Abstract
The MSP domain is a conserved immunoglobulin-like structure that is important for C. elegans reproduction and human motor neuron survival. C. elegans MSPs are the most abundant proteins in sperm, where they function as intracellular cytoskeletal proteins and secreted hormones. Secreted MSPs bind to multiple receptors on oocyte and ovarian sheath cell surfaces to induce oocyte maturation and sheath contraction. MSP binding stimulates oocyte MPK-1 ERK MAP Kinase (MAPK) phosphorylation, but the function and mechanism are not well understood. Here we show that the Shp class protein-tyrosine phosphatase PTP-2 acts in oocytes downstream of sheath/oocyte gap junctions to promote MSP-induced MPK-1 phosphorylation. PTP-2 functions in the oocyte cytoplasm, not at the cell surface to inhibit multiple RasGAPs, resulting in sustained Ras activation. We also provide evidence that MSP promotes production of reactive oxygen species (ROS), which act as second messengers to augment MPK-1 phosphorylation. The Cu/Zn superoxide dismutase SOD-1, an enzyme that catalyzes ROS breakdown in the cytoplasm, inhibits MPK-1 phosphorylation downstream of or in parallel to ptp-2. Our results support the model that MSP triggers PTP-2/Ras activation and ROS production to stimulate MPK-1 activity essential for oocyte maturation. We propose that secreted MSP domains and Cu/Zn superoxide dismutases function antagonistically to control ROS and MAPK signaling.
Keywords: MSP, MAP Kinase, Oocyte Maturation, Shp, RasGAP, Ras, Fertilization, SOD1, Amyotrophic Lateral Sclerosis, VAPB, Reactive oxygen species
Introduction
The major sperm protein (MSP) domain folds into an approximately 120 amino acid immunoglobulin-like seven-stranded beta sandwich that is conserved throughout eukaryotic evolution (Bullock et al., 1996; Han et al., 2009). This domain was initially identified in C. elegans MSPs, the most abundant proteins in sperm (Klass and Hirsh, 1981). MSPs function as secreted hormones (Kosinski et al., 2005; Miller et al., 2001) and intracellular cytoskeletal proteins (Bottino et al., 2002). The ancestral MSP domain-containing proteins are VAPs (VAMP/synaptobrevin-associated proteins), which contain an N-terminal MSP domain, a coiled coil motif, and a transmembrane-spanning region (Han et al., 2009). A P56S substitution in the human VAPB MSP domain is associated with a dominantly inherited form of amyotrophic lateral sclerosis (ALS), an adult onset motor neuron degenerative disorder (Nishimura et al., 2004). We have recently shown that MSPs and VAP MSP domains have an evolutionarily conserved signaling function and that the P56S mutation prevents MSP domain secretion (Tsuda et al., 2008). The most extensively characterized gene associated with ALS is sod1, a cytosolic Cu/Zn superoxide dismutase (Rosen et al., 1993). However, the mechanisms by which sod1 and vapb mutations influence ALS pathogenesis are not clear.
C. elegans oocytes become fertilizable through a process called oocyte maturation, characterized by the transition from meiotic prophase to metaphase I, cytoskeletal rearrangement, and meiotic spindle assembly (Greenstein, 2005). Sperm secrete MSP to induce oocyte maturation and ovarian sheath contraction, which together facilitate fertilization (Kosinski et al., 2005; Miller et al., 2001) (Fig. 1A). MSP signal transduction involves incompletely understood hierarchies in oocytes and sheath cells (Han et al., 2009). MSP binds to multiple receptors on oocyte and sheath surfaces, including the VAB-1 Eph receptor protein-tyrosine kinase (Govindan et al., 2006; Miller et al., 2003). These receptor pathways influence oocyte maturation, as well as other processes such as oocyte growth, microtubule reorganization, and large ribonucleoprotein foci disassembly (Harris et al., 2006; Jud et al., 2008; Nadarajan et al., 2009). Gαs-adenylate cyclase signaling is required in sheath cells to induce oocyte maturation, suggesting that sheath cells are critical MSP sensors (Govindan et al., 2006; Govindan et al., 2009) (Fig. 1A). MSP binding antagonizes gap junctional communication between oocytes and sheath cells (Govindan et al., 2006; Whitten and Miller, 2007). Downstream of sheath/oocyte gap junctions is oocyte MPK-1 ERK MAP Kinase (MAPK) (Church et al., 1995), which becomes phosphorylated throughout the cytosol (Miller et al., 2001). MPK-1 phosphorylation occurs late in the MSP signaling response, just prior to nuclear envelope breakdown (Han et al., 2009).
Figure 1.
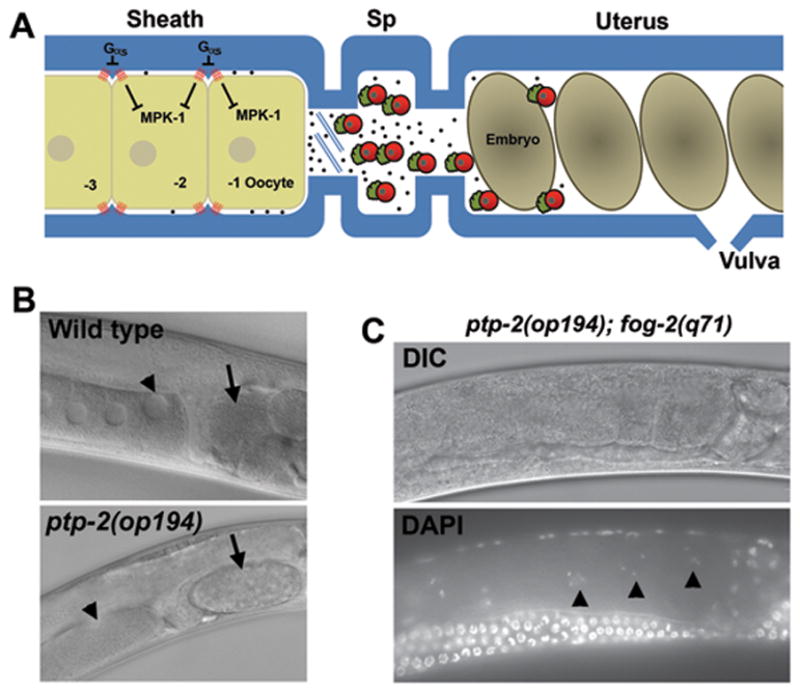
The C. elegans proximal gonad and ptp-2 mutant phenotype. (A) Diagram of the wild-type hermaphrodite proximal gonad. The oocyte closest to the spermatheca (Sp) is called the −1 oocyte. Sperm (red) secrete the protein hormone MSP (black dots) to induce MPK-1 phosphorylation, oocyte maturation, and sheath cell contraction, which together facilitate ovulation. In fog-2(q71) and fog-3(q443) mutant females, all germ cells develop into oocytes, which arrest in diakinesis of meiotic prophase in the absence of male sperm. Oocytes mature sequentially every 20–25 minutes in the presence of sperm/MSP. (B) DIC micrograph of wild-type and ptp-2(op194) mutant gonads. The arrowhead points to the oocyte adjacent to the spermatheca (−1 oocyte). The arrow points to a fertilized embryo. Notice that the single fertilized embryo in the ptp-2 mutant uterus is large and has completed multiple cell divisions, contrasting with the smaller wild-type embryo that has not yet divided. (C) DIC and DAPI micrographs of an unmated ptp-2(op194); fog-2(q71) female. The arrowheads indicate diakinesis stage oocytes.
The MAPK cascade, including KSR-2 Kinase Suppressor of Ras, LET-60 Ras, LIN-45 Raf, MEK-2 MAP kinase kinase, and MPK-1 MAPK has an essential function in promoting progression through meiotic pachytene (Church et al., 1995; Hsu et al., 2002; Ohmachi et al., 2002). MPK-1 is expressed throughout the germ line, where it is primarily regulated by phosphorylation (Lee et al., 2007). Analyses of loss of function and gain of function alleles support roles for the MAPK cascade in regulating oocyte pachytene progression, growth, differentiation, and possibly oocyte maturation. A major hindrance to studying the MAPK cascade in MSP signaling is the earlier roles. For example, loss of function mutations in let-60, lin-45, and mpk-1 prevent progression to diakinesis and oocyte development. Questions such as what is the role of the MAPK cascade during oocyte maturation and how is this cascade regulated have been difficult to address. The PTP-2 SH2 domain-containing tyrosine phosphatase has been identified as an upstream regulator of Ras during oogenesis (Gutch et al., 1998). PTP-2 is homologous to Drosophila Corkscrew (CSW) and mammalian Shp1 and Shp2. These phosphatases comprise a highly conserved subfamily thought to promote sustained MAPK signaling downstream of receptor activation (Feng, 1999; Neel et al., 2003). A null mutation in ptp-2 causes abnormally large oocytes and low brood size (Gutch et al., 1998). We have shown that ptp-2 mutant hermaphrodites have low oocyte maturation rates and MPK-1 phosphorylation levels (Miller et al., 2004). Whether or not these defects are due to impaired MSP signaling or abnormal oogenesis is not clear. Based on these studies, PTP-2 might provide a key entry point for understanding MPK-1 functions and regulation during MSP signaling.
Here, we show that MSP induces PTP-2/Ras activation and reactive oxygen species (ROS) signaling to promote MPK-1 activation. We show that ptp-2 mutant oocytes progress to diakinesis, disassemble the nucleoleus, and initiate chromosome condensation, but fail to phosphorylate MPK-1 and complete maturation in response to MSP. Our results support the model that PTP-2 acts in oocytes downstream of sheath/oocyte gap junctions to antagonize RasGAP activity and promote sustained Ras activation essential for oocyte maturation. In a second mechanism, MSP stimulates ROS production to augment MPK-1 phosphorylation. The Cu/Zn superoxide dismutase SOD-1 inhibits this mechanism downstream of or in parallel to ptp-2. We propose that Cu/Zn superoxide dismutases breakdown cytosolic ROS important for MSP domain signal transduction.
Results
ptp-2 functions in sperm-dependent oocyte maturation, but not sheath contraction
To investigate the mechanism(s) by which MSP regulates MPK-1 activity, we decided to characterize the Shp class phosphatase PTP-2. Previous studies have shown that ptp-2(op194) mutants have oocyte maturation and MPK-1 phosphorylation defects, but it is not clear whether these defects are due to abnormal oogenesis or meiotic progression. The op194 allele deletes the phosphatase domain and is a null allele (Gutch et al., 1998). First, we monitored oocyte development and oocyte maturation by DIC microscopy. The ptp-2(op194) mutant strains used in our studies contain the linked unc-4(e120) allele for genotype scoring (Miller et al., 1992). unc-4 encodes a paired-class homeodomain protein expressed in motor neurons and the e120 allele does not influence oocyte maturation (Table 1, compare lines 1 and 2; P > 0.05). As previously reported (Gutch et al., 1998), proximal gonads of ptp-2(op194) mutants contain developing oocytes, with the most proximal oocyte (−1 position) often significantly larger than the others and wild-type oocytes (Fig. 1A and B). ptp-2 mutant gonads contain fewer oocytes than wild-type gonads, consistent with delayed pachytene progression and oocyte development. Oocyte maturation was directly observed in two cases. Both times it occurred normally and was followed by successful ovulation and fertilization. ptp-2 mutant hermaphrodites typically contain 1–5 fertilized eggs undergoing embryonic development in the uterus, but not unfertilized eggs (Fig. 1B). These observations support the idea that ptp-2 mutant oocytes are competent to undergo oocyte maturation. Proximal gonads of older mutant hermaphrodites sometimes contain degenerating or abnormally developing oocytes.
Table 1.
Oocyte Maturation Rates
| Genotype | Sperm (Y/N) | Maturations per hour | N |
|---|---|---|---|
| 1. Wild type | Y | 2.58±0.33 | 15 |
| 2. unc-4(e120) | Y | 2.53±0.45 | 13 |
| 3. ptp-2(op194) L4+36h | Y | 0.10±0.11 | 14 |
| 4. ptp-2(op194) L4+16h | Y | 0.63±0.24 | 14 |
| 5. fog-2(q71) | N | 0.08±0.09 | 10 |
| 6. fog-2(q71) x fog-2(q71) male | Y | 2.45±0.28 | 9 |
| 7. ptp-2(op194); fog-2(q71) L4+36h x fog-2(q71) male | Y | 0.07±0.09 | 8 |
| 8. ptp-2(op194); fog-2(q71) L4+16h x fog-2(q71) male | Y | 0.36±0.32 | 8 |
| 9. fog-2(q71) + Buffer alone | N | 0.05±0.11 | 10 |
| 10. fog-2(q71) + 200nM MSP | N | 1.13±0.40 | 10 |
| 11. ptp-2(op194); fog-2(q71) + 200nM MSP | N | 0.03±0.08 | 10 |
| 12. let-60(n1046gf) | Y | 2.34±0.94 | 15 |
| 13. ptp-2(op194); let-60(n1046gf) | Y | 2.20±0.97 | 15 |
| 14. let-60(n1046); fog-3(q443) | N | 0.20±0.20 | 12 |
| 15. ptp-2(op194); gap-1(ga133) | Y | 0.29±0.24 | 10 |
| 16. ptp-2(op194); gap-2(tm748) | Y | 0.02±0.04 | 10 |
| 17. ptp-2(op194); gap-1(ga133); gap-2(tm748) | Y | 1.30±0.36 | 10 |
| 18. ptp-2(op194) inx-14 RNAi | Y | 0.11±0.13 | 9 |
| 19. ptp-2(op194) inx-22 RNAi | Y | 0.13±0.19 | 8 |
| 20. fog-2(q71) inx-14 RNAi | N | 0.66±0.35 | 8 |
| 21. fog-2(q71) inx-22 RNAi | N | 0.75±0.44 | 8 |
| 22. ptp-2(op194); unc-43(n498gf) | Y | 0.19±0.10 | 16 |
| 23. sod-1(tm776) | Y | 2.74±0.23 | 15 |
| 24. sod-1(tm776); fog-2(q71) | N | 0.13±0.14 | 10 |
See text for details. Average value ± standard deviation is shown. All ptp-2(op194) strains contain the unc-4(e120) allele. The oocyte maturation rate of unmated fog-3(q443) females is not significantly different than unmated fog-2(q71) females (Corrigan et al., 2005). The fog-2(q71) allele does not affect male reproductive capacity or sperm function.
The oocyte maturation rate of adult ptp-2(op194) mutants is low, much less than the rate of wild-type and unc-4(e120) controls (Table 1, compare line 3 to lines 1 and 2; P < 0.001). The maturation rate of young adult mutants (16–20 hrs post L4) is higher than adult mutants (36–40 hrs post L4), due either to maternally derived ptp-2 or a ptp-2-independent mechanism (Table 1, compare lines 3 and 4). The low oocyte maturation rate of ptp-2 mutants is not rescued by mating to wild-type males, even though abundant male-derived sperm accumulate in the spermatheca (data not shown). We constructed ptp-2(op194); fog-2(q71) double mutant females and measured oocyte maturation rates in the presence and absence of sperm. Unmated ptp-2(op194); fog-2(q71) females accumulate diakinesis-stage oocytes in the proximal gonad, as judged using the DNA stain 4′, 6-diamidino-2-phenylindole (DAPI) (Fig. 1C). Mating the ptp-2 mutant females to fog-2(q71) males, which have fully functional sperm (Schedl and Kimble, 1988) (Table 1, lines 5 and 6), does not promote an oocyte maturation rate increase in adults, but does promote a small increase in young females (Table 1, compare lines 5–8). We conclude that the ptp-2 mutant oocyte maturation defect is not due to defective sperm.
MSP stimulates an increase in the basal sheath contraction rate. ptp-2 is not required for this response, as ptp-2(op194) hermaphrodites and mated ptp-2(op194); fog-2(q71) females have higher basal contraction rates than unmated female controls (Table 2, compare lines 1–4; P < 0.001). In addition to sheath contraction, MSP promotes sustained actomyosin-dependent cytoplasmic streaming that drives oocyte growth (Govindan et al., 2009; Nadarajan et al., 2009). The oocytes of unmated ptp-2(op194); fog-2(q71) females are smaller than those of ptp-2(op194) hermaphrodites (Fig. 1B and C) and resemble oocytes from unmated female controls. These results demonstrate that ptp-2 is not required for basal sheath contraction and oocyte growth. Collectively, the data support the hypothesis that ptp-2 mutants receive MSP signals, but fail to respond with an oocyte maturation rate increase.
Table 2.
Ovarian Sheath Cell Contraction Rates
| Genotype | Sperm (Y/N) | Contractions per minute | N |
|---|---|---|---|
| 1. Wild type | Y | 9.63±1.17 | 10 |
| 2. ptp-2(op194) | Y | 9.83±1.91 | 12 |
| 3. ptp-2(op194); fog-2(q71) | N | 1.50±1.00 | 12 |
| 4. ptp-2(op194); fog-2(q71) x fog-2(q71) male | Y | 8.79±1.61 | 11 |
| 5. fog-2(q71) + Buffer alone | N | 2.03±0.81 | 11 |
| 6. fog-2(q71) + 200nM MSP | N | 7.30±2.08 | 10 |
| 7. ptp-2(op194); fog-2(q71) + 200nM MSP | N | 7.94±2.13 | 14 |
See text for details. Average value ± standard deviation is shown. All ptp-2(op194) strains contain the unc-4(e120) allele, which does not influence sheath contraction. fog-2(q71) females are unmated unless stated otherwise.
ptp-2 mutant oocytes fail to fully activate MPK-1 and exit meiotic prophase rapidly
We used molecular markers to evaluate the developmental status of ptp-2 mutant oocytes. The monoclonal antibody MAPK-YT detects the diphosphorylated, activated form of MPK-1 (dpMPK-1) (Lee et al., 2007; Miller et al., 2001; Page et al., 2001). In wild-type gonads, dpMPK-1 is often seen throughout the cytoplasm and nucleus of the −1 to −3 oocytes, with highest levels in the −1 oocyte. In contrast, we rarely detect dpMPK-1 in oocytes of ptp-2 mutant hermaphrodites or mated ptp-2(op194); fog-2(q71) females (Fig. 2 and data not shown). The low dpMPK-1 levels in ptp-2 mutant hermaphrodite gonads resemble unmated female gonads (Fig. 2). Therefore, ptp-2 is required for elevated MPK-1 activity in the most proximal oocytes.
Figure 2.
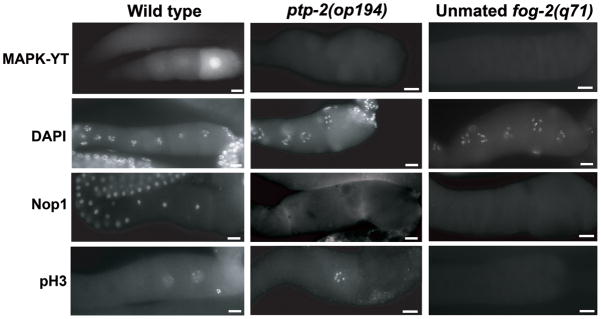
Molecular characterization of wild-type hermaphrodite, ptp-2 mutant hermaphrodite, and unmated female proximal gonads. The proximal gonad is in the same orientation as Fig. 1A, with the spermatheca to the right. The MAPK-YT, anti-Nop1, and anti-pH3 antibodies are described in the text. Bars, 20μm.
DAPI was used to evaluate oocyte meiotic progression. DAPI staining of ptp-2(op194) gonads shows the oocytes progress to diakinesis of meiotic prophase, similar to wild type and unmated females (Fig. 2). Another marker for developmental status is the nucleolus, which is present in developing oocytes transitioning through meiotic prophase (McCarter et al., 1999). The nucleolus disappears about 70 minutes before nuclear envelope breakdown. Nucleolar disappearance is not sperm-dependent, as it occurs in unmated fog-2(q71) females. DIC microscopy and an antibody against the nucleolar marker Nop1 (Aris and Blobel, 1989; Burrows et al., 2006; Henriquez et al., 1990) indicate that the nucleolus disappears in ptp-2 mutant oocytes (Fig. 2). Nop1 distribution in ptp-2(op194) mutant gonads resembles that of unmated females. These data support the hypothesis that ptp-2 mutant oocytes are competent to undergo oocyte maturation.
M-phase entry can be monitored in maturing oocytes using an antibody specific for phosphorylated histone H3 at serine 10 (pH3), a marker for chromosome condensation (Hsu et al., 2000). In wild-type gonads, anti-pH3 antibodies stain chromosomes of the −1 to −3 oocytes in a sperm-dependent manner. We often detected ptp-2 mutant oocytes whose chromosomes stained with anti-pH3 antibodies (Fig. 2), suggesting that ptp-2(op194) mutant oocytes initiate M-phase entry, but are slow to undergo nuclear envelope breakdown and progress to metaphase.
Our data indicate that ptp-2 is required for the sperm-dependent increase in the oocyte maturation rate. Purified recombinant MSP directly microinjected into the reproductive tract of unmated females stimulates oocyte maturation and basal sheath contraction (Miller et al., 2001; Tsuda et al., 2008). MSP diffuses into the proximal gonad, where it binds to cell surface receptors (Miller et al., 2003). To test whether ptp-2 is necessary for MSP-induced oocyte maturation, we microinjected 200nM recombinant MSP into unmated ptp-2(op194) and control females. MSP does not stimulate the oocyte maturation rate of unmated ptp-2(op194); fog-2(q71) females (Table 1, compare lines 9–11; P > 0.05). In contrast, MSP does stimulate an increase in the basal sheath contraction rate of unmated ptp-2(op194); fog-2(q71) females and control fog-2(q71) females (Table 2, compare lines 6 and 7 to line 5; P < 0.001). MSP-induced sheath contraction is observed in the same ptp-2 mutant gonads that fail to undergo oocyte maturation. We considered the possibility that ptp-2 is required for surface expression of MSP receptors. To examine MSP receptor expression, we incubated dissected gonads with 200nM recombinant 6His-MSP conjugated to fluorescein (MSP-FITC). MSP-FITC binding is specific and requires cell surface receptor expression (Miller et al., 2003; Tsuda et al., 2008). In wild-type and ptp-2(op194) gonads, MSP-FITC binds extensively to the oocyte and sheath interface (Fig. S1). Taken together, these results support the model that ptp-2 acts downstream of MSP to promote sustained MPK-1 activity essential for high oocyte maturation rates.
ptp-2 acts upstream of Ras and multiple RasGAPs
The brood size defect of ptp-2(op194) mutants is suppressed by the let-60(n1046) and let-60(n1700) gain of function alleles (Gutch et al., 1998). To examine oocyte maturation, we generated ptp-2(op194); let-60(n1046) double mutants. Observation by DIC microscopy indicates that ptp-2(op194) oocyte maturation and oocyte growth defects are suppressed by the n1046 gain of function allele. The ptp-2(op194); let-60(n1046gf) oocyte maturation rate is much higher than the ptp-2(op194) mutant rate (Table 1, compare line 13 to 3; P < 0.001) and is similar to the let-60(n1046gf) and wild-type rates (Table 1, lines 1, 12, and 13). Next, we examined MPK-1 phosphorylation using the MAPK-YT antibody. ptp-2(op194); let-60(n1046gf) mutants have more dpMPK-1 than ptp-2(op194) mutants, but less than wild type and let-60(n1046gf) mutants (Fig. 3A–D). These data support the hypothesis that ptp-2 functions upstream of or in parallel to let-60 Ras to stimulate MPK-1 activation and oocyte maturation.
Figure 3.
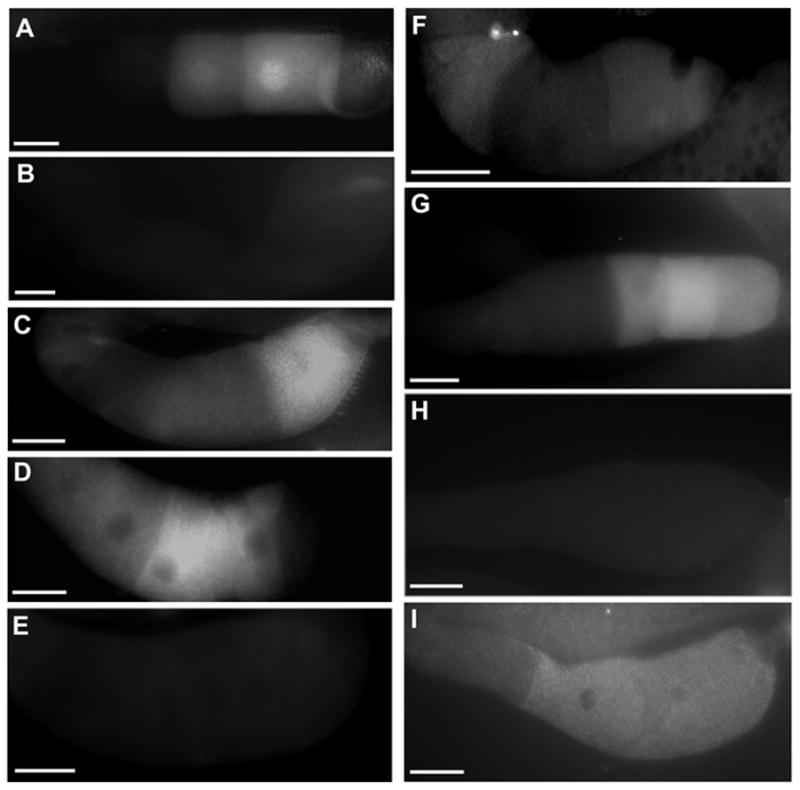
MPK-1 MAPK activity in wild-type and mutant gonads. Dissected proximal gonads were incubated with MAPK-YT antibodies, which recognize the diphosphorylated form of MPK-1. Panels are (A) wild type, (B) ptp-2(op194), (C) ptp-2-(op194); let-60(n1046gf), (D) let-60(n1046gf), (E) unmated let-60(n1046); fog-2(q71) female, (F) ptp-2(op194);gap-1(ga133);gap-2(tm748), (G) vab-1 RNAi, (H) vab-1 RNAi ptp-2(op194), and (I) ptp-2(op194); Pie-1p::gfp::ptp-2. Gonad orientation is with spermatheca to the right. Bars, 20μm.
To test whether Ras activation is sufficient to promote oocyte maturation in the absence of MSP, we generated let-60(n1046gf); fog-3(q443) females. The oocyte maturation rate of unmated let-60(n1046gf); fog-3(q443) females is similar to the rate of unmated control females (Table 1, compare lines 14 and 5). Furthermore, we did not detect elevated dpMPK-1 levels in the most proximal oocytes (Fig. 3E). Mating unmated let-60(n1046gf); fog-3(443) females to wild-type males stimulates the oocyte maturation rate and induces MPK-1 phosphorylation (data not shown). Similar results were observed in mated and unmated ptp-2(op194); let-60(n1046gf); fog-3(443) females. Therefore, increased Ras activity is not sufficient to induce MPK-1 phosphorylation and oocyte maturation in the absence of MSP.
In Drosophila embryos, CSW dephosphorylates a RasGAP binding site on the RTK Torso, preventing RasGAP recruitment (Cleghon et al., 1998). Of the three RasGAPs identified in C. elegans (Hajnal et al., 1997; Stetak et al., 2008), gap-3 is implicated in oocyte pachytene progression. To avoid potential indirect interactions due to pachytene defects, we focused on gap-1 and gap-2 (Stetak et al., 2008). Double and triple mutant strains were constructed for oocyte maturation and MPK-1 activation analyses. The oocyte maturation rate of gap-1(ga133); gap-2(tm748); ptp-2(op194) mutants is significantly higher than the rates of ptp-2(op194) mutants, gap-2(tm748); ptp-2(op194) mutants, and gap-1(ga133); ptp-2(op194) mutants (Table 1, compare line 17 to lines 3, 15, and 16; P < 0.001). The triple mutant strain is viable, contrasting with ptp-2(op194) mutants. Therefore, loss of gap-1 and gap-2 together can partially suppress the oocyte maturation and viability defects of ptp-2 mutants. The gap-1(ga133); ptp-2(op194) maturation rate is slightly higher than the ptp-2(op194) rate (Table 1, compare lines 15 and 3), indicating that loss of gap-1 alone causes a mild suppressive effect. Oocyte dpMPK-1 levels in gap-1(ga133); gap-2(tm748); ptp-2(op194) hermaphrodites are higher than ptp-2(op194) mutants, but less than the wild type (Fig. 3F). These results support the hypothesis that ptp-2 functions upstream of multiple RasGAPs to promote Ras activation and oocyte maturation.
ptp-2 acts downstream of sheath/oocyte gap junctions
The MSP receptor vab-1 negatively regulates MPK-1 phosphorylation in hermaphrodites. vab-1 null mutant and RNAi hermaphrodite gonads have more oocytes containing phosphorylated MPK-1 than wild-type hermaphrodite gonads. To test the genetic relationship between vab-1 and ptp-2, we examined MPK-1 phosphorylation in single and double knockouts. Contrasting with MPK-1 levels in vab-1 RNAi gonads (Fig. 3G), MPK-1 phosphorylation levels in vab-1 RNAi ptp-2(op194) gonads (Fig. 3H) resemble those in ptp-2(op194) gonads (Fig. 3B). These data support the idea that ptp-2 acts downstream of vab-1. The unc-43 CaMKII homolog (Reiner et al., 1999) is an MSP-responsive effector that functions in oocytes and sheath cells (Corrigan et al., 2005). An unc-43(n498) gain of function mutation causes elevated oocyte maturation and MPK-1 activation in the absence of MSP. To examine the genetic interaction between unc-43 and ptp-2, we generated ptp-2(op194); unc-43(n498gf) double mutants. The oocyte maturation rate of the double mutants is similar to ptp-2(op194) single mutants (Table 1, compare line 22 to line 3). unc-43(n498gf) does suppress the ptp-2(op194) oocyte size defect, suggesting that the genetic hierarchies regulating oocyte growth and maturation are different. These results are consistent with the model that ptp-2 acts downstream of vab-1 and unc-43 to regulate MPK-1 activity.
The Gαs heterotrimeric G protein subunit GSA-1 is required in sheath cells to promote MSP-induced oocyte maturation (Govindan et al., 2009). The oocyte maturation defect caused by sheath gsa-1 loss is suppressed by loss of the innexins inx-14 or inx-22 (Govindan et al., 2006; Govindan et al., 2009). inx-14 and inx-22 are thought to form sheath/oocyte gap junctions that negatively regulate oocyte maturation in the absence of MSP (Govindan et al., 2006; Whitten and Miller, 2007). To examine the genetic relationship between ptp-2 and gap junctions, we reduced inx-14 and inx-22 function by RNAi in ptp-2(op194) mutants. inx-14 or inx-22 RNAi does not suppress the ptp-2 mutant oocyte maturation and fertility defects (Table 1, compare lines 18 and 19 to line 3). In control females, inx-14 and inx-22 RNAi caused increased oocyte maturation rates (Table 1, compare lines 20 and 21 to line 5). Next, we tested the genetic relationship between ptp-2 and gap junctions in the absence of MSP. Unmated inx-22(tm1661); fog-2(q71) females have high oocyte maturation rates (Whitten and Miller, 2007). To test whether this defect is dependent on ptp-2, we generated inx-22(tm1661); ptp-2(op194); fog-2(q71) triple mutants. In contrast to unmated inx-22(tm1661); fog-2(q71) females, the oocyte maturation rate of unmated triple mutant females is low and few oocytes accumulate in the uterus (Fig. 4A–C). Taken together, these results support the hypothesis that ptp-2 functions downstream of sheath/oocyte gap junctions.
Figure 4.

Genetic relationship between ptp-2 and the innexin inx-22. (A, B) DIC micrographs of (A) unmated inx-22(tm1661); fog-2(q71) females and (B) unmated ptp-2(op194); inx-22(tm1661); fog-2(q71) females. Arrowheads indicate unfertilized oocytes in the uterus that have undergone oocyte maturation and ovulation. Gonad orientation is with spermatheca to the right. (C) Quantification of oocyte maturation rates. N is shown above error bars, which represent standard deviation.
Germline PTP-2 expression partially rescues ptp-2 null oocyte maturation defects
The ptp-2 gene lies within a large operon containing eight genes with internal promoters. A previous study showed that the ~600 base pairs upstream of the ptp-2 translational start site was sufficient to drive GFP expression in a subset of head neurons, the intestine, spermatheca, and body wall muscle (Vazquez-Manrique et al., 2007). To test whether additional upstream sequences could influence expression, we generated transgenic lines containing genomic DNA ~5 kilobase pairs upstream of the ptp-2 translational start site fused to the GFP coding sequence. These lines express GFP in head neurons, the intestine, and body wall muscle, similar to the smaller genomic construct. We also observed GFP in embryos, the developing vulva, and the adult gonadal sheath cells (Fig. 5).
Figure 5.
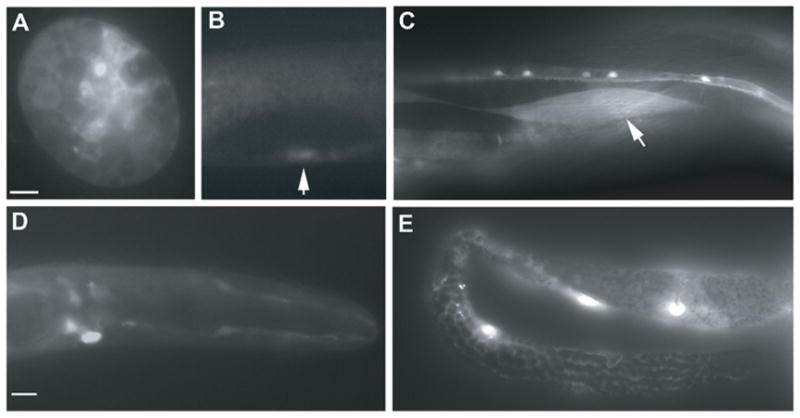
ptp-2 expression. (A–E) Genomic DNA (~5kb) upstream of the ptp-2 translational start site drives GFP expression in embryonic cells (A), the developing vulva (B, arrow), adult body wall muscle (C, arrow), adult head neurons (D), and adult gonadal sheath cells (E). Bars, 10 μm.
ptp-2 mRNAs are abundantly expressed in the germ line (Reinke et al., 2004; Reinke et al., 2000). Our genetic data suggest that PTP-2 functions in oocytes downstream of sheath/oocyte gap junctions and upstream of let-60 Ras. The microinjection method used to generate Ptp-2p::gfp transgenic lines is not reliable for evaluating oocyte expression due to frequent silencing of the high copy arrays in the germ line (Praitis et al., 2001). To test whether PTP-2 expression in oocytes is sufficient to rescue the oocyte maturation defects, we generated transgenic lines by microparticle bombardment that express GFP::PTP-2 under control of the pie-1 germline promoter (Tenenhaus et al., 1998; Tenenhaus et al., 2001). Microparticle bombardment generates low copy transgenic lines that sometimes escape the germ line silencing machinery (Praitis et al., 2001). Two independent lines show faint GFP expression throughout the cytoplasm of germ cells and oocytes, but not in the nuclei (Fig. 6A). Numerous other lines did not contain detectable GFP expression. To examine the subcellular localization in better detail, we dissected gonads out of the animal. GFP::PTP-2 was seen in the cytoplasm, sometimes associated with vesicle-like structures, but not at the cell surface (Fig. 6B). This pattern contrasts with the localization of MSP receptors, which are abundant at the oocyte cell surface (Fig. S1).
Figure 6.

Germline GFP::PTP-2 expression rescues ptp-2(op194) phenotypes. (A, B) GFP::PTP-2 expression, driven with the pie-1 promoter, is faint, but detectable in the germ line of adult hermaphrodites (A). GFP is observed throughout the germ cell and oocyte cytoplasm, but not in the nucleus or at the cell surface. In dissected gonads, GFP appears to localize to vesicles in the oocytes (B). Gonad orientation is with spermatheca to the right. (C) The transgene partially rescues the oocyte maturation rate, embryonic lethality, and larval lethality defects of ptp-2(op194) mutants. N is shown above error bars, which represent standard deviation. Bars, 10 μm.
To determine whether germline GFP::PTP-2 could rescue the ptp-2 mutant defects, we crossed the transgene into the ptp-2(op194) background. The genotype was confirmed by PCR. Germline expression of GFP::PTP-2 is sufficient to partially rescue the ptp-2 null mutant oocyte maturation defect, embryonic lethality, and larval lethality (Fig. 6C). In addition, MPK-1 phosphorylation is detected in the most proximal oocytes (Fig. 3G). These data support to the hypothesis that PTP-2 functions in oocytes to regulate oocyte maturation and MPK-1 activation.
sod-1 negatively regulates MPK-1 phosphorylation
To identify additional mechanisms important for MPK-1 activity, we focused on ROS, which have been implicated in regulating MAPK in cultured cells (Abe et al., 2000; Aikawa et al., 1997; Secondo et al., 2008). The Cu/Zn superoxide dismutase SOD-1 converts ROS to oxygen or hydrogen peroxide. Dominant mutations in sod1 and vapb are associated with ALS, but the genetic relationship between these genes, if any, is uncertain (Nishimura et al., 2004; Rosen et al., 1993). To test whether C. elegans SOD-1 regulates oocyte MPK-1 phosphorylation, we examined sod-1(tm776) null mutants (Yanase et al., 2009). Approximately twice as many oocytes contain dpMPK-1 in sod-1 null gonads compared to wild-type gonads (Fig. 7A and D). Similar results are observed in sod-1 RNAi gonads (Fig. 7D). C. elegans contains a second Cu/Zn superoxide dismutase called sod-5 that can partially compensate for sod-1 loss (Yanase et al., 2009). Silencing of both sod-1 and sod-5 by RNAi results in higher dpMPK-1 levels than sod-1 RNAi alone and wild-type controls (Fig. 7D). The oocyte maturation rate of sod-1(tm776) hermaphrodites is similar to the wild-type rate, indicating that elevated MPK-1 activity does not cause abnormally high maturation rates (Table 1, compare line 23 to line 1). These results indicate that SOD-1 negatively regulates oocyte MPK-1 phosphorylation. To investigate the genetic relationship between sod-1 and mpk-1, we examined sod-1 RNAi ptp-2(op194) gonads. In contrast to ptp-2(op194) gonads (Fig. 4B), MPK-1 phosphorylation is detectable in sod-1 RNAi ptp-2(op194) and sod-1 sod-5 double RNAi ptp-2(op194) gonads (Fig. 7B and C). Therefore, sod-1 inhibits MPK-1 phosphorylation downstream of ptp-2 or in a parallel pathway. Taken together, the data support the hypothesis that ROS are downstream secondary messengers involved in MSP signaling.
Figure 7.
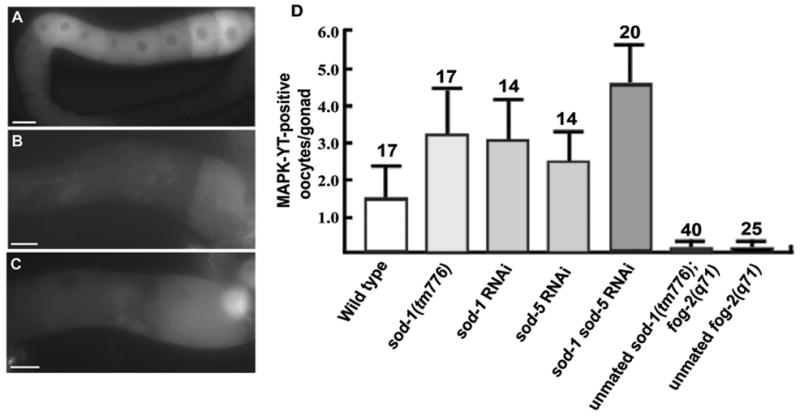
Cu/Zn superoxide dismutase activity antagonizes oocyte MPK-1 phosphorylation. (A) Multiple oocytes in sod-1(tm776) hermaphrodite gonads contain phosphorylated MPK-1, detected using the MAPK-YT antibody. (B–C) In ptp-2(op194) mutants, sod-1 RNAi (B) and sod-1 sod-5 double RNAi (C) partially rescue the MPK-1 phosphorylation defect. Phosphorylated MPK-1 is not observed in ptp-2(op194) gonads (see Fig. 3B and H). Gonad orientation is with spermatheca to the right. (D) Quantification of oocyte MPK-1 phosphorylation levels in proximal gonads. N is shown above error bars, which represent standard deviation. Bars, 20μm.
Sperm/MSP stimulate ROS production
To test whether sperm influence ROS levels, we incubated unmated females and wild-type hermaphrodites with increasing concentrations of paraquat. Paraquat generates intracellular ROS that cause concentration-dependent toxicity depending on endogenous ROS and superoxide dismutase activity. Unmated females are more resistant than wild-type hermaphrodites to paraquat-induced lethality (Fig. 8A). Therefore, sperm presence causes increased ROS, decreased superoxide dismutase activity, or a combination of both. If MSP acts solely to inhibit SOD-1 activity, then sod-1 loss in unmated females should cause increased MPK-1 phosphorylation or oocyte maturation. Oocyte MPK-1 phosphorylation is not detected in unmated sod-1 mutant females (Fig. 7D) and the oocyte maturation rate of unmated sod-1(tm776); fog-2(q71) females is not significantly different than unmated control females (Table 1, compare line 24 to line 5). These results suggest that MSP induces ROS production. To examine ROS levels, we used the ROS indicator 2,7-dichlorodihydrofluorescein-diacetate (H2-DCF-DA), a nonfluorescent dye that becomes trapped in cells and fluoresces following oxidation by ROS. In dissected gonads, the most proximal oocytes of wild-type hermaphrodites have increased fluorescence compared to unmated fog-2(q71) females (Fig. 8B–E). The fluorescence pattern is compartmentalized within individual oocytes, resembling the MPK-1 phosphorylation pattern. Similar results are observed in live animals incubated with H2-DCF-DA for several hours, except the staining frequency is much lower, possibly due to poor dye penetration into the gonad (Fig. 8F and G). sod-1(tm776) mutant gonads incubated with H2-DCF-DA often contained more fluorescent oocytes than wild-type gonads (data not shown). These results support the model that MSP induces ROS production to augment MPK-1 phosphorylation.
Figure 8.
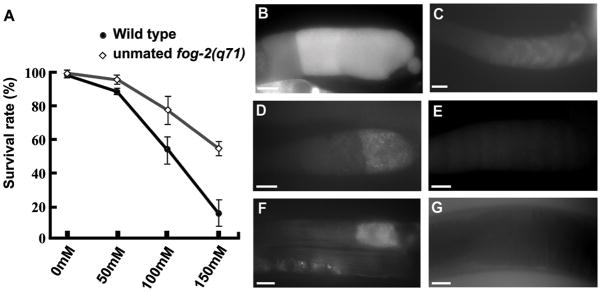
Sperm promote increased reactive oxygen species levels. (A) Unmated females are more resistant than wild-type hermaphrodites to paraquat-induced lethality. Paraquat generates intracellular superoxide that becomes toxic depending on endogenous ROS levels and superoxide dismutase activity. (B–G) The ROS indicator H2-DCF-DA shows enhanced fluorescence in wild-type dissected gonads (B, D) and live animals (F) compared to unmated fog-2(q71) female dissected gonads (C, E) and live animals (G). Dissected gonads in panels D and E were lightly fixed prior to mounting, causing a reduction in fluorescent intensity (see Methods). Gonad orientation is with spermatheca to the right. Bars, 20μm.
ROS such as superoxide are a byproduct of mitochondrial respiration, raising the possibility that MSP stimulates respiration to increase ROS levels. We tested this possibility using Mitotracker CMXRos, a red-fluorescent dye that stains mitochondria undergoing respiration. CMXRos accumulation is dependent upon membrane potential generated from pumping hydrogen ions across the inner membrane during electron transport (Pendergrass et al., 2004; Sherwood et al., 2005). In dissected gonads, mitochondria in the most proximal oocytes and sperm often stain more intensely with Mitotracker CMXRos than those in developing oocytes or germ cells (Fig. 9A and not shown). In unmated fog-2(q71) females, oocyte mitochondrial fluorescence is less intense and uniform throughout the proximal gonad (Fig. 9B). These data are consistent with the possibility that MSP promotes increased respiration in the most proximal oocytes. Next, we measured oxygen consumption in wild-type hermaphrodites containing sperm and unmated fog-2(q71) females. Wild-type hermaphrodites consume more oxygen than unmated females when the data are normalized to worm number (P = 0.02; Fig. 9C). However, there is no significant difference when the data are normalized to protein content (Fig. 9D), indicating that females contain less protein. Unmated females have more oocytes than wild-type hermaphrodites, but no sperm or embryos. The increased oxygen consumption rates in the wild type could, therefore, be due to oocytes, sperm, embryos, or a combination. A previous study has shown that presence of both sperm and oocytes, but not embryos causes anoxia sensitivity, a result consistent with our oxygen consumption data normalized to worm number (Mendenhall et al., 2009). Taken together, most data are consistent with MSP promoting mitochondrial respiration to increase cytosolic ROS levels and MPK-1 phosphorylation. However, the mitochondrial respiration component needs further study to be definitive.
Figure 9.
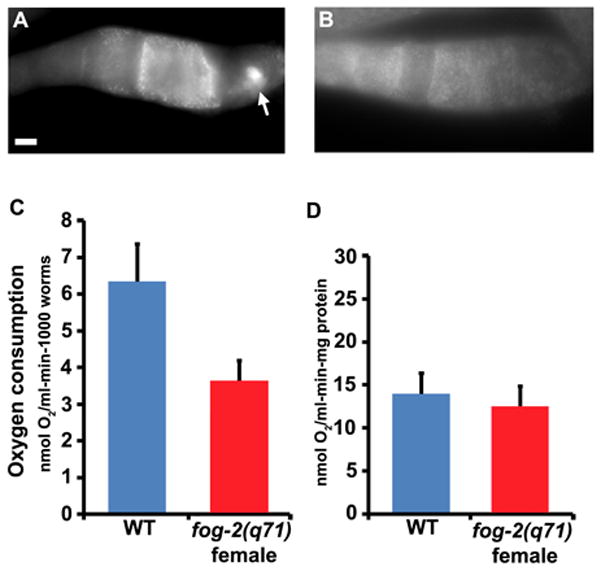
Oocyte mitochondrial function in the presence and absence of sperm. (A, B) Dissected proximal wild-type (A) and unmated fog-2(q71) female (B) gonads stained with Mitotracker CMXRos. Gonad orientation is with spermatheca to the right. The bright fluorescence in the wild-type spermatheca (panel A) is sperm mitochondria (arrow). Bar, 20μm. (C, D) Oxygen consumption rates in wild-type hermaphrodites (WT) and unmated fog-2(q71) females normalized to worm number (C) and protein content (D). The P values calculated using a Student’s T-test are 0.02 for panel C and 0.5 for panel D.
Discussion
MSP regulates a complex network of signaling cascades in sheath cells and oocytes to induce sheath contraction and oocyte maturation. In this study, we show that MSP antagonizes sheath/oocyte gap junctional communication to activate an oocyte cascade comprised of PTP-2 Shp, GAP-1 and GAP-2 RasGAPs, LET-60 Ras, and MPK-1 MAPK. We propose that PTP-2 associates with the cytoplasmic domain of an unknown receptor in an oocyte vesicular compartment to inhibit RasGAP activity, resulting in sustained MPK-1 activation essential for high oocyte maturation rates. In a second mechanism, MSP stimulates production of reactive oxygen species, which act as second messengers to augment MPK-1 phosphorylation. The Cu/Zn superoxide dismutases SOD-1 acts downstream of ptp-2 or in a parallel pathway to attenuate MPK-1 phosphorylation. sEvidence for this model is discussed in the following paragraphs.
We show that PTP-2 is required to promote high oocyte maturation rates in response to MSP. ptp-2 null mutant gonads initiate sheath contraction, oocyte histone phosphorylation, and oocyte growth pathways. However, MPK-1 fails to become fully activated in the proximal oocytes. Genetic data support a model similar to one proposed for Drosophila CSW. During embryonic development, the receptor tyrosine kinase Torso promotes sustained activation of the MAPK cascade to specify terminal structures (Cleghon et al., 1998). CSW physically associates with torso by binding to pY630. CSW recruitment promotes the dephosphorylation of pY918, a RasGAP binding site. The loss of local RasGAP activity promotes sustained Ras activation. During C. elegans oocyte maturation, MSP stimulates the diphosphorylation and activation of oocyte MPK-1. MPK-1 phosphorylation levels are low in ptp-2 null mutant oocytes, despite the presence of MSP and cell surface MSP receptors. A gain-of-function mutation in let-60 Ras or loss of multiple RasGAPs suppresses the ptp-2 mutant oocyte maturation rate defect, as well as the embryonic and larval lethality. Increased MPK-1 phosphorylation is detected in these suppressed mutants, although the levels are reduced compared to the wild type. The results suggest that a small increase in Ras activity is sufficient to restore oocyte maturation rates and viability in ptp-2 mutants. Our data are consistent with those of Lee et al., who showed that a genetic reduction in mpk-1 activity delays oocyte maturation (Lee et al., 2007). ptp-2 mutants do not have the pachytene arrest phenotype observed in let-60 and mpk-1 null mutants (Church et al., 1995; Lee et al., 2007), although meiotic progression may be delayed. Maternal ptp-2 could be sufficient to promote pachytene exit or alternatively, ptp-2 may not be required at this stage. ptp-2 null mutant oocytes are large and resemble those seen in hypomorphic mpk-1 mutant strains. Therefore, it seems likely that MPK-1 activity is reduced, but not eliminated in ptp-2 mutants.
MPK-1 activation is a late event in the MSP signaling response, occurring approximately 30–45 minutes after the microinjection of extracellular MSP (Han et al., 2009; Miller et al., 2001). This delay suggests that the PTP-2/LET-60/MPK-1 cascade is downstream in the oocyte maturation signaling hierarchy. Genetic and transgenic studies provide additional support. Phosphorylated MPK-1 is uniformly distributed throughout the oocyte cytoplasm and similar to the PTP-2 subcellular localization, except that PTP-2 appears to localize to vesicles. Driving GFP::PTP-2 expression specifically in the germ line partially rescues the oocyte maturation and MPK-1 activation defects, as well as embryonic lethality of ptp-2 null mutants. The incomplete rescue could be due to insufficient germline expression levels, the N-terminal GFP interfering with PTP-2 function, or lack of sheath PTP-2 expression. In germ cells and oocytes, GFP::PTP-2 localizes to the cytoplasm, but not at the cell surface or in the nucleus. Genetic analyses are consistent with ptp-2 acting downstream of the MSP receptor vab-1, the CaMKII unc-43, and components of sheath/oocyte gap junctions. ptp-2’s genetic relationship with sheath/oocyte gap junctions contrasts with that of gsa-1, which is required in the sheath cells (Govindan et al., 2006; Govindan et al., 2009). The data support the model that sheath Gαs-adenylate cyclase signaling functions upstream of sheath/oocyte gap junctions to regulate the PTP-2/LET-60/MPK-1 cascade. It is not well understood how MSP couples to GSA-1 and how gap junctions couple to PTP-2.
Ras gain of function females lacking sperm have low oocyte maturation rates and MPK-1 phosphorylation levels, suggesting that MSP regulates additional mechanisms important for MPK-1 activation. Paraquat and ROS indicator studies are consistent with MSP increasing oocyte ROS levels. Genetic studies using the Cu/Zn superoxide dismutases, which function to breakdown ROS in the cytoplasm, add further support. SOD-1 and SOD-5 act together to negatively regulate MSP-induced MPK-1 phosphoryation downstream of ptp-2 or in a parallel pathway. Cu/Zn superoxide dismutase activity is not required in the absence of sperm. ROS are rapidly diffusing second messengers known to positively influence the MAPK cascade in cultured mammalian cells (Abe et al., 2000; Aikawa et al., 1997; Secondo et al., 2008). Rapid diffusion could partially explain how MPK-1 becomes phosphorylated throughout the oocyte cytosol in response to MSP. The mechanism by which MSP induces ROS may involve increased oocyte mitochondrial respiration, generating energy for oocyte maturation and early embryogenesis. ROS are formed during mitochondrial electron transport when oxygen is incompletely reduced. MitoTracker CMXRos and oxygen consumption assays normalized to worm number are consistent with increased oxidative respiration. Additional support comes from previous studies showing that presence of both sperm and oocytes, but not embryos makes hermaphrodites more sensitive to anoxia (Mendenhall et al., 2009). Cells undergoing high rates of mitochondrial respiration are likely to be more sensitive to anoxia. However, oxygen consumption assays normalized to protein content do not support a role for MSP in mitochondrial respiration. It is possible that sperm presence promotes increased respiration and protein synthesis. Alternatively, increased oxygen consumption rates in hermaphrodites could be due to embryos. Further studies are necessary to understand the relationship between MSP domains and mitochondrial function.
Shp class phosphatases are characterized by the presence of two N-terminal SH2 domains that often bind to phosphotyrosines on receptor intracellular regions (Neel et al., 2003). Drosophila CSW acts downstream of the receptor tyrosine kinases Torso, Sevenless, Deranged, Breathless, and Heartless (Allard et al., 1996; Perkins et al., 1996; Perkins et al., 1992). In C. elegans, the MSP receptor VAB-1 is an Eph receptor protein-tyrosine kinase that negatively regulates MPK-1 activation (Miller et al., 2003). Most data do not support the model that VAB-1 and PTP-2 interact. PTP-2 does not appear to localize to the cell surface, contrasting with VAB-1 localization (Cheng et al., 2008; Miller et al., 2003). Furthermore, genetic studies are consistent with ptp-2 acting downstream of unc-43 and sheath/oocyte gap junctions. If there is an interaction between VAB-1 and PTP-2, the mechanism is likely to be transient and involve additional receptors. A more likely scenario is that PTP-2 interacts with an unidentified receptor localizing to endosomes or another intracellular compartment in oocytes. In cultured mammalian cells, the localization of Ras and Erk to various intracellular compartments, including endosomes, is mechanistically linked to sustained MAPK signaling (Bivona and Philips, 2003; Mor and Philips, 2006).
MAPK activation is a key step in vertebrate oocyte maturation, although the regulatory mechanisms and site of action are still debated (Liang et al., 2007). In Xenopus oocytes, progesterone-induced oocyte maturation is accompanied by MAPK activation. Constitutively active MAPK promotes oocyte maturation in the absence of progesterone and chemically inhibiting MAPK prevents maturation (Grigorescu et al., 1994; Posada and Cooper, 1992). MAPK activation is thought to be mediated by the proto-oncogene MOS, a vertebrate germ cell-specific kinase that phosphorylates MAP Kinase Kinase (Liang et al., 2007; Seger and Krebs, 1995). However, MOS inactivation by morpholinos does not prevent oocyte maturation (Dupre et al., 2002). In mice, deletion of mos or erk1 does not prevent luteinizing hormone-induced oocyte maturation, but deletion of erk1 and erk2 in granulosa cells does (Fan et al., 2009; Hashimoto et al., 1994; Liang et al., 2007). Thus, MAPK activity is essential in the granulosa cells, but probably not in the oocytes. Whether Shps play a role in vertebrate oocyte maturation is not known. C. elegans MPK-1 activation does not involve MOS. Although MPK-1 activation is observed in oocytes and surrounding sheath cells, the essential role appears to be in oocytes. These species-specific differences in MAPK regulation may be due to differences in the maturation-inducing hormones and their transduction mechanisms.
There is accumulating evidence that the MSP domain has an evolutionarily conserved signaling function mediated by Eph receptors and other receptors. The human VAPB MSP domain is found in blood serum, possesses extracellular signaling and receptor binding activities in C. elegans gonads, and can bind to the mouse EphA4 receptor (Tsuda et al., 2008). A P56S substitution in the human VAPB MSP domain is associated with ALS, although the pathological mechanism is not understood (Nishimura et al., 2004). P56S acts as a dominant negative mutation in multiple model systems and prevents VAP MSP domain secretion in fly cells (Ratnaparkhi et al., 2008; Teuling et al., 2007; Tsuda et al., 2008). Dominant mutations in sod1 are also associated with ALS and these are gain of function (Rosen et al., 1993; Rothstein, 2009). Our results support the model that MSP and SOD-1 act antagonistically to control ROS and MPK-1 signaling. Therefore, it is possible that MSP domain proteins and Cu/Zn superoxide dismutases are components of an evolutionarily ancient signaling mechanism important for human motor neuron survival.
Materials and Methods
C. elegans genetics and strains
C. elegans variety Bristol, strain N2 is the wild-type strain. Cultures were grown on NGM plates with NA22 bacteria as the food source (Brenner, 1974). fog-2(q71) strains (Schedl and Kimble, 1988) were maintained as male/female stocks, while fog-3(q443) strains (Ellis and Kimble, 1995) were balanced with the translocation hT2(qIs48)I. Strain construction and marker scoring were done as previously described using PCR and phenotypic analyses (Miller et al., 2003). Genes, alleles, and balancer chromosomes are described in WormBase (http://www.wormbase.org). The strains and genetic markers used or generated were as follows: WS841 [ptp-2(op194) unc-4(e120)/mIn1II; him-5(e1490) V] (Gutch et al., 1998), DG1743 [fog-3(q443)/hT2(qIs48)I] (Miller et al., 2003), MT1092 [unc-43(n498)IV] (Reiner et al., 1999), CZ337 [vab-1(dx31)II] (George et al., 1998), HT1593 [unc-119 (ed3)III] (Praitis et al., 2001), MT2124 [let-60(n1046)IV], AH12 [gap-1(ga133)X] (Hajnal et al., 1997), JN147 [gap-2(tm748)X], XM1011 [inx-22(tm1661)I] (Whitten and Miller, 2007), FX776 [sod-1(tm776)II] (Yanase et al., 2009), CB4108 [fog-2(q71)V], [ptp-2(op194) unc-4(e120)/mIn1II; fog-2(q71)V], [fog-3(q443)/hT2(qIs48)I; let-60(n1046)IV], [inx-22(tm1661)I; ptp-2(op194) unc-4(e120)/mIn1II], [ptp-2(op194) unc-4(e120)/mIn1II; let-60(n1046)IV], [ptp-2(op194) unc-4(e120)/mIn1II; unc-43(n498)IV], [ptp-2(op194) unc-4(e120)/mIn1II; gap-1(ga133)X; gap-2(tm748)X], [ptp-2(op194) unc-4(e120)/mIn1II; gap-1(ga133)X], [ptp-2(op194) unc-4(e120)/mIn1II; gap-2(tm748)X], [ptp-2(op194) unc-4(e120)/mIn1II; Ex [Ppie::gfp::ptp-2 + unc-119(+)], and [sod-1(tm776); fog-2(q71)]. The sod-1(tm776) mutation was backcrossed to the wild-type four times.
Phenotypic analysis and rate determination
Oocyte meiotic maturation and ovarian sheath contraction were analyzed in anesthetized animals (0.1% tricaine and 0.01% tetramisole in M9 buffer) on 2% agarose pads as previously described (McCarter et al., 1999). A Zeiss Axioskop 2 plus, MRM Axiocam Hi-Res digital camera, and PC computer were used to observe and record images. DAPI staining of dissected gonads was used for evaluating oocyte meiotic progression. Oocyte maturation and sheath contraction were monitored for each strain by direct observation using DIC optics. For adult hermaphrodites and females, basal sheath contraction rates were measured as previously described (McCarter et al., 1999; Miller et al., 2003). Oocyte maturation rates were determined by monitoring oocyte ovulation in isolated animals on seeded plates for 1.5 to 24 hours, which was consistent with direct observation by DIC microscopy. Because food is required for high maturation rates, monitoring animals on seeded plates is optimal for accurate quantification over time periods > 90 minutes. Tables 1 and 2 show average oocyte maturation or sheath contraction rates ± standard deviation. To test for significance, a two sample T-test was used.
RNA-mediated interference
RNAi was performed by the feeding and soaking methods (Timmons et al., 2001; Timmons and Fire, 1998). RNAi HT115 bacterial feeding strains are from the genome-wide library (Kamath et al., 2003). Each clone was sequenced for confirmation.
Transgenics
For generating transgenic C. elegans, 5kb of genomic DNA upstream of the ptp-2 translational start site was inserted into pPD95.81 upstream of GFP. The primers 5′-CGGGGATCCCGCTTGCATGAAACATGAAATTGGTGG-3′ and 5′-TCTACCGGTACTTCACCATTCACGCGGTAATAG-3′ were used for PCR amplification of genomic DNA. The plasmid (90μg/ml) was mixed with pRF4 [rol-6(d)] (90μg/ml) and injected into the gonads of young adult hermaphrodites. Transgenic progeny were selected based on the roller phenotype. Multiple independent lines for each transgene were analyzed. To generate the Pie-1p::gfp::ptp-2 transgene, the ptp-2 ORF was cloned into pDONR201 (Invitrogen, Carlsbad, CA) before recombining into pID3.01B, which contains the pie-1 promoter upstream of gfp and the cloning site. This construct was sequenced and transformed into unc-119(ed3) hermaphrodites by microparticle bombardment (Praitis et al., 2001). Multiple transgenic lines were generated. One transgenic line with ~95% unc-119(+) animals and visible GFP expression was crossed into ptp-2(op194) unc-4(e120)/mIn1II hermaphrodites. The genotype was confirmed by PCR. The resulting ptp-2(op194) unc-4(e120); Piep::gfp::ptp-2 + unc-119(+) strain was homozygous viable, contrasting with ptp-2(op194) unc-4(e120) mutants. GFP expression in this line was faint, but detectable.
Immunocytochemistry and GFP imaging
Monoclonal MAPK-YT (Sigma), anti-phosphohistone H3 (Upstate Biotechnology, Waltham, MA), anti-NOP1 (Encor Biotech, Alachua, FL) antibodies were incubated with dissected gonads as previously described (Burrows et al., 2006; Miller et al., 2001). Dissected gonads were fixed in 2% neutral-buffered paraformaldehyde at 4°C overnight, washed in PBT, blocked with 1mg/ml BSA for 2 hours, and then incubated with the antibodies for 4 hours at room temperature. The concentrations were as follows: MAPK-YT (1:2000), anti-phospho-histone H3 (1:500), and anti-Nop1 (1:500). Anti-mouse or anti-rabbit FITC-conjugated secondary antibodies were used for detection. DAPI was used to visualize DNA. For evaluating GFP expression of transgenic Pie-1p::gfp::ptp-2 strains, animals were kept in the dark for 24 hours prior to visualization.
MSP microinjection and binding
Recombinant 6His-MSP was expressed and purified under native conditions as previously described (Miller et al., 2001). MSP concentrations were determined by the BCA protein assay. MSP or M9 buffer alone was microinjected through the vulva using a Zeiss Axiovert 200 microscope, hydraulic fine type micromanipulator, and Narishige IM-30 microinjector (Corrigan et al., 2005; Tsuda et al., 2008). To evaluate MSP binding, recombinant 6His-MSP was conjugated to NHS-Fluorescein and purified (Miller et al., 2003). Binding assays were conducted as previously described (Miller et al., 2003; Tsuda et al., 2008). MSP-FITC is biologically active and binding is specific.
Paraquat resistance and reactive oxygen species
Resistance to paraquat was determined as described previously with slight modification (An and Blackwell, 2003; Li et al., 2008). Forty one-day adult wild-type hermaphrodites or unmated fog-2(q71) females were placed in 200 μl of M9 buffer containing 0, 50, 100, or 150 mM paraquat for 24 h at 20°C. After the incubation, 0.5 ml of M9 was added and the worms were transferred to NGM plates and examined for survival. Worms that failed to respond to gentle prodding were scored as dead. Each genotype was analyzed in triplicate.
Intracellular ROS levels were determined using the membrane-permeable nonfluorescent dye 2,7-dichlorodihydrofluorescein-diacetate (H2-DCF-DA) (Anaspec, Fremont, CA). After entry into cells, H2-DCF-DA is converted to membrane-impermeable HDCF. In the presence of ROS, HDCF is oxidized to fluorescent 2,7-dichlorofluorescein (DCF). Dissected adult gonads were soaked in 50μM H2-DCF-DA buffer for 10min and mounted on agarose pads without fixation using Vaseline to prevent the coverslip from crushing the gonads. Unfixed gonads mounted under a coverslip are difficult to image, limiting the number of samples that can be analyzed. To help circumvent this issue, we lightly fixed the gonads in 1% neutral-buffered paraformaldehyde for 5 min before mounting. Fixation decreased the fluorescent intensity, but facilitated analysis of larger gonad numbers. In addition, we soaked live worms in H2-DCF-DA for 7 hours, anesthetized them, and mounted them for microscopy. In live animals, fluorescent oocytes were sometimes observed in wild-type hermaphrodites, but not unmated females. The lower frequency in live hermaphrodites relative to dissected gonads might be due to poor dye penetration into the gonad. Fluorescence was monitored using a Zeiss Axioskop 2 microscope equipped with fluorescence and a GFP filter. Hermaphrodite and unmated female gonads were analyzed in the same solution and independently.
Mitotracker CMXRos and oxygen consumption
Dissected worm gonads were incubated in 50nM MitoTracker Red CMXRos (Molecular Probes, Inc., Eugene, OR) and placed in dark place at room temperature for 10 min. Gonads were lightly fixed in 1% neutral-buffered paraformaldehyde for 5 min before mounting. Hermaphrodite and unmated female gonads were analyzed in the same solution and independently. Oxygen consumption rates were measured as previously described using the oxygraph system (Hansatech, UK) with slight modifications (Braeckman et al., 2002). Synchronized wild-type and unmated fog-2(q71) female worms were grown to L4 stage, transferred to new NGM plates, and incubated for an additional 24 hours at 20°C. 1000 1day old adult worms were individually picked and collected into 1ml M9 buffer prepared at 20°C. Worms were incubated for 40 min at 20°C to remove the intestinal bacteria and washed five times using M9 buffer. 1ml M9 with 1000 worms were placed into the chamber equipped with a S1 Clark type polarographic oxygen electrode disc. The chamber was maintained at 20°C using a constant-temperature circulating water bath and the oxygen concentration was measured for at least 10 minutes. Worms were carefully collected from the chamber for protein quantification. Rates were normalized to either total protein content or the number of worms. We performed at least three independent measurements per strain.
Supplementary Material
Figure S1. MSP-FITC binding to wild-type and ptp-2 mutant proximal gonads. MSP-FITC binding to wild-type and ptp-2 mutant gonads (shown) can be out-competed with a 25-fold molar excess of unlabelled MSP. Binding assays were conducted as previously described (Miller et al., 2003; Tsuda et al., 2008). Gonad orientation is with spermatheca to the right. Bar, 10 μm.
Acknowledgments
We thank Pauline Cottee and Wes Edmonds for comments on the manuscript. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH. Financial support for this study came from an American Cancer Society Research Scholars Grant to M.A.M. (RSG-06-151-01-DDC). The sod-1(tm776) strain was created by the Japanese National Bioresource Project, which is supported by the Ministry of Education, Culture, Science, Sports and Technology.
Addendum to Acknowledgments: Some financial support for this study was provided by the NIH (GM085105).
References
- Abe J, Okuda M, Huang Q, Yoshizumi M, Berk BC. Reactive oxygen species activate p90 ribosomal S6 kinase via Fyn and Ras. J Biol Chem. 2000;275:1739–48. doi: 10.1074/jbc.275.3.1739. [DOI] [PubMed] [Google Scholar]
- Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest. 1997;100:1813–21. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JD, Chang HC, Herbst R, McNeill H, Simon MA. The SH2-containing tyrosine phosphatase corkscrew is required during signaling by sevenless, Ras1 and Raf. Development. 1996;122:1137–46. doi: 10.1242/dev.122.4.1137. [DOI] [PubMed] [Google Scholar]
- An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–93. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aris JP, Blobel G. Yeast nuclear envelope proteins cross react with an antibody against mammalian pore complex proteins. J Cell Biol. 1989;108:2059–67. doi: 10.1083/jcb.108.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivona TG, Philips MR. Ras pathway signaling on endomembranes. Curr Opin Cell Biol. 2003;15:136–42. doi: 10.1016/s0955-0674(03)00016-4. [DOI] [PubMed] [Google Scholar]
- Bottino D, Mogilner A, Roberts T, Stewart M, Oster G. How nematode sperm crawl. J Cell Sci. 2002;115:367–84. doi: 10.1242/jcs.115.2.367. [DOI] [PubMed] [Google Scholar]
- Braeckman BP, Houthoofd K, De Vreese A, Vanfleteren JR. Assaying metabolic activity in ageing Caenorhabditis elegans. Mech Ageing Dev. 2002;123:105–19. doi: 10.1016/s0047-6374(01)00331-1. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TL, Roberts TM, Stewart M. 2.5 A resolution crystal structure of the motile major sperm protein (MSP) of Ascaris suum. J Mol Biol. 1996;263:284–96. doi: 10.1006/jmbi.1996.0575. [DOI] [PubMed] [Google Scholar]
- Burrows AE, Sceurman BK, Kosinski ME, Richie CT, Sadler PL, Schumacher JM, Golden A. The C. elegans Myt1 ortholog is required for the proper timing of oocyte maturation. Development. 2006;133:697–709. doi: 10.1242/dev.02241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Govindan JA, Greenstein D. Regulated trafficking of the MSP/Eph receptor during oocyte meiotic maturation in C. elegans. Curr Biol. 2008;18:705–14. doi: 10.1016/j.cub.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church DL, Guan KL, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development. 1995;121:2525–35. doi: 10.1242/dev.121.8.2525. [DOI] [PubMed] [Google Scholar]
- Cleghon V, Feldmann P, Ghiglione C, Copeland TD, Perrimon N, Hughes DA, Morrison DK. Opposing actions of CSW and RasGAP modulate the strength of Torso RTK signaling in the Drosophila terminal pathway. Mol Cell. 1998;2:719–27. doi: 10.1016/s1097-2765(00)80287-7. [DOI] [PubMed] [Google Scholar]
- Corrigan C, Subramanian R, Miller MA. Eph and NMDA receptors control Ca2+/calmodulin-dependent protein kinase II activation during C. elegans oocyte meiotic maturation. Development. 2005;132:5225–37. doi: 10.1242/dev.02083. [DOI] [PubMed] [Google Scholar]
- Dupre A, Jessus C, Ozon R, Haccard O. Mos is not required for the initiation of meiotic maturation in Xenopus oocytes. Embo J. 2002;21:4026–36. doi: 10.1093/emboj/cdf400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RE, Kimble J. The fog-3 gene and regulation of cell fate in the germ line of Caenorhabditis elegans. Genetics. 1995;139:561–77. doi: 10.1093/genetics/139.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–41. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng GS. Shp-2 tyrosine phosphatase: signaling one cell or many. Exp Cell Res. 1999;253:47–54. doi: 10.1006/excr.1999.4668. [DOI] [PubMed] [Google Scholar]
- George SE, Simokat K, Hardin J, Chisholm AD. The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell. 1998;92:633–43. doi: 10.1016/s0092-8674(00)81131-9. [DOI] [PubMed] [Google Scholar]
- Govindan JA, Cheng H, Harris JE, Greenstein D. Galphao/i and Galphas signaling function in parallel with the MSP/Eph receptor to control meiotic diapause in C. elegans. Curr Biol. 2006;16:1257–68. doi: 10.1016/j.cub.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Govindan JA, Nadarajan S, Kim S, Starich TA, Greenstein D. Somatic cAMP signaling regulates MSP-dependent oocyte growth and meiotic maturation in C. elegans. Development. 2009;136:2211–21. doi: 10.1242/dev.034595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein D. Control of oocyte meiotic maturation and fertilization. WormBook. 2005:1–12. doi: 10.1895/wormbook.1.53.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorescu F, Baccara MT, Rouard M, Renard E. Insulin and IGF-1 signaling in oocyte maturation. Horm Res. 1994;42:55–61. doi: 10.1159/000184146. [DOI] [PubMed] [Google Scholar]
- Gutch MJ, Flint AJ, Keller J, Tonks NK, Hengartner MO. The Caenorhabditis elegans SH2 domain-containing protein tyrosine phosphatase PTP-2 participates in signal transduction during oogenesis and vulval development. Genes Dev. 1998;12:571–85. doi: 10.1101/gad.12.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Whitfield CW, Kim SK. Inhibition of Caenorhabditis elegans vulval induction by gap-1 and by let-23 receptor tyrosine kinase. Genes Dev. 1997;11:2715–28. doi: 10.1101/gad.11.20.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SM, Cottee PA, Miller MA. Sperm and oocyte communication mechanisms controlling C. elegans fertility. Dev Dyn. 2009 doi: 10.1002/dvdy.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Govindan JA, Yamamoto I, Schwartz J, Kaverina I, Greenstein D. Major sperm protein signaling promotes oocyte microtubule reorganization prior to fertilization in Caenorhabditis elegans. Dev Biol. 2006;299:105–21. doi: 10.1016/j.ydbio.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;370:68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- Henriquez R, Blobel G, Aris JP. Isolation and sequencing of NOP1. A yeast gene encoding a nucleolar protein homologous to a human autoimmune antigen. J Biol Chem. 1990;265:2209–15. [PubMed] [Google Scholar]
- Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, Grushcow JM, Brame CJ, Caldwell JA, Hunt DF, Lin R, Smith MM, Allis CD. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–91. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Hsu V, Zobel CL, Lambie EJ, Schedl T, Kornfeld K. Caenorhabditis elegans lin-45 raf is essential for larval viability, fertility and the induction of vulval cell fates. Genetics. 2002;160:481–92. doi: 10.1093/genetics/160.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jud MC, Czerwinski MJ, Wood MP, Young RA, Gallo CM, Bickel JS, Petty EL, Mason JM, Little BA, Padilla PA, Schisa JA. Large P body-like RNPs form in C. elegans oocytes in response to arrested ovulation, heat shock, osmotic stress, and anoxia and are regulated by the major sperm protein pathway. Dev Biol. 2008;318:38–51. doi: 10.1016/j.ydbio.2008.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–7. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Klass MR, Hirsh D. Sperm isolation and biochemical analysis of the major sperm protein from C. elegans. Dev Biol. 1981;84:299–312. doi: 10.1016/0012-1606(81)90398-5. [DOI] [PubMed] [Google Scholar]
- Kosinski M, McDonald K, Schwartz J, Yamamoto I, Greenstein D. C. elegans sperm bud vesicles to deliver a meiotic maturation signal to distant oocytes. Development. 2005;132:3357–69. doi: 10.1242/dev.01916. [DOI] [PubMed] [Google Scholar]
- Lee MH, Ohmachi M, Arur S, Nayak S, Francis R, Church D, Lambie E, Schedl T. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics. 2007;177:2039–62. doi: 10.1534/genetics.107.081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ebata A, Dong Y, Rizki G, Iwata T, Lee SS. Caenorhabditis elegans HCF-1 functions in longevity maintenance as a DAF-16 regulator. PLoS Biol. 2008;6:e233. doi: 10.1371/journal.pbio.0060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CG, Su YQ, Fan HY, Schatten H, Sun QY. Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol. 2007;21:2037–55. doi: 10.1210/me.2006-0408. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111–28. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- Mendenhall AR, LeBlanc MG, Mohan DP, Padilla PA. Reduction in ovulation or male sex phenotype increases long-term anoxia survival in a daf-16-independent manner in Caenorhabditis elegans. Physiol Genomics. 2009;36:167–78. doi: 10.1152/physiolgenomics.90278.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DM, Shen MM, Shamu CE, Burglin TR, Ruvkun G, Dubois ML, Ghee M, Wilson L. C. elegans unc-4 gene encodes a homeodomain protein that determines the pattern of synaptic input to specific motor neurons. Nature. 1992;355:841–5. doi: 10.1038/355841a0. [DOI] [PubMed] [Google Scholar]
- Miller MA, Cutter AD, Yamamoto I, Ward S, Greenstein D. Clustered organization of reproductive genes in the C. elegans genome. Curr Biol. 2004;14:1284–90. doi: 10.1016/j.cub.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–7. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Miller MA, Ruest PJ, Kosinski M, Hanks SK, Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 2003;17:187–200. doi: 10.1101/gad.1028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- Nadarajan S, Govindan JA, McGovern M, Hubbard EJ, Greenstein D. MSP and GLP-1/Notch signaling coordinately regulate actomyosin-dependent cytoplasmic streaming and oocyte growth in C. elegans. Development. 2009;136:2223–34. doi: 10.1242/dev.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–93. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JR, Gillingwater T, Webb J, Skehel P, Zatz M. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75:822–31. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmachi M, Rocheleau CE, Church D, Lambie E, Schedl T, Sundaram MV. C. elegans ksr-1 and ksr-2 have both unique and redundant functions and are required for MPK-1 ERK phosphorylation. Curr Biol. 2002;12:427–33. doi: 10.1016/s0960-9822(02)00690-5. [DOI] [PubMed] [Google Scholar]
- Page BD, Guedes S, Waring D, Priess JR. The C. elegans E2F- and DP-related proteins are required for embryonic asymmetry and negatively regulate Ras/MAPK signaling. Mol Cell. 2001;7:451–60. doi: 10.1016/s1097-2765(01)00193-9. [DOI] [PubMed] [Google Scholar]
- Pendergrass W, Wolf N, Poot M. Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A. 2004;61:162–9. doi: 10.1002/cyto.a.20033. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Johnson MR, Melnick MB, Perrimon N. The nonreceptor protein tyrosine phosphatase corkscrew functions in multiple receptor tyrosine kinase pathways in Drosophila. Dev Biol. 1996;180:63–81. doi: 10.1006/dbio.1996.0285. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Larsen I, Perrimon N. corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992;70:225–36. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- Posada J, Cooper JA. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992;255:212–5. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–26. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnaparkhi A, Lawless GM, Schweizer FE, Golshani P, Jackson GR. A Drosophila model of ALS: human ALS-associated mutation in VAP33A suggests a dominant negative mechanism. PLoS ONE. 2008;3:e2334. doi: 10.1371/journal.pone.0002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Newton EM, Tian H, Thomas JH. Diverse behavioral defects caused by mutations in Caenorhabditis elegans unc-43 CaM kinase II. Nature. 1999;402:199–203. doi: 10.1038/46072. [DOI] [PubMed] [Google Scholar]
- Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131:311–23. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- Reinke V, Smith HE, Nance J, Wang J, Van Doren C, Begley R, Jones SJ, Davis EB, Scherer S, Ward S, Kim SK. A global profile of germline gene expression in C. elegans. Mol Cell. 2000;6:605–16. doi: 10.1016/s1097-2765(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(Suppl 1):S3–9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- Schedl T, Kimble J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119:43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secondo A, De Mizio M, Zirpoli L, Santillo M, Mondola P. The Cu-Zn superoxide dismutase (SOD1) inhibits ERK phosphorylation by muscarinic receptor modulation in rat pituitary GH3 cells. Biochem Biophys Res Commun. 2008;376:143–7. doi: 10.1016/j.bbrc.2008.08.110. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. Faseb J. 1995;9:726–35. [PubMed] [Google Scholar]
- Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–62. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Stetak A, Gutierrez P, Hajnal A. Tissue-specific functions of the Caenorhabditis elegans p120 Ras GTPase activating protein GAP-3. Dev Biol. 2008;323:166–76. doi: 10.1016/j.ydbio.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Tenenhaus C, Schubert C, Seydoux G. Genetic requirements for PIE-1 localization and inhibition of gene expression in the embryonic germ lineage of Caenorhabditis elegans. Dev Biol. 1998;200:212–24. doi: 10.1006/dbio.1998.8940. [DOI] [PubMed] [Google Scholar]
- Tenenhaus C, Subramaniam K, Dunn MA, Seydoux G. PIE-1 is a bifunctional protein that regulates maternal and zygotic gene expression in the embryonic germ line of Caenorhabditis elegans. Genes Dev. 2001;15:1031–40. doi: 10.1101/gad.876201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuling E, Ahmed S, Haasdijk E, Demmers J, Steinmetz MO, Akhmanova A, Jaarsma D, Hoogenraad CC. Motor neuron disease-associated mutant vesicle-associated membrane protein-associated protein (VAP) B recruits wild-type VAPs into endoplasmic reticulum-derived tubular aggregates. J Neurosci. 2007;27:9801–15. doi: 10.1523/JNEUROSCI.2661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–12. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Han SM, Yang Y, Tong C, Lin YQ, Mohan K, Haueter C, Zoghbi A, Harati Y, Kwan J, Miller MA, Bellen HJ. The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. 2008;133:963–77. doi: 10.1016/j.cell.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Manrique RP, Gonzalez-Cabo P, Ortiz-Martin I, Ros S, Baylis HA, Palau F. The frataxin-encoding operon of Caenorhabditis elegans shows complex structure and regulation. Genomics. 2007;89:392–401. doi: 10.1016/j.ygeno.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Whitten SJ, Miller MA. The role of gap junctions in Caenorhabditis elegans oocyte maturation and fertilization. Dev Biol. 2007;301:432–446. doi: 10.1016/j.ydbio.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Yanase S, Onodera A, Tedesco P, Johnson TE, Ishii N. SOD-1 deletions in Caenorhabditis elegans alter the localization of intracellular reactive oxygen species and show molecular compensation. J Gerontol A Biol Sci Med Sci. 2009;64:530–9. doi: 10.1093/gerona/glp020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. MSP-FITC binding to wild-type and ptp-2 mutant proximal gonads. MSP-FITC binding to wild-type and ptp-2 mutant gonads (shown) can be out-competed with a 25-fold molar excess of unlabelled MSP. Binding assays were conducted as previously described (Miller et al., 2003; Tsuda et al., 2008). Gonad orientation is with spermatheca to the right. Bar, 10 μm.


