Abstract
OBJECTIVES:
Despite a documented clinical need, no patient reported outcome (PRO) symptom measure meeting current regulatory requirements for clinically relevant end points is available for the evaluation of treatment benefit in diarrhea-predominant IBS (IBS-D).
METHODS:
Patients (N=113) with IBS-D participated in five study phases: (1) eight concept elicitation focus groups (N=34), from which a 17-item IBS-D Daily Symptom Diary and four-item IBS-D Symptom Event Log (Diary and Event Log) were developed; (2) one-on-one cognitive interviews (N=11) to assess the instrument's comprehensiveness, understandability, appropriateness, and readability; (3) four data triangulation focus groups (N=32) to confirm the concepts elicited; (4) two hybrid (concept elicitation and cognitive interview) focus groups (N=16); and (5) two iterative sets of one-on-one cognitive interviews (N=20) to further clarify the symptoms of IBS-D and debrief a revised seven-item Diary and four-item Event Log.
RESULTS:
Of thirty-six concepts initially identified, 22 were excluded because they were not saturated, not clinically relevant, not critical symptoms of IBS-D, considered upper GI symptoms, or too broad or vaguely defined. The remaining concepts were diarrhea, immediate need (urgency), bloating/pressure, frequency of bowel movements, cramps, abdominal/stomach pain, gas, completely emptied bowels/incomplete evacuation, accidents, bubbling in intestines (bowel sounds), rectal burning, stool consistency, rectal spasm, and pain while wiping. The final instrument included a daily diary with separate items for abdominal and stomach pain and an event log with four items completed after each bowel movement as follows: (1) a record of the bowel movement/event and an assessment of (2) severity of immediacy of need/bowel urgency, (3) incomplete evacuation, and (4) stool consistency (evaluated using the newly developed Astellas Stool Form Scale). Based on rounds of interviews and clinical input, items considered secondary or nonspecific to IBS-D (rectal burning, bubbling in intestines, spasms, and pain while wiping) were excluded.
CONCLUSIONS:
The IBS-D Symptom Diary and Event Log represent a rigorously developed PRO instrument for the measurement of the IBS-D symptom experience from the perspective of the patient. Its content validity has been supported, and future work should evaluate the instrument's psychometric properties.
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder characterized by abdominal pain or discomfort associated with altered bowel habits.1 Subtypes of the disease are further designated by the predominance of constipation, diarrhea, a combination of the two, or as an undifferentiated form (IBS-C, -D, -mixed, -not specified, respectively). Although the symptoms of IBS can collectively be used to make a diagnosis,2 individual symptoms are by themselves neither sensitive nor specific to document treatment benefit,3 complicating the diagnosis as well as the treatment of the disease. Indeed, both the United States Food and Drug Administration (FDA) and European Medicines Agency have noted the challenge of capturing the clinically important symptoms of IBS to measure reliably the treatment benefit in clinical trials.4, 5 While assessment of symptoms currently remains the only avenue of both diagnosis and treatment evaluation in this condition,6 the symptoms of IBS vary greatly among patients and tend to be complex, rendering the development of optimal clinical trial end points for the evaluation of drug efficacy a challenge.
Among these challenges, as noted in the World Gastroenterology Organization Global Guideline (2009), is the symptomatic array composing IBS, which includes symptoms that are not specific to the disease, often taking the form of transient gut phenomena experienced by almost any individual. Further complicating the matter, patients may transition between the various IBS subgroups and thus present with a shifting symptomatology. Moreover, considerable overlap exists with other functional GI disorders, such as gastroesophageal reflux disease, dyspepsia, and functional constipation.
In an attempt to account for the wide and varying array of symptoms in IBS, past attempts to measure treatment benefit have included global items such as self-reported “adequate relief of symptoms in the past seven days” and “satisfactory relief of symptoms in the past seven days.”7 While these measures have been approved by the FDA as primary end points in trials for the treatment of IBS, they are no longer considered valid or reliable for this purpose. These single-item reports of overall symptom change lack the specificity required by the FDA—namely, the ability of an instrument to measure improvement and decrements in the critical signs and symptoms that are important to patients and that are clinically relevant.4 In addition, at issue with these measures is the need for respondents to average over the cluster of symptoms associated with IBS, inherently difficult in this condition, and over a time period of 1 week.
Of the qualitative instruments currently available to assess IBS, only three evaluate IBS symptoms (The Irritable Bowel Severity Scoring System (IBSSS), The Bristol Stool Form Scale (BSFS), and the IBS (GSRS-IBS));8, 9, 10 the remaining measures evaluate HRQoL,11, 12, 13, 14, 15 psychological impact,16 or work productivity.17 Although the Irritable Bowel Syndrome Quality of Life Instrument was used to support a labeling claim for the FDA-approved diarrhea-predominant IBS (IBS-D) drug alosetron hydrochloride (Lotronex) in 2000,18 the instrument is unlikely to be successful in supporting claims in the current regulatory environment owing to deviations from guidance recommendations, such as the instrument's 30-day recall period.13 The IBSSS9 also implements an over-long recall period, ranging from 1 week to as long as a year. Also problematic is that the instrument includes both symptoms and HRQoL in one measure, and that no patient involvement was documented as part of its development. The BSFS10 was also developed without documented patient input, and has moreover only been validated with a clinical indicator—namely, stool transit time. The GSRS-IBS, a modified version of the GSRS, similarly did not include patient input in its development.8
Furthermore, none of the available measures is subtype specific. This is of particular concern in light of the challenge of diagnosing IBS and the variability in subtype symptomatology, and both the FDA and European Medicines Agency have suggested that patient reported outcome (PRO) instruments specific to particular IBS subtypes may be potentially necessary to measure effectively the therapeutic benefit.4
This report describes the development of an IBS-D-specific PRO symptom severity measure specifically tailored to measure treatment effects and to be used as a clinical trial end point in IBS-D. The multidisciplinary research team, which included a GI specialist, undertook five qualitative research studies to inform development as well as document the content validity of this new PRO instrument. The resulting measure, comprising a seven-item IBS-D Daily Symptom Diary and four-item IBS-D Symptom Event Log (hereafter referred to as the Diary and the Event Log, respectively), represents the first IBS-D qualitative symptom measure for evaluating treatment benefit developed in compliance with FDA regulatory guidance. Specific to communications with the FDA, six interactions (including two of face-to-face meetings) between the developers and the Study Endpoint and Labeling Development (SEALD) and the GI Division at the FDA were held for advice.
METHODS
Centralized Institutional Review Boards (New England Institutional Review Board in 2007 and Copernicus Group Independent Review Board from 2008 to 2011) approved the studies and iterative submission of study documents (study protocol, interview guides, patient information and informed consent form, and health information and demographic form). Written informed consent was obtained from all patients before their participating in the study. The study was performed in accordance with the Declaration of Helsinki and US 21 Code of Federal Regulations.19 All patients also signed a Health Insurance Portability and Accountability Act form.20 Patients received a stipend for their participation.
Recruitment of patients and inclusion/exclusion criteria
Patients were eligible if they were between 18 and 75 years of age, fluent in US English, and capable of comprehending and signing an informed consent form for participation, and willing and able to participate in a 90-min focus group or cognitive interview. Patients' eligibility according to the Rome III criteria2 was confirmed on a Case Report Form (which included the Rome III definition) filled out by the patient's physician before inclusion in the study. Excluded patients included those with an organic disease or functional GI syndrome, other than IBS, potentially affecting digestive tract passage or colonic function, including stricture, obstruction, or ileus; benign polyps or colonic diverticulosis judged to have an influence on the digestive tract passage or colonic function; a history of surgical resection of the stomach, small intestine, or large intestine (excluding resection of the appendix or benign polyps); a history of ischemic colitis or unexplained blood passage by rectum; uncontrolled lactose intolerance; or abdominal disease requiring radiotherapy.
All data were collected at US non-medical facilities in Boston, Chicago, New Orleans, St Louis, and Philadelphia, and were deidentified before analysis. Patients were recruited by two commercial recruitment agencies through databases of clinicians (including primary care physicians and GI specialists). Before the patients' entry into the study, the patients' treating clinicians were asked to confirm the diagnosis of IBS-D (based on the Rome III criteria) and eligibility on a signed and dated Case Report Form for all patients.
Study design and overall methods
Rigorous and appropriate qualitative research data collection methods based on grounded theory were used to conduct all focus groups and the concept elicitation components of cognitive interviews.21 The consolidated criteria for reporting qualitative research (COREQ) were adhered to for all interview and focus group activities.22 Data collection was carried out according to the principles of grounded theory, and all qualitative exercises sought to produce spontaneously elicited, rich descriptions of the symptoms and impacts of IBS-D. In the grounded theory approach, concepts emerge from patient input, allowing the voice of the patient to be heard rather than applying an a priori theoretical model or construct to interpret the data.23, 24, 25, 26, 27
A focus group methodology was chosen for concept elicitation, as breadth rather than depth of concepts was desired, and an open-ended, semistructured interview guide was used to generate discussion among focus group members. Focus groups were gender specific by design to elicit more candid feedback from participants. Each focus group was facilitated by a trained moderator and a comoderator. Each focus group was conducted in English, across several regions of the United States.
All cognitive interviews were conducted face-to-face to confirm content validity, comprehensibility, relevance, and readability, and that the fit between item stems and responses, as well as the recall period, were appropriate.28 Patients were asked to complete the Diary and Event Log to measure IBS-D symptoms and provide their feedback using the “think aloud” method.29 Patients were asked to identify words, terms, or concepts that they did not understand and/or to give their interpretation of particular items. They were also asked if revisions should be made to the Diary and Event Log to make them more appropriate, comprehensive, and interpretable. All focus groups and cognitive interviews were audio and video recorded and transcribed verbatim.
Analysis of qualitative data
All study transcripts were coded and analyzed using the Atlas.ti software program.27 A standardized coding scheme was initially derived from the interview guide and patients' words describing the symptoms and impacts of IBS-D; the scheme was then refined based on discussion and consensus reconciliation of discrepancies between four researchers' independent coding of an initial transcript. When the coding scheme was finalized, the remaining transcripts were single-coded. New codes were included as they emerged, and conceptually equivalent codes were merged, by agreement among the full coding team. If new codes emerged in ongoing review of transcripts, the four coders alerted each other, and if agreed upon, the codes were included in the coding scheme. All coded transcripts were reviewed by two senior researchers to ensure consistency and accuracy. This coding process was used in all phases of the study.
Following this qualitative analysis of the concept elicitation focus groups, the concepts identified as most salient to the participants' experience of IBS-D were then formulated into a conceptual framework. Revisions to both the conceptual framework and the instrument were considered following each subsequent stage. To ensure the clinical relevance of the concepts included, input was sought throughout the evolution of the conceptual framework from clinical experts.
Saturation30 was assessed to confirm the adequacy of the sample size as well as the sufficiency of the data to support the elaboration of the concepts and their dimensions (e.g., frequency, duration, or severity).
Phase I: concept elicitation (N=34)
Instrument development began with a concept elicitation phase, during which focus group interviews were used to gather spontaneously elicited descriptions of patients' experiences with IBS-D. The FDA PRO Draft Guidance (Of note, research began before finalization of the 2009 FDA PRO Guidance. In these instances, Astellas closely followed the 2006 Draft FDA Guidance. Astellas followed the 2009 FDA Guidance upon its release.)31 recommendations regarding content validity were rigorously adhered to throughout the development process. Focus group meetings were conducted in English between December 2007 and February 2008 at sites across the United States. Each session lasted approximately 90 min.
Item generation (initial draft instrument)
Following concept elicitation, an item generation meeting of clinicians, statisticians, and PRO experts was held in March of 2008, subsequent to which a draft conceptual framework and instrument were developed by the core research team. All spontaneously elicited concepts were assessed by frequency, clinical relevancy, whether they were considered most bothersome, and saturation (defined as the point after which no new relevant information emerges in subsequent interviews). Concepts that were clear and simple (as opposed to complex and multidimensional), clinically relevant, and had achieved saturation were chosen for inclusion.
Based on these analyses, the initial drafts of the 17-item IBS-Daily Symptom Diary and four-item IBS-D Symptom Event Log were developed (©2012 Astellas Pharma Global Development, Inc. (“APGD”). Reprinted in Clinical and Translational Gastroenterology with permission of APGD. All rights reserved. To seek permission to reprint or distribute copies of the IBS-Daily Symptom Diary and/or IBS-D Symptom Event Log, e-mail copyright@astellas.com.): (1) record of every bowel movement/event and assessment of (2) severity of “immediacy of need”/bowel urgency, (3) incomplete evacuation, and (4) stool consistency. A stool form scale that had been developed to measure diarrhea in patients with HIV32 was selected to assess stool consistency. This stool form scale was an adapted version of the BSFS (hereafter “adapted BSFS”) and was chosen over the widely used BSFS10 as the pictorial representations of the former better matched patients' spontaneous descriptions of stool consistency. Ultimately, both the adapted BSFS and BSFS were cognitively debriefed in phase IV of this study.
Phase II: cognitive interviews (N=11)
Additional patients were recruited to assess patient understanding of the initial draft instrument's instructions, items, and response options in individual face-to-face cognitive interviews (November 2008).
Phase III: data triangulation to confirm concepts elicited in phases I and II (N=32)
Data from four additional gender-specific concept elicitation focus groups that had been conducted for an independent study of patients with IBS-D were triangulated with the data from phases I and II to confirm the initial group of elicited symptoms.33 Triangulation refers to the combination of data sources, different researchers, multiple perspectives on a phenomenon of interest, or the use of multiple methods to study a phenomenon.33, 34 Patients met inclusion/exclusion criteria similar to the other phases of qualitative research, with the primary exception that patients over 70 years of age were excluded to more closely match the target population. Specifically, the age range of inclusion was changed to align with the population of a separate, ongoing clinical trial at Astellas.
Phase IV: concept clarification and cognitive interview of stool images and symptom severity (N=16)
Further clarification of the meaning of the signs and symptoms included in the IBS-D Daily Symptom Diary and IBS-D Event Log was sought via two gender-specific concept clarification/content validation focus groups conducted in April of 2011. Methodologic changes in phase IV included the addition of more directly focused queries along with non-leading, probing follow-up questions. During the cognitive debriefing portion of the focus groups, patients were asked to provide feedback on four handouts to assess the content validity of the IBS-D Symptom Event Log stool form scale images (the adapted BSFS)32 and the BSFS, both shown first without and then with written descriptions. To assess the content validity of the IBS-D Daily Symptom Diary's severity items, patients were asked to compare another set of two handouts that varied the relevant question by asking patients to either rate the “severity” or the “worst severity” of each symptom. Patients were asked to choose an answer and explain why they chose their answer on both handouts.
Phase V: final cognitive interviews (N=20)
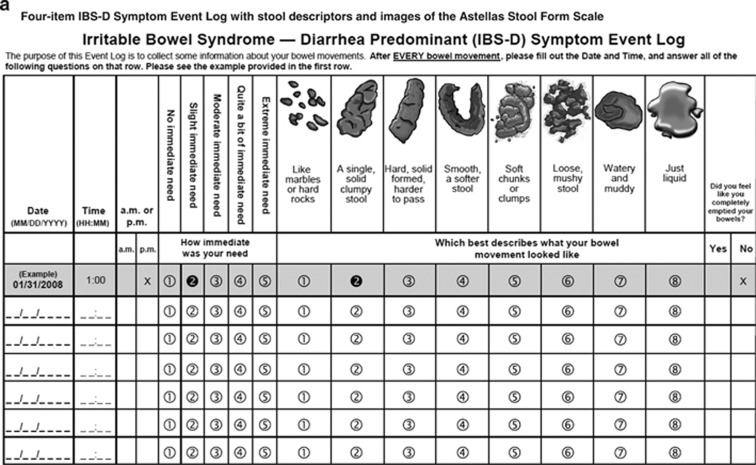
The final two sets of cognitive interviews (N=11 and N=9, respectively) were conducted and analyzed as described previously. The Event Log used in phase V included the newly developed Astellas Stool Form Scale (ASFS) to measure stool consistency; during an item reconciliation meeting, it was decided to revise the written descriptions of stool consistency to better match patients' verbal descriptions, to add two new images to the Event Log stool consistency continuum, and to replace existing images with new ones created by an artist. The development of the ASFS will be more fully described in a forthcoming publication.
RESULTS
Study population
The patient and demographic characteristics of the 113 patients who participated in the five qualitative research phases of the study are presented in Table 1. Approximately 63% of patients were women. The mean ages of participants of both the initial and latter round of focus groups and interviews were roughly comparable, ranging from 44.7±15.5 to 52.7±7.9 years (Table 1). Other demographic characteristics were similarly comparable between focus groups. Condition severity ranged across patients; however, most reported their IBS-D to be mild or moderate, as would be expected based on IBS epidemiology.35 Information associated with screening failures was not collected and therefore is not included in the results of this study.
Table 1. Patient demographics.
| Concept elicitation focus groups ( N =34) | Initial cognitive interviews ( N =11) | Triangulation with additional focus groups ( N =32) | Concept clarification focus groups ( N =16) | Final cognitive interviews ( N =20) | |
|---|---|---|---|---|---|
| Male, n (%) | 12 (35%) | 5 (45%) | 8 (25%) | 8 (50%) | 9 (45%) |
| Female, n (%) | 22 (65%) | 6 (55%) | 24 (75%) | 8 (50%) | 11 (55%) |
| Mean age (s.d.) | 44.7 (15.5) | 50.7 (13.1) | 45 (10.8) | 52.7 (7.9) | 49 (12.1) |
| Black/African American | 4 (12%) | 2 (18%) | 4 (13%) | 4 (25%) | 12 (60%) |
| Hispanic/Latino of any race | 1 (3%) | 1 (9%) | 1 (3%) | 2 (12%) | 0 (0%) |
| White/Caucasian | 26 (76%) | 6 (55%) | 26 (81%) | 10 (63%) | 8 (40%) |
| Othera | 3 (9%) | 2 (18%) | 1 (3%) | 0 (0%) | 0 (0%) |
| Education | |||||
| High school diploma (or GED) or less | 5 (15%) | 0 (0%) | 6 (19%) | 0 (0%) | 6 (30%) |
| Some college or certificate program | 8 (23%) | 11 (100%) | 18 (56%) | 4 (25%) | 3 (15%) |
| College or university degree (2–4 years) | 16 (47%) | 0 (0%) | 6 (19%) | 7 (44%) | 10 (50%) |
| Graduate degree | 5 (15%) | 0 (0%) | 2 (6%) | 5 (31%) | 1 (5%) |
| Health status (patient report) | |||||
| Excellent | 4 (12%) | 2 (18%) | 0 (0%) | 3 (19%) | 1 (5%) |
| Very good | 9 (26%) | 1 (9%) | 6 (19%) | 5 (31%) | 5 (25%) |
| Good | 14 (41%) | 5 (45%) | 20 (62%) | 8 (50%) | 10 (50%) |
| Fair | 5 (15%) | 3 (27%) | 6 (19%) | 0 (0%) | 4 (20%) |
| Poor | 2 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Severity of IBS-D (patient report) | |||||
| Very mild | 4 (12%) | 1 (9%) | 1 (3%) | 0 (0%) | 1 (5%) |
| Mild | 8 (23%) | 4 (36%) | 4 (12%) | 4 (25%) | 3 (15%) |
| Moderate | 19 (56%) | 4 (36%) | 18 (56%) | 9 (56%) | 13 (65%) |
| Severe | 3 (9%) | 1 (9%) | 8 (25%) | 3 (19%) | 2 (10%) |
| Very severe | 0 (0%) | 1 (9%) | 1 (3%) | 0 (0%) | 1 (5%) |
GED, general equivalency diploma; IBS-D, diarrhea-predominant irritable bowel syndrome; s.d., standard deviation.
Note: Patients were only eligible to participate in one phase of the study.
The “other” category does not include Asian Americans. No Asian Americans were part of this study.
Phase I: concept elicitation (N=34)
The most common symptom spontaneously mentioned by patients during the concept elicitation focus group sessions was diarrhea (n=29); the second most common was the immediate need to have a bowel movement (n=18). Patients at times described “immediate need” as the sensation of needing to have a bowel movement but not necessarily being able to empty their bowels. Cramps and increased frequency of bowel movements were spontaneously mentioned by 16 patients each. Four of the 16 patients who reported cramps stated that cramping was a precursor to diarrhea. Patients tended to differentiate between bloating, pressure, and gas; bloating was described as the buildup of gas in the abdomen and/or referred to as a visual distension, whereas pressure was consistently described as an internal sensation in relation to a need to defecate, and gas was reported in relation to the actual expulsion of gas through the rectum.
The most bothersome symptom mentioned by patients during the concept elicitation focus group sessions was abdominal/stomach pain (n=10), followed by frequency of bowel movements (n=7). Overall, 36 concepts were elicited in total. A summary of the 22 most frequently elicited concepts, rationale for their inclusion or exclusion in the final draft of the PRO, and an exemplary quote for each concept are presented in Table 2. Of note, 12 subjects mentioned constipation as a symptom, which speaks to the severity continuum of the disease, and suggests that even among patients diagnosed as having the diarrhea-predominant subtype of IBS, there can still be occasional constipation.36 Concepts 23–36 (pain following a BM, no signs before episode, loss of appetite, increased time spent during each visit to bathroom, pain when going to the bathroom, achiness/non-GI-related pain, digestive pain when eating certain foods, weight loss, no control over condition, can't eat certain foods, spasms, soreness in rectum, vomiting, and weight gain) were reported by very few patients (n<4) and were either not considered relevant, vague/ill-defined, or related to other concepts already mentioned.
Table 2. Details of the 22 concepts most commonly reported during concept elicitation and rationale for their inclusion or exclusion in the PRO.
| No. | Concept elicited –ranked by frequency in focus groups | Spontaneously elicited concept in focus groups total ( N =34) | Most bothersome ( N =34) | Decision and rationale for inclusion or exclusion in final PRO | Exemplary quote |
|---|---|---|---|---|---|
| 1. | Diarrhea | 29 | 5 | Included; saturated; bothersome | (…) I went for nine straight days. Four and five times. Towards the end it went down to like three times but it was all diarrhea. |
| 2. | Immediate need (urgency) | 18 | 3 | Included; saturated; bothersome | (…) Once it flares up, you got to go. Like right then and then if you don't, it's going to come whether you go or not. |
| 3. | Frequency of BMs | 16 | 7 | Included; saturated; bothersome | Once in awhile you got to go like ten times. As soon as you come out of the bathroom, you got to go back in. |
| 4. | Cramps | 16 | 5 | Included; saturated; bothersome | I've figured out the worst thing about this is the cramps. I can deal with the diarrhea, I can deal with the flatulence, I'm just—man, them cramps, they just—the pain is just about as bad as a migraine. |
| 5. | Bloating/pressure | 14 | 3 | Included; saturated; bothersome | Like I know if I don't go for days that I will get bloated and then I get the pain and then when I do go, it all goes away. |
| 6. | Abdominal/stomach pain | 13 | 10 | Included; saturated; bothersome | Yeah, when I have it I have severe pain across the whole bottom of my stomach |
| 7. | Constipation | 12 | 0 | Excluded; usually described as prior to IBS-D; may have had IBS-C or mixed IBS first; ultimately included in the ASFS as hard stools end of severity continuum because reported in concept clarification groups and confirmed in last sets of cis. The number of bowel movement is also capture by the event log | Yeah, I would get nauseous, too a lot of the time. I started off getting constipated or a flare-up and then I'd get really kind of nauseous, when I kind of went into that diarrhea phase, but I can relate with that. |
| 8. | Gas | 10 | 5 | Included; saturated; bothersome; changed to “pass gas” after last interviews based on patient meaning of concept. | I get the cramps and that, I get a lot of gas. I feel bloated. My stomach is rumbling and I pass gas a lot. |
| 9. | Tired/weakness | 10 | 0 | Excluded; not specific to IBS-D | I think if you have a lot of days of having the diarrhea, it naturally makes you fatigued. I mean, because you're losing so much liquids and whatever, and I think it can cause tiredness. |
| 10. | Completely emptied bowels/incomplete evacuation | 9 | 0 | Included; saturated; confirmed as core concept and bothersome; included as yes/no on event log | I'll have two or three, four movements and still feel like I'm not quite emptied out yet. |
| 11. | Nausea | 9 | 0 | Excluded; upper GI symptom | When I first started I thought I had the flu because you had the diarrhea and the nauseous and that kind of thing. So I thought I was having the flu. However, it kept continuing for a long time. But I have a sensitive stomach, too. |
| 12. | Accident | 7 | 0 | Included; saturated; appeared to be the severe end of the BM frequency and stool consistency severity continuum of IBS-D | I was once in traffic in New York and what is it—the Washington Bridge? … Up there at the top in traffic like you couldn't move anywhere…and I had to go. … I went. |
| 13. | Lack of control | 7 | 0 | Excluded; saturated, but more an emotional impact than symptom (lack of control over life); concept appears to overlap with accident | (…) It does. That's why I say, it controls so much of where you go, what you do. |
| 14. | Bleeding from rectum/blood in stool | 6 | 2 | Excluded; more an impact than symptom; secondary to IBS-D | When I saw that blood, I was like this is not good. So I was thinking there's something with my stomach. So it's like once I saw the blood, I was like man, I'm getting blood in my stool |
| 15. | Bubbling in intestines/bowel sounds | 6 | 0 | Excluded; saturated but further analysis found concept was not specific to IBS-D | I guess that's one other symptom, is the growling noise that you hear. The gurgling. … Usually it comes with the cramps. Sometimes when you hear growling you feel the cramps. |
| 16. | Rectal burning | 5 | 0 | Excluded; saturated but further analysis found concept was secondary to IBS | This is my little trick of the trade. And it's got the little Wet Ones that you can flush and then I carry with me Gynecort because—or Vagisil or something, because when it begins to end, it starts to burn. |
| 17. | Heartburn | 5 | 0 | Excluded; upper GI symptom; not specific to IBS-D | I experience heartburn. |
| 18. | Sweats | 5 | 0 | Excluded; not a lower GI symptom; not specific to IBS-D | This—sweating, yeah. Instant hot. Head to toe. |
| 19. | Rectal spasm | 4 | 1 | Excluded; saturated and bothersome but further analysis found secondary to frequent BMS; not specific to IBS-D | (…)When I say a spasm, I'm talking about, it actually feels like it's in my rectum. … Just sort of pulsating, kind of trying to get something to work. |
| 20. | Stool consistency | 4 | 0 | Included; saturated; clinically relevant | But looking at the physical stool you know that something is not right. It's very—sometimes it's chunky liquid. |
| 21. | Inflamed rectum | 4 | 0 | Excluded; concept captured in “pain while wiping” and further data collection and analysis found secondary to IBS-D | So now it's in that point, now it's probably a little bit inflamed over there, and it's red over there. |
| 22. | Pain while wiping | 4 | 0 | Excluded; saturated but further data collection and analysis found secondary to IBS-D | And so it's just rough … and it's also you get really sore from using toilet paper and all. … And it really worked well, because the time before that, I was in such pain from all the toilet paper. So I just said, well I'm not using toilet paper ever again. I'm going to a baby wipe guy. |
BM, bone marrow; IBS-D, diarrhea-predominant irritable bowel syndrome; PRO, patient reported outcome.
Item generation (initial draft instrument)
Of the 36 symptoms identified during concept elicitation, 14 were retained based on concept saturation, bothersomeness, and reported frequency for the first draft of the 17-item Instrument: diarrhea (frequency and severity), immediate need (frequency and severity), stomach and abdominal pain (severity), abdominal cramp (severity), pressure in the abdomen (severity), feeling full (frequency), bloating (severity), gas (frequency), gurgling signaling diarrhea (frequency), complete evacuation (frequency), rectal spasm (severity), rectal burning after bowel movement (severity), pain after wiping (severity), and accidents (frequency). Rectal symptoms were initially incorporated into the instrument; however, other GI symptoms (e.g., heartburn) or general concepts (e.g., feeling tired) were excluded. An event log format intended to be used following every bowel movement was used for symptoms associated with bowel movements (examining stool consistency), and a daily diary format was used for other symptoms (e.g., abdominal pain). Response categories for the daily diary used an 11-point numeric rating scale, while the event log used either a five-point or six-point ordinal (Likert-type) scale. The first version of the conceptual framework was developed at this point.
Phase II: cognitive interviews (N=11)
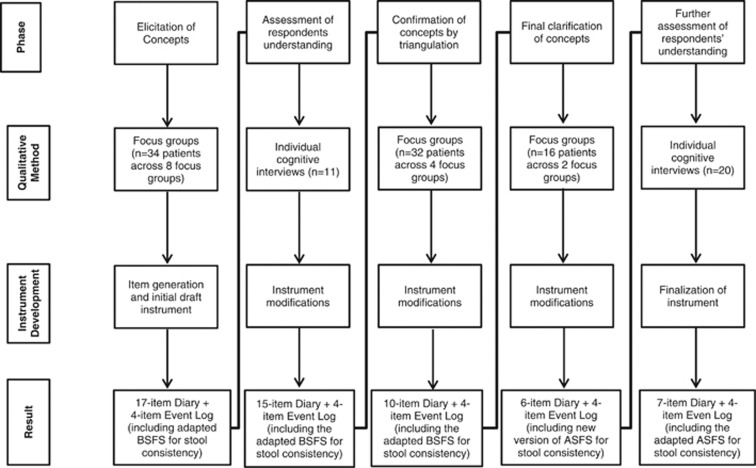
All 11 patients participating in cognitive interviews of the initial draft instrument were found to understand the instructions and items as intended, with the exception of two questions: immediate need, which was deleted as it was already included in the Event Log, and fullness or early satiety, which was subsequently deleted (resulting in a 15-item Diary); the Event Log was left unchanged. See Table 3 for an inventory of the major revisions in the development of the Diary and Event Log across research activities and studies.
Table 3. Instrument evolution by development phase.
| Phase | Qualitative method | Instrument development | Result |
|---|---|---|---|
| Elicitation of concepts | Focus groups (n=34 patients across eight focus groups) | Item generation and initial draft instrument | 17-Item Diary+4-item Event Log (including adapted BSFS for stool consistency) |
| Assessment of respondents understanding | Individual cognitive interviews (n=11) | Instrument modifications | 15-Item Diary+4-item Event Log (including the adapted BSFS for stool consistency) |
| Confirmation of concepts by triangulation | Focus groups (n=32 patients across four focus groups) | Instrument modifications | 10-Item Diary+4-item Event Log (including the adapted BSFS for stool consistency) |
| Final clarification of concepts | Focus groups (n=16 patients across two focus groups) | Instrument modifications | 6-Item Diary+4-item Event Log (including new version of the ASFS for stool consistency) |
| Further assessment of respondents understanding | Individual cognitive interviews (n=20) | Finalization of instrument | 7-Item Diary+4-item Event Log (including the ASFS for stool consistency) |
ASFS, Astellas Stool Form Scale; BSFS, Bristol Stool Form Scale; Diary, IBS-D Daily Symptom Diary; Event Log, IBS-D Symptom Event Log.
Phase III: data triangulation for concept confirmation (N=32)
Data from the additional focus groups were used to confirm the concepts in the initial draft of the PRO with regard to spontaneous reporting and saturation. No new concepts regarding IBS-D symptoms were reported, confirming that concepts and their meanings from both data sources were consistent.
Patient feedback and internal review led to the deletion of five items from the 15-item IBS-D Daily Symptom Diary. Items on the frequency and severity of diarrhea were removed from the Diary, as the potential accuracy and reliability of bowel movement frequency and stool consistency would be better assessed in the Event Log. The severity of diarrhea item was also deleted from the Event Log. Another immediate need item was similarly removed from the Diary because it was redundant with an item in the Event Log and was thought to be better captured at each event. The incomplete evacuation question was deleted from the diary and added to the event log because these data were considered to be more accurate if captured at each event. Pain after wiping was also deleted, as it was considered secondary to bowel movement frequency.
Further modifications included changes to the response option for the item on incomplete evacuation, which was revised to a dichotomous yes/no and integrated into the Event Log. In addition, the response option for the item concerning accidents was changed from a frequency rating scale to a dichotomous yes/no. Finally, a five-point Likert-type scale (five response choices containing the following: None of the time, A little of the time, Some of the time, Most of the time, and All of the time), rather than an 11-point numeric rating scale, was chosen as a better response option for all frequency questions based on internal review and regulatory advice.
Overall, these modifications resulted in a 10-item Diary: stomach pain (severity), abdominal pain (severity), abdominal cramping (severity), pressure in the abdomen (severity), bloating (severity), gas (frequency), sounds signaling diarrhea (frequency), rectal spasm (severity), rectal burning after bowel movement (severity), accident (yes/no); and a four-item Event Log: (1) timing of each bowel movement, (2) assessment of immediate need, (3) incomplete evacuation, and (4) stool consistency). Both stomach pain and abdominal pain were kept as separate items at this stage, as both were reported separately and distinctly by patients.
Phase IV: concept clarification and cognitive interview of stool images and symptom severity (N=16)
The most commonly experienced symptom in the phase IV set of two hybrid focus groups was bloating (n=14). Most patients described bloating as both the sensation of being overly full and the sign of bowels getting ready to release. One patient described the sensation as, “Well, for me, it just feels like I'm swollen and full, like I just had this—maybe drank a gallon of soda, you know? I'm just—feel like I'm going to explode.” Abdominal or stomach pain was mentioned by 13 patients. One patient described abdominal or stomach pain as, “the pain is the worst. Yes, it is. And you curl up like a little baby, you know?”
Thirteen patients also reported experiencing immediate need. Most patients described immediate need as having to use the bathroom but not always being able to get to one fast enough. In the words of one patient, “It's like when you've got to go, but you can't find somewhere to go fast enough, so you're holding it because you can't.” Ten of the 16 patients also associated immediate need with accident: “Um, it's sometimes hard to not be close to a bathroom. And um I've had to wear protective underwear. So that's kind of embarrassing for myself.”
Twelve patients each spontaneously mentioned diarrhea, cramps, constipation, and accident with quotes similar to those reported in the previous focus groups and cognitive interviews. Eleven patients each mentioned abdominal pressure and gas. Nine patients mentioned rectal burning, usually due to frequent bowel movements and wiping. Eight patients mentioned rectal spasms, which were typified by extreme pain: “For me, it's like a shooting pain … that is very, very painful.” Six patients mentioned incomplete evacuation, describing it as either a sensation of not having completely emptied their bowels, or having to repeatedly return to the bathroom after they thought a bowel movement had been completed. One patient elaborated, “You empty your bowel and you … thought it was completely empty…. I'll wash and then I'll go to the living room … And grrrr, right back.” No new concepts or subconcepts regarding symptoms of IBS-D were reported in the last two focus groups compared with the previous focus groups, again confirming saturation and frequency (count of concepts).
Following the concept confirmation focus groups, an item reconciliation meeting of the entire multidisciplinary research team was held. Further internal review of the draft instrument, in conjunction with regulatory guidance, led to the following additional instrument modifications: items on rectal spasms, burning sensation, and abdominal sounds were deleted, as they were deemed secondary to diarrhea; the anchors for abdominal/stomach pain, abdominal pressure, and abdominal cramps were changed from “worst” to “most severe” to maintain consistency with item stem wording; and one item, “accidents,” was reformatted to appear in the same table format as the other items for consistency (even though it differed by response option).
In addition, pictorial descriptions of the stool form scale in the (Figure 1) Event Log were replaced with original artwork developed specifically for the instrument (Figure 2) to better match patients' verbal descriptions and to encompass all the stool forms described by them. Once the new stool form descriptions were finalized, a graphic artist was provided with images from the BSFS,10 the adapted BSFS, and the King's Stool Chart37, 38, 39 as models to generate new stool illustrations. Based on comparisons of patient descriptions, the final stool descriptors selected were: “like marbles or hard rocks;” “a single, solid clumpy stool;” “hard, solid formed, harder to pass;” “smooth, a softer stool, almost snake-like;” “soft chunks or clumps;” “loose, mushy stool;” “watery and muddy;” and “just liquid.” Based on these descriptors, two new stool images were defined for the stool consistency continuum. The graphic artist designed a set of eight new images in total (two new images plus new depictions of six existing images) based on the patient descriptions and images found in the public domain. The original descriptors for stool consistency were also revised based on further analysis of accounts provided by focus group patients.
Figure 1.
Instrument evolution by development phase.
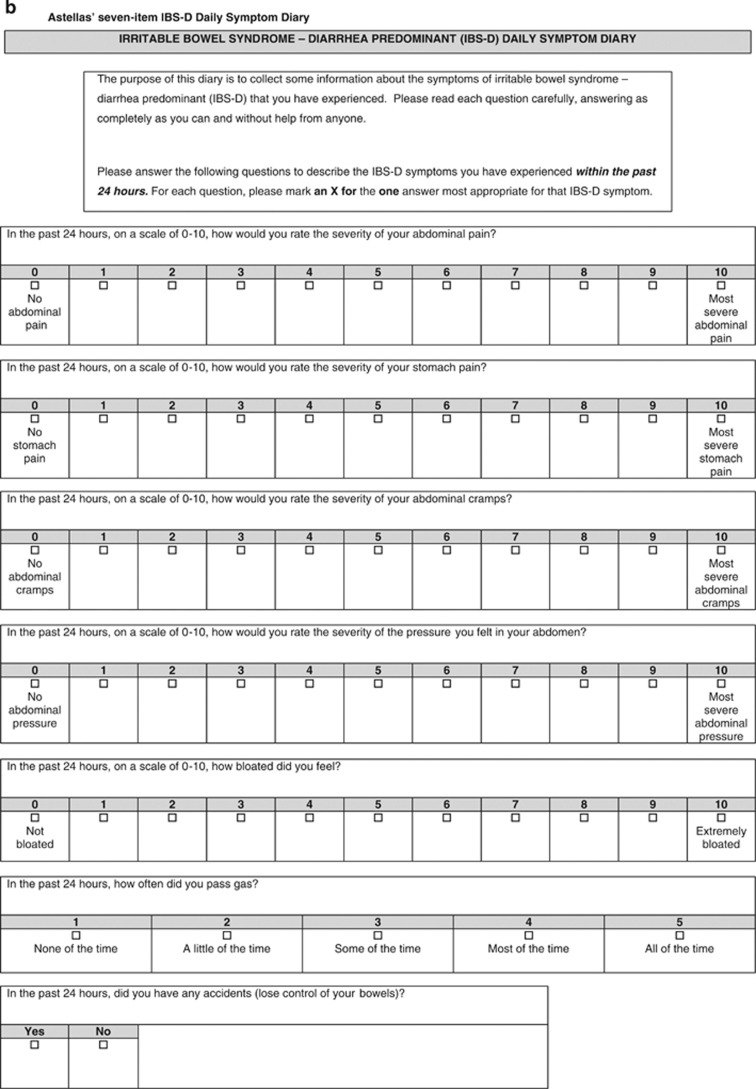
Figure 2.
Final four-item IBS-D Symptom Event Log with stool descriptors and images of the Astellas Stool Form Scale (a) and seven-item IBS-D Daily Symptom Diary (b).
Phase V: cognitive interviews of the seven-item IBS-D Daily Symptom Diary and four-item IBS-D Symptom Event Log (N=20)
Only minor changes were made to the Diary following the final sets of cognitive interviews: the phrase “Irritable bowel syndrome” was added to the instructions, as some patients were unfamiliar with the IBS-D acronym; originally, the abdominal and stomach pain concepts were merged into a single item, but the stomach/abdominal pain item was then divided into two items because patients reported both and described the pain differently and in different locations (specifically, half of the patients (n=10, 50%) referred to abdominal/stomach pain as stomach pain, two (10%) referred to it as abdominal pain, three (15%) referred to it as both, and three used alternative terms (intestinal pain or cramps); the cramps item was moved to follow the abdominal pain item after the latter set of cognitive interviews to help determine whether and how patients differentiate between the two symptoms; and the instructions were changed to clarify that every bowel movement was to be recorded, whether or not it appeared to be related to IBS-D.
All items retained for the final version of the instrument (Figure 2) were found to be relevant, readable, comprehensible, and important to patients during the final round of cognitive interviews, further supporting the content validity of the instrument.
Conceptual framework resulting from all stages of the qualitative research
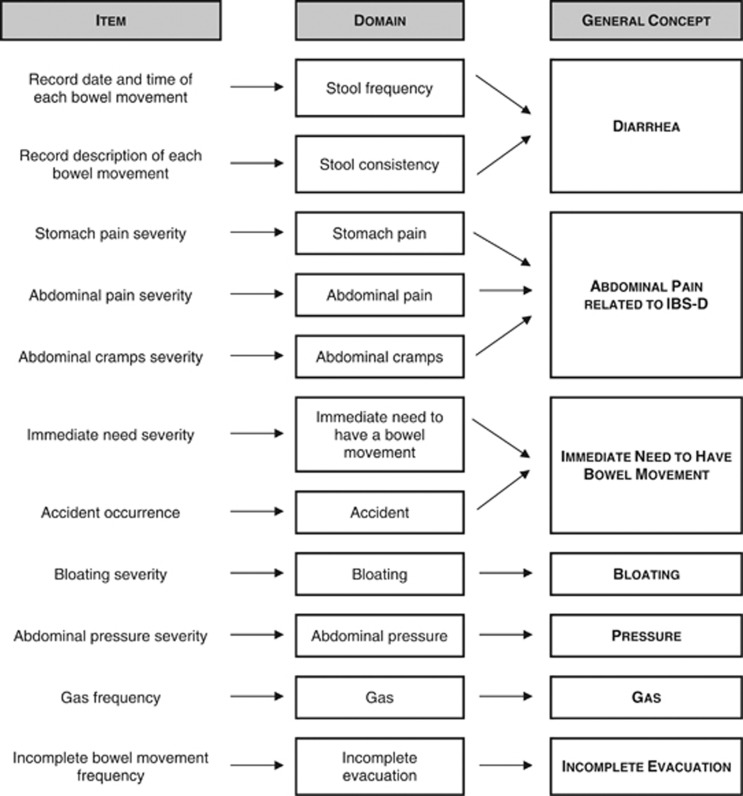
A conceptual framework was first developed during the initial item generation and subsequently revised based on feedback captured during study phases III and IV, which resulted in the deletion of the abdominal sounds, rectal spasm, and rectal burning items and domains.
The conceptual framework for the IBS-D Daily Symptom Diary and the IBS-D Symptom Event Log that emerged from all of the qualitative research described here is provided in Figure 3. The framework is divided into three columns outlining items, domains, and general concepts from left to right. Based on the qualitative research, it is proposed that the stool frequency and stool consistency items from the event log should be combined to form a single diarrhea score. Similarly, it is hypothesized that the three items relating to pain in the abdominal area (stomach pain, abdominal pain, abdominal cramps) should be combined to form a single score assessing abdominal pain related to IBS-D. It is suggested that the items measuring the immediate need to have a bowel movement and the occurrence of accidents could be combined into a single score, reflecting the fact that the two concepts are closely related. It is hypothesized that the remaining concepts should be scored as single item scores.
Figure 3.
Conceptual framework resulting from qualitative research.
DISCUSSION
Neither a biomarker nor a validated PRO that conforms to current regulatory guidance is currently available for the assessment of treatment benefit in IBS-D. The end results of the five-phase qualitative research study described in this report—a multidisciplinary collaboration of clinicians, PRO experts, and industry representatives, together with guidance from regulatory authorities—are the seven-item IBS-D Daily Symptom Diary and four-item IBS-D Symptom Event Log (Figure 2), the first IBS-D qualitative symptom measure for evaluating treatment benefit in interventional trials or in a real-world clinical setting developed in compliance with modern US regulatory guidance. Specifically targeted for use as a clinical trial end point, the measure's development has also benefited from the evaluation and recommendations of the FDA's GI Division and the SEALD group.
While the original draft instrument initially incorporated the adapted BSFS,32 a widely used stool form scale and FDA-recommended coprimary end point for IBS-D trials,4 the new ASFS was eventually created in response to the failure of the BSFS to capture adequately all stool forms described by patients in the opening round of focus groups. The ASFS, in contrast to the BSFS,10 was developed in an IBS-D-only population and includes constipation representations and a finer gradation of diarrhea, reflecting the variations of diarrhea seen in IBS-D. A full description of the development of the ASFS is in preparation.
Other modifications of note to the original draft include changes to the abdominal pain severity item. Initially developed based on the results of the first round of focus groups, the scale was designed as a simple measure of the severity of abdominal pain. Although this is counter to the FDA's guidance with regard to IBS,4 which recommends assessment of abdominal pain at its worst, testing of both versions of the abdominal pain severity item in phase IV focus groups found the more appropriate wording to be: “In the past 24 h, on a scale of 0–10, how would you rate the severity of your abdominal pain?” The final set of cognitive interviews, just as the first, also confirmed the relevance and comprehensibility of the initial form of the severity item. Moreover, it should be noted that the recommendations in the FDA IBS guidance are acknowledged to be interim recommendations, to stand until instruments developed to the standard required in the FDA PRO guidance became available.
While efforts were made to enroll a diverse sample of IBS-D sufferers, not all demographic groups were necessarily well represented during development activities. In particular, relatively few Hispanics and no patients of Asian ethnic background participated in the study (Table 1). In all phases, patients self-reported their severity; clinicians' reported severity was also collected in phases III and IV as part of inclusion of the study. Additionally, patients with severe and very severe IBS-D (as based on patient self-report on a five-point patient global impression of severity item noted on Table 1) were potentially under-represented. However, saturation was achieved for all concepts spontaneously elicited by the initial round of focus groups, and the concepts selected for inclusion were confirmed in subsequent cognitive interviews and focus groups, indicating that the concepts underpinning the newly developed instrument are robust and comprehensive in the sampled population. Nevertheless, subsequent validation work should confirm the instrument's applicability in these (as well as other) populations.
An additional potential limitation concerns the triangulation data set, which excluded patients above the age of 70 years. This adjustment of the age criteria was made to more closely match the epidemiology of the disease, as it occurs predominantly in patients between 25 and 64 years of age; seventy-five percent of IBS patients fall within this age range, with peak prevalence occurring between the ages of 20 and 30 years, afflicting individuals most severely during the period of productive work life and thereby significantly increasing the patient and societal burden of disease.37 However, the change in criteria was of minor consequence to the study, as it was only in phases 1 and 2 that patients 70 years and over were initially eligible to participate; phase I enrolled no patient over the age of 70 years, and phase II, only one patient (aged 72 years).
It is also worth noting that the PRO instrument developed in the course of these activities was based on feedback from a US-only population. However, literature describing the symptomatology of IBS in Asian populations, for example, suggests this instrument may effectively capture relevant symptoms in other populations, as well.40 A rigorous, cross-cultural validation of the measure would be necessary to support its content validity before use in non-US populations, and this represents a necessary future step in the instrument's development. To date, the PRO has been linguistically validated for 10 countries and is in the process of cultural validation for several of these. This process and its results will be described in a forthcoming publication.
Mention should also be made of the inclusion of constipation as one of the symptom concepts assessed. While its inclusion may be potentially viewed with concern (as it is not a diagnostic criteria of IBS-D), from phase I onward a considerable number of patients mentioned that they would occasionally experience constipation. This is consistent with evidence that patients often transition between subtypes36 as well as the Rome III definition of IBS-D.
Future development of the IBS-D Daily Symptom Diary and Symptom Event Log will require an assessment of the instrument's psychometric properties. A hypothesized conceptual framework was developed based on the initial concept elicitation research and revised following the subsequent stages of qualitative research. The next step in the evaluation of this newly developed IBS-D instrument is to assess the appropriateness of this proposed framework through quantitative analysis of the instrument's measurement properties. This process will include potentially culling additional items based on further consideration of the qualitative findings reported herein, the clinical relevance of items, and the initial psychometric performance of the items and proposed scores. Thus, it is likely that the conceptual framework and instrument may evolve further. The resulting instrument will then be subjected to full psychometric testing to evaluate its reliability, validity, and responsiveness to determine whether it would be suitable as a clinical trial end point. The ultimate value of the IBS-D Daily Symptom Diary and IBS-D Symptom Event Log will be determined by application in clinical trials and in the real-world clinical setting.
In conclusion, the IBS-D qualitative research and instrument development reported herein has been completed in close adherence with regulatory guidance, and has captured the fundamental and patient-relevant symptomatology of IBS-D. The newly developed Astellas IBS-D Daily Symptom Diary and IBS-D Daily Event Log jointly represent the first IBS-D qualitative symptom measure for evaluating treatment benefit developed in compliance with FDA regulatory guidance. Nevertheless, development of scoring and quantitative validation is necessary before the development of the instrument can be considered complete.
Study Highlights

Acknowledgments
We thank Astellas colleagues: Maggie Ayers, Jana Cummings, Ingrid Gagainis, Rosanne Jordan, Amy Johnson, Maggie Liosatos, Salim Mujais, MD, Allam Fakhoury, PharmD, and Adelphi Values colleagues: Benjamin Banderas, Jonathan Stokes, Nina Galipeau, Crystal Tellefsen, Allison Kornstein, and Jessica Santiccioli for their contribution to the development of this instrument. We also thank Ramon Iovin, PhD, for his editorial contributions.
Guarantor of the article: Leticia Delgado-Herrera, RPh, MS.
Specific author contributions: P.M. provided initiation and senior leadership of the study; design of the manuscript; approval of the final version of the manuscript. K.E.L. designed the research and had overall responsibility for the conduct of the study reported within this manuscript, development of the discussion guide that was used to collect the data for the focus groups and individual interviews, conducted some of the interviews, oversaw and developed the methods to analyze the data; wrote the original draft of the manuscript; and approved the final draft of the submitted manuscript. L.D.H. participated in the planning and review of the protocol for the conduct of the study, and provided comments to the overall strategy and execution; participated in the review and interpretation of the data, and aided with next protocols in the development of the Astellas' PRO IBS-D; helped prepare the paper and provided extensive comments/revisions to the draft versions of the paper; reviewed and approved the final draft submitted to the journal. S.K. provided input on the design of this research and conduct of the study; critically reviewed the discussion guide, study protocol, deidentified patient transcripts and analysis plan; provided input on the original outline, draft, and final version of the manuscript. A.L. provided interpretation of data/analysis, design of the manuscript, and approval of the final version of the manuscript. C.L. participated in planning of study, interpretation of data, and review of manuscript. G.S. participated in the planning and review of the protocol for the conduct of the study, and in the review and interpretation of the data; helped prepare the paper and provided extensive comments/revisions to the draft versions of the paper; and approved the final draft submitted. A.N. participated in the review of the protocol for the conduct of the study; reviewed the final draft submitted to the journal. L.T.W. oversaw and participated in the collection and analysis of the data, collaborated in the development of the IBS-D Daily Symptom Diary and 4-item IBS-D Symptom Event Log, coauthored the report of the study findings, which preceded the manuscript; reviewed and revised drafts of the manuscript; and approved the final draft of the manuscript that has been submitted. E.P. provided support in study design, data collection, analysis, and interpretation. K.R. was involved in the development of the tool, the planning of the studies, interpretation of data and in drafting the manuscript, and has approved the final draft. B.Z. provided oversight of the Astellas IBS-D patient-reported outcome development program, including review and input to protocols, study reports, and this manuscript.
Financial support: Funding for the study and this publication was provided to Adelphi Values by Astellas Global Pharma Development.
Potential competing interests: L.D.H., C.L., G.S., A.N., and B.Z. are employees of Astellas Pharma Global Development. S.K. was an employee of Astellas Pharma at the time of the research and is presently used elsewhere. K.R. is listed as an inventor on the patent for the tool, but has no financial stake. L.T.W. was an employee of Adelphi Values at the time of this research. P.M., K.E.L., A.L., and E.P. have no potential competing interests to declare.
References
- Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol. 2010;25:691–699. doi: 10.1111/j.1440-1746.2009.06120.x. [DOI] [PubMed] [Google Scholar]
- Gilkin RJ., Jr The spectrum of irritable bowel syndrome: a clinical review. Clin Ther. 2005;27:1696–1709. doi: 10.1016/j.clinthera.2005.11.012. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Food and Drug Adminstration, Center for Drug Evaluation and Research Guidance for Industry: Irritable Bowel Syndrome—Clinical Evaluation of Products for Treatment, Silver Spring, MD,2012
- Committee for Medicinal Products for Human Use Concept paper on the revision of the CHMP points to consider on the evaluation of medicinal products for the treatment of irritable bowel syndrome (CPMP/EWP/785/97)European Medicines Agency, May2012
- US Department of Health and Human Services Guidance for Industry: Irritable Bowel Syndrome—Clinical Evaluation of Products for Treatment. Draft Guidance: Silver Spring, MD2010
- Muller-Lissner S, Koch G, Talley NJ, et al. Subject's Global Assessment of Relief: an appropriate method to assess the impact of treatment on irritable bowel syndrome-related symptoms in clinical trials. J Clin Epidemiol. 2003;56:310–316. doi: 10.1016/s0895-4356(03)00027-1. [DOI] [PubMed] [Google Scholar]
- Wiklund IK, Fullerton S, Hawkey CJ, et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947–954. doi: 10.1080/00365520310004209. [DOI] [PubMed] [Google Scholar]
- Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990;300:439–440. doi: 10.1136/bmj.300.6722.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassany O, Marquis P, Scherrer B, et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527–533. doi: 10.1136/gut.44.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groll D, Vanner SJ, Depew WT, et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962–971. doi: 10.1111/j.1572-0241.2002.05616.x. [DOI] [PubMed] [Google Scholar]
- Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- The EuroQol Group EuroQol-5D: a new facility for the measurement of health-related quality of life. Health Pol. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Bushnell DM, Martin ML, Ricci JF, et al. Performance of the EQ-5D in patients with irritable bowel syndrome. Value Health. 2006;9:90–97. doi: 10.1111/j.1524-4733.2006.00086.x. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- PRO Labels-Patient Reported Outcomes Labels. [electronic article]. Available at: http://www.mapi-prolabels.org/2010. accessed: November 2013.
- World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. Nurs Ethics. 2002;9:105–109. doi: 10.1191/0969733002ne486xx. [DOI] [PubMed] [Google Scholar]
- United States Health Insurance Portability and Accountability Act of 1996 Public Law 104-191. US Statut Large. 1996;110:1936–2103. [PubMed] [Google Scholar]
- Lasch KE, Marquis P, Vigneux M, et al. PRO development: rigorous qualitative research as the crucial foundation. Qual Life Res. 2010;19:1087–1096. doi: 10.1007/s11136-010-9677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- Strauss A, Corbin J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Sage: London, UK; 1998. [Google Scholar]
- K Charmaz. Good Days Bad Days: the Self in Chronic Illness and Time. Rutgers University Press: New Brunswick, NJ; 1991. [Google Scholar]
- Charmaz K.Grounded theoryIn Smith JA, Harre R, Van Langenhove L, (eds)Rethinking Methods in Psychology Sage: London, UK; 199527–49. [Google Scholar]
- Glaser B, Strauss AL. The Constant Comparative Method of Qualitative Analysis. Discovery of Grounded Theory: Strategies for Qualitative Research. Aldine de Gruyter: New York, NY; 1967. pp. 101–116. [Google Scholar]
- Muhr T.User's Manual for Atlas.ti 5.0 Berlin: Atlas.ti. [5.0] Berlin2004
- US Department of Health and Human Services, Food and Drug Administration Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims, Silver Spring, MD,2009
- Willis GB. Cognitive Interviewing in Practice: Think-Aloud, Verbal Probing, and Other Techniques. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Sage Publishers: Thousand Oaks, CA; 2005. pp. 42–63. [Google Scholar]
- Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10:269–281. doi: 10.1586/erp.10.30. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services Draft Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Food and Drug Administration: Silver Spring, MD,2006
- Mertz HR, Beck CK, Dixon W, et al. Validation of a new measure of diarrhea. Dig Dis Sci. 1995;40:1873–1882. doi: 10.1007/BF02208649. [DOI] [PubMed] [Google Scholar]
- Janesick VJ. The Dance of Qualitative Research: Metaphor, Methodology, and Meaning. Handbook of Qualitative Research. Sage Publications: London, UK; 1994. pp. 209–218. [Google Scholar]
- Patton M. Qualitative Research & Evaluation Methods. Sage Publications: London, UK; 2002. [Google Scholar]
- Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: a Rome Foundation Working Team Report. Am J Gastroenterol. 2011;106:1749–1759. doi: 10.1038/ajg.2011.201. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Morris CB, Hu Y, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005;128:580–589. doi: 10.1053/j.gastro.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Whelan K, Judd P, Taylor M. Defining and reporting diarrhoea during enteral tube feeding: do health professionals agree. J Hum Nutr Diet. 2003;16:21–26. doi: 10.1046/j.1365-277x.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- Whelan K, Judd P, Taylor M. Assessment of fecal output in patients receiving enteral tube feeding: validation of a novel chart. Eur J Clin Nutr. 2004;58:130–137. doi: 10.1038/sj.ejcn.1601927. [DOI] [PubMed] [Google Scholar]
- Whelan K, Judd P, Preedy V, et al. Covert assessment of concurrent and construct validity of a chart to characterize fecal output and diarrhea in patients receiving enteral nutrition. J Parent Enteral Nutr. 2008;32:160–168. doi: 10.1177/0148607108314769. [DOI] [PubMed] [Google Scholar]
- Gwee KA, Bak YT, Ghoshal UC, et al. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1189–1205. doi: 10.1111/j.1440-1746.2010.06353.x. [DOI] [PubMed] [Google Scholar]