant in many cell types. However, complete ablation of any of the three branches causes embryonic/perinatal death in mice, suggesting their unique and essential role at the physiological level. See “ER Stress and the UPR” section for details. ATF6, activating transcription factor 6; PERK, pancreatic ER eIF2α kinase; UPR, unfolded protein response.
IRE1, a type I transmembrane protein, is the most conserved ER stress sensor with both an Ser/Thr kinase domain and an endoribonuclease (RNase) domain in its cytosolic portion. On release from BiP, the luminal domain of IRE1α dimerizes in the plane of the ER membrane, leading to trans-autophosphorylation and activation of its kinase and RNase activities. Activated IRE1α cleaves and removes a 26-base intron from an mRNA and produces a translational frameshift that is translated to produce the active CREB/ATF basic leucine zipper-containing (bZIP) transcription factor X-box-binding protein 1 (XBP1). XBP1 is an essential transcriptional activator of many UPR genes that control ER protein folding, intracellular trafficking, ERAD, phospholipid biosynthesis, and ER membrane expansion. In addition to the cleavage of Xbp1 mRNA, the RNase domain of IRE1 also degrades a subset of ER-localized mRNAs during ER stress, a process called regulated IRE1-dependent decay of mRNA (RIDD). Recently, IRE1 was shown to cleave several microRNAs, and this is linked to the activation of inflammatory and apoptotic signaling (96, 169). In addition, the kinase domain of IRE1α also integrates ER stress with pro-inflammatory responses through direct binding with adaptor protein tumor necrosis factor alpha (TNFα) receptor-associated factor 2 (TRAF2) and subsequent activation of the nuclear factor-kappaB (NF-κB) and c-Jun N-terminal kinase (JNK) pathways (20, 75, 176). In mammals, there are two IRE1 genes, IRE1α and IRE1β. Deletion studies demonstrated that the IRE1α-XBP1 pathway is necessary for murine embryonic development and critical for the differentiation, function, and survival of many cell types which secrete large amounts of protein. IRE1α is ubiquitously expressed, whereas IRE1β is selectively expressed in intestinal and respiratory epithelial cells (111). Mice deleted of Ire1β display increased sensitivity to experimentally induced colitis and disrupted mucin secretion in the colon and respiratory tract (9, 111, 166).
PERK is a type I transmembrane protein with a cytosolic Ser/Thr kinase domain. During ER stress, PERK is activated in a similar manner as IRE1. Activated PERK phosphorylates Ser51 on the α subunit of eIF2α, which attenuates global protein synthesis, thereby reducing the ER protein-folding burden. In mammalian cells, three cytosolic kinases also phosphorylate eIF2α at Ser51, which are activated by different stress conditions: general control nonrepressed 2 kinase (GCN2) activated by amino-acid deprivation, dsRNA-activated protein kinase (PKR) activated by dsRNA, and heme-regulated eIF2α kinase (HRI) activated by heme depletion and oxidative stress. The concerted action of these four eIF2α kinases, all of which are encoded by single genes, regulate mRNA translation initiation in a process termed the integrated stress response (142). eIF2α phosphorylation is conserved in all nucleated cells from protozoa to plants and humans. Therefore, it was surprising that mice with whole-body knock-in mutation of a Ser51Ala non-phosphorylatable eIF2α develop normally, although they die at 1 day after birth, due to hypoglycemia associated with defective gluconeogenesis in the liver (147). This observation was the first that linked stress response signaling and protein synthesis to metabolic control in metazoans. Typically, eIF2α phosphorylation-mediated translation attenuation is transient due to the activities of GADD34 and CReP, two regulatory targeting subunits of protein phosphatase PPP1, which directs eIF2α dephosphorylation to restore protein synthesis. In addition to global translational attenuation, phosphorylated eIF2α is required for selective translation of a subset of mRNAs, including the activating transcription factor 4 (ATF4), a potent bZIP transcription factor that activates genes encoding transcription factors, ER chaperones and trafficking machinery, amino-acid biosynthesis, antioxidative stress responses, and autophagy (68). Among the downstream targets of ATF4 is CHOP/GADD153, a bZIP transcription factor that plays a crucial role in ER stress-induced apoptosis (20, 142).
The ER stress sensor ATF6 is a type II transmembrane protein harboring a CREB/ATF bZIP domain at its N-terminus. During ER stress, release of the chaperone BiP from the luminal domain permits trafficking of ATF6 to the Golgi apparatus, where it is sequentially cleaved by site-1 protease (S1P) and S2P at the transmembrane site to release a cytosolic fragment that migrates to the nucleus to activate gene transcription. S1P and S2P are the same processing enzymes which are responsible for cleavage of sterol-regulatory element-binding proteins that control lipid and cholesterol biosynthesis (185). There are two ATF6 genes in mammals, ATF6α and ATF6β. Where mice without ATF6α or ATF6β survive under normal conditions, double deletion of Atf6α and Atf6β causes very early embryonic lethality although the mechanism is unknown. The released ATF6α cytosolic fragment migrates to the nucleus and transactivates numerous ER chaperone genes, including BiP, Grp94, and P58IPK, as well as some ERAD components. ATF6α is required to optimize protein folding, maturation, and secretion in response ER stress, and, as a consequence, Atf6α−/− cells cannot survive chronic ER stress. Genes that require ATF6β for transcription have yet to be defined. In addition to ATF6, several other transcription factors that are activated by regulated intramembrane proteolysis exist in mice, including CREBH, Luman, and OASIS, which serve diverse and important biological functions in different cell types (20, 176).
Production of ROS in the Cell
In eukaryotic cells, ROS can be generated in multiple organelles, including the ER and mitochondria as a byproduct of oxidative protein folding, mitochondrial respiration, and detoxification. As a highly regulated process, ROS production profoundly affects cell function and homeostasis in all organisms.
Oxidative protein folding and production of ROS
In the lumen of the ER, correct folding of most membrane and secretory proteins requires the generation of disulfide bonds between cysteine residues to stabilize tertiary and quaternary structures. Disulfide bond formation is a reversible process that is achieved by a thiol-disulfide exchange reaction (31). In eukaryotic cells, oxidative protein folding is catalyzed by a number of ER oxidoreductases, including protein disulfide isomerases (PDI), ERp72, and ERp57. In addition, ER protein folding is kinetically and thermodynamically regulated by the redox state of the microenvironment, which is under the control of redox buffers, including thiol-disulfide pairs and reduced/oxidized pyridine nucleotides in the ER lumen (167). Glutathione (GSH), the most abundant non-protein thiol in eukaryotic cells, can be oxidized to glutathione disulfide (GSSG). A balance between GSH and GSSG maintains the redox homeostasis in the cell. The cytosol is a reducing environment with a GSH/GSSG ratio ranging from 30:1 to 100:1, while the GSH/GSSG ratio in ER lumen is as high as 1:1–3:1 (83). The highly oxidized environment in the ER is essential for oxidative protein folding.
PDI is a multifunctional oxidoreductase and chaperone that catalyzes the formation, isomerization, and reduction of disulfide bonds in the ER. During disulfide bond formation, cysteine residues in the active site of PDI accept two electrons from the cysteine residues in polypeptide substrates, leading to the reduction of PDI and oxidation of the substrate. Then, PDI transfers the electrons to an acceptor to start another cycle of disulfide bond formation. The ER oxidoreductase 1 (Ero1), a flavin adenine dinucleotide-binding protein, functions and accepts electrons from PDI both in vitro and in vivo (52, 132). While Ero1p is required for oxidative protein folding in yeast, mammalian cells lacking both Ero1α and Ero1β, two homologs of Ero1p, do not display dramatic defects in disulfide bond formation (194). So far, several ER enzymes can mediate ERO1-independent disulfide bond formation during oxidative protein folding, including vitamin K epoxide reductase, quiescin sulfhydryl oxidase (QSOX), and peroxiredoxin IV (Fig. 1) (64).
Evidence suggests that oxidative protein folding is an important resource of ROS production in the cell. After accepting electrons from PDI, ERO1 transfers the electrons to molecular oxygen (O2) and produces H2O2, the major ROS produced in the ER lumen. The QSOX family enzymes generate H2O2 during oxidative protein folding via a similar mechanism (145). Based on the amount of H2O2 generated during ER oxidative protein folding, it was estimated that approximately 25% of all ROS produced in yeast results from Ero1p-mediated disulfide bond formation (167). Most eukaryotic cells have a variety of antioxidative stress responses. However, some evidence suggests that the ER has limited enzymatic antioxidant protection under basal conditions (145), which could predispose the ER to oxidative stress under conditions of an increased protein folding load.
Mitochondrial respiration and production of ROS
Mitochondrial respiration produces ATP that is coupled with ROS production, mostly in the form of superoxide (O2−•) after one electron reduction of molecular oxygen. In mammalian mitochondria, there are seven known sites of superoxide production: pyruvate and 2-oxoglutarate dehydrogenases, glycerol 3-phosphate dehydrogenase, the flavin in complex I, ubiquinone-binding sites in complex I and complex III, and the electron transferring flavoprotein:Q oxidoreductase in fatty acid β-oxidation (16).
Mitochondrial ROS profoundly impact cellular physiology and pathogenesis, and it is under multilayer control. First, the proton motive force of the electron transport chain (ETC) produces ROS. Mitochondrial ROS can be eliminated by a number of mitochondrial and cytosolic enzymatic antioxidants, including superoxide dismutases (SODs), glutathione peroxidases, peroxiredoxins, and catalase. ROS production in mitochondria is under direct and indirect regulation by a number of signaling pathways, including Ca2+ influx, energy demand, cellular redox status, hypoxia, ER stress, inflammation, immune responses, autophagy, and mitochondrial biogenesis (13, 150). Other important ROS producers in the cell include NADPH oxidase (NOX), xanthine oxidase, 5-lipoxygenase, and cyclooxygenase (42).
ER Stress and Oxidative Stress in Cellular Homeostasis and Apoptosis
ER stress in apoptosis
UPR signaling is an important adaptive mechanism in response to protein misfolding in the ER. However, prolonged ER stress leads to activation of the pro-apoptotic UPR, which plays critical roles in certain physiological and many pathological conditions.
In response to ER stress, eIF2α phosphorylation attenuates global protein synthesis, which serves to reduce the ER protein-folding load and simultaneously selectively increases translation of adaptive response mRNAs, including that of ATF4, to restore ER homeostasis. Subsequently, ATF4 activates transcription of CHOP. In vitro and animals studies demonstrated that CHOP is a master regulator of ER stress-induced apoptosis (143). During ER stress, CHOP increases the level of pro-apoptotic BH3-only protein Bim through CHOP-C/EBPα-mediated transcriptional activation (135). In neuronal cells, CHOP may transactivate BIM and the p53 upregulated modulator of apoptosis (PUMA) on ER stress through cooperation with the transcription factor forkhead box, class O, 3a (59). CHOP can also inhibit pro-survival protein Bcl-2 through transcriptional suppression, which may require liver inhibitory protein, an isoform of C/EBPβ (26). In addition, CHOP can activate transcription of other pro-apoptotic genes, including telomere repeat binding factor 3 and death receptor 5, both of which are important mediators of ER stress-induced apoptosis in several cancer cells (161). Studies also implicate a role for CHOP in promoting protein synthesis to cause oxidative stress, leading to apoptosis (107). Recently, chromatin immunoprecipitation and mRNA deep sequencing analyses demonstrated that CHOP and ATF4 form a heterodimer to induce transcription of genes that encode protein synthetic machinery (65). Indeed, forced expression of ATF4 with CHOP increases protein synthesis and causes oxidative stress that is required for cell death. One component of the ATF4/CHOP-stimulated increase in protein synthesis is mediated through transcriptional activation of GADD34, the regulatory subunit of PPP1 that directs eIF2α dephosphorylation to restore global mRNA translation. An increase in protein synthesis under conditions where protein folding is defective causes more misfolding and further exacerbates cell death signaling through oxidative stress (65, 107). CHOP has also been involved in oxidative stress induction, which will be discussed in the next section “Cross-talk between ER stress and oxidative stress in apoptosis.”
IRE1 is the ER stress sensor conserved from yeast to mammals. The IRE1-XBP1 pathway is required for murine embryonic development as well as for optimal UPR activation and normal function in many secretory cells, including pancreatic acinar and β cells, plasma cells, and intestinal Paneth cells (20). However, increasing evidence suggests that IRE1 may contribute to apoptotic cell death, especially on chronic ER stress. First, IRE1α can contribute to apoptotic cell death by activating the JNK pathway through a direct interaction with TRAF2. Activated JNK can induce cell death in different ways, including the phosphorylation of Bcl-2, which inhibits its anti-apoptotic function in regulating Ca2+ flux from the ER and inhibiting pro-apoptotic BH3-only-containing Bcl2 family members such as Bax and Bak. The IRE1α-TRAF2 complex may also activate caspase-12 on ER stress. In addition, IRE1 may cause cell death by interacting with factors involved in apoptosis. In mammalian cells, activated IRE1α can bind Bax and Bak on the ER membrane and initiate the mitochondrial-dependent apoptotic cascade. IRE1α is also linked to the activation of PUMA and BH3 interacting-domain death agonist (Bid), two pro-apoptotic proteins. During ER stress, RIDD could help mitigate the ER protein-folding burden and restore ER homeostasis. However, this process may contribute to cell death during prolonged ER stress by degrading mRNAs encoding pro-survival functions (75, 161). Recently, IRE1α was shown to degrade specific microRNAs that target the mRNA encoding caspase-2, thereby boosting caspase-2-dependent apoptosis during ER stress (169).
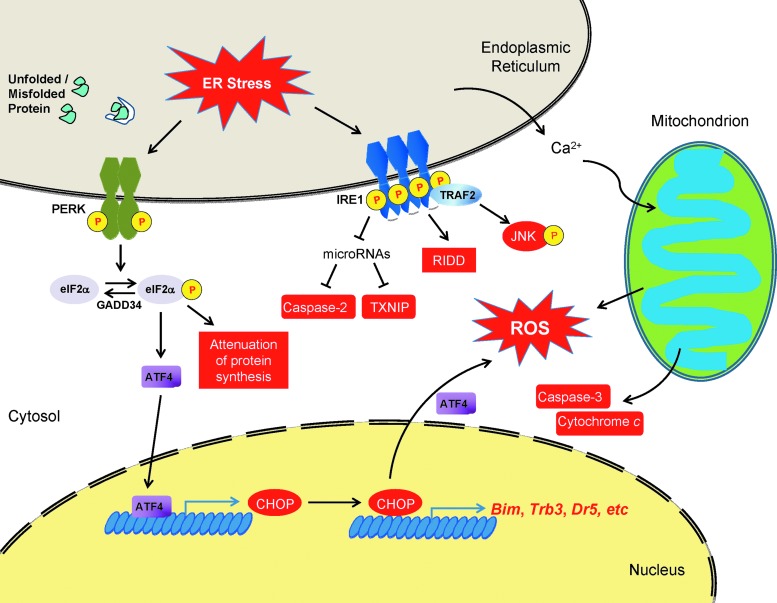
One key event of ER stress-induced apoptosis is the processing of caspases. So far, the activation of caspases-2, -3, -4, -6, -7, -8, -9, and -12 has been reported in different in vitro and/or in vivo models of ER stress. Among these caspases, the deletion of caspase genes 3, -7, -9, or -12, as well as APAF1 protects against ER stress-induced cell death (62). However, the activation cascade of some caspase pathways during the execution phase of ER stress is still elusive (Fig. 3).
FIG. 3.
ER stress-mediated cell death. ER stress leads to apoptotic cell death at transcriptional, post-transcriptional, translational, and post-translational levels. Pro-apoptotic components induced during ER stress are labeled red. See “ER Stress and Oxidative Stress in Cellular Homeostasis and Apoptosis” for details.
Cross-talk between ER stress and oxidative stress in apoptosis
Both ER stress and oxidative stress are involved in a variety of physiological and pathophysiological conditions. Studies in the past decade indicate that these two cellular stresses are closely linked events in cell homeostasis and apoptosis.
First, some forms of ROS can disturb ER protein folding and induce ER stress. Exogenous oxidants such as ROS producers, peroxides, metal ions, and lipid oxidation products may activate some aspects of the UPR. 7-ketocholesterol, a major oxidation product of cholesterol in atherosclerotic plaque, induces the full UPR in macrophages and vascular smooth muscle cells (97, 131). The 7-ketocholesterol-activated UPR is suppressed by N-acetyl-cysteine, an antioxidant, suggesting that this ER stress induction is oxidative stress dependent. However, other forms of ROS, such as H2O2, can only stimulate mild or specific components of the UPR (145). Therefore, a general conclusion that can be drawn from these findings is the nature of the oxidative stress; for example, strength and location may determine whether it is sufficient to induce potent ER stress. Given the importance of redox state in ER homeostasis, the sensing of altered redox is essential to ER protein folding machinery. A recent study demonstrated that non-selenocysteine containing phospholipid hydroperoxide glutathione peroxidase (NPGPx), a member of glutathione peroxidase family, senses oxidative stress in the ER lumen, then forms a disulfide bond with BiP, and promotes its chaperone activity. The loss of NPGPx in mice led to oxidative stress-induced tissue damage, increased tumorigenesis, and impaired longevity (179).
Given the role of disulfide bond formation in the ER as an important source of ROS, protein misfolding in the ER could contribute to oxidative stress. GSH can reduce disulfide bonds in proteins with improperly paired disulfide bonds to enable proper disulfide bond formation by the PDI-Ero1 cycle. When the microenvironment of ER protein folding is severely disrupted, or when a misfolding-prone protein is expressed in the cell, a futile cycle of disulfide bond formation and reduction could lead to oxidative stress by generating a large amount of H2O2 and depleting ER GSH levels (72). As an adaptive response during ER stress, ERAD requires the breakage of disulfide bonds by disulfide reductase ERdj5 before retrotranslocation and degradation of substrate protein, which may also compromise ER redox balance (171). In spite of these relevant data of ER protein misfolding in the induction of oxidative stress, recent studies used novel ER-redox sensors to show that some ER stress inducers did not alter ER redox state, or even rendered the ER lumen more reduced (149). These discrepancies might result from differences in the strength and/or timing of the ER stress, as well as by the different experimental systems utilized, such as cell type and method of redox measurement.
As a major pro-apoptotic factor of the UPR, CHOP also induces oxidative stress in different manners. In mammalian cells, Ero1α is transcriptionally activated by CHOP and can increase ROS production during ER stress. In addition, Ero1α causes inositol-1,4,5-trisphosphate receptor (IP3R)-mediated Ca2+ leakage from the ER, which activates Ca2+ sensing kinase CaMKII in the cytosol, leading to the activation of pro-apoptotic pathways, including Fas and mitochondrial membrane permeability transition (20, 75, 161). CaMKII also induces NOX subunit Nox2 and causes oxidative stress, which results in PKR-dependent CHOP induction as a positive feed-forward cycle during ER stress (97). CHOP contributes to cell death by restoring global mRNA translation during ER stress, which may lead to protein misfolding and mitochondrion-dependent induction of oxidative stress (7, 65, 107).
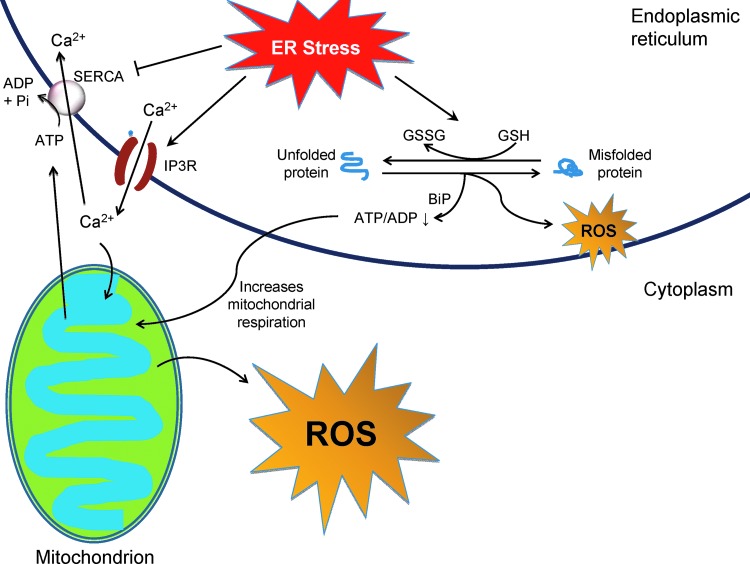
Mitochondria are another important site for ROS production during ER stress. In ER-stressed cells, Ca2+ released from the ER is taken up by mitochondria, leading to opening of the permeability transition pore to release cytochrome c from the mitochondrial matrix. The loss of cytochrome c inhibits complex III of the ETC and enhances ROS production by increasing the ubisemiquinone radical intermediate. In addition, increased Ca2+ in the mitochondria stimulates Krebs cycle dehydrogenases, thereby boosting oxygen consumption and ROS production. Mitochondrial Ca2+ also activates nitric oxide synthase, whose product disturbs the ETC and enhances ROS generation (16, 159). During ER stress, Ca2+ release from the ER and mitochondrial ROS production creates a vicious cycle that impairs cellular homeostasis and induces apoptosis. ROS opens the ER Ca2+ channels IP3Rs and ryanodine receptors and releases more ER Ca2+, which further disturbs ER protein folding and induces mitochondrial oxidative stress and dysfunction. ER and mitochondria are interconnected physically and functionally by mitochondria-associated ER membranes (MAMs), a structure that may be important for Ca2+ uptake by the mitochondria (17). Recent studies showed that ER Ca2+ channels, including the IP3Rs and the mitochondrial voltage-dependent anion channel, are enriched in MAMs, which might facilitate Ca2+ flow between the two organelles (160). In addition, the ER stress sensor PERK resides in MAMs and helps maintain the ER-mitochondria junction that plays a critical role in mitochondrial dysfunction and apoptosis (174). In addition to Ca2+-mediated mitochondrial ROS production, the futile cycle of disulfide bond formation and breakage during ER stress could deplete cellular energy and stimulate mitochondrial respiration, which may also increase ROS production in mitochondria (Fig. 4).
FIG. 4.
ER stress-induced ROS production in the cell. ROS are usually generated by cellular processes, including oxidative protein folding and mitochondrial respiration, which can be augmented to disrupt cell function and survival during ER stress. See “Cross-talk between ER stress and oxidative stress in apoptosis” for details. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Given the deleterious impact of oxidative stress induced during protein misfolding in the ER, eukaryotic cells have evolved antioxidative stress responses to restore cellular redox homeostasis. The PERK branch of the UPR induces ATF4 and NRF2, two transcription factors that transactivate antioxidative stress response genes, including SODs, heme oxygenase-1, glutathione transferase, and uncoupling mitochondrial protein 2 (145). In addition, small-molecular antioxidants, such as butylated hydroxyanisole (BHA), can prevent ER stress-induced apoptosis and promote proper protein folding and secretion (104, 105), which further demonstrates the crucial role of oxidative stress in protein misfolding-related cellular dysfunction.
ER Stress and Oxidative Stress in Human Diseases
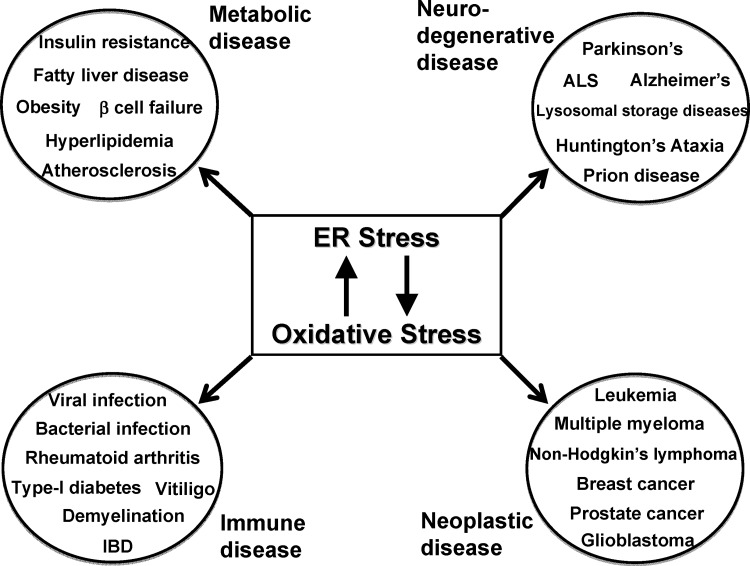
The previous decade has witnessed an increase in our knowledge of protein misfolding-induced human diseases through animal studies and clinical investigations. The pathogenesis is further complicated by the fact that oxidative stress is usually induced during ER stress and contributes dramatically to cell dysfunction and apoptosis (Fig. 5). Next, we discuss how understanding the two cellular stresses develop and cross-talk in human disease will provide insights for novel prevention and treatment strategies. We have not covered neurodegenerative disease, as this topic was recently reviewed (30, 39, 80, 115).
FIG. 5.
ER stress and oxidative stress in human diseases. ER stress and oxidative stress are linked to multiple human pathologies, including metabolic, neurodegenerative, immune/inflammatory, and neoplastic diseases. Studies on the two cellular stresses have not only contributed to our understanding of the pathogenesis, but also opened new avenues to next-generation therapies for these debilitating illnesses. See “ER Stress and Oxidative Stress in Human Diseases” for details.
Metabolic disease
Diabetes
Pancreatic β cells are the primary source of insulin, which accounts for about half of the protein production in these professional secretory cells. High protein secretion predisposes β cells to the challenge of ER stress. Meanwhile, β cells are sensitive to oxidative stress due to their high energy consumption and low levels of antioxidant enzymes (108). Both type 1 and type 2 diabetes involve β-cell dysfunction and/or apoptosis, which are associated with ER stress and oxidative stress (6, 178). Several pathological, environmental, and genetic causes are proposed to induce ER stress and oxidative stress in β cells, including glucotoxicity, lipotoxicity, and inflammatory challenge (51, 178). Indeed, a high-fat diet alone increases proinsulin misfolding in C57Bl6/J mice (148). Chronic high-glucose challenge (glucotoxicity) induces the pro-apoptotic UPR, including CHOP and IRE1α-JNK, and oxidative stress in β cells in both in vitro and in vivo models. Free fatty acids, such as palmitate, induce ER stress and oxidative stress and cause apoptosis in β cells (6, 51, 99). Proinflammatory cytokines, including interferon-γ and interleukin (IL)-1β, also induce ER stress in β cells and contribute to the pathogenesis of type 1 diabetes (24).
Since oxidative stress is usually coupled with ER stress, the UPR has evolved to handle both protein folding defects and oxidative challenge. The PERK-eIF2α pathway plays a crucial role in β-cell function and survival. Patients with Wolcott–Rallison syndrome, an autosomal-recessive disease, harbor loss-of-function mutations in the PERK gene and suffer from infancy-onset β-cell failure (35, 74, 191). Later, murine studies demonstrated that deletion of Perk or expression of non-phosphorylatable Ser51Ala mutation in eIF2α (AA) impairs adaptive UPR signaling, disturbs intracellular trafficking from the ER to the Golgi, reduces expression of β-cell-specific genes, diminishes insulin granule number, and increases apoptosis in pancreatic β cells (67, 147). In addition, β cells with a defective PERK-eIF2α pathway also exhibit oxidative stress (7). This phenotype may be explained by at least two different mechanisms: (i) unregulated protein synthesis in Perk−/− and AA β cells exacerbates ER protein misfolding and increases ROS production; (ii) a compromised ATF4-dependent antioxidative stress response in the absence of a functional PERK-eIF2α pathway. The importance of oxidative stress in β-cell pathology in the AA model was demonstrated by showing that feeding of antioxidant BHA alone alleviates β-cell dysfunction and restores glucose homeostasis in these mice. In contrast to Perk, Chop deletion improves β-cell function and reduces apoptosis in genetic- and diet-induced diabetes in mice (157). Similarly, the protective effect of Chop deletion in β cells is phenocopied in murine diabetic models by feeding of BHA, which is consistent with previous findings that CHOP disrupts cellular homeostasis by inducing oxidative stress. The IRE1α-XBP1 pathway is required for the biosynthesis, folding, and maturation of proinsulin (92, 100). IRE1α directly attenuates the translation of insulin mRNA through RIDD, which may protect β cells from ER stress and oxidative stress induced by hyperactivated insulin synthesis [2].
Insulin resistance
Insulin resistance (IR), a major characteristic of obesity and type 2 diabetes, is caused by impaired insulin signaling in multiple organs, including the liver, adipose tissue, and muscle. Animal and clinical studies indicate that ER stress and oxidative stress may be important contributors to IR (50). In mammals, the liver orchestrates the homeostasis of glucose and lipid metabolism. Hepatic IR leads to hyperglycemia and hyperlipidemia by inducing gluconeogenesis and lipogenesis, respectively (178). ER stress and the UPR contribute to hepatic IR through different mechanisms. IRE1α induces hepatic IR through activation of JNK and IKK, which blocks insulin signaling by serine phosphorylation of insulin receptor substrate 1 and 2 (6, 18, 162, 170). In addition to IRE1α, ER stress can also activate JNK through CAMKII and PKR and induce hepatic IR (122, 163). Free fatty acids are an important metabolic signal that induces both ER stress and oxidative stress in hepatocytes and adipocytes, which then leads to increased lipolysis, induction of proinflammatory mediators and IR. However, the underlying mechanism is still poorly understood. ROS play a causative role in IR; while antioxidants, including N-acetylcysteine, SOD, and catalase, can improve insulin sensitivity in cultured muscle cells (81).
Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is characterized by excessive accumulation of triacylglycerol in the liver due to increased influx of free fatty acids and/or de novo lipogenesis in the absence of significant alcohol consumption. ER stress is linked to multiple hepatic dysfunctions, including IR, lipotoxcity, inflammation, cell death, and steatosis (53, 103) (Fig. 2). The presence of ER stress and oxidative stress was demonstrated in the livers of animal models of nonalcoholic steatohepatitis (NASH) as well as in the livers of patients with NAFLD or NASH (128, 134). Recent studies using genetic murine models suggest that ER stress plays a causal role in the pathogenesis of hepatosteatosis. Induction of ER stress caused hepatosteatosis in mice, and this hepatosteatosis was much more severe in mice that had mutations in the three UPR pathways, Ire1α, Atf6α, as well as Ser51Ala eIF2α (144, 190). A point mutation in Sec61α1, which encodes the aqueous channel for protein translocation into the ER, disrupted the ER secretory pathway and increased the susceptibility to hepatic steatosis and fibrosis on metabolic challenge in mice (101). Forced expression of Gadd34 in murine liver reduced eIF2α phosphorylation and protected against ER stress and hepatosteatosis on the feeding of a high-fat diet (126). Recently, the IRE1α-XBP1 pathway was shown to play an important role in the assembly and secretion of very-low-density lipoprotein in the liver. Mice deleted in Ire1α in hepatocytes displayed more severe steatosis on fasting or challenge of a high fructose diet (177). The IRE1α-XBP1 pathway was required to induce expression of PDI that is essential for microsomal triglyceride transfer protein activity to promote triglyceride assembly with ApoB. Finally, the hepatocyte-specific ATF6 family member CREBH regulates liver lipid metabolism and patients with nonsynomenous mutant hypomorphic alleles of CREBH exhibit severe hypertriglyceridemia (95, 188). Several animal and clinical studies showed strong correlations between oxidative stress such as serum oxidative markers and hepatic lipid peroxidation and severity of NAFLD/NASH. During the pathogenesis of NAFLD, accumulation of lipid in hepatocytes induces β oxidation of fatty acids, which overwhelms the ETC in the mitochondria and promotes ROS production. Oxidative damage of mitochondrial membrane proteins, such as the components of ETC, impairs mitochondrial function and exacerbates ROS overproduction. Accumulation of fatty acids also stimulates extramitochondrial fatty acid oxidation in organelles, including peroxisomes and microsomes, which also contributes to ROS production and oxidative stress. In addition, a reduced antioxidative stress response, such as coenzyme Q10, Cu-Zn SOD, and catalase, was observed in both patients and animal models of NASH (140). A recent study linked fatty acid oxidation to ER stress by showing that pharmacological inhibition of fatty acid oxidation in hepatocytes increases cellular redox potential and protects against ER stress (168). However, the functional interaction between ER stress and oxidative stress in hepatocyte homeostasis and pathogenesis of NAFLD/NASH is still elusive.
Inflammatory disease
ER stress and oxidative stress are observed in several inflammatory diseases, including inflammatory bowel disease (IBD), chronic obstructive pulmonary disease, chronic kidney disease, alcoholic liver disease, hepatitis, pancreatitis, and rheumatoid arthritis (1, 2, 36, 47, 57, 70, 85, 89, 103, 124, 129, 138, 186, 187). These two stresses can contribute to inflammatory pathologies in various organ systems by causing cellular dysfunction and inducing “cell autonomous” inflammation. Accumulated evidence suggests that the UPR is a crucial inducer of pro-inflammatory signals in the cell. The IRE1-XBP1 pathway, the most conserved UPR signaling, promotes cellular inflammation through several different mechanisms. The kinase domain of IRE1α can activate JNK-AP1 and NF-κB signaling through a physical interaction with adaptor protein TRAF2 (86, 170). XBP1s can transactivate pro-inflammatory genes Tnfα and Il6 in macrophages by directly binding to their promoter/enhancer regions (112). The PERK-eIF2α-CHOP pathway also plays important roles in the inflammatory response. Phosphorylation of eIF2α facilitates the nuclear translocation of NF-κB by attenuating the synthesis of IκB protein (183). In dendritic cells, CHOP induces the transcription of gene encoding IL-23, an inflammatory cytokine that stimulates the maturation of Th17 cells (61). In macrophages, CHOP is important for the induction of caspase-11 mRNA and subsequent activation of pro-caspase-1 and the inflammasome (44). Recently, the IRE1α and PERK pathways were linked to inflammasome activation in pancreatic β cells through increasing thioredoxin interacting protein (TXNIP) to inhibit thioredoxin and cause oxidative stress (125). In addition, ATF6α and CREBH also contribute to inflammatory response. ER stress induces cleavage and activation of ATF6α and CREBH that act to increase transcription of the systemic arm of the inflammatory response, the acute phase response (APR) genes in the liver (189). ROS production has long been associated with cellular inflammation. ROS function as second messengers and activate a number of signal transduction pathways, including JNK, p38 MAPK, ERK, PI3K/Akt, PKC, Src family kinases, and growth factor tyrosine kinase receptor pathways, all of which can lead to the induction of inflammatory genes. Furthermore, ROS also stimulate redox-sensitive transcription factors, including NF-κB, AP1, and hypoxia-inducible factor-1 (HIF-1), which play crucial roles in inflammatory responses (94). In addition to inducing inflammation at the cellular level, ER stress and oxidative stress can also contribute to the pathogenesis of inflammatory disorders in various tissue/organ systems in a “non-cell autonomous” manner. IBD is an example where ER stress and ROS induce inflammatory disease in both “cell autonomous” and “non-cell autonomous” manners.
IBD, including Crohn's disease and ulcerative colitis, is a group of inflammatory conditions in the gastrointestinal tract. Murine and human intestines harbor four intestinal epithelial cells (IEC), including Paneth and goblet cells, which secrete large amounts of antimicrobial peptides and mucins, respectively, and are crucial for intestinal barrier function and mucosal homeostasis. Accumulated evidence suggests that IECs, particularly Paneth and goblet cells, are sensitive to alterations in ER protein folding homeostasis due to environmental challenge and/or genetic defects (46, 114). ER stress markers are induced in the mucosal tissues of patients with IBD as well as in several murine models of colitis and Crohn's ileitis (12, 23, 73, 82, 90, 152). Mice that express a misfolding-prone mutant of MUC2 mucin, the major mucin in the large intestine, displayed ER stress, goblet cell dysfunction, and spontaneous colitis (73). The anti-inflammatory cytokine IL-10 ameliorated the misfolding of mutant MUC2 mucin, reduced ER stress, and improved mucin secretion in colonic goblet cells both in vitro and in vivo (71). Glucocorticoids, a family of drugs that have been used in IBD therapies for decades, were recently shown to reduce ER stress in colonic epithelial cells by transactivating ER chaperones and ERAD components (33). So far, several studies using murine genetic models have demonstrated the crucial role of specific UPR components in IEC function and mucosal homeostasis. The IEC-specific ablation of Xbp1 caused progressive Paneth cell death, reduced goblet cell number, as well as spontaneous inflammation in the ileum of ∼60% of the mice (90). In addition, several nonsynonymous SNPs in the coding region of XBP1 were identified by deep sequencing of IBD patients and control individuals, which raised the possibility that hypomorphic function of Xbp1 may contribute to IBD by impairing IEC homeostasis and intestinal barrier function (90). The deletion of Chop, a pro-apoptotic transcription factor, is protective against dextran sodium sulfate (DSS)-induced colitis in mice (123). Deletion of Ire1β exacerbates epithelial cell death and mucosal inflammation on the challenge with DSS (9). Recent studies suggest that IRE1β degrades Muc2 mRNA in colonic goblet cells in mice, thereby optimizing the biosynthesis of Muc2 mucin. In the absence of IRE1β, ER stress was induced in colonic epithelial cells, probably due to increased translation of Muc2 mRNA that overwhelms ER protein folding capacity (166). In contrast, IRE1β promotes mucin production in respiratory epithelial cells by mediating Xbp1 splicing (111). PKR can be activated by ER stress and multiple inflammatory stimuli (56). PKR protects against DSS colitis by activating eIF2α-phosphorylation-mediated UPR signaling and prosurvival components, including STAT3 and AKT in colonic epithelial cells (22). A recent study demonstrated that ER cochaperone P58IPK and ATF6α, a master transactivator of ER chaperone genes, are important for the function and survival of colonic epithelial cells by reducing ER stress and suppressing the pro-apoptotic UPR on DSS challenge (19, 23). So far, there have been limited studies that provide insights into how ER stress and the UPR affect cell-autonomous inflammation in IECs and inflammatory cells in the pathogenesis of IBD (25). However, previous findings support the general conclusion that ER stress-induced epithelial dysfunction may be sufficient to cause intestinal inflammation by compromising mucosal homeostasis and barrier function in the gut.
Increased production of ROS and reactive nitrosative species (RNS) were observed in the mucosal tissues of both chemical-induced and genetic models of IBD, as well as in patients with IBD. Oxidative and nitrosative stress cause damage to macromolecules in cells, as indicated by the formation of lipid peroxidation products and protein modifications, including carbonyls in the mucosa. During the initiation and progression of mucosal inflammation, multiple cell types in the gut can generate ROS/RNS. Neutrophils, macrophages, and IEC can produce a large amount of superoxide and nitric oxide via the activation of NOXs and inducible nitric oxide synthase, respectively, in the pathogenesis of IBD. Overproduction of ROS/RNS contributes to intestinal inflammation by causing epithelial cell death and mucosal tissue injury, as well as by stimulating cell-autonomous inflammation in both IEC and inflammatory cells (192). Depletion of antioxidants is also observed in inflamed mucosal tissues in both human and animal models. The endogenous antioxidative stress defense plays a critical role in the homeostasis of the intestinal mucosa, highlighted by studies of mice deficient in glutathione peroxidase-1/2 or Nrf2 (48, 91). In a small case-controlled study, a polymorphism in the paraoxonase 1 gene (PON1 R192 allele) was associated with both Crohn's disease and ulcerative colitis in an Ashkenazi Jewish population from Israel (87). In addition, polymorphisms in genes encoding Mn-SOD, epoxide hydrolase, NAD(P)H:quinone oxidoreductase, and Hrf2 are associated with ulcerative colitis in different populations (192). Nrf2, a transcription factor that plays a central role in the antioxidative stress response in the gut, is activated by both ER stress and oxidative stress. On ER stress, Nrf2 is phosphorylated and activated by PERK and then migrates into the nucleus to induce antioxidative stress response genes (32). Given the coexistence of ER stress and oxidative stress in inflamed mucosa, it is still to be determined whether the two cellular stresses reciprocally induce each other in the gut, or whether either one is sufficient as an initiating event in the induction of IBD.
Neoplastic disease
One important hallmark of neoplastic disease is the uncontrolled growth of transformed cells in the body. Carcinogenesis is a process in which precancerous and cancerous cells resist multiple stresses, including ER stress and oxidative stress during their growth and expansion. Primary human tumor cells of various origins, including breast, lung, liver, colon, prostate, and brain, show increased UPR signaling, while peritumoral cells do not. Limited supplies of oxygen and nutrients due to poor vascularization constantly challenge solid tumor cells in vivo. Hypoxia activates UPR components, including BiP, XBP1, ATF4, and CHOP, in multiple tumor cell types. In transgenic mice with spontaneous mammary carcinogenesis, splicing of Xbp1 mRNA correlates with the degree of hypoxia in the tumor (40). In addition, a high rate of glycolysis in cancer cells and insufficient blood supply combine together to limit the glucose available to solid tumors (158). BiP is an essential ER chaperone for both normal cells and transformed cells. In primary human melanoma, liver, colon, and breast cancer tissues, the level of BiP was found to positively correlate with tumor progression. The physiological significance of BiP was demonstrated by studies that Bip heterozygosity significantly reduces the cancer cell proliferation, survival, as well as angiogenesis in breast tumors (37). In addition, conditional knockout of Bip in the prostate of mice with Pten inactivation suppressed prostate cancer growth (54). Finally, sublitase, a bacterial cytotoxin that selectively cleaves BiP, can kill glioblastoma cells (133).
The IRE1-XBP1 pathway also plays an important role in carcinogenesis. Knocking down Xbp1 in human fibrosarcoma cells inhibits their growth and angiogenesis in a xenograft model (141). Later, it was shown that IRE1α is essential for the expression of vascular endothelial growth factor A (VEGF-A) and lung cancer growth both in vitro and in vivo (40). The importance of PERK in tumorigenesis is supported by the findings that loss of PERK in mouse fibroblasts and human colon cancer cells reduced tumor growth and angiogenesis when grafted into immunodeficient mice (10, 11). The PERK-eIF2α-ATF4 pathway can promote cancer cell survival and expansion by inducing the hypoxic response and autophagy (69, 173). The UPR is linked to the production of several proinflammatory, tumorigenic cytokines, including IL-6, TNFα, and IL-23. On ER stress challenge, murine lymphoma cells showed transcriptional induction of several inflammatory genes, including Il-6, Tnfα, Il-23, Tlr2, and Cebpβ (180). More interestingly, macrophages cultured in the conditioned medium of ER-stressed cancer cells displayed induction of the UPR and proinflammatory signals, including IL-6, TNFα, IL-23, MIP-1α, and MIP-1β, which suggests that “transmissible” ER stress initiated in cancer cells may be exploited to modify the tumor microenvironment through activation of inflammatory cells (102).
Oxidative stress plays an important role in almost every hallmark of cancer as defined by Hanahan and Weinberg (49, 66). A review of recent studies suggests that ER stress and oxidative stress have overlapping as well as intertwined functions in carcinogenesis. Both ER stress and oxidative stress promote epithelial mesenchymal transition, a key step of metastasis and tissue invasion of many tumor cells. Both stresses activate VEGF signaling and angiogenesis. HIF-1, an essential transcription factor during the hypoxic response, is regulated by both ER stress and oxidative stress. While the tumor suppressor PTEN can be inactivated by oxidative stress during tumorigenesis, PTEN activity requires PKR-eIF2α signaling (120). Moreover, the detachment of mammary epithelial cells from extracellular matrix activates the PERK-eIF2α-ATF4-CHOP branch of the UPR, which protects mammary tumor cells from anoikis by stimulating both autophagy and antioxidative stress responses (5). Later, it was found that oxidative stress causes induction of c-Myc and n-Myc, which improve cancer cell survival through PERK/eIF2α/ATF4-dependent induction of autophagy (69). Oxidative stress induces mutations and aerobic glycolysis by disrupting mitochondrial function, leading to the Warburg effect, which is characterized by increased glycolysis and altered lipid metabolism, by activating mitophagy and inhibiting mitochondrial respiration in cancer cells (58, 109, 110, 172). Pharmacological inhibition of fatty acid oxidation protects cells against ER stress (168); however, its link to the Warburg effect has not been determined. In addition, ER stress and oxidative stress have profound impacts on cell autonomous and non-cell autonomous inflammation, which are critical to both tumor cell expansion and anti-tumor immunity (49). In spite of these inter-related functions, it is still unknown how ER stress and oxidative stress cross-talk with each other during the different stages of carcinogenesis.
Therapeutic Implications
Previous studies indicate that ER stress and oxidative stress form a vicious cycle in many human pathologies, including metabolic, neurodegenerative, and inflammatory diseases (104). Therefore, therapies that target both stresses may be more effective to treat these diseases. Previous findings suggest that antioxidants can suppress both oxidative stress and ER stress for these diseases (21). The over-expression of the misfolding-prone coagulation factor VIII in cultured cells and mouse liver leads to the induction of ER stress, oxidative stress, and cell death. BHA, an antioxidant wildly used in the food industry, alleviated ER stress, oxidative damage, and apoptosis, and enhanced the folding and secretion of factor VIII both in vitro and in vivo (105). In addition, BHA protected β cells that were genetically engineered for increased proinsulin synthesis which caused both ER stress and oxidative stress (7). Similarly, Mitoquinone and MitoTempol, two mitochondrial-targeted antioxidants, reduced mitochondrial oxidative stress, ER stress, energy depletion, and cell death in β cells with glucotoxicity or glucolipotoxicity. Clinical trials demonstrated that Mitoquinone protects against Parkinson's disease and cardiac ischemia-reperfusion injury with excellent safety profile in patients (98, 154).
Tauroursodeoxycholate (TUDCA) and 4-phenylbutyrate (PBA), two small-molecular “chemical chaperones,” impede protein misfolding and aggregation as well as promote intracellular trafficking and secretion. The Food and Drug Administration (FDA) approved UDCA, the unconjugated form of TUDCA, and PBA for the treatment of primary biliary cirrhosis and urea-cycle disorders, respectively. Recently, TUDCA and PBA compounds demonstrated therapeutic potential in preclinical/clinical studies for multiple diseases associated with ER stress, including IR (45, 88, 127, 128, 136, 184), alcoholic/nonalcoholic liver disease (84), Alzheimer's disease (139, 181), neuronal cell apoptosis (116), as well as acinar cell death and pancreatitis (151). Many of these diseases are also associated with oxidative stress, which may play a causal role in the pathogenesis. Given the excellent safety profiles of TUDCA and PBA in humans, these compounds deserve further exploration for mono-therapies or combinatorial therapies with antioxidants for diseases associated with both ER stress and oxidative stress (106).
In addition to antioxidants and chemical chaperones, small molecules that activate endogenous components of the adaptive UPR and antioxidative stress response may exhibit therapeutic potential for these diseases. The PERK-ATF4 branch of the UPR not only induces ER chaperones and intracellular trafficking machinery, but also activates antioxidative stress defenses in the cell. Therefore, pharmacological activation of the PERK-ATF4/Nrf2 pathway may improve cellular homeostasis by suppressing both ER stress and oxidative stress. Salubrinal and guanabenz, two structurally unrelated small-molecular compounds, have been identified to prevent the dephosphorylation of eIF2α by disrupting PP1 complex, thereby sustaining PERK-ATF4 signaling during ER stress (15, 165). Salubrinal alleviated protein aggregation and apoptosis in cell culture models of Alzheimer's, Huntington's, and Parkinson's disease by inducing the eIF2α phosphorylation-mediated adaptive UPR (93, 155, 156). In several rodent models of Parkinson's disease and α-synucleinopathies, salubrinal reduced the accumulation of α-synuclein in the ER and delayed the onset of disease (27, 28). In a mouse model of familial ALS, salubrinal reduced the accumulation of poly-ubiquitinated protein inside motor neurons, improved motor neuron and muscle function, and prolonged survival (146). In addition, salubrinal protected against neuronal injury on excitotoxic challenge in the rat brain (156). In several other disease models, salubrinal improved cellular homeostasis by suppressing oxidative stress, ER stress, and mitochondrial dysfunction (38, 182, 193). Guanabenz is an FDA-approved α2-adrenergic agonist that is used to treat hypertension for decades. Before its UPR-regulating function was discovered, guanabenz was shown to reduce the accumulation of prion in both yeast and mammalian cells in vivo (164). In Akita mice that express mutant, misfolding-prone insulin, guanabenz reduced β-cell dysfunction and apoptosis (165). In a Drosophila model of oculopharyngeal muscular atrophy, guanabenz prevented nuclear inclusion formation and muscular degeneration by inhibiting the aggregation and toxicity of poly(A) binding protein nuclear 1 (8). In addition, guanabenz ameliorated cell death and preserved light detection function in a murine model of ciliopathies, a rare genetic disorder caused by protein trafficking defects (118).
The killing power of ER stress and oxidative stress has demonstrated therapeutic potential for several cancers. Ras-driven tumors are refractory to conventional treatments as well as to monotherapy of the ER stress inducers tunicamycin and thapsigargin. However, combining tunicamycin or thapsigargin with rapamycin showed a rapid suppression of tumor growth in mice. It was demonstrated that rapamycin inhibits the synthesis of glutathione, an essential antioxidant in the cell, by repressing the transcription of glucose 6-phosphate dehydrogenase of the pentose phosphate pathway. A combination of an HSP90 inhibitor IPI-504, an ER stress inducer currently in clinical trials, and rapamycin, which exacerbates oxidative stress, suppressed the growth of two Ras-driven tumors both in vitro and in vivo by stimulating progressive ER stress and mitochondrial damage (34). The findings suggest that some cancer therapies may require both ER stress and oxidative stress to be successful. Recently, a novel PERK kinase inhibitor (GSK2656157) was shown to inhibit the growth of tumor xenografts in mice (3). Several IRE1 endoribonuclease inhibitors, which prevent Xbp1 mRNA splicing and RIDD, exhibited a therapeutic effect against multiple myeloma in vitro and/or in vivo (117, 130, 137). In future, it will be worthwhile to test the combinatorial effects of the oxidative stress inducer, for example, rapamycin, and ER stress stimulators such as bortezomib, brefeldin A, and sublitase, as well as novel UPR regulators, including PERK and IRE1 inhibitors, in cancer therapy (173).
Conclusions
Recent insights indicate that ER stress and oxidative stress are highly inter-related biological processes which regulate a wide range of signaling pathways in the cell. This is not only demonstrated by the fact that the two stresses coexist and induce each other, but also reflected by the multi-functional stress responses which target both ER protein misfolding and redox imbalance. The two cellular stresses profoundly impact normal physiology as well as many human pathologies, including metabolic, neurodegenerative, inflammatory, and neoplastic diseases. Recently, studies suggest that the two stresses and their downstream signaling pathways are promising targets for novel therapeutics. In spite of the exciting findings in the past decade, a number of questions still remain: (i) how do ER stress and oxidative stress act on components at the molecular and cellular level to alter physiology? (ii) how do these stress responses cross-talk with each other in different cell types and disease models? and (iii) how can we design compounds and therapies that selectively target ER stress and/or oxidative stress in specific tissues/organs? Current efforts to disentangle these puzzles are impeded by the complex nature of both ER stress and oxidative stress. More mechanistic and physiological studies using newly developed high-throughput technologies, a real-time in vitro and in vivo imaging system, and novel conditional genetic animal models are needed.
Abbreviations Used
- APR
acute phase response
- ATF6
activating transcription factor 6
- BHA
butylated hydroxyanisole
- DSS
dextran sodium sulfate
- eIF2α
eukaryotic translation initiation factor 2
- ER
endoplasmic reticulum
- ERAD
ER-associated protein degradation
- Ero1
ER oxidoreductase 1
- ETC
electron transport chain
- FDA
Food and Drug Administration
- GSH
glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- HIF-1
hypoxia-inducible factor-1
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cells
- IL
interleukin
- IP3R
inositol-1,4,5-trisphosphate receptor
- IR
insulin resistance
- JNK
c-Jun N-terminal kinase
- MAM
mitochondria-associated ER membrane
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NF-κB
nuclear factor-kappaB
- NOX
NADPH oxidase
- NPGPx
non-selenocysteine containing phospholipid hydroperoxide glutathione peroxidase
- PBA
4-phenylbutyrate
- PDI
protein disulfide isomerases
- PERK
pancreatic ER eIF2α kinase
- PKR
dsRNA-activated protein kinase
- PUMA
p53 upregulated modulator of apoptosis
- QSOX
quiescin sulfhydryl oxidase
- redox
reduction–oxidation
- RNS
reactive nitrosative species
- ROS
reactive oxygen species
- S1P
site-1 protease
- SOD
superoxide dismutase
- TNFα
tumor necrosis factor alpha
- TRAF2
TNFα receptor-associated factor 2
- TUDCA
tauroursodeoxycholate
- TXNIP
thioredoxin interacting protein
- UPR
unfolded protein response
- VEGF
vascular endothelial growth factor
- XBP1
X-box-binding protein 1
Acknowledgments
The authors acknowledge the grant support from NIH HL057346, HL052173, DK042394, and DK088227 and the Crohn's and Colitis Foundation of America (R.J.K.).
References
- 1.Almenier HA, Al Menshawy HH, Maher MM, and Al Gamal S. Oxidative stress and inflammatory bowel disease. Front Biosci (Elite Ed) 4: 1335–1344, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Ambade A. and Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int J Hepatol 2012: 853175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, Choudhry AE, Alsaid H, Jucker BM, Axten JM, and Kumar R. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res 73: 1993–2002, 2013 [DOI] [PubMed] [Google Scholar]
- 4.This reference has been deleted.
- 5.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, and Aguirre-Ghiso JA. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol 31: 3616–3629, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Back SH. and Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem 81: 767–793, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, and Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab 10: 13–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbezier N, Chartier A, Bidet Y, Buttstedt A, Voisset C, Galons H, Blondel M, Schwarz E, and Simonelig M. Antiprion drugs 6-aminophenanthridine and guanabenz reduce PABPN1 toxicity and aggregation in oculopharyngeal muscular dystrophy. EMBO Mol Med 3: 35–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, West AB, and Ron D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest 107: 585–593, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi MX, Naczki C, Koritzinsky M, Fels D, Blais J, Hu NP, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, and Koumenis C. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J 24: 3470–3481, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, Koumenis C, Harding HP, Ron D, Holcik M, and Bell JC. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol 26: 9517–9532, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogaert S, De Vos M, Olievier K, Peeters H, Elewaut D, Lambrecht B, Pouliot P, and Laukens D. Involvement of endoplasmic reticulum stress in inflammatory bowel disease: a different implication for colonic and ileal disease? PLoS One 6: e25589, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolisetty S. and Jaimes EA. Mitochondria and reactive oxygen species: physiology and pathophysiology. Int J Mol Sci 14: 6306–6344, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.This reference has been deleted.
- 15.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, and Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307: 935–939, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol 45: 466–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bravo R, Gutierrez T, Paredes F, Gatica D, Rodriguez AE, Pedrozo Z, Chiong M, Parra V, Quest AF, Rothermel BA, and Lavandero S. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol 44: 16–20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, and Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao SS. and Kaufman RJ. PKR in DSS-induced colitis: a matter of genetic background and maternal microflora? Inflamm Bowel Dis 19: E49–E50, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Cao SS. and Kaufman RJ. Unfolded protein response. Curr Biol 22: R622–R626, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Cao SS. and Kaufman RJ. Targeting endoplasmic reticulum stress in metabolic disease. Expert Opin Ther Targets 17: 437–448, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Cao SS, Song B, and Kaufman RJ. PKR protects colonic epithelium against colitis through the unfolded protein response and prosurvival signaling. Inflamm Bowel Dis 18: 1735–1742, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao SS, Zimmermann EM, Chuang BM, Song B, Nwokoye A, Wilkinson JE, Eaton KA, and Kaufman RJ. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology 144: 989–1000, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, and Eizirik DL. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 54: 452–461, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Chang JS, Ocvirk S, Berger E, Kisling S, Binder U, Skerra A, Lee AS, and Haller D. Endoplasmic reticulum stress response promotes cytotoxic phenotype of CD8alphabeta+ intraepithelial lymphocytes in a mouse model for Crohn's disease-like ileitis. J Immunol 189: 1510–1520, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Chiribau CB, Gaccioli F, Huang CC, Yuan CL, and Hatzoglou M. Molecular symbiosis of CHOP and C/EBP beta isoform LIP contributes to endoplasmic reticulum stress-induced apoptosis. Mol Cell Biol 30: 3722–3731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, Schneider BL, and Lee MK. Endoplasmic reticulum stress is important for the manifestations of alpha-synucleinopathy in vivo. J Neurosci 32: 3306–3320, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colla E, Jensen PH, Pletnikova O, Troncoso JC, Glabe C, and Lee MK. Accumulation of toxic alpha-synuclein oligomer within endoplasmic reticulum occurs in alpha-synucleinopathy in vivo. J Neurosci 32: 3301–3305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.This reference has been deleted.
- 30.Cornejo VH. and Hetz C. The unfolded protein response in Alzheimer's disease. Semin Immunopathol 35: 277–292, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Creighton TE, Hillson DA, and Freedman RB. Catalysis by protein-disulphide isomerase of the unfolding and refolding of proteins with disulphide bonds. J Mol Biol 142: 43–62, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Cullinan SB. and Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol 38: 317–332, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Das I, Png CW, Oancea I, Hasnain SZ, Lourie R, Proctor M, Eri RD, Sheng Y, Crane DI, Florin TH, and McGuckin MA. Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins. J Exp Med 210: 1201–1216, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, Maertens O, Jeong SM, Bronson RT, Lebleu V, Kalluri R, Normant E, Haigis MC, Manning BD, Wong KK, Macleod KF, and Cichowski K. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell 20: 400–413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, and Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 25: 406–409, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Dickhout JG. and Krepinsky JC. Endoplasmic reticulum stress and renal disease. Antioxid Redox Signal 11: 2341–2352, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, Mao C, Ye R, Wang M, Pen L, Dubeau L, Groshen S, Hofman FM, and Lee AS. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res 68: 498–505, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Dou G, Sreekumar PG, Spee C, He S, Ryan SJ, Kannan R, and Hinton DR. Deficiency of alphaB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med 53: 1111–1122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doyle KM, Kennedy D, Gorman AM, Gupta S, Healy SJ, and Samali A. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J Cell Mol Med 15: 2025–2039, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drogat B, Auguste P, Nguyen DT, Bouchecareilh M, Pineau R, Nalbantoglu J, Kaufman RJ, Chevet E, Bikfalvi A, and Moenner M. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-a expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res 67: 6700–6707, 2007 [DOI] [PubMed] [Google Scholar]
- 41.This reference has been deleted.
- 42.Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 43.This reference has been deleted.
- 44.Endo M, Mori M, Akira S, and Gotoh T. C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol 176: 6245–6253, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, and Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 15: 1383–1391, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eri RD, Adams RJ, Tran TV, Tong H, Das I, Roche DK, Oancea I, Png CW, Jeffery PL, Radford-Smith GL, Cook MC, Florin TH, and McGuckin MA. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol 4: 354–364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esrefoglu M. Oxidative stress and benefits of antioxidant agents in acute and chronic hepatitis. Hepat Mon 12: 160–167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esworthy RS, Aranda R, Martin MG, Doroshow JH, Binder SW, and Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol 281: G848–G855, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Fiaschi T. and Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol 2012: 762825, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flamment M, Hajduch E, Ferre P, and Foufelle F. New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab 23: 381–390, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Fonseca SG, Gromada J, and Urano F. Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol Metab 22: 266–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frand AR. and Kaiser CA. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell 1: 161–170, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Fu S, Watkins SM, and Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab 15: 623–634, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, and Lee AS. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci U S A 105: 19444–19449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.This reference has been deleted.
- 56.Garcia MA, Meurs EF, and Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie 89: 799–811, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, and Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med 18: 589–598, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Gasparre G, Porcelli AM, Lenaz G, and Romeo G. Relevance of mitochondrial genetics and metabolism in cancer development. Cold Spring Harb Perspect Biol 1;5(2), 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghosh AP, Klocke BJ, Ballestas ME, and Roth KA. CHOP potentially co-operates with FOXO3a in neuronal cells to regulate PUMA and BIM expression in response to ER stress. PLoS One 7: e39586, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.This reference has been deleted.
- 61.Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, and Gaston JS. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci U S A 107: 17698–17703, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorman AM, Healy SJ, Jager R, and Samali A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol Ther 134: 306–316, 2012 [DOI] [PubMed] [Google Scholar]
- 63.This reference has been deleted.
- 64.Hagiwara M. and Nagata K. Redox-dependent protein quality control in the endoplasmic reticulum: folding to degradation. Antioxid Redox Signal 16: 1119–1128, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Han J, Back SH, Hur J, Lin Y-H, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, and Kaufman RJ. Endoplasmic reticulum stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15: 481–490, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanahan D. and Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, and Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell 7: 1153–1163, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, and Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, Li Y, Gao Y, Liu H, Li C, Maity A, Thomas-Tikhonenko A, Perl AE, Koong A, Fuchs SY, Diehl JA, Mills IG, Ruggero D, and Koumenis C. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest 122: 4621–4634, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasnain SZ, Lourie R, Das I, Chen AC, and McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol 90: 260–270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasnain SZ, Tauro S, Das I, Tong H, Chen AC, Jeffery PL, McDonald V, Florin TH, and McGuckin MA. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 144: 357–368, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Haynes CM, Titus EA, and Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 15: 767–776, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, Adams R, Kato M, Nelms KA, Hong NA, Florin TH, Goodnow CC, and McGuckin MA. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 5: e54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herbach N, Rathkolb B, Kemter E, Pichl L, Klaften M, de Angelis MH, Halban PA, Wolf E, Aigner B, and Wanke R. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes 56: 1268–1276, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13: 89–102, 2012 [DOI] [PubMed] [Google Scholar]
- 76.This reference has been deleted.
- 77.This reference has been deleted.
- 78.This reference has been deleted.
- 79.This reference has been deleted
- 80.Hoozemans JJ. and Scheper W. Endoplasmic reticulum: the unfolded protein response is tangled in neurodegeneration. Int J Biochem Cell Biol 44: 1295–1298, 2012 [DOI] [PubMed] [Google Scholar]
- 81.Houstis N, Rosen ED, and Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440: 944–948, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Hu S, Ciancio MJ, Lahav M, Fujiya M, Lichtenstein L, Anant S, Musch MW, and Chang EB. Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology 133: 1893–1904, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hwang C, Sinskey AJ, and Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257: 1496–1502, 1992 [DOI] [PubMed] [Google Scholar]
- 84.Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, and Lee AS. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology 54: 229–239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jun M, Venkataraman V, Razavian M, Cooper B, Zoungas S, Ninomiya T, Webster AC, and Perkovic V. Antioxidants for chronic kidney disease. Cochrane Database Syst Rev 10: CD008176, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaneko M, Niinuma Y, and Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull 26: 931–935, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Karban A, Hartman C, Eliakim R, Waterman M, Nesher S, Barnett-Griness O, and Shamir R. Paraoxonase (PON)1 192R allele carriage is associated with reduced risk of inflammatory bowel disease. Dig Dis Sci 52: 2707–2715, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, and Klein S. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59: 1899–1905, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaser A, Adolph TE, and Blumberg RS. The unfolded protein response and gastrointestinal disease. Semin Immunopathol 35: 307–319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, and Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134: 743–756, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, and Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66: 11580–11584, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Lee AH, Heidtman K, Hotamisligil GS, and Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A 108: 8885–8890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee do Y, Lee KS, Lee HJ, Kim do H, Noh YH, Yu K, Jung HY, Lee SH, Lee JY, Youn YC, Jeong Y, Kim DK, Lee WB, and Kim SS. Activation of PERK signaling attenuates Abeta-mediated ER stress. PLoS One 5: e10489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee IT. and Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol 84: 581–590, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Lee JH, Giannikopoulos P, Duncan SA, Wang J, Johansen CT, Brown JD, Plutzky J, Hegele RA, Glimcher LH, and Lee AH. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nat Med 17: 812–815, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, Heintz N, Greengard P, Hui S, Tang Q, Trusina A, Oakes SA, and Papa FR. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab 16: 250–264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li G, Scull C, Ozcan L, and Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol 191: 1113–1125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim S, Rashid MA, Jang M, Kim Y, Won H, Lee J, Woo JT, Kim YS, Murphy MP, Ali L, Ha J, and Kim SS. Mitochondria-targeted antioxidants protect pancreatic beta-cells against oxidative stress and improve insulin secretion in glucotoxicity and glucolipotoxicity. Cell Physiol Biochem 28: 873–886, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Lin N, Chen H, Zhang H, Wan X, and Su Q. Mitochondrial reactive oxygen species (ROS) inhibition ameliorates palmitate-induced INS-1 beta cell death. Endocrine 42: 107–117, 2012 [DOI] [PubMed] [Google Scholar]
- 100.Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, and Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab 4: 245–254, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Lloyd DJ, Wheeler MC, and Gekakis N. A point mutation in Sec61alpha1 leads to diabetes and hepatosteatosis in mice. Diabetes 59: 460–470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, and Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A 108: 6561–6566, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malhi H. and Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol 54: 795–809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malhotra JD. and Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, and Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A 105: 18525–18530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malo A, Kruger B, Seyhun E, Schafer C, Hoffmann RT, Goke B, and Kubisch CH. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 299: G877–G886, 2010 [DOI] [PubMed] [Google Scholar]
- 107.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, and Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18: 3066–3077, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martin-Gronert MS. and Ozanne SE. Metabolic programming of insulin action and secretion. Diabetes Obes Metab 14Suppl 3: 29–39, 2012 [DOI] [PubMed] [Google Scholar]
- 109.Martinez-Outschoorn UE, Lin Z, Trimmer C, Flomenberg N, Wang C, Pavlides S, Pestell RG, Howell A, Sotgia F, and Lisanti MP. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: implications for PET imaging of human tumors. Cell Cycle 10: 2504–2520, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martinez-Outschoorn UE, Pestell RG, Howell A, Tykocinski ML, Nagajyothi F, Machado FS, Tanowitz HB, Sotgia F, and Lisanti MP. Energy transfer in “parasitic” cancer metabolism: mitochondria are the powerhouse and Achilles' heel of tumor cells. Cell Cycle 10: 4208–4216, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, Ron D, O'Neal WK, and Ribeiro CM. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol 6: 639–654, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martinon F, Chen X, Lee AH, and Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 11: 411–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.This reference has been deleted.
- 114.McGuckin MA, Eri RD, Das I, Lourie R, and Florin TH. Intestinal secretory cell ER stress and inflammation. Biochem Soc Trans 39: 1081–1085, 2011 [DOI] [PubMed] [Google Scholar]
- 115.Mercado G, Valdes P, and Hetz C. An ERcentric view of Parkinson's disease. Trends Mol Med 19: 165–175, 2013 [DOI] [PubMed] [Google Scholar]
- 116.Mimori S, Okuma Y, Kaneko M, Kawada K, Hosoi T, Ozawa K, Nomura Y, and Hamana H. Protective effects of 4-phenylbutyrate derivatives on the neuronal cell death and endoplasmic reticulum stress. Biol Pharm Bull 35: 84–90, 2012 [DOI] [PubMed] [Google Scholar]
- 117.Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, Hu Y, Fabre C, Minami J, Ohguchi H, Kiziltepe T, Ikeda H, Kawano Y, French M, Blumenthal M, Tam V, Kertesz NL, Malyankar UM, Hokenson M, Pham T, Zeng Q, Patterson JB, Richardson PG, Munshi NC, and Anderson KC. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood 119: 5772–5781, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mockel A, Obringer C, Hakvoort TB, Seeliger M, Lamers WH, Stoetzel C, Dollfus H, and Marion V. Pharmacological modulation of the retinal unfolded protein response in Bardet-Biedl syndrome reduces apoptosis and preserves light detection ability. J Biol Chem 287: 37483–37494, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.This reference has been deleted.
- 120.Mounir Z, Krishnamoorthy JL, Robertson GP, Scheuner D, Kaufman RJ, Georgescu MM, and Koromilas AE. Tumor suppression by PTEN requires the activation of the PKR-eIF2alpha phosphorylation pathway. Sci Signal 2: ra85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.This reference has been deleted.
- 122.Nakamura T, Cho DH, and Lipton SA. Redox regulation of protein misfolding, mitochondrial dysfunction, synaptic damage, and cell death in neurodegenerative diseases. Exp Neurol 238: 12–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Namba T, Tanaka K, Ito Y, Ishihara T, Hoshino T, Gotoh T, Endo M, Sato K, and Mizushima T. Positive role of CCAAT/enhancer-binding protein homologous protein, a transcription factor involved in the endoplasmic reticulum stress response in the development of colitis. Am J Pathol 174: 1786–1798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Neofytou E, Tzortzaki EG, Chatziantoniou A, and Siafakas NM. DNA damage due to oxidative stress in chronic obstructive pulmonary disease (COPD). Int J Mol Sci 13: 16853–16864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oslowski CM, Hara T, O'Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, and Urano F. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab 16: 265–273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, and Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab 7: 520–532, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr., and Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 9: 35–51, 2009 [DOI] [PubMed] [Google Scholar]
- 128.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, and Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pandol SJ, Gorelick FS, Gerloff A, and Lugea A. Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig Dis 28: 776–782, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, and Koong AC. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood 117: 1311–1314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O'Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, and Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24: 10703–10717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pollard MG, Travers KJ, and Weissman JS. Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol Cell 1: 171–182, 1998 [DOI] [PubMed] [Google Scholar]
- 133.Prabhu A, Sarcar B, Kahali S, Shan Y, and Chinnaiyan P. Targeting the unfolded protein response in glioblastoma cells with the fusion protein EGF-SubA. PLoS One 7: e52265, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, and Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134: 568–576, 2008 [DOI] [PubMed] [Google Scholar]
- 135.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, and Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129: 1337–1349, 2007 [DOI] [PubMed] [Google Scholar]
- 136.Raciti GA, Iadicicco C, Ulianich L, Vind BF, Gaster M, Andreozzi F, Longo M, Teperino R, Ungaro P, Di Jeso B, Formisano P, Beguinot F, and Miele C. Glucosamine-induced endoplasmic reticulum stress affects GLUT4 expression via activating transcription factor 6 in rat and human skeletal muscle cells. Diabetologia 53: 955–965, 2010 [DOI] [PubMed] [Google Scholar]
- 137.Ri M, Tashiro E, Oikawa D, Shinjo S, Tokuda M, Yokouchi Y, Narita T, Masaki A, Ito A, Ding J, Kusumoto S, Ishida T, Komatsu H, Shiotsu Y, Ueda R, Iwawaki T, Imoto M, and Iida S. Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood Cancer J 2: e79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ribeiro CM, and O'Neal WK. Endoplasmic reticulum stress in chronic obstructive lung diseases. Curr Mol Med 12: 872–882, 2012 [DOI] [PubMed] [Google Scholar]
- 139.Ricobaraza A, Cuadrado-Tejedor M, Marco S, Perez-Otano I, and Garcia-Osta A. Phenylbutyrate rescues dendritic spine loss associated with memory deficits in a mouse model of Alzheimer disease. Hippocampus 22: 1040–1050, 2012 [DOI] [PubMed] [Google Scholar]
- 140.Rolo AP, Teodoro JS, and Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 52: 59–69, 2012 [DOI] [PubMed] [Google Scholar]
- 141.Romero-Ramirez L, Cao HB, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, Le QT, and Koong AC. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res 64: 5943–5947, 2004 [DOI] [PubMed] [Google Scholar]
- 142.Ron D. and Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 143.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, and Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4: e374, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, and Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell 15: 829–840, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Santos CX, Tanaka LY, Wosniak J, and Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11: 2409–2427, 2009 [DOI] [PubMed] [Google Scholar]
- 146.Saxena S, Cabuy E, and Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci 12: 627–636, 2009 [DOI] [PubMed] [Google Scholar]
- 147.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, and Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7: 1165–1176, 2001 [DOI] [PubMed] [Google Scholar]
- 148.Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, and Kaufman RJ. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med 11: 757–764, 2005 [DOI] [PubMed] [Google Scholar]
- 149.Schuiki I, Zhang L, and Volchuk A. Endoplasmic reticulum redox state is not perturbed by pharmacological or pathological endoplasmic reticulum stress in live pancreatic beta-cells. PloS One 7: e48626, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sena LA. and Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48: 158–167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Seyhun E, Malo A, Schafer C, Moskaluk CA, Hoffmann RT, Goke B, and Kubisch CH. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, acinar cell damage, and systemic inflammation in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 301: G773–G782, 2011 [DOI] [PubMed] [Google Scholar]
- 152.Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, and Haller D. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology 132: 190–207, 2007 [DOI] [PubMed] [Google Scholar]
- 153.This reference has been deleted.
- 154.Smith RA. and Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci 1201: 96–103, 2010 [DOI] [PubMed] [Google Scholar]
- 155.Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, Dawson VL, Dawson TM, and Ross CA. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet 14: 3801–3811, 2005 [DOI] [PubMed] [Google Scholar]
- 156.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, and Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci 27: 901–908, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song B, Scheuner D, Ron D, Pennathur S, and Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118: 3378–3389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Spiotto MT, Banh A, Papandreou I, Cao H, Galvez MG, Gurtner GC, Denko NC, Le QT, and Koong AC. Imaging the unfolded protein response in primary tumors reveals microenvironments with metabolic variations that predict tumor growth. Cancer Res 70: 78–88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.St-Pierre J, Buckingham JA, Roebuck SJ, and Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 160.Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, and Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175: 901–911, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tabas I. and Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13: 184–190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tam AB, Mercado EL, Hoffmann A, and Niwa M. ER stress activates NF-kappaB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One 7: e45078, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, and Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest 119: 2925–2941, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tribouillard-Tanvier D, Beringue V, Desban N, Gug F, Bach S, Voisset C, Galons H, Laude H, Vilette D, and Blondel M. Antihypertensive drug guanabenz is active in vivo against both yeast and mammalian prions. PLoS One 3: e1981, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tsaytler P, Harding HP, Ron D, and Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 332: 91–94, 2011 [DOI] [PubMed] [Google Scholar]
- 166.Tsuru A, Fujimoto N, Takahashi S, Saito M, Nakamura D, Iwano M, Iwawaki T, Kadokura H, Ron D, and Kohno K. Negative feedback by IRE1beta optimizes mucin production in goblet cells. Proc Natl Acad Sci U S A 110: 2864–2869, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tu BP. and Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 164: 341–346, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tyra HM, Spitz DR, and Rutkowski DT. Inhibition of fatty acid oxidation enhances oxidative protein folding and protects hepatocytes from endoplasmic reticulum stress. Mol Biol Cell 23: 811–819, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, Papa FR, and Oakes SA. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic caspase-2. Science 338: 818–822, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, and Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666, 2000 [DOI] [PubMed] [Google Scholar]
- 171.Ushioda R, Hoseki J, Araki K, Jansen G, Thomas DY, and Nagata K. ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science 321: 569–572, 2008 [DOI] [PubMed] [Google Scholar]
- 172.Vander Heiden MG, Cantley LC, and Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Verfaillie T, Garg AD, and Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett 332: 249–264, 2013 [DOI] [PubMed] [Google Scholar]
- 174.Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, and Agostinis P. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 19: 1880–1891, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.This reference has been deleted.
- 176.Walter P. and Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086, 2011 [DOI] [PubMed] [Google Scholar]
- 177.Wang S, Chen Z, Lam V, Han J, Hassler J, Finck BN, Davidson NO, and Kaufman RJ. IRE1alpha-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab 16: 473–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang S. and Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol 197: 857–867, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wei PC, Hsieh YH, Su MI, Jiang X, Hsu PH, Lo WT, Weng JY, Jeng YM, Wang JM, Chen PL, Chang YC, Lee KF, Tsai MD, Shew JY, and Lee WH. Loss of the oxidative stress sensor NPGPx compromises GRP78 chaperone activity and induces systemic disease. Mol Cell 48: 747–759, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wheeler MC, Rizzi M, Sasik R, Almanza G, Hardiman G, and Zanetti M. KDEL-retained antigen in B lymphocytes induces a proinflammatory response: a possible role for endoplasmic reticulum stress in adaptive T cell immunity. J Immunol 181: 256–264, 2008 [DOI] [PubMed] [Google Scholar]
- 181.Wiley JC, Pettan-Brewer C, and Ladiges WC. Phenylbutyric acid reduces amyloid plaques and rescues cognitive behavior in AD transgenic mice. Aging Cell 10: 418–428, 2011 [DOI] [PubMed] [Google Scholar]
- 182.Wu LL, Russell DL, Norman RJ, and Robker RL. Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Mol Endocrinol 26: 562–573, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu S, Tan M, Hu Y, Wang JL, Scheuner D, and Kaufman RJ. Ultraviolet light activates NFkappaB through translational inhibition of IkappaBalpha synthesis. J Biol Chem 279: 34898–34902, 2004 [DOI] [PubMed] [Google Scholar]
- 184.Xiao C, Giacca A, and Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes 60: 918–924, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, and Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6: 1355–1364, 2000 [DOI] [PubMed] [Google Scholar]
- 186.Yoo SA, You S, Yoon HJ, Kim DH, Kim HS, Lee K, Ahn JH, Hwang D, Lee AS, Kim KJ, Park YJ, Cho CS, and Kim WU. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J Exp Med 209: 871–886, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zadak Z, Hyspler R, Ticha A, Hronek M, Fikrova P, Rathouska J, Hrnciarikova D, and Stetina R. Antioxidants and vitamins in clinical conditions. Physiol Res 58Suppl 1: S13–S17, 2009 [DOI] [PubMed] [Google Scholar]
- 188.Zhang C, Wang G, Zheng Z, Maddipati KR, Zhang X, Dyson G, Williams P, Duncan SA, Kaufman RJ, and Zhang K. Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice. Hepatology 55: 1070–1082, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, and Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124: 587–599, 2006 [DOI] [PubMed] [Google Scholar]
- 190.Zhang K, Wang S, Malhotra J, Hassler JR, Back SH, Wang G, Chang L, Xu W, Miao H, Leonardi R, Chen YE, Jackowski S, and Kaufman RJ. The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J 30: 1357–1375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang W, Feng D, Li Y, Iida K, McGrath B, and Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab 4: 491–497, 2006 [DOI] [PubMed] [Google Scholar]
- 192.Zhu H. and Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med (Maywood) 237: 474–480, 2012 [DOI] [PubMed] [Google Scholar]
- 193.Zhu S, Wang Y, Jin J, Guan C, Li M, Xi C, Ouyang Z, Chen M, Qiu Y, Huang M, and Huang Z. Endoplasmic reticulum stress mediates aristolochic acid I-induced apoptosis in human renal proximal tubular epithelial cells. Toxicol In Vitro 26: 663–671, 2012 [DOI] [PubMed] [Google Scholar]
- 194.Zito E, Chin KT, Blais J, Harding HP, and Ron D. ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J Cell Biol 188: 821–832, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]