Abstract
Hydrogen sulfide (H2S) is the most recent endogenous gasotransmitter that has been reported to serve many physiological and pathological functions in different tissues. Studies over the past decade have revealed that H2S can be synthesized through numerous pathways and its bioavailability regulated through its conversion into different biochemical forms. H2S exerts its biological effects in various manners including redox regulation of protein and small molecular weight thiols, polysulfides, thiosulfate/sulfite, iron-sulfur cluster proteins, and anti-oxidant properties that affect multiple cellular and molecular responses. However, precise measurement of H2S bioavailability and its associated biochemical and pathophysiological roles remains less well understood. In this review, we discuss recent understanding of H2S chemical biology, its relationship to tissue pathophysiological responses and possible therapeutic uses.
Keywords: sulfide, cysteine, nitric oxide, cardiovascular, oxidative stress
1. Introduction
Hydrogen sulfide (H2S) has emerged as an important gaseous signaling molecule playing numerous roles in health and disease, along with CO and NO [1; 2; 3; 4; 5; 6]. It acts as a relaxant of smooth muscle and thus a vasodilator, a regulator of cardiac function and N-methyl D-aspartate (NMDA) receptor in the brain, and cytoprotectant and mediator for cardiovascular therapeutic approaches [7; 8; 9; 10; 11; 12]. Understanding precise pathophysiological signaling mechanisms and the metabolism of H2S is a topic of active research. And unraveling its interactions within different tissues, with other biochemical molecules and various signaling mediators is becoming ever more complex. Thus, in this rapidly growing field it is important to appreciate key findings and their potential implications regarding H2S chemical biology that are now known, as well as to identify critical areas for further understanding and clarification. In this review, we address numerous salient issues regarding biochemical synthesis and metabolism of H2S, measurement techniques used to detect levels of H2S both in vitro and in vivo, biological functions regulated by H2S and the potential of therapeutic approaches employing H2S based therapies for various clinical applications.
2. H2S biosynthesis: enzymatic and non-enzymatic
The production of H2S can occur via two pathways - enzymatic and non-enzymatic. Enzymatic synthesis of H2S occurs through three enzymes that are cystathionine gamma-lyase (CGL also abbreviated as CSE), cystathionine beta-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (3-MST). These enzymes have been reported to be organ-specific depending on the type of enzyme. CBS is predominantly found in the brain, nervous system, and liver [8], while CSE is mostly found in the vasculature and liver, and 3-MST can be found in the brain and vasculature [13]. However, all three enzymes are distributed across many tissues and are often jointly present such as CBS and CSE being most prominently found in the liver and kidney [13]. Importantly, while CSE and CBS are hemeproteins primarily located in the cytosol, 3-MST is a zinc-dependent protein found in both the mitochondria and cytosol.
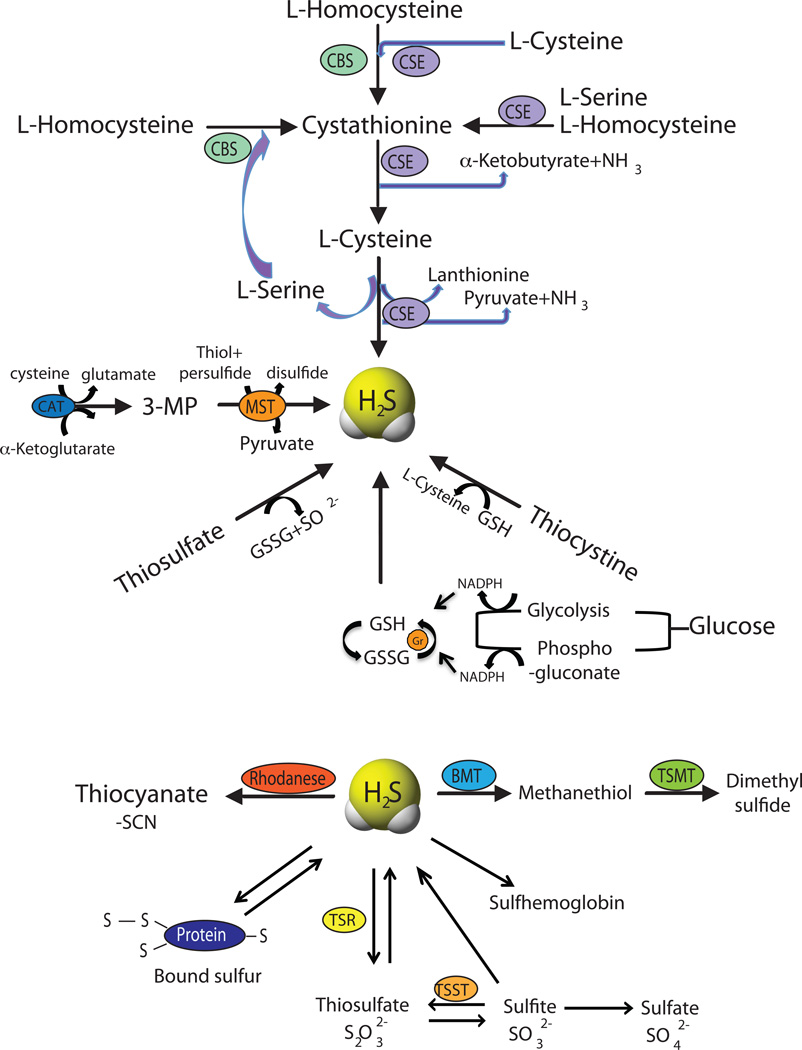
Cystathionine is a critical intermediate metabolite involved in many sulfur-containing amino acids, formed by CBS through condensation of homocysteine along with serine. CSE is also involved in a reaction that converts L-cystathionine and cysteine to form L-cysteine and α-ketoglutarate (α-KG). CSE and CBS ultimately produce H2S through a reaction involving the generation of L-cysteine, pyruvate, and ammonia. Whereas, 3-MST produces H2S through a reaction involving the generation of pyruvate from 3-mercaptopyruvate (3-MP). It has recently been shown that 3-MST might also cleave mercaptopyruvate to form pyruvate and H2S, or catalyze the transsulfuration of a thiol to a persulfide, which can subsequently join a second thiol to form a disulfide and release H2S [14]. 3-MP substrate is provided through the metabolism of cysteine and α-KG by cysteine aminotransferase (CAT). Figure 1 illustrates how these enzymes coordinately regulate transsulfuration activity controlling physiological H2S levels in a complex and overlapping manner.
Figure 1. Enzymatic and non-enzymatic synthesis of H2S and its metabolism.
Enzymatic synthesis of H2S involves three enzymes in the mammalian systems, Cystathionine β-synthase (CBS), Cystathionine γ-lyase (CGL or CSE) and 3-mercaptopyruvate sulfurtransferase (MST) to form H2S. Non-enzymatic synthesis occurs through glucose, glutathione and polysulfides. H2S further metabolized into thiocyanate, methanethiol and thiosulfate catalyzed by rhodanese, bisulfide methyltransferase (BMT) and thiosulfate reductase (TSR) enzymes respectively. Thiosulfate can oxidize to sulfite through thiosulfate sulfurtransferase (TSST) and subsequently to sulfate. H2S reacts with hemoglobin to form sulfhemoglobin and with proteins in the tissues in the form of bound sulfur pool.
Apart from enzymatic synthesis pathways, endogenous production of H2S can also occur through other non-enzymatic processes that are less well understood. Non-enzymatic production of H2S occurs through glucose, glutathione, inorganic and organic polysulfides (present in garlic) and elemental sulfur. H2S can be generated from glucose either via glycolysis or from phosphogluconate via NADPH oxidase. Glucose reacts with methionine, homocysteine or cysteine to produce gaseous sulfur compounds – methanethiol and hydrogen sulfide. H2S is also produced through direct reduction of glutathione and elemental sulfur. Reduction of elemental sulfur to H2S is mediated through reducing equivalents of the glucose oxidation pathway like NADH, or NADPH [15]. H2S formation from thiosulfate results from a reductive reaction involving pyruvate, which acts as a hydrogen donor. Thiosulfate is an intermediate of sulfur metabolism from cysteine and a metabolite of H2S that can also lead to the production of H2S [3; 16; 17]. Though the involvement of mitochondria in oxidizing sulfide has been well known [18], the specific affinity of the enzyme 3-MST to thiosulfate and in its production has been recently reported [19; 20]. Further, rhodanese is involved in metabolizing thiosulfate into H2S and sulfite [17]. Figure 1 summarizes various enzymatic and non-enzymatic H2S synthesis pathways that have been described.
3. Different biochemical forms of H2S
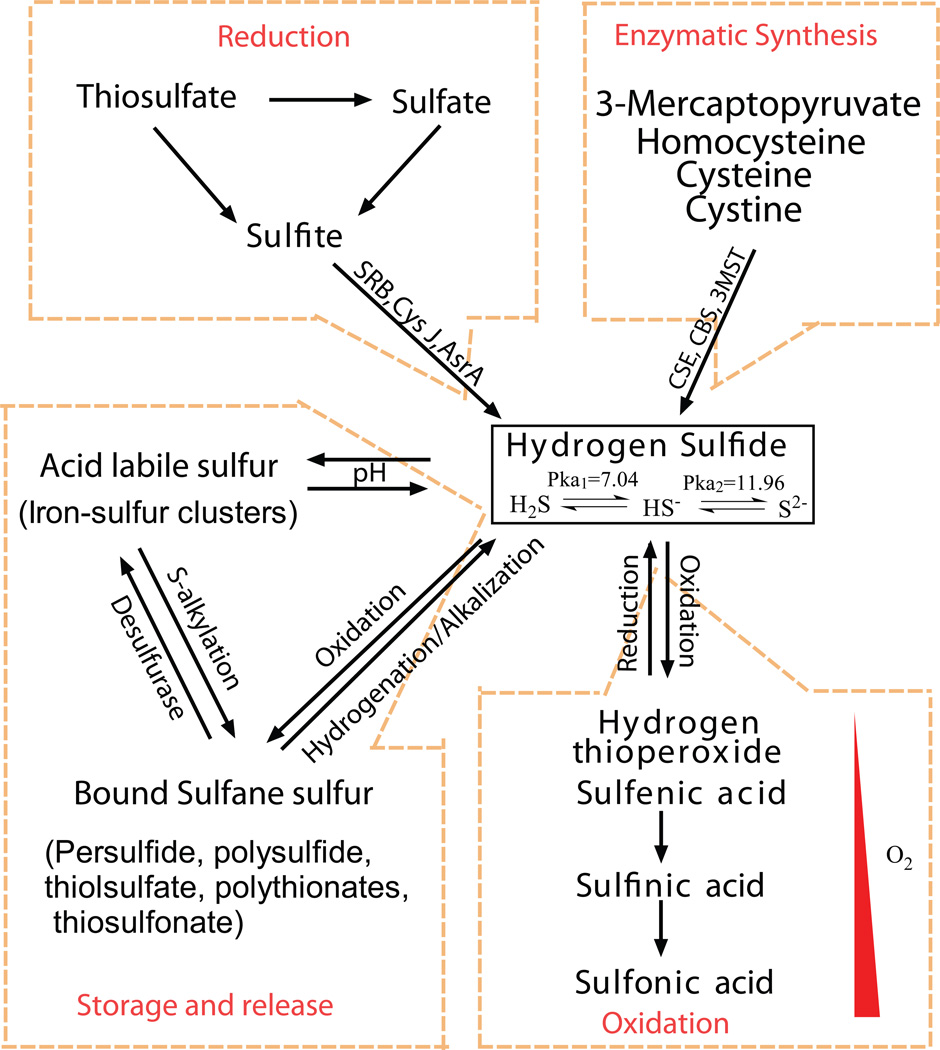
Apart from its free state, H2S can react with different biochemical molecules establishing different bioavailable pools including stable, acid-labile and bound sulfide forms (figure 2). In the stable pool, sulfur atoms are not readily reactive or liberated upon chemical treatment with acid or dithiothreitol [3]. These compounds exist in a reduced divalent form like H2S or oxidized hexavalent form such as sulfate anion. Sulfide can also be categorized based on the form in which they are stored in a biological system such as free sulfides or in bound forms like acid-labile forms and bound sulfane sulfur [21]. Free or unbound sulfide exists as S2−, HS− or H2S, where its acidic dissociation constants (pKa) range between >12 and 7.0, respectively (figure 2). Acid-labile sulfide is mainly in the form of iron–sulfur (Fe-S) complexes and persulfides, which play a critical role in redox reactions in cytoplasm and mitochondria. The critical pH below which H2S is released from acid-labile sulfur like Fe-S is 5.4 [22]. Conversely, bound sulfane sulfur exists as a compound containing sulfur-bonded sulfur [23]. This includes compounds like polysulfides, thiosulfate, polythionates, thiosulfonates bisorganyl-polysulfanes or monoarylthiosulfonates and elemental sulfur. Bound-sulfane sulfur compounds like polysulfides release H2S under reducing conditions suggesting that cellular redox state is important for regulating its bioavailability [24]. Moreover, free H2S can be incorporated into proteins as bound sulfane sulfur, where its divalent sulfur form binds only to the elemental sulfur, persulfides and polysulfides [25]. These various biochemical forms represent complex and diverse ways in which H2S bioavailability can be maintained. However, the movement of H2S bioequivalents from these pools and the pathophysiological conditions in which they are biochemically converted remains poorly understood.
Figure 2. Storage and release of H2S and factors involved.
H2S can be interconverted between gaseous and other complex storage compounds involving various factors like pH, oxidation, reduction, hydrogenation, and alkalization, microorganisms like sulfate reducing bacteria (SRB), apart from enzymatic catalyses through CSE, CBS, 3MST, sulfite reductase α-sub unit (CysJ), anaerobic sulfide reductase A (AsrA).
H2S interacts with membrane and cytosolic proteins to produce reactive and unstable persulfides. These persulfides can be further converted to other biochemical forms including thiocysteine, thiotaurine, protein–SSH, thiocystine, mercaptopyruvate and others. Sulfide donors may impart structural changes in proteins through persulfide related sulfuration and sulfhydration reactions of proteins. Oxidized GSSG can non-enzymatically react with H2S to form glutathione persulfide [26]. Likewise, reduced GSH also plays a catalytic role in elemental sulfur activation. Thus, numerous sulfide compounds may exist biologically that participate in multiple biochemical interactions with other molecular targets. These complex interactions of sulfide pose challenging obstacles in determining important biochemical forms that are present and active in biological systems. However, future systematic experimental approaches discretely aimed at understanding these complex sulfide variants should provide significant insight into specific roles of these molecules.
4. Detection methods for H2S
Recent studies show that H2S plays physiological, pathological and toxicological roles directly related to its bioavailable levels in vitro and in vivo. Though physiologically the role of H2S seems to be protective in nature, there is abundant evidence where seemingly slight elevations in H2S mediate toxic affects. As such, there is a continued interest in the research community to understand the pathophysiological roles of H2S in relation to its biological concentration [8; 27; 28; 29; 30]. The protective versus adverse effects of H2S have been one of the most contentious, with conflicting arguments for both effects being reported in the literature [31; 32; 33]. Importantly, a similar historical research path was observed with the study of NO having a duplicitous role as a cytoprotectant and cytotoxicant. Thus, it is imperative that accurate measurement of H2S in its free and various biochemical forms is accomplished within biological systems to advance the understanding of this important molecule in pathophysiological settings.
The detection of sulfide and its pools has gained significant attention from analytical and biological communities. The detection methods for sulfide have evolved from simple non-specific colorimetric assays to the more recently used techniques such as high pressure liquid chromatography (HPLC), gas chromatograph (GC), mass spectrometry (MS), and polarographic, electrochemical and chemiluminescent detection that are highly specific and sensitive with much lower detection limits. However, each technique has certain advantages and limitations that require careful consideration before attempting to measure bioavailable sulfide and its various forms. Below we discuss several factors influencing detection of sulfide in its various forms along with specific detection techniques and their associated strengths and weaknesses.
Factors affecting H2S stability
pH is one of the key determining factors of H2S stability due to its distinct acidic disassociation constants and ability to interact with metals. As mentioned previously, H2S may exist as different biochemical species (acid labile forms) depending on the surrounding pH conditions. The kinetic properties of sulfide distribution and oxidation changes under different pH and temperature conditions [34; 35]. At physiological pH (7.4) and 37 °C, ~20% of sulfide exists as H2S gas, while at pH 7.4 and 25 °C ~40% of sulfide exists as H2S gas. At alkaline pH (e.g. 9.5), sulfide predominantly exists as HS− anion [36]. The reactivity of dissociated HS− is high, as it is more readily oxidized than H2S. In vivo free bioavailable sulfide primarily exists in two forms as H2S gas and its highly reactive anion, HS− [18], thus deviations in pH from the physiological range can liberate bound sulfide pools contributing to what would be detected as free H2S/HS−concentrations in biological specimens. The rate of sulfide oxidation is also increased at physiological pH reaching the maximum at pH 8; whereas, reactivity rates are decreased at both low and high pH’s reaching a nadir at pH 9 that gradually increases as the pH rang increases to 11 [34]. These examples illustrate the importance of pH as a regulatory factor of sulfide chemical reactions that clearly influence its measurement.
Another regulating factor that influences H2S stability is oxygen (O2). From previous studies in non-biological [34; 37] and biological samples [38], it has been shown that oxygen influences the production and availability of sulfide. Olson et al. have shown in lamprey aortae and rat and bovine arteries that H2S production is increased in hypoxic conditions and inhibited upon subjecting it to O2[38]. Our group has further demonstrated that oxygen concentration and pH affects sulfide stability and derivatization within biological samples [39; 40]. We found that at a pH of 9.5 at 21% oxygen the maximum sulfide stability was only 70%, improving to 80% stability at 10% O2, and greater than 90% stability at 1% O2. Together, these findings reveal that the stability and detection of sulfide is critically affected by pH and oxygen tension and its measurement would be most reliable with thoroughly deoxygenated, pH-optimized reagents used under atmospherically controlled conditions.
Specific measurement methods
Current literature reports several measurement techniques for H2S from the basic to more analytically complex such as spectrophotometry, chromatography, ion-selective electrode and nanoparticles that detect forms of sulfide from different non-biological and biological sources like sewage, marine water, urine, feces, blood, serum, tissues, and breath [2; 3]. Continuous refinement of these techniques has improved detection specificity and lowered threshold limitations. However, there are several technical, practical and biological uncertainties with several of these measurement techniques.
The zinc trap/methylene blue and spectrophotometric assays
The colorimetric detection of the end product methylene blue (MB) often referred to as the ‘zinc-trap’ method has been one of the most commonly applied techniques for the detection of H2S. This assay relies on trapping sulfide with a metal (e.g. zinc acetate) with subsequent acidification and reaction with N, N-dimethyl-p-phenylenediamine (DMPD) to form methylene blue that is measured using a spectrophotometer or plate reader at 670 nm. In 1883, Fischer et al. initially reported this method primarily aimed to measure sulfide levels in aquatic samples [41]. Many modifications of this method have been made over the years for analyzing non-biological samples—mainly sewage and seawater [42; 43; 44; 45; 46; 47]. Guenther et al. also developed a spectrophotometry based direct method for sulfide detection at pH 8.0 using direct ultraviolet detection with a limit of <1µM [48]. Likewise, Kovatsis and Tsougas have reported similar detection approaches of atmospheric H2S at pH 5.6 using a lead acetate solution [49]. While, these techniques have been reported to be useful for sulfide detection from aquatic or environmental samples they are not best suited for blood, tissue and other biological specimens. There are many critical issues with this method when applied to complex biological specimens including:
interference with colored substances formed as sulfide reacts with N,N-dimethyl-p-phenylenediamine sulfate in the presence of the oxidizing agent Fe3+,
strong chemical pretreatments (i.e. acids) of this method lead to liberation of H2S from other forms of sulfide (e.g. acid labile pools) that can contribute to erroneous measurements,
lack of sensitivity to low (physiological) H2S concentrations and absence of distinct absorption peaks at 670 nm,
dimer and trimer formation of methylene blue with high absorption spectra at 667 nm where it does not obey Beer’s law contributing to erroneous results,
time dependent nature of color formation and intensity that requires consistent and careful monitoring,
and that this method does not control for factors influencing sulfide stability such as pH or O2 concentrations.
Despite these limitations and resulting disparate sulfide measurements, it is surprising that this method is still widely used to measure H2S levels.
Serious concerns have been raised by several groups working in the field over application of the methylene blue based assay that has been critically addressed in recent publications on sulfide detection methods [36; 39; 40; 50; 51]. Work from our group and others have rigorously evaluated the methylene blue assay of sulfide detection in biological samples [36; 39; 40]. Results from our work revealed that the methylene blue method does not produce a bona fide hydrogen sulfide peak at low expected physiological concentrations unlike other analytical detection methods (e.g. HPLC detection of sulfide-di-bimane) [39; 40]. Using the methylene blue method, the sulfide specific product is detectable by a distinct wavelength between the range of 660–680 nm and only at high concentrations of H2S (e.g. 1mM). However, at a lower concentration of H2S (50 µM) in phosphate buffered saline solution no detectable peak was observed. This demonstrates that the methylene blue method may only be suitable for measuring larger concentrations of H2S. These findings confirm that this assay does not reliably measure lower concentrations of H2S in the micromolar range and seriously questions the utility of the methylene blue method for detection of sulfide in biological specimens. Fortunately, other analytical techniques have shown promise in measuring biological H2S levels including gas/ion chromatography, HPLC, polarographic sulfide detectors, mass spectrometry and fluorescent probes.
Sulfide-specific ion-selective electrodes (ISEs)
ISEs have also been in use to detect H2S level in biological samples, with a detection range of 1–10 µM. Though this method is readily available and easy to operate, it too has its flaws in detecting biological sulfide levels. ISEs measure the S2− form of sulfide that requires an alkaline environment (antioxidant buffer) to favor the S2− equilibrium, which may contribute to erroneous sulfide readings. Whitfield et al. have demonstrated this erroneous affect of ISEs using Trout plasma and bovine serum albumin (BSA) samples [4]. They showed that sulfide levels of BSA and trout plasma samples placed in alkaline buffer rapidly increased over time reaching 1mM by 12 h. Searcy and Peterson reported that the lower detection limit could go to 0.5 µM and claimed that the electrode can work in oxygenated and deoxygenated buffers equally [15]. However, its application to biological samples is questionable considering the potential for erroneous measurements due to strong chemical pretreatment of samples.
Polarographic electrodes
Polarographic H2S sensor detection is well documented and a reliable method to measure H2S levels as reported by Kraus and colleagues and the Olson laboratory. Polarographic H2S detection can be employed for real-time measurement of H2S gas from biological samples, with a lower detection limit in the nM range under anoxic conditions [52]. With this approach, endogenous sulfide levels have been reported in the low µM range (<5 µM) in rat whole blood and in rat aorta (5–40 µM) [53]. However, using the polarographic H2S sensor approach Whitfield et al. reported that sulfide was undetectable in plasma and blood collected from various animal sources. They observed that plasma and blood levels were relatively undetectable (<100 nM total sulfide) and only observed a slight increase in H2S (low µM), upon exogenous application of a sulfide donor that was rapidly cleared. This is in stark contrast to other reports estimating physiological sulfide concentrations between 10 and 300 µM using the methylene blue method. From a historical perspective, speaking with different investigators employing polarographic electrodes early on, many of these custom built electrodes used a unique silicone polycarbonate blend membrane (most likely M213 membrane initially designed by General Electric silicones) that is no longer commercially available. While it is not clear whether current commercially available electrodes use a similar membrane, it is apparent from talking with investigators in the field that the sensitivity and longevity of newer polarographic electrodes are somewhat different from that of previous reports. Nonetheless, a recent report by Faccenda et al. has sought to alternative approaches and demonstrated that polydimethylsiloxane (PDMS) membranes are quite permeable to H2S and useful in constructing devices for continuous measurement of H2S [54]. Though the H2S polarographic sensor method is very sensitive and accurate, it is not capable of detecting other pools of sulfide (e.g. acid labile and bound sulfide). Additionally, processing of samples to preserve the sulfide content for later polarographic measurement has not been routinely reported as this method appears best suited for immediate real time measurement of H2S production.
Chromatography approaches
Chromatography detection methods for H2S include gas, ion-exchange chromatography and variants of HPLC for detection of various pools of sulfide from biological samples. Tangerman et al. have reported a GC based technique for measuring volatile sulfur compounds in human breath using a specific sulfur detector with a detection limit of about 0.2 ng/l (0.1 ppb) [2]. Likewise, Ubuka et al devised a technique to measure sulfide pools using gas chromatography with a flame photometric detector (GC-FPD) and ion chromatography (IC), and measured hydrogen sulfide and acid-labile sulfur in rat liver and heart tissues [24]. They reported the acid-labile sulfide in the range of 100 nmol/g tissue, but no detectable free sulfide. Goodwin et al. have measured sulfide levels in human and rat brain tissue through gas dialysis/ion chromatography in a range of 100 µM [55]. Furne et al. have used a combination of gas chromatography with a chemiluminescence sulfur detector to detect free H2S levels with a low detection limit in the nM range [56]. With this method, very low levels of free H2S were measured in mouse brain (14 nM) and liver tissue (17 nM) and in human blood at an estimated 100 pM. Ishigami et al. have developed a GC based method using silver particles to trap free H2S from the rat brain homogenate, with a low µM (9 µM) detection limit [22]. In addition they also reported other storage forms of sulfide, acid-labile and bound, in the µM range. Lastly, Levitt et al. has reported the concentration of mouse blood H2S in the range of 15 nM using a combined GC/chemiluminscent detection method [57]. These approaches are clearly sensitive and specific, but may be viewed as less ‘user-friendly’ due to the analytical equipment needed impacting the likelihood of these methods being used. Moreover, different specimen collection and processing methods likely contribute to the reported levels as these methods primarily facilitate detection of H2S gas alone versus other bioavailable forms of sulfide (e.g. HS−, acid labile sulfide and bound sulfane sulfur).
High pressure liquid chromatography analysis has been employed by several laboratories to measure bioavailable HS in various forms. Using RP-HPLC detection of methylene blue, Savage and Gould used zinc acetate to trap and measure H2S levels in brain tissue homogenized under acidic conditions [58]. Ogasawara also previously reported a fluorescence-based HPLC approach, derivatized with p-phenylenediamine and Fe3+ to measure H2S levels by converting it to thionine [21]. Monobromobimane (MBB), a thiol sensitive fluorescent probe, was reported Togawa et al. and Newton and Fahey to measure bioavailable H2S [59; 60]. In this assay the H2S/HS− specific sulfide-diamine product is measured through HPLC coupled with fluorescence detection. This method is very sensitive and specific but does require the use of HPLC equipment and procedures that may be unavailable to some investigators.
Recent reports have further examined the MBB assay for H2S detection providing greater detailed information regarding this approach as a highly useful method for sulfide detection. Wintner et al employed the MBB protocol under derivatization conditions at pH 8.0, and observed free H2S levels in rat blood to be around 0.7 µM [61]. Our group has refined and optimized the MBB assay identifying ideal optimal pH and oxygen concentration ranges that enable consistent and reliable measurement of H2S bioavailability with a detection limit of 2 nM [40; 61]. Using this protocol that carefully controls for pH, oxygen tension, and the presence of metals; we have reported that human plasma free H2S/HS− levels are in the mid nanomolar range (300–500 nM) and that mouse plasma free H2S/HS− is in the low micromolar range ~1.0 µM. It is important to understand that the MBB assay measures all bioavailable sulfide in the gas and anion form (H2S/HS−), which are both biochemically active, yielding a comprehensive measurement of H2S bioequivalents in biological specimens. We have further adapted the MBB assay to measure other biochemical forms of bioavailable H2S contained within acid labile and bound sulfane sulfur pools [39]. Using this new method, we found the acid labile pool of sulfide (which contains Fe-S cluster proteins and persulfides) to be the most abundant in plasma (~2–4 µM) in both mice and man; whereas the free and bound sulfane sulfur pools were considerably lower in the nanomolar range. These combined approaches employing MBB derivatization of sulfide from various biochemical forms represents a powerful and very useful method with which to evaluate biological H2S bioavailability under different pathophysiological conditions.
Fluorescent probes for H2S detection
Due to numerous cellular signaling and therapeutic implications of H2S, significant attention has been given to devising novel methods for sensitive and real-time H2S detection within specific tissue and cellular compartments. Recently, single-walled carbon nanotube (SWNT) networks and Quantum Dot (QD) fluorescence techniques have been reported to detect H2S as low as 3 ppb [62] and 0.15 µM [63], respectively in non-biological systems. Both of these approaches are highly selective and sensitive to H2S/HS−. Thus, use of this type of detection approach may be informative for evaluating cellular H2S production. Along these same lines, Liu et al. and Peng et al. have developed a fluorescence-based strategy for detection of H2S [64; 65]. In their method, Liu et al. have measured the concentration of H2S in plasma by assessing the fluorescence signal from benzodithiolone product formation; whereas, Peng et al. synthesized a novel reduction-sensitive, stable non-fluorescent chemoprobe, dansyl azide (DNS-Az), that becomes fluorescent upon reacting with sulfide. Though these fluorescent H2S detection methods are very simple and cost-effective, possible limitations may exist in the sensitivity of H2S detection (micromolar) and the time needed to sufficiently detect changes in H2S levels (several minute to hour). Moreover, some of the reported fluorescent dyes have excitation-emission wavelengths (490–700 nm) near tissue autofluroescence wavelengths that could make sensitive and/or selective detection of low sulfide concentrations difficult. Finally, this technical approach is currently not able to measure sulfide levels from different biochemical pools within the sample or account for the fact that H2S can be quickly scavenged by proteins [39; 40], that could influence the signal obtained from biological samples. However, the non-reactive nature of these dyes with other biological thiols (e.g. cysteine and glutathione) is a strength along with the ease of sample analysis highlighting various beneficial aspects of these approaches.
Final points of consideration
In the past, remarkably high levels of H2S have been reported using techniques that are increasing understood to be prone to error and artifact (e.g. the methylene blue assay). However, as with any approach or method, specific experimental details or questions must be carefully considered before embarking upon a study. Although, as we have discussed above, there are strengths and weaknesses to the various methods used to measure H2S bioavailability. Table 1 provides a distilled comparison between these methods along with useful references for the reader. However, there are additional important experimental details for consideration that include but are not limited to: 1) sensitivity- what levels of H2S detection are required or desired? 2) technical capability- what equipment or resources are available to perform various measurement techniques? 3) specimen type- biological fluids, tissue, exhaled gases or breath? 4) temporal requirements- is constant real time measurement necessary or will a single time point suffice? It is important to remember that the measurement method used will also influence results such that using a method that only measures H2S in the gaseous phase will not accurately represent bioavailable sulfide equivalents that may exist in the anion form (HS−) that are still biologically active and measurable using different approaches. Considering both experimental details along with understanding specific method capabilities and limitations will significantly aid both the investigator and the field of H2S research in its quest to learn more about this gasotransmitter.
Table 1.
Comparisons of commonly used H2S detection methods
| Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Direct Spectophotometric measurement |
|
|
[48; 49] |
| Gas Chromatography/ flame photometric detector (FPD) |
|
|
[3; 56; 191] |
| Ion Chromatography |
|
|
[3; 55] |
| Polaragraphic Sensor (H2S electrode) |
|
|
[52; 53] |
| Gas Chromatography/ Chemiluminescene |
|
|
[2; 191] |
| Methylene Blue Assay |
|
|
[192] |
| Fluorescent Probe of H2S |
|
|
[193; 194; 195; 196] |
| Monobromobimane Derivatization/HPLC |
|
|
[197; 198] |
5. Biological functions of sulfide
Knowledge of the biological roles for endogenous H2S is constantly expanding. Many studies in the literature indicate that H2S executes physiological effects at a wide range of concentrations between 10 and 300 µM [66]. However, it is important to keep in mind that many of these levels were determined using older detection methods versus newer, sophisticated analytical approaches. Nonetheless, it is clear that H2S is involved in modulating various physiological responses including anti-inflammation [67], reducing oxidative stress [68], neuromodulation [8], vasoregulation [69], protection from reperfusion injury after myocardial infarction [70], and inhibition of insulin resistance [71]. Moreover, many researchers continue to explore this signaling molecule for its involvement in various aspects of cell function, cytoprotection and cellular signaling.
Vasodilation and anti-hypertensive effects
Nitric oxide (NO) is a well established signaling molecule acting as an endothelium-derived relaxing factor (EDRF) of the vasculature. However, recent studies have shown evidence that H2S also acts as a relaxing factor of blood vessels involving alternation in K+ channel activity and elevated cGMP levels in vascular smooth muscle cells (SMCs) similar to NO [72; 73]. Li et al. demonstrated in a renal hypertension rat model that the H2S donor NaHS relaxes vascular smooth muscle, causes rapid and reversible relaxation of isolated aortic rings, and dilates the perfused renal vasculature by opening KATP channels of vascular smooth muscles cells. They also observed reduced hypertension levels mediated by the H2S donor in a dose dependent manner [74]. Moreover, other investigators have reported that H2S mediates vasorelaxation through KATP channels in vascular SMCs such that inhibtion by glibenclamide blocks H2S-induced dilation of aortic rings, the mesenteric artery, and hepatic microvasculature [75; 76; 77]. Bucci et al. have also attributed H2S vasodilatory effects to the cGMP pathway [78]; while, Zhao et al. indicated that cGMP pathways were not involved in this response [75]. Although specific mechanisms remain unknown, it is clear from these studies that H2S plays a prominent role in regulating endothelium-dependent vasoactive activities.
Consistent with the above findings, diminished H2S levels are associated with constriction of blood vessels, thus increasing blood pressure. Yang et al. have reported that genetic deletion of the H2S generating enzyme cystathionine γ-lyase (CSE) leads to the development of hypertension [72]. Supporting this study, Benavides et al. have shown that garlic relaxes rat aortic rings through an endothelium-independent and H2S dependent mechanism [79]. They demonstrated that garlic-derived organic polysulfides via glucose and thiol-dependent mechanisms release H2S, which causes the vasorelaxant effect in rat aortic rings. Similar conclusions were drawn from the work of Ashraf et al. demonstrating an endothelium-dependent vasodilator response by garlic in a NO/cGMP dependent manner [80]. These findings are consistent with previous studies showing that low doses of the H2S donor NaHS produced short-lived relaxation to mesenteric artery and intestinal contractility [76; 81]. Interestingly, however, higher doses of NaHS caused a biphasic relaxation/constriction response, a response that warrants further investigation [76]. In summary, it is clear that H2S acts as an effective vasodilator and subsequent reduction of blood pressure, yet more studies are needed to understand specific cellular and signalling mechanisms regulating these responses.
Anti-inflammatory effects
It is well known that nonsteroidal anti-inflammatory drugs (NSAIDs) induce gastroenteropathy [82]. Research suggests that NSAIDs suppressed endogenous H2S synthesis by reducing expression of CSE. The accompanying reduction of H2S synthesis may in turn contribute to an increase in leukocyte adherence resulting in gastric injury that is seen after NSAID administration [83; 84; 85]. Similarly, administration of exogenous H2S reduced the ability of these agents to cause gastric injury. Fiorucci et al. has also found that exogenously supplied H2S suppressed NSAID-induced granulocyte infiltration, expression of endothelial and leukocyte adhesion molecules, and expression of tumor necrosis factor α (TNF- α) [82]. It was found that leukocyte adhesion to the vascular endothelium induced by aspirin injury was decreased after increasing H2S bioavailablity and that CSE inhibition with propargylglycine exacerbated asprin mediated mucosal injury and inflammation [82]. This study also observed that leukocyte expression of LFA-1 was suppressed by exogenous H2S. Interestingly, this article also showed a molecular aspect of H2S induced anti-inflammatory effects, such that H2S donors decreased aspirin-induced leukocyte adhesion through the activation of KATP channels and inhibition of CSE activity that promotes leukocyte adhesion. Additional studies demonstrated that coadministration of an H2S donor with an NSAID resulted in inhibition of NSAID-induced leukocyte adherence and reduction of the severity of gastric damage [86; 87]. In contrast, irreversible inhibition of CSE can attenuate the severity of experimental pancreatitis [88] and endotoxemia [89]. These results demonstrate that H2S donors can down-regulate adhesion molecule and proinflammatory cytokine expression, therefore identifying H2S, its synthesis enzymes, and molecular targets (e.g. KATP channels) as potential targets for novel anti-inflammatory therapies.
Anti-oxidant effects
Cells can be protected from oxidative stress through numerous mechanisms with intracelluar glutathione dependent or independent pathways serving as primary mediators [90]. Evidence in simple uni or multicellular organisms reveals that sulfur containing substances dimethylsulphoniopropionate and dimethylsulfide act as endogenous scavangers of reactive oxygen species in marine algae [91]. However, in more complex organisms it has be observed that H2S exerts its antioxidant effect not directly but through induction of glutathione metabolism responses [1]. This is confirmed by a recent study by Hamar et al demonstrating that H2S itself is a rather poor antioxidant compared to other antioxidant defenses [92]. Jha et al. has also shown that hydrogen sulfide protects hepatocytes from ischemia reperfusion (I/R) injury through up regulation of intracellular antioxidants [11]. Likewise, Calvert et al has reported that exogenous H2S confers cardioprotection against I/R injury through Nrf-2 induction [29]. Conversely, it has been shown that oxidative stress is increased by decreased endogenous production of H2S in hypoxic pulmonary hypertensive rats [93]. Moreover, H2S enhances the activity of cysteine and cystine transporters to increase the level of substrates for glutathione (GSH) production. H2S produced by 3-mercaptopyruvate sulfurtransferase (3-MST) along with catalase may also directly suppress oxidative stress in mitochondria. H2S also attenuates oxidative injury in astrocytes by H2O2 by increasing glutamate uptake [94]. H2S can also inhibit peroxynitrite-mediated tyrosine nitration of neuronal proteins, suggesting that H2S has the potential to act as an inhibitor of peroxynitrite-mediated processes in vivo [70]. Evidence also indicates that H2S increases the ability of the antioxidant enzyme superoxide dismutase to scavenge superoxide and increase the level of GSH biosynthetic enzyme γ-glutamylcysteine synthase [95]. Lastly, it is well known that H2S modulates mitochondria function, as it is a potent and reversible inhibitor of cytochrome c oxidase. Through this ability to blunt cellular respiration, which in turn reduces mitochondrial ROS production and decreases mitochondrial uncoupling, H2S can elict cytoprotection [96]. However, the protective dose range of H2S against mitochondrial uncoupling and ROS formation compared to NO is much narrower likely due to the fact that posttranslational modifications due to H2S/HS− are more difficult to biochemically reverse compared to nitros(yl)ation. Together, the majority of evidence suggests that H2S production and bioavailability potently regulates cellular redox status through antioxidant defense responses versus direct antioxidant activity.
Cytoprotection/ Anti-apoptosis effects
Hydrogen sulfide exerts anti-apoptotic effects in different organs but many studies of this nature have performed in I/R injury of the heart. It has been shown that H2S attenuated cardiac myocyte apoptosis subjected to myocardial I/R in vivo [29]. Sodha and colleagues reported that H2S exerts its anti-apoptotic effect by inactivation of caspase-9 caused by I/R [97]. Sivarajah et al. has further demonstrated that administration of 5-hydroxydecanoate (5-HD), a KATP channel blocker, abolished the attenuation in cardiac myocyte apoptosis and caspase-9 activity caused by H2S [70]. Complementary to the attenuation in caspase-9 activity, H2S also ameliorated the expression of Bcl-2 protein caused by regional myocardial I/R that was abrogated by 5-HD. Thus, these results indicate that H2S mediates significant anti-apoptotic effects, which may be secondary to the opening of putative mitochondrial KATP channels [70]. Likewise, inhalation of H2S before ischemia has been shown to decrease retinal I/R injury and cell loss through attenuation of caspase-3 cleavage and activity, thereby decreasing apoptosis [98]. Lastly, Shi et al. have shown that the attenuation of cardiomyocyte apoptosis by H2S is associated with early inhibition of JNKs during reperfusion, increased Bcl-2/Bax expression ratio, and diminished cytochrome c release. Thus, all of the above findings demonstrate that H2S induces cytoprotection by an anti-apoptotic pathway [99].
Fibrinolytic activity
Inhibition of fibrinolytic activity or deficiency of the factors involved might upset the hemostatic balance and allow excessive fibrin deposition especially in diabetes, hypertension and hypercholesterolemia. One study has shown that a marked rise in blood coagulation of hypercholesterolemic rabbits was significantly reduced by the essential oils of garlic. They also observed that fibrinolytic activity was actually increased even above the normal control levels, which were mediated by the essential oils of garlic [100]. The plasma fibrinolytic activity in rabbits, which was decreased upon cholesterol feeding, was considerably increased when this diet was supplemented with garlic [101]. Studies on fibrinolytic activity of garlic in humans have shown increased fibrinolytic activity and inhibition of platelet aggregation [102]. Acute as well as chronic intake of garlic oil and raw garlic increased fibrinolytic activity. Earlier studies from Bordia et al. also demonstrated that garlic oil increased fibrinolytic activity after 3 hours of administration. They further reported that chronic administration of garlic oil increased fibrinolytic activity significantly in healthy as well as acute myocardial infarction patients [100; 103]. Other studies have similarly suggested that both raw and fried garlic significantly enhances fibrinolytic activity in human plasma [104; 105].
Anti-platelet activation and aggregation effects
Platelet aggregation superimposed on an atherosclerotic vessel is an antecedent event causing total blockage of blood flow leading to myocardial infarction and thromboembolic diseases such as peripheral arterial disease. In animal studies, pre-treatment of rabbits with an aqueous extract of garlic significantly inhibited thromboxane-B2 (TXB2) synthesis (a potent platelet aggregator) and protected against thrombocytopenia induced by collagen or arachidonate infusion. These observations indicate that garlic and its associated H2S may be beneficial in the prevention of thrombosis [106]. Aqueous extract of garlic was found to inhibit platelet aggregation induced by ADP, epinephrine, collagen and arachidonate in a dose-dependent manner in vitro and to inhibit biosynthesis of prostacyclin in rat aorta [107]. A dose-dependent inhibition of cyclooxygenase activity and collagen-induced platelet aggregation was observed in rabbit platelets treated with raw garlic in vitro while boiled garlic was found to have little effect [108]. Garlic extract containing diallyl disulfide and diallyl trisulfide, prevent acute platelet thrombus formation in stenosed canine coronary arteries [109]. Fresh garlic extract is effective in reducing thromboxane formation by platelets both in vivo and in vitro in models of thrombosis. It was observed that garlic inhibits thrombin-induced platelet synthesis of TXB2 in a dose and time-dependent manner in rabbits. The rapid recovery of platelet cyclooxygenase activity after infusion of a single dose of garlic suggested that garlic be consumed frequently in order to achieve beneficial effects of limiting thrombosis [110]. In human studies, a positive response to garlic inhibition of thrombosis has also been observed. Like enhancement of fibrinolysis, garlic also attenuates platelet adhesion or aggregation in humans. Bordia and his colleagues first showed the dose-dependent inhibition of platelet aggregation by garlic [111]. Raw garlic, garlic oil and other extracts of garlic have been shown to inhibit platelet aggregation in vitro induced by ADP, collagen, arachidonate, epinephrine and calcium ionophore in humans [112]. Chronic intake of garlic powder and garlic oil also inhibits platelet aggregation in humans [113]. Moreover, the effect of garlic on platelet function is also acute in that a single dose of garlic can inhibit platelet aggregation in humans [114]. Recently, Allison et al. have demonstrated that garlic extract inhibition of platelet activation and aggregation can be attributed to increased platelet cAMP levels and inhibition of GPIIb/IIIa (αIIbβ3), an integrin that promotes platelet-platelet aggregation [115]. These findings together with the fibrinolytic effects of garlic and its extracts suggests that H2S bioavailability may play an important role in regulating platelet function and thrombosis. However, it is not yet precisely clear whether garlic dervied H2S is truly responsible for the beneficial effects observed or whether endogenous H2S metabolism responses exert equally important roles in regulating platelet function and thrombosis.
Pro-angiogenic effects
Angiogenesis is a regulated process of microvascular growth that is often invoked to revascularize ischemic tissues of many organ systems. Likewise, angiogenic activity may also serve pathological roles through its regulation of chronic inflammation and tumorigenesis [116; 117]. Our laboratory and other investigators showed that H2S modulates angiogenesis responses in vitro by enhancing endothelial cell proliferation and migration [118; 119; 120]. In these studies, researchers found that low micromolar concentrations of H2S in the form of Na2S or NaHS increases endothelial cell growth, migration and formation of tube-like structures in cultured endothelial cells [118; 119; 120]. Bir et al. demonstrated that H2S stimulates endothelial cells more potently under hypoxia than normoxia suggesting that H2S plays a more dominant role for angiogenesis under hypoxic conditions [118]. Similarly, NaHS treatment also seems to increase the proliferation and migration of vascular smooth muscle cells under hypoxic conditions [121].
Hydrogen sulfide also promoted neovascularization in vivo in the mouse, as well as in chicken chorioallantoic membranes [119; 120]. Subsequent studies from our group and other laboratories found that H2S significantly promoted angiogenesis and improved regional blood flow in ischemic limbs, which indicate microvascular growth in ischemic tissues [9]. This effect of H2S is mediated through up-regulation of HIF-1/VEGF/Akt mediated pathway in vascular endothelial cells [118; 119; 121]. Our study showed that H2S restores blood flow in ischemic tissue via a NO/HIF-1/VEGF dependent pathway [118]. Recent study by Tao et al. further showed that VEFR2 acts as a receptor for H2S during angiogenesis [122]. Likewise, it has also been reported that down regulation of VEGFR2 during ischemia was reversed by H2S with specific phosphorylation at Tyr 996 of the receptor [9]. Cai et al. first suggested that H2S exerts its angiogenic effect by enhancing Akt phosphorylation, which was attenuated by PI-3 kinase inhibitor suggesting that PI3K is upstream of Akt in underlying H2S signaling mechanism [119]. Further study showed that H2S upregulates the phosphorylation of ERK 1/2 and p38 and this effect was attenuated by MAPK indicating the involvement of MAPK/ERK pathway in H2S mediated angiogenesis [120]. It has also been reported that KATP channels partially mediate the angiogenic effects of H2S [9; 118] in that selective and non-selective KATP channel blockers attenuated p38 and hsp27 phosphorylation that are during angiogenic stimulation [120].
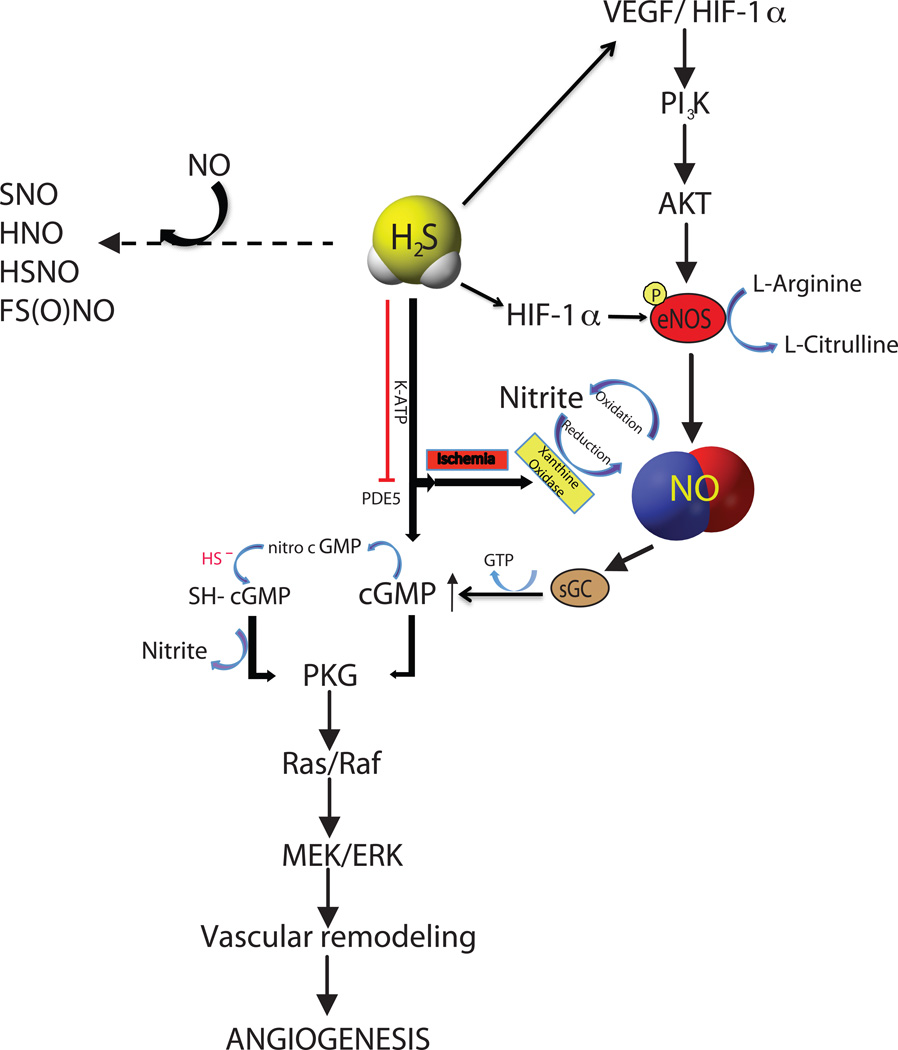
H2S may also regulate angiogenesis through other mechanisms such that selective potentiation of other molecules, such as NO and CO may contribute to this effect [123]. Our study and reports from others showed that H2S stimulates angiogenesis via enzymatic and non-enzymatic NO generation dependent pathways [118; 124]. Moreover, Bucci et al. reported that H2S increases cGMP in smooth muscle cells through inhibition of phosphodiesterase (PDE) activity. This effect of H2S subsequently induces both endothelial proliferation and migration under hypoxic conditions [78]. Figure 3 summarizes the various signalling pathways whereby H2S can regulate angiogenic activity. However, further studies are needed to better understand cooperative mechanisms necessary for proangiogenic effects of H2S.
Figure 3. Interactions of H2S and NO signaling pathways.
H2S and NO have common signaling that include upstream molecules like VEGF, HIF-1α, AKT, eNOS and XO mediated nitrite reduction to NO under ischemia; downstream signaling involves cGMP/PKG, Ras/Raf and MEK/ERK pathways leading to vascular remodeling. NO and H2S possibly react chemically to form novel compounds like S-nitrosothiol (SNO), nitroxyl (HNO), simple form of S-nitrosothiol (HSNO) and sulfinyl nitrite FS(O)NO.
Cardioprotective effects (MI and I/R)
Several studies in vitro and in vivo have suggested that H2S has a cardioprotective effect [70; 96; 97; 125]. DL-propargylglycine (PAG), a CSE inhibitor, blocks this protective effect by inhibiting endogenous H2S production. There is an inverse relationship with H2S and MI. Zhu and his colleagues have demonstrated that decreased infarct size and mortality are linked to elevated plasma H2S concentrations after MI, and the opposite, where decreased H2S levels in the plasma were associated with an increased infarct size and mortality [6]. Their study also showed that inhibition of endogenous H2S had a negative consequence on the cell viability. A similar study by Bian et al. showed that exogenously administered H2S improved cardiac rhythm, cell viability and function in MI rats compared to the vehicle, whereas PAG administration worsened these effects [126].
H2S has been shown to be cytoprotective in multiple organ systems. It protects the heart during I/R injury, both in pre- and post-conditioning. In post-conditioning, H2S protects the heart by different mechanisms. Zhang et al. reported that H2S protects the heart against I/R injury by opening K-ATP channels, which is supported by two similar studies [6; 70; 127]. Another report has suggested that ischemic preconditioning with H2S also involves PKC and K-ATP channel [126]. H2S also protects the heart by activating Nrf-1 and Nrf-2 mediated Akt phosphorylation that ultimately reduces the oxidative stress in cardiomyocytes [128]. Besides, H2S also involves eNOS related Akt phosphorylation that stimulates angiogenesis resulting in cardioprotection [119; 129]. Likewise, H2S protects heart by increasing NO bioavailability and blocking of nitric oxide synthase attenuates H2S induced cardioprotection in mice [130; 131; 132].
Recent study by Qipshidze et al. showed that H2S induces cytoprotection and angioprotection during MI to protect heart by increasing the expression of VEGF, flk-1, flt-1 and simultaneously decreasing endostatin, angiostatin and parstatin expression [133]. Anti-apoptosis signaling such as modulation of Bcl-2, decreased expression of Beclin-1 and alteration of phosphorylation of stress activated proteins are also involved in H2S mediated cardioprotection [134]. Research also suggests that H2S preserves both the structure and function of mitochondria and inhibits cellular respiration by blocking cytochrome c oxidase leading to protection against myocardial ischemic injury [135; 136]. It was also observed that H2S protects cardiomyocytes through Ca2+ channel and cAMP/PKA pathway mediated inhibition of myocardial contractility [137]. Similarly, H2S induces cardioprotection by preventing Ca2+ overload in ischemic heart by suppressing Na+/H+ exchanger -1 involving PI-3K/ Akt/PKG dependent pathway [138]. PDE-5 inhibitor, tadalafil induces cardioprotection by PKG dependent production of H2S in ischemic heart [139]. Preconditioning with H2S in I/R injury also protects against myocardial injury. Decreased c-Fos protein, improved cytosolic Ca2+ in a PKC dependent pathway, activated Nrf2 signaling, activated ERK and PI3k/Akt pathways are observed mechanisms of H2S induced (preconditioned with H2S) cardimyocyte protection during I/R injury [29; 140; 141; 142]. Thus, therapeutic application of H2S may be beneficial in various cardiac diseases.
Atherogenesis
As discussed above, CBS is a primary enzyme producing H2S. Deficiency of CBS enzymatic activity leads to decreased production of H2S as well as development of hyperhomocysteinemia. Studies demonstrate that hyper-homocysteinemia in turn leads to formation of premature atherosclerosis via the promotion and sustained injury of endothelial cells, and inducing vascular smooth muscle cell proliferation [143; 144]. In addition, increased homocysteine may impair generation and bioavailability of EDRF/NO, endothelium-dependent vasodilation; interfere with many transcription factors and signal transduction, and oxidize low-density lipoproteins that lead to formation of atherosclerotic plaque [145; 146]. Hyperhomocysteinemia has a strong correlation with premature peripheral, cerebral and coronary arterial disease following atherosclerosis [147]. Moreover, these effects of hyperhomocysteinemia can be reversed by H2S therapy highlighting the importance of this molecule in regulating different pathophysiological events in this process [148; 149].
Importantly, H2S levels have been reported to be inversely related to plasma lipid levels in healthy individuals [150]. However, the precise levels of plasma and plaque H2S levels and its biochemical forms remain poorly understood in patients with various forms of vascular disease that requires further study. Nonetheless, H2S can inhibit calcification and osteoblast differentiation of vascular smooth muscle cells, resulting in prevention of atherosclerosis that leads to vascular occlusive disease [151]. H2S also inhibits foam cell formation by down-regulating the expression of CD36, SR-A and ACAT1 via the KATP/ERK1/2 pathway and inhibiting oxLDL uptake in human monocyte-derived macrophages [152]. H2S containing aspirin therapy prevents the progression of atherosclerosis by down-regulation of CX3CR1 in macrophages via PPAR-γ dependent pathway in apoE deficient mice [153]. Studies from different laboratories showed that H2S inhibits adhesion molecules (i.e. ICAM, VCAM, E-selectin) involving NF-kB, and protein kinase A and B signaling pathways, and regulates development and progression of atherosclerosis [154; 155]. H2S also protects from oxLDL induced vascular insults and formation of atherosclerotic plague by inhibiting eNOS degradation, restoring eNOS-caveolin complex formation, and PKB dependent eNOS activation [156]. Clearly, the field of H2S and atherogenesis is an exciting and challenging area that will continue to grow and undoubtedly change the way we understand the pathophysiological role of H2S for cardiovascular health.
6. H2S interactions with NO and other biochemical molecules
Modulation of enzymatic activity by gaseous molecules
As previously discussed, the functions and targets of NO and H2S are closely related both physiologically and pathologically. Likewise, both NO and H2S are produced enzymatically by three enzymes– nNOS/iNOS/eNOS for NO and CSE/CBS/3-MST for H2S, with heme moieties playing a role in regulating functions of NOS isoforms and CBS. These enzymes have specific ligands and cofactors; and recent evidence suggests that there are interactions between NO, H2S and their enzymatic systems.
Hydrogen sulfide donors, diallyl disulfide (DADS) and diallyl trisulfide (DATS), can induce eNOS activity through phosphorylation of Ser 1177 and PKB, subsequently increase NO production. This study further revealed that DADS and DATS also prevented Ox-LDL mediated proteosome dependent eNOS degradation [156]. Similarly, H2S stimulates the AKT/eNOS pathway to preserve the cardiac contractile function during ischemic post-conditioning [129]. Moreover, H2S activates the eNOS/NO/P-38 MAPK pathway to prevent I/R injury [157]. In another study, H2S was shown to potentiate the expression of iNOS following stimulation with IL-1β in cultured rat vascular smooth muscle cells [158]. In contrast, other studies revealed that H2S inhibits nNOS and eNOS likely by altering interactions with BH4 and inhibiting iNOS through additional mechanisms [159; 160]. Likewise, both in vitro and in vivo experimental studies showed that H2S down-regulates the vascular L-arginine/NOS/NO pathway [161; 162]. Consistent with these reports, H2S has been reported to alleviate pulmonary vascular remodeling by down-regulating eNOS protein expression and decreasing NOS activity [163]. Together, these data highlight that H2S regulation of NOS activity is complex requiring further investigation into specific molecular mechanisms.
Interestingly, NOS substrate or NO donors can up-regulate the expression or activity of H2S producing enzymes [164; 165]. Eto and Kimura have demonstrated that exogenously applied SNP to ex vivo rat brain cell suspensions increases CBS activity, although independent of NO production [165]. L-arginine attenuates pulmonary artery pressure by augmenting the expression of lung tissue CSE mRNA as well as the activity of CSE in lung tissue [166]. In contrast, studies also reveal that metabolites of NO (nitrite and nitrate) can reduce CBS enzyme activity during I/R of rat kidneys and that CBS activity was restored upon treatment with cPTIO, an NO scavenger [167]. Additional studies are needed to better understand how H2S and NO may reciprocally regulate one anothers enzyme synthesis pathways.
Although, the relationship between NO and H2S may occur in a reciprocal fashion, it is also evident that NOS/NO and CSE/CBS/H2S pathways can mutually affect specific biochemical, cellular and physiological functions in different organs. However, clear mechanistic insight on H2S effects on NO/NOS system activity and vice-versa remains poorly defined requiring further study.
Molecular Target Interactions of NO and H2S
Several reports indicate a potential synergistic effect between NO and H2S in controlling various biological responses of vascular function. Hosoki et al. showed that NO and H2S interact in a co-ordinate fashion in regulating vascular tone [152]. Coletta et al. found that NO and H2S mutually affect PKG to promote proliferation and angiogenesis in vitro [153]. This study revealed that inhibition of NO production by blocking eNOS attenuated H2S-mediated angiogenic activity under normoxic conditions and reduced H2S-dependent vasorelaxation highlighting the importance of NO in vascular H2S signaling. Conversely, inhibition of H2S-production through pharmacological inhibition of cystathionine-γ-lyase has been reported to diminish NO-stimulated cGMP accumulation and angiogenesis, and attenuate acetylcholine-induced vasodilation suggesting a role for H2S in the vascular activity of NO [124]. Moreover, low concentrations of H2S (low micromolar) significantly enhance smooth muscle relaxation induced by NO in the thoracic aorta indicating the role of endogenous H2S in regulation of smooth muscle tone in synergy with NO [168]. Similarly, H2S may act in concert with NO in Stonustoxin-mediated vasorelaxation, and the NO donor sodium nitroprusside can increase H2S synthesis both in vitro and in vivo. [165; 169]. NO donor treatment of endothelial cells also augments cysteine uptake in a dose dependent manner involving increased transporter synthesis and activity revealing how NO can regulated cellular H2S substrate bioavailability in cells [170]. These findings combined with those above suggesting reciprocal regulation make it difficult to predict a priori how H2S and NO would affect physiological and cellular responses although it is clear that these two gasotransmitters can work in cooperative or antagonistic fashion.
Biochemistry of NO-H2S interactions
Hydrogen sulfide and NO may react under specific conditions to form complex/novel species that display distinct biological functions. A recent study revealed that NO donors, as well as NO gas and a synthetic peroxynitrite (ONOO−) react with H2S in vitro to form nitrosothiol [171]. The authors posited that simultaneous NO and H2S production in LPS treated rats could produce nitrosothiol in vivo. Likewise, studies showed that administration of NaHS or inducing endogenous H2S production via cysteine/pyridoxal phosphate reduces plasma NO2−/NO3−, suggesting consumption of NO possibly through formation of nitosothiol [161; 171]. Similarly, H2S reacts with S-nitrosothiols to form thionitrous acid (HSNO). These results suggest that, at the cellular level, HSNO can be metabolized to generate NO+, NO, and NO− species, all of which have distinct physiological consequences of their own. HSNO also can freely diffuse through membranes, facilitating trans-nitrosation of proteins such as hemoglobin. Moreover, HSNO can lead to the generation of HNO− (nitroxyl anion), which has significant cardiopharmacological potential [172]. Likewise, Yong et al. indicate that H2S interacts with NO to form HNO− that exhibits positive inotropic and lusitropic effects on the heart during inflammation-induced arrhythmia [173]. H2S can attenuate isoproterenol induced cardiac injury and improves survival after cardiac arrest and cardiopulmonary resuscitation in mice in a NO dependent pathway [132]. Peroxynitrite rapidly interacts with H2S and generates sulfinyl nitrite [HS(O)NO] which abolishes the pro-apoptotic, oxidative and nitrative properties of peroxynitrite. Furthermore, the thionitrate isomer formed is capable of releasing NO in a pH-dependent manner that likely contributes to the observed cytoprotective effects [174]. These studies highlight the varied reactions between NO-H2S and their diverse biochemical products and functions that are just beginning to be explored.
H2S effects on other reactive oxygen species
H2S may also alter the production of various other reactive oxygen species (ROS). H2S can interfere with NOX driven superoxide production in vascular smooth muscle cells by inhibiting NOX1 expression and Rac1 activity [175]. Likewise, the effect of peroxynitrite can be neutralized by H2S in brain endothelial cells during oxidative stress and in liver, heart and lung during I/R injury [11; 176], possibly through chemical mechanisms discussed above. Along this line, it has been suggested that a combination of NO and H2S therapy may be a better option for treatment of I/R injury after organ transplantation to effectively balance the impact of each molecule on ROS toxicity [177]. However, additional studies are needed to more comprehensively examine the effect of H2S on ROS formation and associated defense pathways, as it is likely that both are affected. Future studies should also address if and how combined H2S/NO based therapeutic approaches are truly more beneficial for disease states involving redox stress pathophysiology.
Interaction with hemeproteins and non-hemeproteins
Biological compounds containing unpaired electrons such as sGC, hemoglobin, and cytochrome c oxidase (CcOx) react rapidly with NO, leading to some of the best-understood physiological effects of NO [178]. The resulting effect is highly dependent on their heme protein. For example, reaction of NO with sGC results in conformational changes in sGC and increases the rate of conversion of GTP to cGMP by which NO exerts its classical signaling function. Conversely, sGC is deactivated rapidly after NO removal [178].
H2S also reacts with hemeproteins in distinct ways: i) by incorporation of H2S into one of the pyrrole rings of the heme, ii) generating the sulfheme derivative, iii) binding to alternate sites of hemeproteins such as cysteine, copper, and zinc ions; or to the ferric iron with subsequent reduction of the heme, and iv) through coordination with the ferric heme iron without inducing reduction or sulfheme production [179]. Likewise, H2S can be liberated from sulfide containing heme-protein by various reduction processes under different conditions. H2S can also bind to both Hb and Mb in the ferric state as a heme ligand with lower affinity, and reduce heme rapidly with the subsequent production of the deoxy ferrous or oxy ferous-O2 compounds [179; 180]. H2S inhibits cytochrome C activity by directly binding and reducing its ferric heme a3 and CuB centers, and under moderate concentration of H2S affinity of heme a3 for O2 decreases, which can stimulate muscle relaxation by diminishing cellular ATP formation. [181]. Importantly, NO can react rapidly with metalloproteins such as iron–sulfur clusters or compounds containing them to generate dinitrosyl iron complexes [182] that influence formation of S-nitrosothiols inside the cells via transnitrosation of NO [183]. Thus, H2S interaction with hemeproteins represents another pathway whereby H2S and NO chemistry and biology collide although much less is know about the importance of these reactions under pathophysiological conditions.
NO, XO and H2S interaction
Xanthine oxidase (XO) stimulates biological NO release from S-nitrosothiols such as S-nitrosocysteine (CysNO) and S-nitrosoglutathione (GSNO) under aerobic and anaerobic conditions [184]. Hypoxanthine (HX)/XO interacts with NO to form nitrated uric acid derivatives that slowly release NO and potentiate the action of NO for relaxation of the circular muscle of the gastric fundus [185]. Nitric oxide and superoxide anions produced by XO may also lead to the production of peroxynitrite, which acts as a vasoactive radical in the fetal-placental vasculature [186]. Nitrite, but not nitrate, protects against myocardial infarction by a mechanism involving the xanthine oxidoreductase-signaling pathway [187]. Similarly, a study showed that under hypoxic conditions, XO reduces nitrate to nitrite and nitrite to NO at the molybdenum site of the enzyme in presence of xanthine [188]. These findings together with work from our group indicate that H2S can potentiate XO activity facilitating nitrite reduction to NO under hypoxic conditions where the function of NOS is impaired [118; 188]. It is known that NO or peroxynitrite inhibits XO activity in a dose dependent manner and attenuates superoxide generation primarily by oxidative disruption of the molybdenum catalytic site [189]. However, NO inactivated XO activity can be recovered in the presence of a sulfide-generating system under anaerobic conditions suggesting reciprocal regulation by NO and sulfide on XO activity in the hypoxic state [190]. Additional studies examining the nature of H2S versus NO regulation of XO activity are needed and may reveal important mechanistic insight into how these gasotransmitters work to regulate cellular redox balance.
7. Conclusion
It is interesting to note that the physiological functions of H2S are comparable to that of NO, as well as controversy surrounding their cytoprotective roles. Throughout the literature on H2S it is reported that the cytoprotective and antioxidant effects occur in the micromolar range; whereas higher H2S exposures, i.e. in the millimolar range, potentiate redox stress and are cytotoxic. In the coming future, it is most likely that the field will come to realize the cellular and signalling function and physiological potency of low nanomolar concentrations of H2S, and that various biochemical forms of the molecule serve important roles in regulating H2S bioavailability and cellular redox balance. From the information above, it is safe to say that H2S serves as a proverbial “double-edged sword,” where it can be extremely beneficial or harmful depending on its concentration and cellular location. These observations also reveal how crucial it is moving forward to accurately determine and control for the levels of H2S in experimental settings, reinforcing the need for rigorous and reliable measurement techniques to monitor the biological levels of H2S. Finally, increased clarity regarding sulfide cellular signaling will also alleviate confusion and lead to a better understanding of the effects of H2S administration in biological systems. The future of H2S biochemistry, chemical biology and pathophysiology represent fertile territory in which to better understand redox processes that will ultimately be important for human health and disease.
Acknowledgments
Funded by NIH HL11331 to C.G.K and Feist Cardiovascular Fellowships to G.K.K. and S.C.B.
References
- 1.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 2.Tangerman A, Meuwese-Arends MT, van Tongeren JH. New methods for the release of volatile sulfur compounds from human serum: its determination by Tenax trapping and gas chromatography and its application in liver diseases. J Lab Clin Med. 1985;106:175–182. [PubMed] [Google Scholar]
- 3.Ubuka T, Abe T, Kajikawa R, Morino K. Determination of hydrogen sulfide and acid-labile sulfur in animal tissues by gas chromatography and ion chromatography. J Chromatogr B Biomed Sci Appl. 2001;757:31–37. doi: 10.1016/s0378-4347(01)00046-9. [DOI] [PubMed] [Google Scholar]
- 4.Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 5.Zhong G, Chen F, Cheng Y, Tang C, Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, Moore PK. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol. 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 7.Pearson RJ, Wilson T, Wang R. Endogenous hydrogen sulfide and the cardiovascular system-what's the smell all about? Clin Invest Med. 2006;29:146–150. [PubMed] [Google Scholar]
- 8.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 10.Bos EM, Leuvenink HG, Snijder PM, Kloosterhuis NJ, Hillebrands JL, Leemans JC, Florquin S, van Goor H. Hydrogen sulfide-induced hypometabolism prevents renal ischemia/reperfusion injury. J Am Soc Nephrol. 2009;20:1901–1905. doi: 10.1681/ASN.2008121269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 13.Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 15.Searcy DG, Lee SH. Sulfur reduction by human erythrocytes. J Exp Zool. 1998;282:310–322. doi: 10.1002/(sici)1097-010x(19981015)282:3<310::aid-jez4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Cooper AJ. Biochemistry of sulfur-containing amino acids. Annu Rev Biochem. 1983;52:187–222. doi: 10.1146/annurev.bi.52.070183.001155. [DOI] [PubMed] [Google Scholar]
- 17.Koj A, Frendo J, Janik Z. [35S]thiosulphate oxidation by rat liver mitochondria in the presence of glutathione. Biochem J. 1967;103:791–795. doi: 10.1042/bj1030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beauchamp RO, Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 19.Nagahara N, Nishino T. Role of amino acid residues in the active site of rat liver mercaptopyruvate sulfurtransferase. CDNA cloning, overexpression, and site-directed mutagenesis. J Biol Chem. 1996;271:27395–27401. doi: 10.1074/jbc.271.44.27395. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- 21.Ogasawara Y, Ishii K, Togawa T, Tanabe S. Determination of trace amounts of sulphide in human red blood cells by high-performance liquid chromatography with fluorimetric detection after derivatization with p-phenylenediamine and iron(III) Analyst. 1991;116:1359–1363. doi: 10.1039/an9911601359. [DOI] [PubMed] [Google Scholar]
- 22.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 23.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 24.Ubuka T. Assay methods and biological roles of labile sulfur in animal tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:227–249. doi: 10.1016/s1570-0232(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 25.Westley AM, Westley J. Biological sulfane sulfur. Anal Biochem. 1991;195:63–67. doi: 10.1016/0003-2697(91)90295-5. [DOI] [PubMed] [Google Scholar]
- 26.Rohwerder T, Sand W. The sulfane sulfur of persulfides is the actual substrate of the sulfur-oxidizing enzymes from Acidithiobacillus and Acidiphilium spp. Microbiology. 2003;149:1699–1710. doi: 10.1099/mic.0.26212-0. [DOI] [PubMed] [Google Scholar]
- 27.Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 2011;121:459–488. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gero D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A. 2011;108:13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medani M, Collins D, Docherty NG, Baird AW, O'Connell PR, Winter DC. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm Bowel Dis. 2011;17:1620–1625. doi: 10.1002/ibd.21528. [DOI] [PubMed] [Google Scholar]
- 31.Baskar R, Li L, Moore PK. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J. 2007;21:247–255. doi: 10.1096/fj.06-6255com. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y, Adhikari S, Ang AD, Moore PK, Bhatia M. Mechanism of induction of pancreatic acinar cell apoptosis by hydrogen sulfide. Am J Physiol Cell Physiol. 2006;291:C503–C510. doi: 10.1152/ajpcell.00547.2005. [DOI] [PubMed] [Google Scholar]
- 33.Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol. 2007;81:1322–1332. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]
- 34.Chen KY, Morris JC. Kinetics of oxidation of aqueous sulfide by oxygen. Environ. Sci. Technol. 1972;6:529–537. [Google Scholar]
- 35.Nielsen AH, Vollertsen J, Hvitved-Jacobsen T. Kinetics and stoichiometry of aerobic sulfide oxidation in wastewater from sewers-effects of pH and temperature. Water Environ Res. 2006;78:275–283. doi: 10.2175/106143005x94367. [DOI] [PubMed] [Google Scholar]
- 36.Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen AH, Vollertsen J, Hvitved-Jacobsen T. Determination of kinetics and stoichiometry of chemical sulfide oxidation in wastewater of sewer networks. Environ Sci Technol. 2003;37:3853–3858. doi: 10.1021/es034035l. [DOI] [PubMed] [Google Scholar]
- 38.Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, Madden JA. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol. 2006;209:4011–4023. doi: 10.1242/jeb.02480. [DOI] [PubMed] [Google Scholar]
- 39.Shen X, Peter EA, Bir S, Wang R, Kevil CG. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol Med. 2012;52:2276–2283. doi: 10.1016/j.freeradbiomed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med. 2011;50:1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer E. Bildung von Methylenblauals Reaction auf Schwefelwasserstoff. Chem.Ber. 1883;26:2234–2236. [Google Scholar]
- 42.Almy LH. A method for the estimation of hydrogen sulfide in proteinaceous food products. Am. Chem. Sot. 1925;47:1381–1390. [Google Scholar]
- 43.Budd MS, Bewick HA. Photometric determination of sulfide and reducible sulfur in alkalies. Anal. Chem. 1952;24:1536–1540. [Google Scholar]
- 44.Fogo JK, Popowsky M. Spectrophotometric determination of hydrogen sulfide. Anal. Chem. 1949;21:732–734. [Google Scholar]
- 45.Sands AE, Grafius MA, Wainwright HW, WILSON MW. The determination of low concentrations of hydrogen sulfide in gas by the methylene blue method. U.S. Bur. Mines Rept. Invest. 1949;4547:1–18. [Google Scholar]
- 46.Sheppard SE, Geddes AL. Effect of solvents upon the absorption spectra of dyes. V. Water as solvent: Quantitative examination of the dimerization hypothesis. J. Am. Chem. Sot. 1944;66:2003–2009. [Google Scholar]
- 47.Pomeroy R. The determination of sulphides in sewage. Sewage Works J. 1936;8:572–591. [Google Scholar]
- 48.Guenther EA, Johnson KS, Coale KH. Direct ultraviolet spectrophotometric determination of total sulfide and iodide in natural waters. Anal Chem. 2001;73:3481–3487. doi: 10.1021/ac0013812. [DOI] [PubMed] [Google Scholar]
- 49.Kovatsis A, Tsougas M. Determination of hydrogen sulfide (H2S) in environment by indirect atomic adsorption spectroscopy. Bull Environ Contam Toxicol. 1976;15:412–420. doi: 10.1007/BF01685064. [DOI] [PubMed] [Google Scholar]
- 50.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal. 2012;17:32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olson KR. Is hydrogen sulfide a circulating "gasotransmitter" in vertebrate blood? Biochim Biophys Acta. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 52.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR, Jr, Darley-Usmar VM, Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 53.Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR, Jr, Doeller JE, Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol. 2007;292:H1953–H1960. doi: 10.1152/ajpheart.01193.2006. [DOI] [PubMed] [Google Scholar]
- 54.Faccenda A, Wang J, Mutus B. Polydimethylsiloxane permeability-based method for the continuous and specific detection of hydrogen sulfide. Anal Chem. 2012;84:5243–5249. doi: 10.1021/ac3008863. [DOI] [PubMed] [Google Scholar]
- 55.Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia MW, Reiffenstein RJ, Dowling G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol. 1989;13:105–109. doi: 10.1093/jat/13.2.105. [DOI] [PubMed] [Google Scholar]
- 56.Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 57.Levitt MD, Abdel-Rehim MS, Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal. 2011;15:373–378. doi: 10.1089/ars.2010.3525. [DOI] [PubMed] [Google Scholar]
- 58.Savage JC, Gould DH. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J Chromatogr. 1990;526:540–545. doi: 10.1016/s0378-4347(00)82537-2. [DOI] [PubMed] [Google Scholar]
- 59.Newton GL, Fahey RC. Determination of biothiols by bromobimane labeling and high-performance liquid chromatography. Methods Enzymol. 1995;251:148–166. doi: 10.1016/0076-6879(95)51118-0. [DOI] [PubMed] [Google Scholar]
- 60.Togawa T, Ogawa M, Nawata M, Ogasawara Y, Kawanabe K, Tanabe S. High performance liquid chromatographic determination of bound sulfide and sulfite and thiosulfate at their low levels in human serum by pre-column fluorescence derivatization with monobromobimane. Chem Pharm Bull (Tokyo) 1992;40:3000–3004. doi: 10.1248/cpb.40.3000. [DOI] [PubMed] [Google Scholar]
- 61.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol. 2010;160:941–957. doi: 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mubeen S, Zhang T, Chartuprayoon N, Rheem Y, Mulchandani A, Myung NV, Deshusses MA. Sensitive detection of H2S using gold nanoparticle decorated single-walled carbon nanotubes. Anal Chem. 2010;82:250–257. doi: 10.1021/ac901871d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang BH, Wu FY, Wu YM, Zhan XS. Fluorescent method for the determination of sulfide anion with ZnS:Mn quantum dots. J Fluoresc. 2010;20:243–250. doi: 10.1007/s10895-009-0545-0. [DOI] [PubMed] [Google Scholar]
- 64.Liu C, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M. Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew Chem Int Ed Engl. 2011;50:10327–10329. doi: 10.1002/anie.201104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng H, Cheng Y, Dai C, King AL, Predmore BL, Lefer DJ, Wang B. A fluorescent probe for fast and quantitative detection of hydrogen sulfide in blood. Angew Chem Int Ed Engl. 2011;50:9672–9675. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 67.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 68.Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, Masuko T, Nishikawa H, Kawabata A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2007;241:11–18. doi: 10.1016/j.tox.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 69.Laggner H, Hermann M, Esterbauer H, Muellner MK, Exner M, Gmeiner BM, Kapiotis S. The novel gaseous vasorelaxant hydrogen sulfide inhibits angiotensin-converting enzyme activity of endothelial cells. J Hypertens. 2007;25:2100–2104. doi: 10.1097/HJH.0b013e32829b8fd0. [DOI] [PubMed] [Google Scholar]
- 70.Sivarajah A, Collino M, Yasin M, Benetti E, Gallicchio M, Mazzon E, Cuzzocrea S, Fantozzi R, Thiemermann C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31:267–274. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- 71.Ali MY, Whiteman M, Low CM, Moore PK. Hydrogen sulphide reduces insulin secretion from HIT-T15 cells by a KATP channel-dependent pathway. J Endocrinol. 2007;195:105–112. doi: 10.1677/JOE-07-0184. [DOI] [PubMed] [Google Scholar]
- 72.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang R. Hydrogen sulfide: a new EDRF. Kidney Int. 2009;76:700–704. doi: 10.1038/ki.2009.221. [DOI] [PubMed] [Google Scholar]
- 74.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 75.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 77.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–548. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 78.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 79.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ashraf MZ, Hussain ME, Fahim M. Endothelium mediated vasorelaxant response of garlic in isolated rat aorta: role of nitric oxide. J Ethnopharmacol. 2004;90:5–9. doi: 10.1016/j.jep.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 81.Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace JL. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 83.Wallace JL, Ignarro LJ, Fiorucci S. Potential cardioprotective actions of no-releasing aspirin. Nat Rev Drug Discov. 2002;1:375–382. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- 84.Beck PL, McKnight W, Lee SS, Wallace JL. Prostaglandin modulation of the gastric vasculature and mucosal integrity in cirrhotic rats. Am J Physiol. 1993;265:G453–G458. doi: 10.1152/ajpgi.1993.265.3.G453. [DOI] [PubMed] [Google Scholar]
- 85.Perini R, Fiorucci S, Wallace JL. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastrointestinal injury and repair: a window of opportunity for cyclooxygenase-inhibiting nitric oxide donors. Can J Gastroenterol. 2004;18:229–236. doi: 10.1155/2004/890585. [DOI] [PubMed] [Google Scholar]
- 86.Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 87.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 88.Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI, Yaghoubi M, Goldschmidt-Clermont PJ, Farber HW, Cohen R, Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats, Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- 90.Sagara Y, Schubert D. The activation of metabotropic glutamate receptors protects nerve cells from oxidative stress. J Neurosci. 1998;18:6662–6671. doi: 10.1523/JNEUROSCI.18-17-06662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sunda W, Kieber DJ, Kiene RP, Huntsman S. An antioxidant function for DMSP and DMS in marine algae. Nature. 2002;418:317–320. doi: 10.1038/nature00851. [DOI] [PubMed] [Google Scholar]
- 92.Hamar J, Solymar M, Tanai E, Cseplo P, Springo Z, Berta G, Debreceni B, Koller A. Bioassay-comparison of the antioxidant efficacy of hydrogen sulfide and superoxide dismutase in isolated arteries and veins. Acta Physiol Hung. 2012;99:411–419. doi: 10.1556/APhysiol.99.2012.4.5. [DOI] [PubMed] [Google Scholar]
- 93.Wei HL, Zhang CY, Jin HF, Tang CS, Du JB. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol Sin. 2008;29:670–679. doi: 10.1111/j.1745-7254.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 94.Lu M, Hu LF, Hu G, Bian JS. Hydrogen sulfide protects astrocytes against H(2)O(2)-induced neural injury via enhancing glutamate uptake. Free Radic Biol Med. 2008;45:1705–1713. doi: 10.1016/j.freeradbiomed.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 95.Searcy DG, Whitehead JP, Maroney MJ. Interaction of Cu,Zn superoxide dismutase with hydrogen sulfide. Arch Biochem Biophys. 1995;318:251–263. doi: 10.1006/abbi.1995.1228. [DOI] [PubMed] [Google Scholar]
- 96.Lavu M, Bhushan S, Lefer DJ. Hydrogen sulfide-mediated cardioprotection: mechanisms and therapeutic potential. Clin Sci (Lond) 2011;120:219–229. doi: 10.1042/CS20100462. [DOI] [PubMed] [Google Scholar]
- 97.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Sellke FW. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008;33:906–913. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Biermann J, Lagreze WA, Schallner N, Schwer CI, Goebel U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol Vis. 2011;17:1275–1286. [PMC free article] [PubMed] [Google Scholar]
- 99.Shi S, Li QS, Li H, Zhang L, Xu M, Cheng JL, Peng CH, Xu CQ, Tian Y. Anti-apoptotic action of hydrogen sulfide is associated with early JNK inhibition. Cell Biol Int. 2009;33:1095–1101. doi: 10.1016/j.cellbi.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 100.Bordia A, Verma SK, Vyas AK, Khabya BL, Rathore AS, Bhu N, Bedi HK. Effect of essential oil of onion and garlic on experimental atherosclerosis in rabbits. Atherosclerosis. 1977;26:379–386. doi: 10.1016/0021-9150(77)90092-2. [DOI] [PubMed] [Google Scholar]
- 101.Mirhadi SA, Singh S, Gupta PP. Effect of garlic supplementation to atherogenic diet on collagen biosynthesis in various tissues of rabbits. Indian Heart J. 1990;42:99–104. [PubMed] [Google Scholar]
- 102.Banerjee SK, Maulik SK. Effect of garlic on cardiovascular disorders: a review. Nutr J. 2002;1:4. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bordia AK, Joshi HK. Garlic on fibrinolytic activity in cases of acute myocardial infarction. Part II. J Assoc Physicians India. 1978;26:323–326. [PubMed] [Google Scholar]
- 104.Sainani GS, Desai DB, Gorhe NH, Natu SM, Pise DV, Sainani PG. Dietary garlic, onion and some coagulation parameters in Jain community. J Assoc Physicians India. 1979;27:707–712. [PubMed] [Google Scholar]
- 105.Arora RC, Arora S. Comparative effect of clofibrate, garlic and onion on alimentary hyperlipemia. Atherosclerosis. 1981;39:447–452. doi: 10.1016/0021-9150(81)90002-2. [DOI] [PubMed] [Google Scholar]
- 106.Ali M, Thomson M, Alnaqeeb MA, al-Hassan JM, Khater SH, Gomes SA. Antithrombotic activity of garlic: its inhibition of the synthesis of thromboxane-B2 during infusion of arachidonic acid and collagen in rabbits. Prostaglandins Leukot Essent Fatty Acids. 1990;41:95–99. doi: 10.1016/0952-3278(90)90060-x. [DOI] [PubMed] [Google Scholar]
- 107.Srivastava KC. Aqueous extracts of onion, garlic and ginger inhibit platelet aggregation and alter arachidonic acid metabolism. Biomed Biochim Acta. 1984;43:S335–S346. [PubMed] [Google Scholar]
- 108.Ali M, Bordia T, Mustafa T. Effect of raw versus boiled aqueous extract of garlic and onion on platelet aggregation. Prostaglandins Leukot Essent Fatty Acids. 1999;60:43–47. doi: 10.1054/plef.1998.0006. [DOI] [PubMed] [Google Scholar]
- 109.DeBoer LW, Folts JD. Garlic extract prevents acute platelet thrombus formation in stenosed canine coronary arteries. Am Heart J. 1989;117:973–975. doi: 10.1016/0002-8703(89)90641-8. [DOI] [PubMed] [Google Scholar]
- 110.Thomson M, Mustafa T, Ali M. Thromboxane-B(2) levels in serum of rabbits receiving a single intravenous dose of aqueous extract of garlic and onion. Prostaglandins Leukot Essent Fatty Acids. 2000;63:217–221. doi: 10.1054/plef.2000.0212. [DOI] [PubMed] [Google Scholar]
- 111.Bordia A. Effect of garlic on human platelet aggregation in vitro. Atherosclerosis. 1978;30:355–360. doi: 10.1016/0021-9150(78)90129-6. [DOI] [PubMed] [Google Scholar]
- 112.Srivastava KC. Evidence for the mechanism by which garlic inhibits platelet aggregation. Prostaglandins Leukot Med. 1986;22:313–321. doi: 10.1016/0262-1746(86)90142-3. [DOI] [PubMed] [Google Scholar]
- 113.Kiesewetter H, Jung F, Pindur G, Jung EM, Mrowietz C, Wenzel E. Effect of garlic on thrombocyte aggregation, microcirculation, and other risk factors. Int J Clin Pharmacol Ther Toxicol. 1991;29:151–155. [PubMed] [Google Scholar]
- 114.Boullin DJ. Garlic as a platelet inhibitor. Lancet. 1981;1:776–777. doi: 10.1016/s0140-6736(81)92644-1. [DOI] [PubMed] [Google Scholar]
- 115.Allison GL, Lowe GM, Rahman K. Aged garlic extract inhibits platelet activation by increasing intracellular cAMP and reducing the interaction of GPIIb/IIIa receptor with fibrinogen. Life Sci. 2012;91:1275–1280. doi: 10.1016/j.lfs.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 116.Bir SC, Kolluru GK, Fang K, Kevil CG. Redox balance dynamically regulates vascular growth and remodeling. Semin Cell Dev Biol. 2012;23:745–757. doi: 10.1016/j.semcdb.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chidlow JH, Jr, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293:G5–G18. doi: 10.1152/ajpgi.00107.2007. [DOI] [PubMed] [Google Scholar]
- 118.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen Sulfide Stimulates Ischemic Vascular Remodeling Through Nitric Oxide Synthase and Nitrite Reduction Activity Regulating Hypoxia-Inducible Factor-1α and Vascular Endothelial Growth Factor–Dependent Angiogenesis. J Am Heart Assoc. 2012 doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 120.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoefer IE. Something is rotten in the state of angiogenesis -- H2S as gaseous stimulator of angiogenesis. Cardiovasc Res. 2007;76:1–2. doi: 10.1016/j.cardiores.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 122.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhu Y, Zhu YC. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang MJ, Cai WJ, Zhu YC. Mechanisms of angiogenesis: role of hydrogen sulphide. Clin Exp Pharmacol Physiol. 2010;37:764–771. doi: 10.1111/j.1440-1681.2010.05371.x. [DOI] [PubMed] [Google Scholar]
- 124.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Can J Physiol Pharmacol. 2007;85:1248–1253. doi: 10.1139/Y07-120. [DOI] [PubMed] [Google Scholar]
- 126.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 127.Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol. 2008;587:1–7. doi: 10.1016/j.ejphar.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 128.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yong QC, Lee SW, Foo CS, Neo KL, Chen X, Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Heart Circ Physiol. 2008;295:H1330–H1340. doi: 10.1152/ajpheart.00244.2008. [DOI] [PubMed] [Google Scholar]
- 130.Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, Lefer DJ, Bloch KD, Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation. 2009;120:888–896. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, Lefer DJ. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2012;302:H2410–H2418. doi: 10.1152/ajpheart.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sojitra B, Bulani Y, Putcha UK, Kanwal A, Gupta P, Kuncha M, Banerjee SK. Nitric oxide synthase inhibition abrogates hydrogen sulfide-induced cardioprotection in mice. Mol Cell Biochem. 2012;360:61–69. doi: 10.1007/s11010-011-1044-6. [DOI] [PubMed] [Google Scholar]
- 133.Qipshidze N, Metreveli N, Mishra PK, Lominadze D, Tyagi SC. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int J Biol Sci. 2012;8:430–441. doi: 10.7150/ijbs.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hausenloy DJ, Yellon DM. Cardioprotective growth factors. Cardiovascular Research. 2009;83:179–194. doi: 10.1093/cvr/cvp062. [DOI] [PubMed] [Google Scholar]
- 135.Forgan LG, Forster ME. Oxygen consumption, ventilation frequency and cytochrome c oxidase activity in blue cod (Parapercis colias) exposed to hydrogen sulphide or isoeugenol. Comp Biochem Physiol C Toxicol Pharmacol. 2010;151:57–65. doi: 10.1016/j.cbpc.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 136.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res. 2008;79:632–641. doi: 10.1093/cvr/cvn140. [DOI] [PubMed] [Google Scholar]
- 138.Hu LF, Li Y, Neo KL, Yong QC, Lee SW, Tan BK, Bian JS. Hydrogen sulfide regulates Na+/H+ exchanger activity via stimulation of phosphoinositide 3-kinase/Akt and protein kinase G pathways. J Pharmacol Exp Ther. 2011;339:726–735. doi: 10.1124/jpet.111.184754. [DOI] [PubMed] [Google Scholar]
- 139.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation. 2009;120:S31–S36. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu XY, Yan XH, Chen SJ. H(2)S protects myocardium against ischemia/reperfusion injury and its effect on c-Fos protein expression in rats. Sheng Li Xue Bao. 2008;60:221–227. [PubMed] [Google Scholar]
- 141.Pan TT, Neo KL, Hu LF, Yong QC, Bian JS. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am J Physiol Cell Physiol. 2008;294:C169–C177. doi: 10.1152/ajpcell.00282.2007. [DOI] [PubMed] [Google Scholar]
- 142.Hu Y, Chen X, Pan TT, Neo KL, Lee SW, Khin ES, Moore PK, Bian JS. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch. 2008;455:607–616. doi: 10.1007/s00424-007-0321-4. [DOI] [PubMed] [Google Scholar]
- 143.de Valk HW, van Eeden MK, Banga JD, van der Griend R, de Groot E, Haas FJ, Meuwissen OJ, Duran M, Smeitink JA, Poll-The BT, de Klerk JB, Wittebol-Post D, Rolland MO. Evaluation of the presence of premature atherosclerosis in adults with heterozygosity for cystathionine-beta-synthase deficiency. Stroke. 1996;27:1134–1136. [PubMed] [Google Scholar]
- 144.Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, Andria G, Boers GH, Bromberg IL, Cerone R, et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- 145.Guilland JC, Favier A, Potier de Courcy G, Galan P, Hercberg S. Hyperhomocysteinemia: an independent risk factor or a simple marker of vascular disease?. 1. Basic data. Pathol Biol (Paris) 2003;51:101–110. doi: 10.1016/s0369-8114(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 146.Thambyrajah J, Townend JN. Homocysteine and atherothrombosis--mechanisms for injury. Eur Heart J. 2000;21:967–974. doi: 10.1053/euhj.1999.1914. [DOI] [PubMed] [Google Scholar]
- 147.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 148.Distrutti E, Mencarelli A, Santucci L, Renga B, Orlandi S, Donini A, Shah V, Fiorucci S. The methionine connection: homocysteine and hydrogen sulfide exert opposite effects on hepatic microcirculation in rats. Hepatology. 2008;47:659–667. doi: 10.1002/hep.22037. [DOI] [PubMed] [Google Scholar]
- 149.Sen U, Sathnur PB, Kundu S, Givvimani S, Coley DM, Mishra PK, Qipshidze N, Tyagi N, Metreveli N, Tyagi SC. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. Am J Physiol Cell Physiol. 2012;303:C41–C51. doi: 10.1152/ajpcell.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jain SK, Micinski D, Lieblong BJ, Stapleton T. Relationship between hydrogen sulfide levels and HDL-cholesterol, adiponectin, and potassium levels in the blood of healthy subjects. Atherosclerosis. 2012;225:242–245. doi: 10.1016/j.atherosclerosis.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zavaczki E, Jeney V, Agarwal A, Zarjou A, Oros M, Katko M, Varga Z, Balla G, Balla J. Hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells. Kidney Int. 2011;80:731–739. doi: 10.1038/ki.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao ZZ, Wang Z, Li GH, Wang R, Tan JM, Cao X, Suo R, Jiang ZS. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med (Maywood) 2011;236:169–176. doi: 10.1258/ebm.2010.010308. [DOI] [PubMed] [Google Scholar]
- 153.Zhang H, Guo C, Zhang A, Fan Y, Gu T, Wu D, Sparatore A, Wang C. Effect of S-aspirin, a novel hydrogen-sulfide-releasing aspirin (ACS14), on atherosclerosis in apoE-deficient mice. Eur J Pharmacol. 2012;697:106–116. doi: 10.1016/j.ejphar.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 154.Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, Tang X, Ren Y, Tang C, Du J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 155.Lei YP, Chen HW, Sheen LY, Lii CK. Diallyl disulfide and diallyl trisulfide suppress oxidized LDL-induced vascular cell adhesion molecule and E-selectin expression through protein kinase A- and B-dependent signaling pathways. J Nutr. 2008;138:996–1003. doi: 10.1093/jn/138.6.996. [DOI] [PubMed] [Google Scholar]
- 156.Lei YP, Liu CT, Sheen LY, Chen HW, Lii CK. Diallyl disulfide and diallyl trisulfide protect endothelial nitric oxide synthase against damage by oxidized low-density lipoprotein. Mol Nutr Food Res. 2010;54(Suppl 1):S42–S52. doi: 10.1002/mnfr.200900278. [DOI] [PubMed] [Google Scholar]
- 157.Yusof M, Kamada K, Kalogeris T, Gaskin FS, Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol. 2009;296:H868–H876. doi: 10.1152/ajpheart.01111.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jeong SO, Pae HO, Oh GS, Jeong GS, Lee BS, Lee S, Kim du Y, Rhew HY, Lee KM, Chung HT. Hydrogen sulfide potentiates interleukin-1beta-induced nitric oxide production via enhancement of extracellular signal-regulated kinase activation in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;345:938–944. doi: 10.1016/j.bbrc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 159.Kubo S, Kurokawa Y, Doe I, Masuko T, Sekiguchi F, Kawabata A. Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase. Toxicology. 2007;241:92–97. doi: 10.1016/j.tox.2007.08.087. [DOI] [PubMed] [Google Scholar]
- 160.Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, Jeon SB, Jeon WK, Chae HJ, Chung HT. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 161.Geng B, Cui Y, Zhao J, Yu F, Zhu Y, Xu G, Zhang Z, Tang C, Du J. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1608–R1618. doi: 10.1152/ajpregu.00207.2006. [DOI] [PubMed] [Google Scholar]
- 162.Kubo S, Doe I, Kurokawa Y, Nishikawa H, Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology. 2007;232:138–146. doi: 10.1016/j.tox.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 163.Li XH, Du JB, Bu DF, Tang XY, Tang CS. Sodium hydrosulfide alleviated pulmonary vascular structural remodeling induced by high pulmonary blood flow in rats. Acta Pharmacol Sin. 2006;27:971–980. doi: 10.1111/j.1745-7254.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- 164.Wang YF, Mainali P, Tang CS, Shi L, Zhang CY, Yan H, Liu XQ, Du JB. Effects of nitric oxide and hydrogen sulfide on the relaxation of pulmonary arteries in rats. Chin Med J (Engl) 2008;121:420–423. [PubMed] [Google Scholar]
- 165.Eto K, Kimura H. A novel enhancing mechanism for hydrogen sulfide-producing activity of cystathionine beta-synthase. J Biol Chem. 2002;277:42680–42685. doi: 10.1074/jbc.M205835200. [DOI] [PubMed] [Google Scholar]
- 166.Shi L, Du JB, Pu DF, Qi JG, Tang CS. Regulation of endogenous cystathionine-gamma-lyase gene expression in high pulmonary flow by nitric oxide precursor. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2006;22:343–347. [PubMed] [Google Scholar]
- 167.Prathapasinghe GA, Siow YL, Xu Z, O K. Inhibition of cystathionine-beta-synthase activity during renal ischemia-reperfusion: role of pH and nitric oxide. Am J Physiol Renal Physiol. 2008;295:F912–F922. doi: 10.1152/ajprenal.00040.2008. [DOI] [PubMed] [Google Scholar]
- 168.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 169.Liew HC, Khoo HE, Moore PK, Bhatia M, Lu J, Moochhala SM. Synergism between hydrogen sulfide (H(2)S) and nitric oxide (NO) in vasorelaxation induced by stonustoxin (SNTX), a lethal and hypotensive protein factor isolated from stonefish Synanceja horrida venom. Life Sci. 2007;80:1664–1668. doi: 10.1016/j.lfs.2007.01.058. [DOI] [PubMed] [Google Scholar]
- 170.Li H, Marshall ZM, Whorton AR. Stimulation of cystine uptake by nitric oxide: regulation of endothelial cell glutathione levels. Am J Physiol. 1999;276:C803–C811. doi: 10.1152/ajpcell.1999.276.4.C803. [DOI] [PubMed] [Google Scholar]
- 171.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- 172.Filipovic MR, Miljkovic J, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovic-Burmazovic I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J Am Chem Soc. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yong QC, Hu LF, Wang S, Huang D, Bian JS. Hydrogen sulfide interacts with nitric oxide in the heart: possible involvement of nitroxyl. Cardiovasc Res. 2010;88:482–491. doi: 10.1093/cvr/cvq248. [DOI] [PubMed] [Google Scholar]
- 174.Filipovic MR, Miljkovic J, Allgauer A, Chaurio R, Shubina T, Herrmann M, Ivanovic-Burmazovic I. Biochemical insight into physiological effects of H(2)S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem J. 2012;441:609–621. doi: 10.1042/BJ20111389. [DOI] [PubMed] [Google Scholar]
- 175.Muzaffar S, Shukla N, Bond M, Newby AC, Angelini GD, Sparatore A, Del Soldato P, Jeremy JY. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res. 2008;45:521–528. doi: 10.1159/000129686. [DOI] [PubMed] [Google Scholar]
- 176.Tyagi N, Moshal KS, Sen U, Vacek TP, Kumar M, Hughes WM, Jr, Kundu S, Tyagi SC. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal. 2009;11:25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Siriussawakul A, Chen LI, Lang JD. Medical gases: a novel strategy for attenuating ischemia-reperfusion injury in organ transplantation? J Transplant. 2012;2012:819382. doi: 10.1155/2012/819382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Toledo JC, Jr, Augusto O. Connecting the chemical and biological properties of nitric oxide. Chem Res Toxicol. 2012;25:975–989. doi: 10.1021/tx300042g. [DOI] [PubMed] [Google Scholar]
- 179.Pietri R, Roman-Morales E, Lopez-Garriga J. Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid Redox Signal. 2011;15:393–404. doi: 10.1089/ars.2010.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nicholls P. The formation and properties of sulphmyoglobin and sulphcatalase. Biochem J. 1961;81:374–383. doi: 10.1042/bj0810374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Collman JP, Ghosh S, Dey A, Decreau RA. Using a functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc Natl Acad Sci U S A. 2009;106:22090–22095. doi: 10.1073/pnas.0904082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Toledo JC, Jr, Bosworth CA, Hennon SW, Mahtani HA, Bergonia HA, Lancaster JR., Jr Nitric oxide-induced conversion of cellular chelatable iron into macromolecule-bound paramagnetic dinitrosyliron complexes. J Biol Chem. 2008;283:28926–28933. doi: 10.1074/jbc.M707862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bosworth CA, Toledo JC, Jr, Zmijewski JW, Li Q, Lancaster JR., Jr Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc Natl Acad Sci U S A. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Trujillo M, Alvarez MN, Peluffo G, Freeman BA, Radi R. Xanthine oxidase-mediated decomposition of S-nitrosothiols. J Biol Chem. 1998;273:7828–7834. doi: 10.1074/jbc.273.14.7828. [DOI] [PubMed] [Google Scholar]
- 185.Colpaert EE, Lefebvre RA. Interaction of hypoxanthine/xanthine oxidase with nitrergic relaxation in the porcine gastric fundus. Br J Pharmacol. 2000;130:359–366. doi: 10.1038/sj.bjp.0703317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Holcberg G, Kossenjans W, Miodovnik M, Myatt L. The interaction of nitric oxide and superoxide in the human fetal-placental vasculature. Am J Obstet Gynecol. 1995;173:528–533. doi: 10.1016/0002-9378(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 187.Baker JE, Su J, Fu X, Hsu A, Gross GJ, Tweddell JS, Hogg N. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and K(ATP) channels. J Mol Cell Cardiol. 2007;43:437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 189.Lee CI, Liu X, Zweier JL. Regulation of xanthine oxidase by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:9369–9376. doi: 10.1074/jbc.275.13.9369. [DOI] [PubMed] [Google Scholar]
- 190.Ichimori K, Fukahori M, Nakazawa H, Okamoto K, Nishino T. Inhibition of xanthine oxidase and xanthine dehydrogenase by nitric oxide. Nitric oxide converts reduced xanthine-oxidizing enzymes into the desulfo-type inactive form. J Biol Chem. 1999;274:7763–7768. doi: 10.1074/jbc.274.12.7763. [DOI] [PubMed] [Google Scholar]
- 191.Levitt MD, Abdel-Rehim MS, Furne J. Free and Acid-labile Hydrogen Sulfide Concentrations in Mouse Tissues: Anomalously High Free Hydrogen Sulfide in Aortic Tissue. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3525. [DOI] [PubMed] [Google Scholar]
- 192.Siegel LM. A Direct Microdetermination for Sulfide. Anal Biochem. 1965;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- 193.Lippert AR, New EJ, Chang CJ. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J Am Chem Soc. 133:10078–10080. doi: 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]
- 194.Liu C, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M. Capture and Visualization of Hydrogen Sulfide by a Fluorescent Probe. Angew Chem Int Ed Engl. doi: 10.1002/anie.201104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Peng H, Cheng Y, Dai C, King AL, Predmore BL, Lefer DJ, Wang B. A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood. Angew Chem Int Ed Engl. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang BH, Wu FY, Wu YM, Zhan XS. Fluorescent method for the determination of sulfide anion with ZnS:Mn quantum dots. J Fluoresc. 20:243–250. doi: 10.1007/s10895-009-0545-0. [DOI] [PubMed] [Google Scholar]
- 197.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med. 50:1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol. 160:941–957. doi: 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]