Abstract
Varicella-zoster virus (VZV) is a medically important human alphaher-pesvirus that causes varicella and zoster. VZV initiates primary infection by inoculation of the respiratory mucosa. In the course of primary infection, VZV establishes a life-long persistence in sensory ganglia; VZV reactivation from latency may result in zoster in healthy and immunocompromised patients. The VZV genome has at least 70 known or predicted open reading frames (ORFs), but understanding how these gene products function in virulence is difficult because VZV is a highly human-specific pathogen. We have addressed this obstacle by investigating VZV infection of human tissue xenografts in the severe combined immunodeficiency mouse model. In studies relevant to the pathogenesis of primary VZV infection, we have examined VZV infection of human T cell (thymus/liver) and skin xenografts. This work supports a new paradigm for VZV pathogenesis in which VZV T cell tropism provides a mechanism for delivering the virus to skin. We have also shown that VZV-infected T cells transfer VZV to neurons in sensory ganglia. The construction of infectious VZV recombinants that have deletions or targeted mutations of viral genes or their promoters and the evaluation of VZV mutants in T cell and skin xenografts has revealed determinants of VZV virulence that are important for T cell and skin tropism in vivo.
1 Introduction
Varicella-zoster virus (VZV) is the causative agent of varicella, which is recognized by its characteristic vesicular exanthem. In the course of primary infection, VZV reaches cranial nerve and dorsal root sensory ganglia (DRG) where it establishes latency; VZV reactivation from latency may result in the clinical syndrome of herpes zoster in healthy and immunocompromised patients (Cohen et al. 2007; Zerboni and Arvin 2008). Since VZV is highly host-restricted, events in VZV pathogenesis have been deduced from clinical observations about primary and recurrent infections in its native human host (Gilden et al. 2000; Arvin 2001a). Epidemiologic studies indicate that primary infection is initiated by mucosal inoculation, which is followed by a viremic phase, allowing viral transport to skin sites of replication. Clinical evidence that VZV viremia is cell-associated consists of recovery of the virus from peripheral blood mononuclear cells (PBMC) obtained just before and after the appearance of the varicella rash (Arvin 2001a). In our early studies, VZV DNA was detected by in situ hybridization in one in 30,000–100,000 PBMC from healthy individuals with acute varicella, and infected cells appeared to be lymphocytes (Koropchak et al. 1989). The capacity of VZV to cause viremia can result in viral dissemination to the lungs, liver, and other organs and in life-threatening varicella in immunocompromised children, unless antiviral therapy is given.
To overcome the challenge of the host restriction of VZV, we have developed methods to study VZV pathogenesis using human tissue xenografts, including thymus/liver (T cell), skin, and DRG, in mice with severe combined immunodeficiency (SCID) (reviewed in Ku et al. 2005; Arvin et al. 2006; Zerboni et al. 2005a, b; Zerboni and Arvin 2008). VZV cannot infect mouse tissues and the foreign tissue grafts are maintained without rejection in these animals. This system also allows the investigation of VZV infection of differentiated human cells in their tissue microenvironment in vivo in the absence of any adaptive immune response. In the first report using this model, Moffat et al. showed that VZV inoculation of skin xenografts produced infection of epidermal and dermal cells similar to those observed in clinical biopsies of VZV lesions; these experiments also demonstrated that VZV was highly infectious for human T cells in thymus/liver xenografts (Moffat et al. 1995). Importantly, this T cell tropism differentiates VZV from the other human alphaherpesviruses, herpes simplex virus (HSV) 1 and 2, which cause mucocutaneous lesions without evidence of systemic spread through a cell-associated viremia.
Studies of VZV pathogenesis in the SCID mouse model have been enhanced by the parallel development of methods to introduce targeted mutations into the VZV genome using cosmids consisting of overlapping fragments of the VZV genome (Cohen and Seidel 1993; Mallory et al. 1997; Kemble et al. 2000; Niizuma et al. 2003); VZV mutagenesis is also now accomplished using bacterial artificial chromosome (BAC) techniques (Zhang et al. 2007; Tischer et al. 2007). When deletions or nucleotide substitutions in the region of interest are not lethal for VZV replication, VZV recombinants can be exploited to identify determinants of VZV virulence in human tissue xenografts in the SCID mouse model and to assess differential requirements for particular gene products and functional domains within protein and promoter elements in T cell, skin, and DRG xenografts.
VZV T cell tropism in T cell xenografts in SCID mouse model
Our initial experiments in human T cell xenografts in SCID mice in vivo demonstrated the synthesis of VZV DNA and viral proteins in both CD4 and CD8 T cell subpopulations (Moffat et al. 1995). VZV infection of T cells in vivo also results in robust virion formation and the appearance of complete virus particles on T cell surfaces (Schaap et al. 2005; Schaap-Nutt et al. 2006). Important characteristics of T cell tropism that emerged from these studies are that VZV-infected T cells do not fuse with adjacent uninfected T cells and the progression of VZV infection in T cell xenografts was associated with the formation of complete VZ virions and release of infectious virus (Fig. 1). This pattern differs from the polykaryocyte or syncytia formation, which is the hallmark of VZV replication in skin and cultured cells in vitro (Fig. 2). Thus, VZV infection of T cells, in contrast to skin, appears to require efficient virion formation and egress for transfer to uninfected cells.
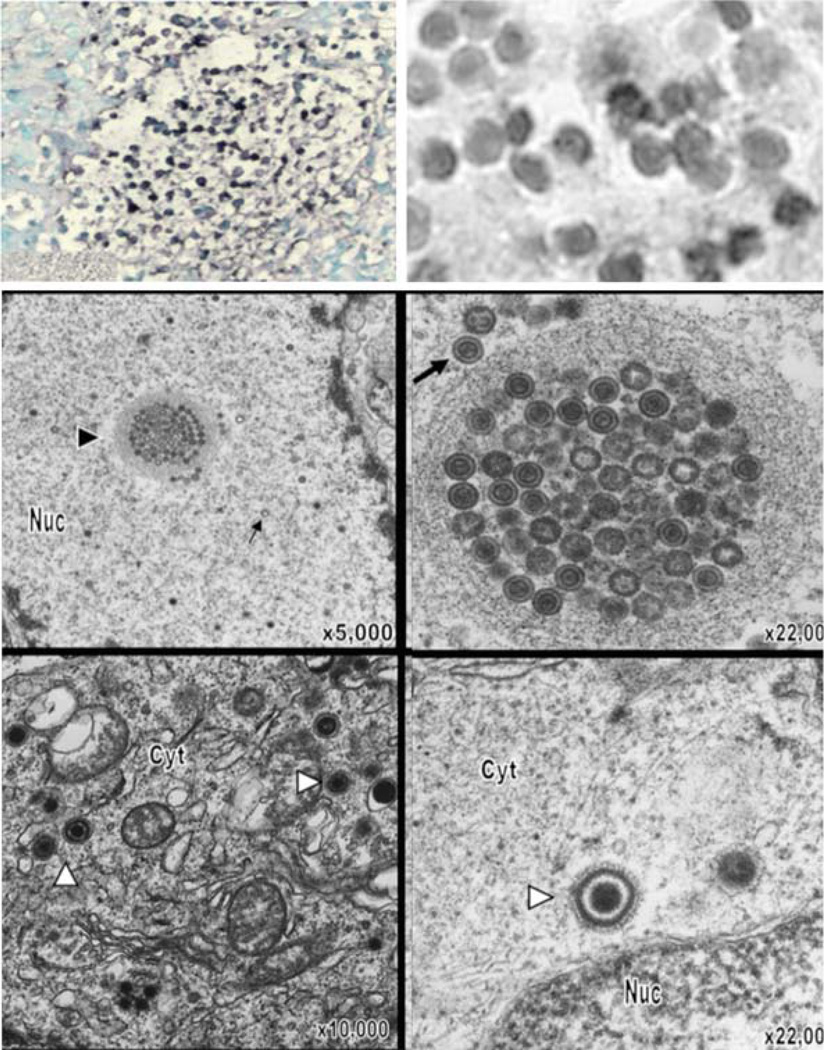
Fig. 1.
VZV infection of T cells in thymus/liver (T cell) xenografts in the SCID mouse model. On day 7 after infection, infected T cell xenografts were tested for VZV DNA by in situ hybridization; darkly stained cells indicate VZV DNA in T cells visualized at lower (left) and higher magnification (right; ×786). By electron microscopy on day 14 after infection, VZV nucleocapsids were present in the nuclei with most containing a dark VZV DNA core (large arrows); some empty capsids were also seen (small arrows). Virions were abundant in T cell nuclei, both individually and in clusters (arrowhead), also shown at higher magnification. Complete virions were found in the cytoplasm. Magnification and locations of cytoplasm (cyt) and nucleus (nuc) are as indicated. From Moffat et al. 1995 and Schaap et al. 2005; reproduced with permission
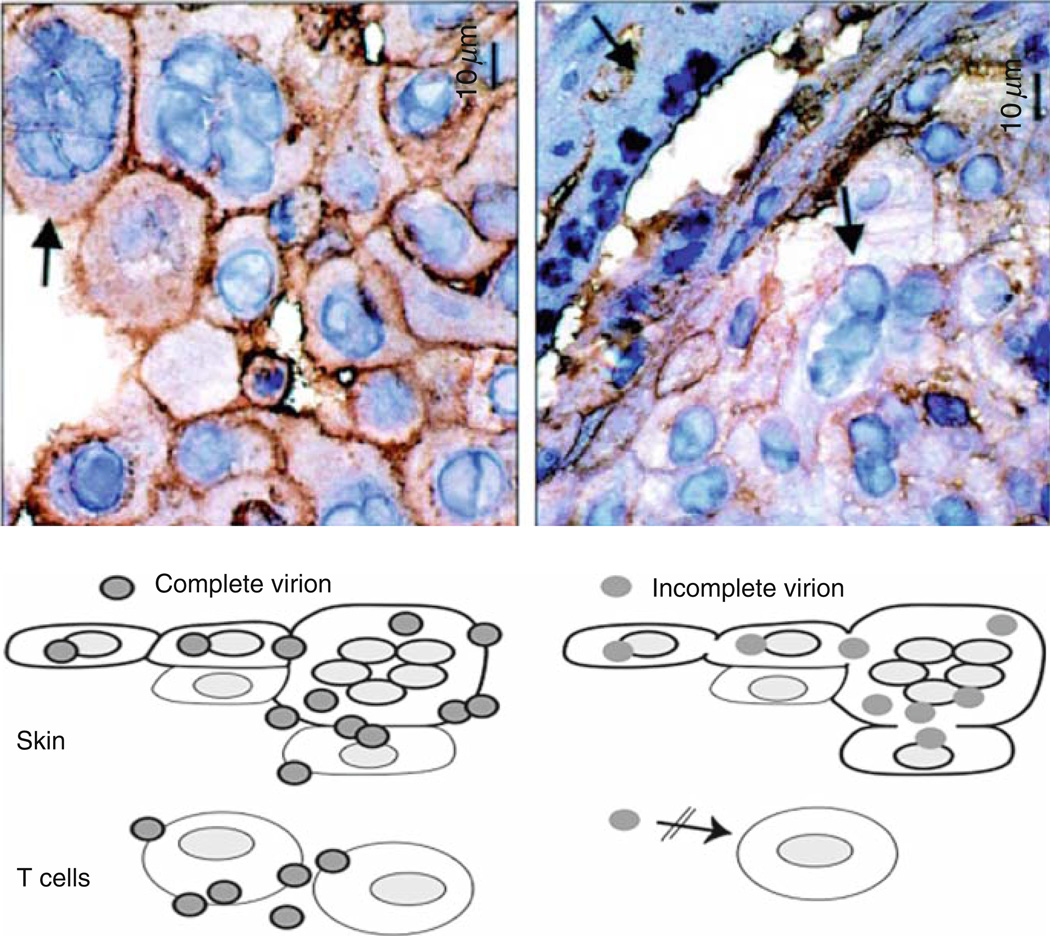
Fig. 2.
Role of cell fusion and polykaryocyte formation in VZV infection of skin xenografts. The upper panels show virion formation in skin xenografts infected with VZV (left panel) and kinase-defective ORF47 mutant (right panel) and examined at day 21 by staining with antibody to VZV gE and hematoxylin. Transmission microscopy demonstrated many intact viral particles in rOkainfected skin cells. Viral particles were incomplete and fewer in rOka47D-N infected skin cells. Arrows indicate polykaryocytes with dark staining of gE in plasma membranes of rOka-infected cells and persistent formation of polykaryocytes in skin infected with the ORF47 kinase mutant despite minimal gE localization to plasma membranes. As illustrated, virus-induced cell fusion, with the characteristic formation of polykaryocytes, supports VZV infection in skin but VZV infection does induce cell fusion. ORF47 kinase mutants do not infect T cells, suggesting that VZV T cell tropism depends upon efficient assembly and release of complete virions. (Adapted from Besser et al. 2004; figures reproduced with permission)
VZV tropism for tonsil T cells in vitro
While T cells can become infected with VZV when PBMC cultures are inoculated with the virus, the percentage of infected cells is low (Koropchak et al. 1989; Soong et al. 2000). Based on the evidence that VZV pathogenesis begins with inoculation of mucosal epithelial cells of the respiratory tract, we speculated that VZV could gain access to T cells in the lymphoid tissue that comprises the tonsils and other components of Waldeyer’s ring (Fig. 3) and that these T cells would be more susceptible to VZV infection than circulating T cells. More than 20% of mononuclear cells in the tonsils are CD3 T cells. In addition, tonsils have a surface layer of respiratory epithelial cells, these cells penetrate into the tonsillar crypts, and T cells as well as B cells migrate across the epithelial cell layer into and out of the tonsils. As predicted, tonsil T cells were highly susceptible to VZV infection in vitro (Ku et al. 2002). Thus, VZV targets tonsil T cells in a manner that is analogous to the tropism of Epstein-Barr virus for tonsil B cells. Of interest, the tonsil T cell populations that were most likely to be infected were activated CD4 T cells expressing CD69 and other activation markers and were predominantly memory T cells; 20–25% of CD4 T cells were infected compared to 10–15% of CD8 T cells. During natural infection, this pattern of tropism for CD4 T cells in tonsils would be expected to result in higher absolute numbers of infected CD4 T cells because two-thirds of tonsil T cells are in this subpopulation. Activated memory CD4 T cells are also common in tonsil T cell populations, presumably because of continuous exposure to various antigens and the cytokine-rich milieu. When infected T cells were treated with phorbol ester, the frequency of VZV-positive T cells increased twofold, indicating that VZV DNA was present and that expression of VZV genes of the putative α, β, and γkinetic classes was inducible by T cell activation. VZV also preferentially infected memory T cell subpopulations that expressed the skin homing markers, cutaneous leukocyte antigen (CLA), and chemokine receptor 4 (CCR4). Targeting memory T cells that are programmed for immune surveillance, and particularly those that have skin homing potential, would be expected to enhance T cell-mediated transport of VZV from the initial site of inoculation in the naïve host to the skin.
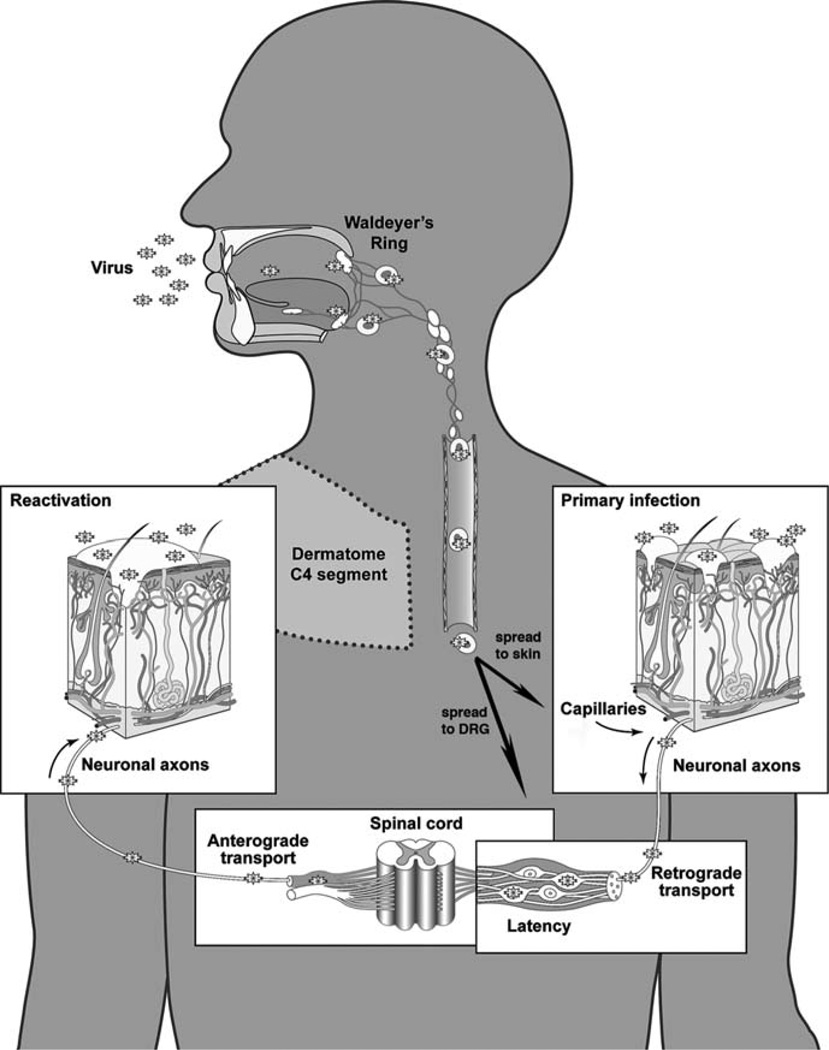
Fig. 3.
A model of the pathogenesis of primary and recurrent VZV infection. VZV infection is acquired by inoculation of mucosal epithelial cells via the respiratory route, transfer across epithelial layers allows infection of T cells in tonsils and other lymphoid tissue that comprise Waldeyer’s ring and allows transport to the skin via a T cell-associated viremia. Infection of skin produces the vesicular rash characteristic of varicella. VZV may reach sensory ganglia by T cell viremia or during skin infection, VZ virions may gain access to the sensory nerve cell body by retrograde axonal transport; lifelong latent infection is established in sensory ganglia in the course of primary VZV infection. Clinical reactivation of latent VZV results in herpes zoster, during which VZ particles gain access to skin via anterograde axonal transport. Adapted from Zerboni and Arvin 2008
Transport of VZV by infected T cells to skin and the formation of skin lesions
Subsequent experiments combining our SCID mouse skin xenograft model with the method to infect tonsil T cells in vitro confirmed that VZV-infected tonsil T cells that are introduced into the mouse circulation by tail vein inoculation can migrate into skin xenografts and deliver infectious virus to epidermal cells in which replication and lesion formation ensues (Ku et al. 2004). T cells were detected in skin xenografts, particularly near hair follicles, within 24 h after being introduced intravenously. Memory CD4 T cells predominated in skin xenografts after infusion of uninfected or infected cells, further supporting the concept that VZV exploits immune surveillance by migratory T cells for viral transport to skin. Thus, the cell-associated viremia necessary for the pathogenesis of primary VZV infection and the occurrence of the characteristic varicella skin lesions appears to depend upon VZV T cell tropism and is enhanced by preferential infection of T cell subpopulations that are most likely to cross the capillary endothelium into skin by the diapedesis mechanisms that these cells use for immune surveillance functions (Fig. 4).
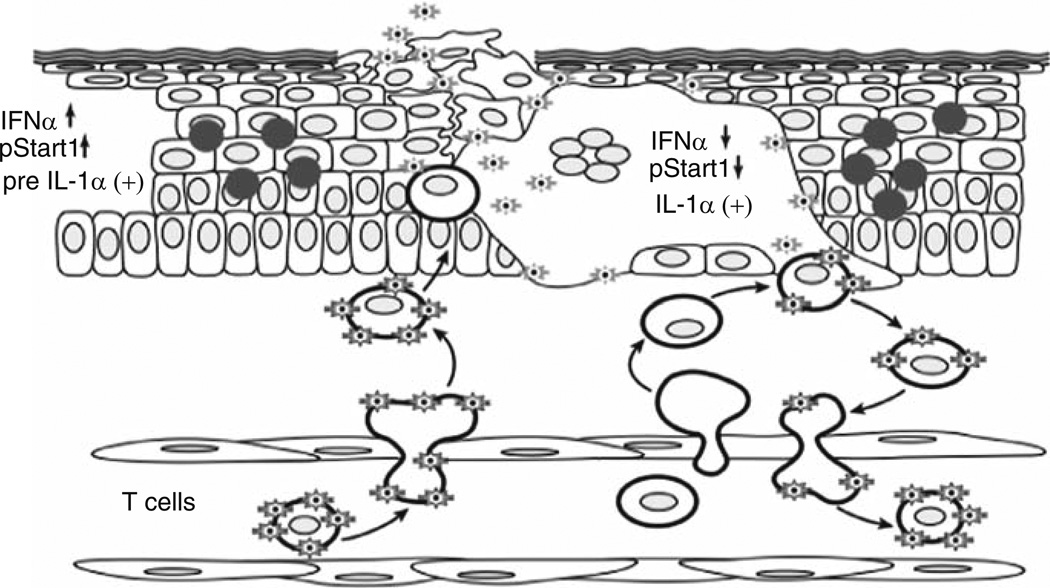
Fig. 4.
Events in the pathogenesis of VZV skin infection. According to the model of primary VZV pathogenesis, T cells within the lymphoid tissues of Waldeyer’s ring become infected by VZV transfer following the initial inoculation of respiratory epithelial cells with the virus. Infected T cells enter the circulation and rapidly transport VZV to the skin, exiting from capillaries across endothelial cells by diapedesis, as occurs during the usual trafficking of T cells through tissues. VZV is then released by the infected T cells at skin sites of replication. The 10–21 day incubation period observed after exposure of naïve individuals to VZV is the interval required for VZV to overcome the innate IFN-α response mounted by epidermal cells and create a lesion that reaches the skin surface, IFN-α production by epidermal cells that surround those infected with VZV prevents a rapid, uncontrolled cell–cell spread that would otherwise incapacitate the host. Viremia may be amplified by trafficking of uninfected T cells through areas where skin lesions are forming. (Adapted from Ku et al. 2005; figure reproduced with permission)
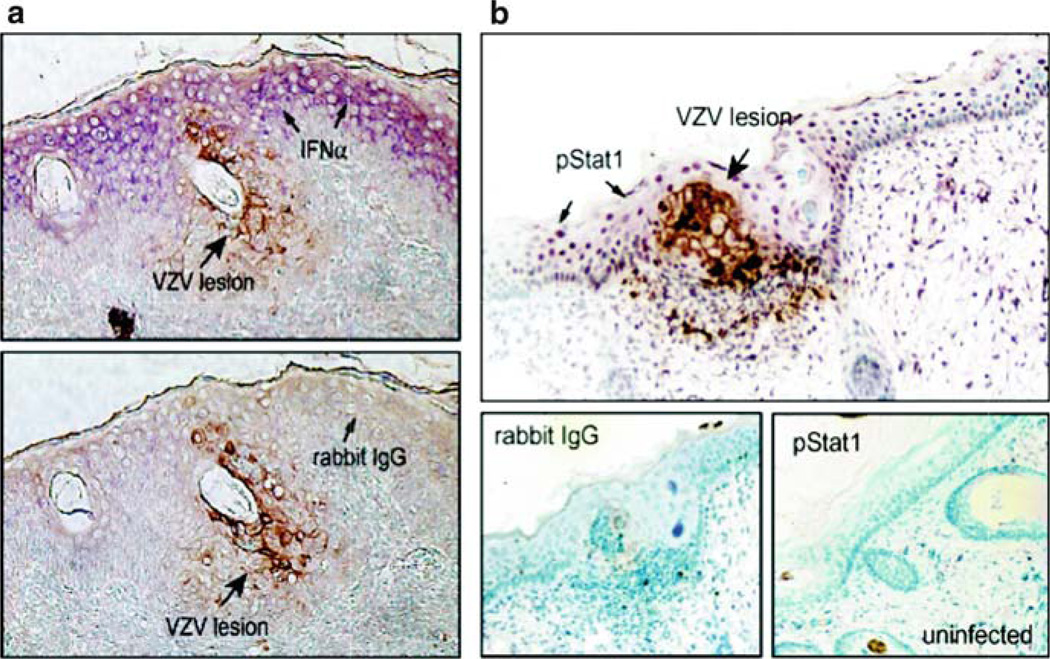
When the progression of VZV infection in skin xenografts after transfer of virus by T cells was examined, we observed that the appearance of skin lesions at the surface of the skin xenografts required an interval of 10–21 days. Infected epidermal cells were detected in small foci that enlarged gradually over this prolonged period, which was in marked contrast to the typical lysis of VZV-infected cells in vitro within 48–72 h. Since SCID mice lack the capacity to mount an adaptive immune response, we predicted that this phenomenon might be the result of innate host antiviral responses. Induction of the interferon (IFN) pathway is well known to be highly restrictive of herpes viruses. Evaluation of VZV-infected skin xenografts revealed that IFN-α was not expressed in the clusters of epidermal cells that were actively infected, as shown by expression of VZV protein markers (Ku et al. 2004) (Fig. 5). In contrast, IFN-α production was prominent in epidermal cells within the xenograft that did not exhibit cyopathic changes or viral protein synthesis; upregulation of the IFN pathway in these cells was also documented by the phosphorylation and nuclear translocation of Stat1. The relevance of this response for the pathogenesis of VZV skin infection was documented by inoculating skin xenografts with VZV and treating the mice with antibody to block the human IFN-α/b receptor or a control antibody. Interference with the up-regulation of the IFN response was associated with a tenfold increase in infectious virus recovery and with accelerated and extensive skin lesion formation in treated animals. In other experiments, we showed that VZV also inhibits the NF-kB-mediated innate response in VZV-infected skin cells while this pathway is up-regulated in adjacent uninfected cells (Jones and Arvin 2003, 2006).
Fig. 5.
IFN-α expression and Stat1 phosphorylation in VZV-infected and uninfected epidermal cells. Formalin-fixed, paraffin-embedded sections of VZV-infected skin xenografts were pretreated and double stained with anti-VZV IgG, detected with DAB (brown), and anti-IFNα or anti-pStat1 antibody detected with Vector VIP (purple). Sections are shown at magnification ×200. (a) Infected cells were identified by VZV protein (brown) expression. In double labeled skin sections, IFN-α (purple) expression was prominent in adjacent uninfected cells but not in VZV-infected cells (top) compared with sections stained with rabbit IgG as a control for IFN-α (bottom). (b) In double labeled sections, phosphorylated Stat1 (purple) was up-regulated in adjacent uninfected cells but absent in VZV-infected cells (top); pStat1 was not detected in uninfected skin (bottom right) compared with rabbit IgG control stain (bottom left). From Ku et al. 2004; reproduced with permission
The analysis of VZV-infected skin xenografts made it possible to evaluate whether VZV replication up-regulated the expression of endothelial cell adhesion molecules known to enhance the entry of effector cells that mediate the adaptive immune response. Comparing levels of E-selectin, ICAM-1, VCAM-1, and chemoattractant receptors in cells within VZV-infected skin xenografts with expression in cells in lesion biopsies taken from healthy individuals with varicella or zoster showed that these proteins were detected abundantly in VZV lesion biopsy specimens but not in skin xenografts. Thus, while virus-infected T cells deliver VZV to skin, initial VZV replication in skin does not trigger expression of molecules that would immediately attract immune cells with the capacity to lyse infected cells and interrupt cell–cell spread. Nevertheless, the usual migration of immune monitoring T cells through skin could amplify the numbers of VZV-infected T cells that then traffic to new skin sites before adaptive immunity is elicited (Fig. 4). Amplification of cell-associated viremia may also occur in other reticuloendothelial tissues, for example, liver and spleen, during this interval.
The role of T cell tropism in the pathogenesis of VZV infection
These observations suggest that VZV infects T cells in tonsils and other regional lymphoid tissues of the upper respiratory tract, and that these infected T cells can transport VZV to skin within a short time after entering the circulation. These experiments make it possible to attribute the relatively prolonged interval between exposure of the naïve host to VZV and the first appearance of the varicella rash to the potent innate responses of epidermal cells that inhibit the cell-to-cell spread of VZV in skin; after VZV is delivered to skin, it must overcome these barriers before lesions are evident at the skin surface (Figs. 3 and 4). By this model, the detection of cell-associated viremia in the last few days of the incubation period does not result in a very rapid formation of skin lesions; instead, it is likely to represent the infection of T cells as they enter and exit from skin sites of active VZV replication during the period that VZV is spreading from cell to cell towards the skin surface. This highly modulated process can be expected to facilitate and enhance the persistence of VZV in the population, since an infection that incapacitates the host would be predicted to reduce its opportunities for transmission to other susceptibles.
2 Defining Determinants of VZV Tropism for T cells and Skin in the SCID Model In Vivo
Combining the SCID mouse model to study VZV pathogenesis in human T cell and skin xenografts with the capacity to make targeted mutations in the VZV genome has allowed investigations of the genetic requirements for VZV virulence in these target cells within their tissue microenvironment in vivo. As summarized later, we have evaluated how mutations that block expression alter the levels of expression or disrupt functional domains of the VZV glycoprotein, gB, the tegument/regulatory proteins, IE62 and IE63, the viral kinases encoded by ORF47 and ORF66, and genes of the conserved ORF9–12 cluster, as well as mutations of VZV gene promoter elements affect VZV tropism for T cells and skin (Table 1). These mutants were also characterized for their effects for VZV replication in cultured cells, which are described in detail in each report cited; consequences for growth kinetics and plaque size are summarized in Table 1. We have also used the in vitro T cell assay to identify gene products required for T cell entry in experiments with these mutants, as indicated in Table 1. The contributions of VZV gH was examined using anti-gH antibody-mediated interference with its functions in skin pathogenesis. Mutational analyses of the functions of gE, gI, and their promoters and their consequences for VZV replication in vitro and virulence in skin and T cells in vivo are described in the chapter on VZV gE and gI.
Table 1.
Effects of selected mutations in VZV genes and promoters on the tropism of varicellazoster virus for T cells and skin in the SCID mouse model
| Cell culture in vitro | SCIDhu mice in vivo | |||||
|---|---|---|---|---|---|---|
| Virus | Growth kinetics |
Plaque size |
T cell entry |
Skin xenografts |
T cell xenografts |
|
| Recombinant Oka | + | +++ | Normal | ++ | ++ | |
| Glycoproteins | ||||||
| ΔgB-491-RSSR-494 | + | +++ | Normal | + | ||
| gB 428-GSGG-431 | + | +++ | Normal | + | ||
| Viral kinases | ||||||
| ORF47- ΔC (C-terminal deletion) | + | ++ | Normal | + | − | |
| ORF47P-S1 (kinase motif mutant) | + | ++ | Normal | ++ | ||
| ORF47P-S2 (kinase motif mutant) | + | ++ | Normal | ++ | ||
| ORF47D-N (kinase motif mutant) | + | ++ | Normal | + | − | |
| ORF66S (stop codon mutant) | + | + | + | |||
| ORF66 S48A (kinase domain) | + | |||||
| ORF66 S331A (kinase domain) | + | |||||
| ORF66 G102A (kinase domain) | + | ++ | + | +/− | ||
| ORF66 S250P (kinase domain) | + | ++ | ++ | ++ | ||
| Tegument/regulatory proteins | ||||||
| ΔORF62 (complete deletion) | + | +++ | Normal | ++ | ||
| + | +++ | Normal | ++ | |||
| ΔORF71 (complete deletion) | ||||||
| ΔORF62/71 (double deletion) | − | |||||
| Δ63/70 (double deletion) | − | |||||
| Δ63 (single copy deletion) | + | ++ | Normal | ++ | ++ | |
| ORF63 T171 (phosphorylation) | + | + | Small | + | ++ | |
| ORF63 S181 (phosphorylation) | + | + | Small | + | ++ | |
| ORF63 S185 (phosphorylation) | + | + | Small | + | ++ | |
| ORF9-10 cluster | ||||||
| ORF9 | − | |||||
| ΔORF10 (complete deletion) | + | +++ | Normal | + | ++ | |
| ORF10 P28A (acidic domain) | + | +++ | Normal | ++ | ||
| ORF10 P28S (acidic domain) | + | +++ | Normal | ++ | ||
| ΔORF11 | + | +++ | Normal | + | +/− | |
| ΔORF12 | + | +++ | Normal | + | ++ | |
| ΔORF10/11 | + | +++ | Normal | +/− | ||
| ΔORF10/11/12 | + | +++ | Normal | + | +/− | |
| ΔORF11/12 | + | +++ | Normal | +/− | ||
| Promoter mutants | ||||||
| ORF10-proΔ+39/+63 | + | +++ | Normal | ++ | ||
| ORF10-proΔUSF | + | +++ | Normal | + | ||
3 The Role of VZV Glycoproteins in T cell and Skin Infection
VZV gB
Information about VZV gB is quite limited. However, we found that modeling predicts a structure similar to HSV gB (Oliver et al. 2009). Nevertheless, in contrast to HSV gB, VZV gB has a furin recognition motif, which is predicted to result in gB cleavage. Substitutions in the primary fusion loop, W180G and Y185G, the deletion Δ491RSRR494, and point mutations 491GSGG494 in the furin recognition motif did not affect gB expression or cellular localization. VZV was recovered from pOka BACs that had either the Δ491RSRR494 or 491GSGG494 mutations (Table 1) but not when the point mutations W180G and Y185G were introduced. Thus, mutagenesis demonstrated that residues in the primary fusion loop of gB were essential but gB cleavage was not. Virion morphology, protein localization, and replication were unaffected for the gBΔ491RSRR494 and gB491GSGG494 viruses in vitro. Nevertheless, deleting the furin recognition motif and blocking gB cleavage caused attenuation in skin xenografts in vivo. Viral titers were lower at both 10 and 21 days after inoculation of skin xenografts with pOka-gBΔ491RSRR494 compared to wild-type pOka; the titer reductions associated with mutagenesis of the cleavage site were 1.5 log10 pfu (31-fold; day 10) and 1.0 log10 pfu (tenfold; day 21). These experiments provided the first evidence that cleavage of a herpes virus fusion protein contributes to pathogenesis in vivo, as has been observed for fusion proteins in other virus families.
VZV gH
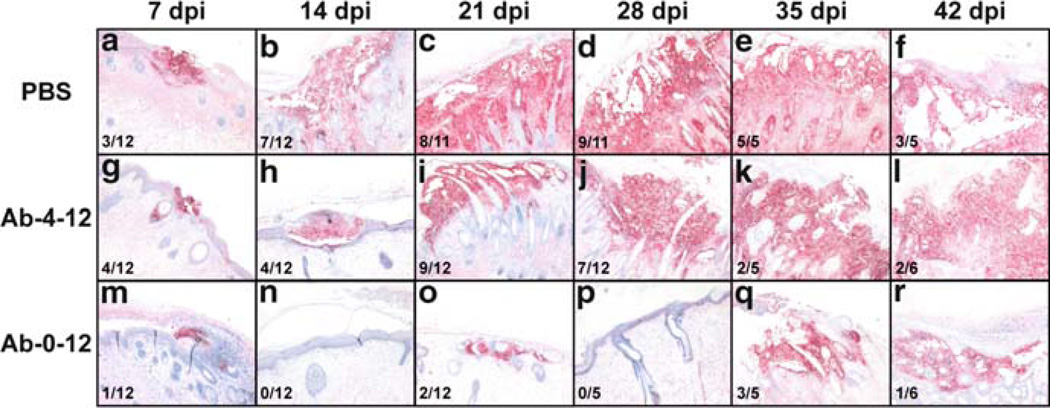
The glycoprotein gH is a highly conserved herpes virus protein that has been shown to have functions in virus entry and cell–cell spread when evaluated in other herpes viruses. Little is known about the functions of VZV gH. In experiments to study gH functions in VZV pathogenesis, we took advantage of the availability of the murine monoclonal antibody against gH, MAb 206, which is known to neutralize VZV and inhibit cell fusion in vitro. When given to SCID mice that had skin xenografts infected with VZV before treatment, we found that anti-gH antibody administration starting 6 h after inoculation and continuing for 12 days reduced the number of skin xenografts that became infected by 60%; in xenografts that became infected, virus titers, genome copies, and lesion extent were reduced (Vleck et al. 2010) (Fig. 6). In contrast, initiating anti-gH antibody 4 days after inoculation suppressed but did not block VZV replication. In vitro, anti-gH antibody bound to plasma membranes and to surface virions. Notably, anti-gH antibody was also internalized into vacuoles within infected cells, associated with intracellular virions and colocalized with markers for early endosomes and the multivesicular body pathway but not the trans-Golgi network. Thus, antibody-mediated interference with gH has the capacity to block virion transfer into uninfected cells and cell–cell fusion and anti-gH antibody can enter cells where it may bind to gH or virions, which may be targeted for degradation. As a consequence, anti-gH antibody can prevent (if given very early) or modulate VZV skin infection in vivo.
Fig. 6.
The formation of lesions in VZV-infected human skin xenografts treated with the anti-gH mAb 206 for 0–12 days or 4–12 days post inoculation. Lesions in skin xenografts inoculated with pOka-infected HELF were identified by VZV gE expression, and were counterstained with hematoxylin. Representative lesions are shown at each timepoint: 7 dpi, A, G, M. 14 dpi, B, H, N. 21 dpi, C, I, O. 28 dpi, D, J, P. 35 dpi, E, K, Q. 42 dpi, F, L, R. 42 The total number of xenografts with lesions is shown in the lower 1 left of each panel. Lesions were identified in xenografts from the PBS (A–F) and Ab-4-12 (G–L) groups at all time points. Lesions were only observed in xenografts from the Ab-0-12 group (M–R) at 7, 21, 35 and 42 dpi. Representative uninfected xenografts are shown for the Ab-0-12 group at 14 and 28 dpi. Syncytia were seen in all lesions. Magnification: ×50. From Vleck et al. 2010, reproduced with permission
The role of VZV regulatory/tegument proteins in VZV pathogenesis
Like other herpes viruses, the VZV tegument contains important regulatory proteins that are available to initiate replication when the infectious particle is uncoated after entering the target cell. Of these, we have examined the contributions of IE62 and IE63 to T cell and skin tropism.
VZV IE62
IE62 is a major immediate early transactivating protein that induces many VZV genes that have been evaluated; it is encoded by duplicated genes, ORFs 62 and 71 (Cohen et al. 2007). When the effects of mutations in these genes on VZV replication in vitro were evaluated, transfections using pOka cosmids from which ORF62, ORF71, or the ORF62/71 gene pair were deleted showed that at least one copy of ORF62 was required (Sato et al. 2003). Inserting ORF62 from pOka or vOka into a non-native site in US allowed VZV growth in cell culture in vitro, although plaque size and virus titers were decreased (Table 1). Targeted mutations in binding sites reported to affect interactions with IE4 or a putative ORF9 protein binding site were compatible with replication. In single deletions of ORF62 or ORF71, recombination events repaired the defective repeat region in some progeny viruses in vitro and in some skin xenografts. Although insertion of ORF62 into the non-native AvrII site permitted growth in cell culture, IE62 expression from its native gene locus was necessary for cell–cell spread in skin xenografts in vivo.
VZV IE63
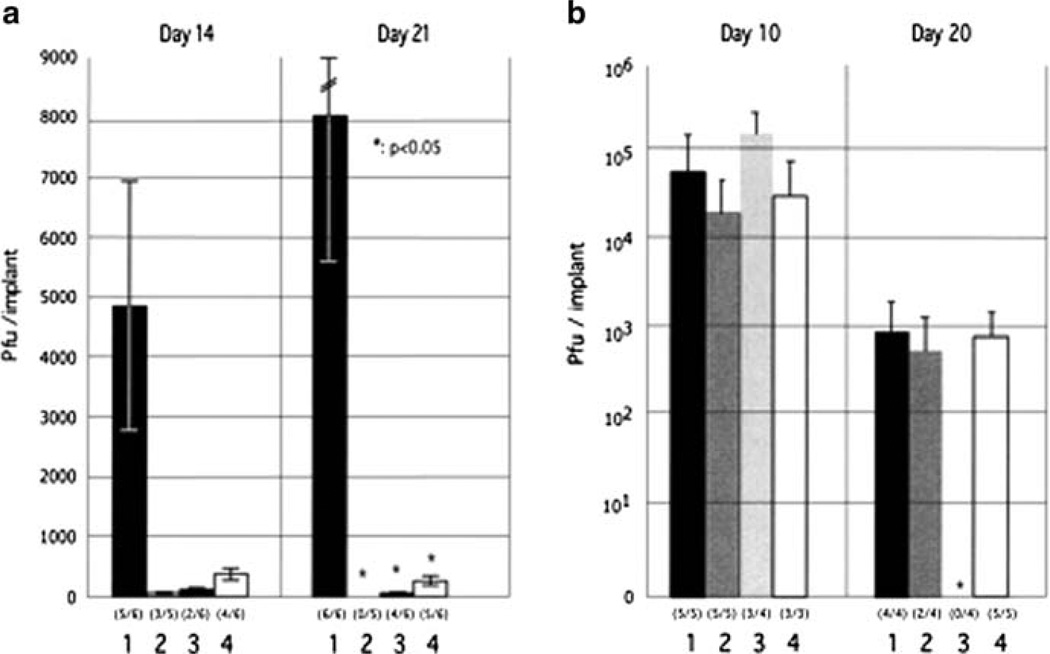
In addition to IE62, we have used cosmid mutagenesis to analyze the functional domains of IE63, also an immediate early regulatory/tegument protein, which is encoded by duplicate genes, ORFs 63, and 70, (Baiker et al. 2004). In this work, 22 ORF63 mutations were also evaluated for effects on IE63 binding to IE62 and on IE63 phosphorylation and nuclear localization in transient transfections. By sequence, IE63 was shown to resemble the HSV-1 US1.5 protein, which is expressed colinearly with ICP22 (US1). The IE62 binding site was mapped to IE63 aa55–67, with R59/L60 being critical residues. Alanine substitutions within the IE63 center region showed that S165, S173, and S185 were phosphorylated by cellular kinases. Four mutations that changed two putative NLS sequences altered IE63 distribution to a cytoplasmic/nuclear pattern. Only three of 22 mutations in ORF63 were compatible with recovery of infectious VZV, although IE63 is not absolutely essential when IE62 is provided as an ORF62 plasmid (Cohen et al. 2005). The three viable IE63 mutants had alanine substitutions altering T171, S181, or S185 (Table 1). These mutants produced less infectious virus and had decreased plaque sizes in vitro. ORF47 kinase and gE synthesis was reduced, showing that IE63 contributes to optimal expression of early and late gene products. The virulence of the three IE63 mutants was markedly attenuated in skin xenografts but not in T cell xenografts, indicating that the contribution of the IE63 tegument/regulatory protein depends upon the differentiated human cell type that is being targeted within the intact tissue microenvironment during VZV pathogenesis (Fig. 7).
Fig. 7.
Replication of IE63 mutant viruses in skin and T cell xenografts in SCID-hu mice. Skin xenografts in SCID mice were injected with (left to right) rOKA, rOKA/ORF63rev[T171], rOKA/ ORF63rev[S181], or rOKA/ORF63rev[S185] having equivalent inoculum titers. Virus titers in skin xenografts were assessed after harvest at day 14 (A, left panel) and day 21 (A, right panel) after inoculation and were graphed as mean titers for xenografts that yielded infectious virus, with lines indicating standard errors. The number of xenografts from which infectious virus was recovered per number that were inoculated is given in parentheses below the horizontal axis. The P values were <0.05 when titers of rOka and each of the IE63 mutant viruses were compared at day 21. Replication of VZV recombinants in T cells was assessed at days 10 and 20 (B). Lines indicate the standard errors. From Baiker et al. 2004; reproduced with permission
The role of the VZV kinases in T cell and skin tropism
VZV encodes two viral kinases, ORF47 and ORF66. ORF47 is a conserved herpes virus gene and ORF66 is present only in the alphaherpesviruses. Deletion of both of these viral kinases is compatible with VZV replication in cultured cells (Cohen et al. 2007); however, both have required functions for VZV pathogenesis in T cells and skin. Initial experiments with ORF47 and ORF66 stop codon mutants demonstrated that inoculation of T cell and skin xenografts showed that ORF47 protein was required for viral growth in human T cells and skin; virulence was restored when ORF47 was expressed from a non-native site in the VZV genome (Moffat et al. 1998a, b). Blocking ORF66 expression impaired VZV infection of T cells but not skin compared with intact VZV.
ORF47 protein
We investigated the role of the ORF47 protein kinase further by constructing VZV recombinants with targeted mutations in conserved motifs of ORF47 and expressing a truncated ORF47 (Table 1) (Besser et al. 2003). Both the C-terminal truncation mutant, rOka47ΔC, and the mutation disrupting the DYS kinase motif in rOKa47Δ-N eliminated ORF47 kinase activity and caused extensive nuclear retention of ORF47 and IE62 proteins in vitro. Disrupting ORF47 kinase function also resulted in a marked decrease in VZV replication and cutaneous lesion formation in skin xenografts in vivo. However, infection in vivo was not blocked completely as long as ORF47 protein binding to IE62 protein was preserved; this function was mapped to the ORF47 N-terminus. These experiments indicated that ORF47 kinase activity is critically important for VZV infection and cell–cell spread in human skin in vivo, but suggested that complex formation with IE62, rather than kinase activity, is the essential contribution of ORF47 protein to VZV replication in vivo.
In subsequent experiments, we found a differential requirement for cell fusion and virion formation in the pathogenesis of VZV infection in skin and T cells (Besser et al. 2004). The rOka47ΔC and rOka47D-N mutants did not infect human T cell xenografts but remained infectious in skin. Epidermal cell fusion persisted and some VZV polykaryocytes were generated in skin infected with rOka47ΔC and rOka47D-N (Fig. 2). Virion assembly was impaired in vitro, but cell fusion continued, causing characteristic VZV syncytia in cultured cells infected with rOka47ΔC or rOka47D-N. Intracellular trafficking of envelope gE and the ORF47 and IE62 proteins was aberrant without ORF47 kinase activity. In skin, ORF47 mutants exhibited cell–cell spread even though EM studies showed markedly defective virion formation. In contrast, VZV-infected T cells do not undergo cell fusion, and the impaired virion assembly due to ORF47 mutations effectively eliminated T cell infection. Thus, we concluded that skin infection can proceed if some cell fusion occurs, whereas VZV T cell tropism is much more dependent on full assembly of infectious virus particles.
ORF66 protein
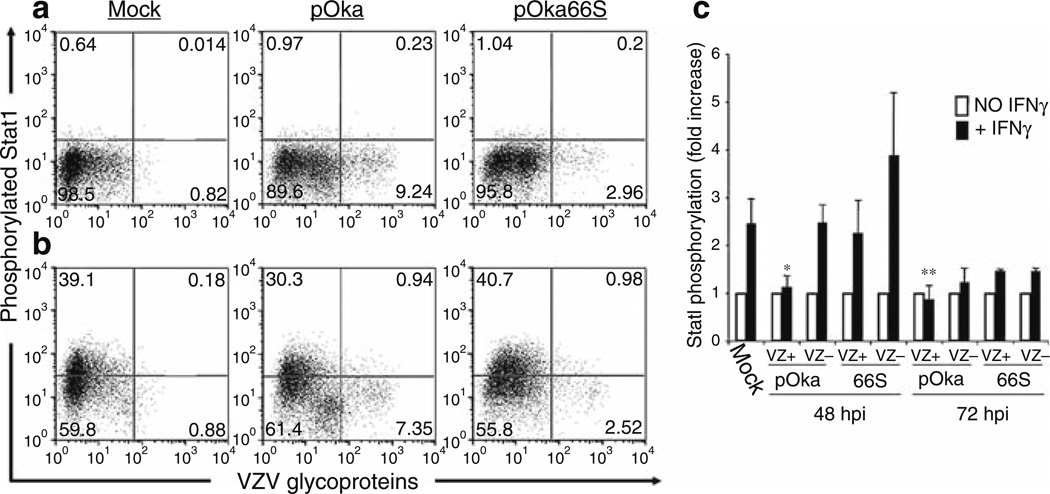
As noted earlier, VZV infection of T cells is associated with robust virion production and modulation of apoptosis and IFN pathways (Schaap et al. 2005). The serine/threonine protein kinase encoded by ORF66 is needed for efficient vOka replication in T cells (Table 1). Preventing ORF66 expression by a stop codon impaired pOka growth in T cell xenografts in vivo, reduced VZ virion formation in T cells, increased the susceptibility of infected T cells to apoptosis, and reduced the capacity of the virus to interfere with induction of the IFN signaling pathway following exposure to IFNγ(Fig. 8). However, preventing ORF66 protein expression reduced growth in cultured cells only slightly and did not diminish virion formation in vitro. The ORF66 stop codon mutant also showed only a slight growth defect in skin compared with pOka. These observations suggested that ORF66 kinase has a unique role during infection of T cells and supports VZV T cell tropism by enhancing survival of infected T cells and contributing to immune evasion.
Fig. 8.
Flow cytometric analysis of Stat1 phosphorylation in human tonsil T cells stimulated with IFN-γ. Column-purified human tonsil T cells were cocultured with VZV-infected HEL monolayers. After 48 h, cells were removed from the monolayer and either left unstimulated (a) or stimulated with recombinant human IFN-γfor 10 min at 37°C (b). Cells were fixed in paraformaldehyde, stained with antibodies and fluorescent conjugates to VZV proteins, permeabilized in methanol, and then stained with antibodies to phospho-Stat1 and CD3. FACS plots show antiphospho-Stat1 versus anti-VZV staining of uninfected (left plots), pOka-infected (middle plots), and pOka66S-infected (right plots) tonsil T cells (gated on CD3+ cells) without stimulation (a) or following stimulation with IFN-γ(b). (c) Data from T cells cultured for 48 or 72 h with infected HEL monolayers are shown as the average fold increase in phospho-Stat1 fluorescence intensity following IFN-γtreatment in VZV-infected and uninfected T cells for four independent experiments done after 48 h and two experiments combined for 72 h. One asterisk indicates a fold increase that is significantly different from that of uninfected cells from the same culture with P = 0.01, while two asterisks indicate a difference with P = 0.02. From Schaap et al. 2005; reproduced with permission
Subsequent experiments showed that an ORF66 mutant with a G102A substitution had defective kinase function, which was demonstrated as a block in autophosphorylation (Schaap-Nutt et al. 2006). Blocking ORF66 kinase function also prevented late IE62 localization to the cytoplasm in vitro, whereas an S250P substitution had no effect on either of these ORF66 functions. Both kinase domain mutants replicated to titers equivalent to pOka in vitro (Table 1). pOka66G102A had slightly reduced growth in skin, which was comparable to the reduction observed with the stop codon mutant, pOka66S. In contrast, infection of T cell xenografts with pOka66G102A was associated with a significant decrease in infectious virus production equivalent to the impaired T cell tropism found with pOka66S. Of interest, disrupting kinase activity with the G102A mutation did not alter IE62 cytoplasmic localization in VZV-infected T cells, suggesting that decreased T cell tropism is due to other ORF66 protein functions. The G102A mutation reduced the antiapoptotic effects of VZV infection of T cells. Thus, VZV T cell tropism depends upon ORF66 kinase activity.
Contributions of the ORF9–12 gene cluster to VZV pathogenesis
VZV has a conserved gene cluster found in other herpes viruses that includes ORF9, ORF10, ORF11, and ORF12. ORF9 is an essential gene, but deleting the other genes in the cluster, either alone or in combination, is compatible with VZV replication in vitro.
ORF10 protein
VZV ORF10 encodes a tegument protein that enhances transactivation of VZV genes and has some homology to HSV-1 VP16 (α-TIF). While VP16 is essential for HSV replication, ORF10 is dispensable in vitro. To analyze ORF10 functions, we made pOkaΔ10 and mutated the acidic activation domain and the putative motif for binding human cellular factor-1 (HCF-1) (Che et al. 2006). No effects on VZV replication, IE gene transcription, or virion assembly occurred in vitro, but deleting ORF10 reduced infection in skin xenografts in vivo (Table 1). Epidermal cells infected with pOkaΔ10 had fewer DNA-containing nucleocapsids and complete virions; extensive aggregates of intracytoplasmic viral particles were also observed. In contrast, deleting ORF10 did not impair VZV T cell tropism in vivo. Altering the activation or putative HCF-1 domains of ORF10 protein had no consequences in vivo. Thus, ORF10 protein is required for efficient VZ virion assembly and is a determinant of VZV virulence in skin in vivo. Mutagenesis of the ORF10 promoter showed that an USF binding site was important for skin tropism, although no effect on VZV replication was observed in cultured cells (Che et al. 2007).
Further analysis of the ORF9–12 gene cluster showed that recombinants lacking ORF10/11, ORF11/12, or ORF10/11/12 had normal growth, virion assembly, and infectivity in tonsil T cells (Table 1) (Che et al. 2008). Deleting ORF12 had no effect in skin but ORF11, 10/11, and 11/12 mutants were attenuated and virus was not recovered after deleting ORF10/11/12, indicating that the intact ORF10-ORF12 cluster is important in skin in vivo (Fig. 9). ORF9 was found to be incorporated into virion tegument and coprecipitated with gE. Work in progress indicates that ORF11 is an even more critical gene product for the pathogenesis of skin infection than ORF10, whereas ORF12 appears to be completely dispensable.
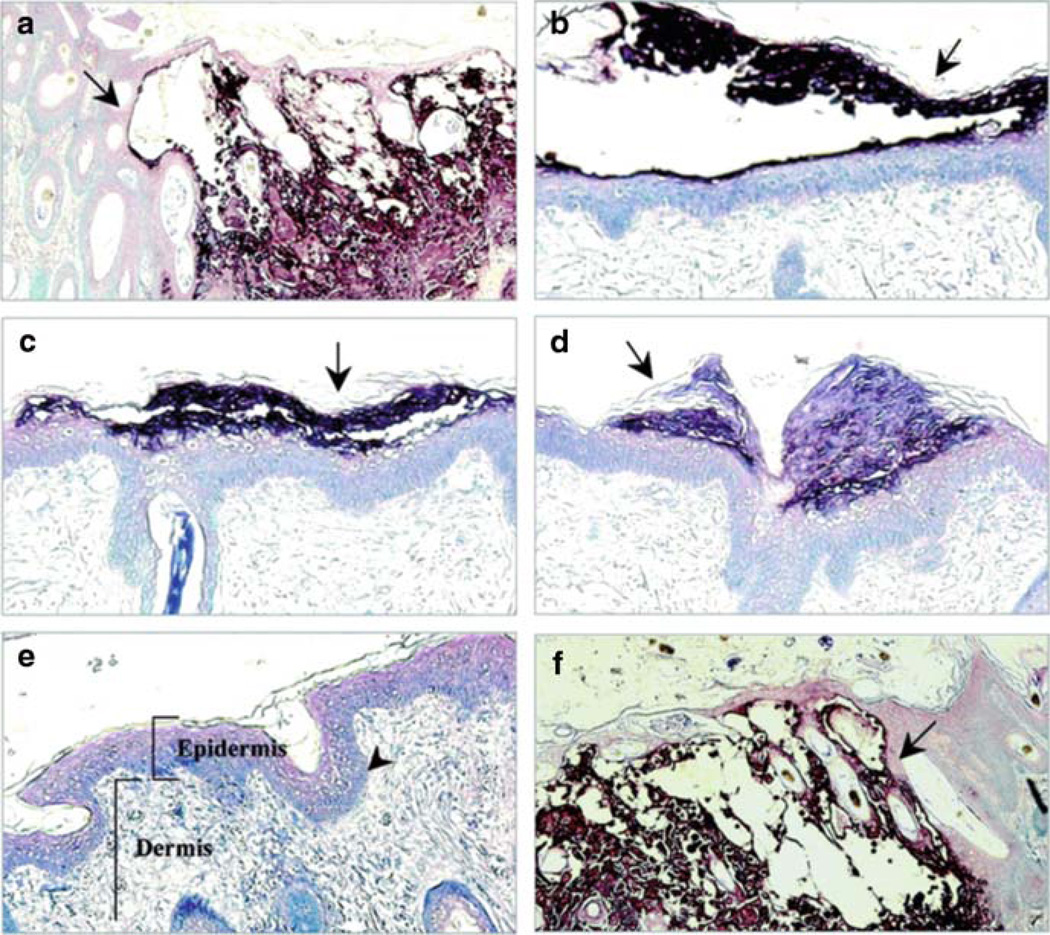
Fig. 9.
Lesion formation in skin xenografts infected with ORF10-to-ORF12 cluster gene mutants. Deparaffinized skin sections harvested at days 21 after infection were incubated with human polyclonal anti-VZV IgG and stained with biotinylated secondary antibody (magnification, ×10). Sections representative of skin xenografts infected with each virus are shown. Large lesions were observed for skin infected with POKA (a) and POKAΔ12 (f). Small lesions (arrows) restricted to the epidermal layer were observed for skin infected with POKAΔ11 (b), POKAΔ10/11 (c), and POKAΔ11/12 (d). POKAΔ10/11/12 (e) did not produce detectable skin lesions. The normal structures of the epidermis, the dermis, and the basement membrane that separates the epidermis from the underlying dermis are indicated in this section. From Che et al. 2008; reproduced with permission
Comparison of the T cell and skin tropims of parent Oka and vaccine Oka viruses
VZV is the only human herpes virus for which licensed vaccines are available. These vaccines are made from the parent Oka (pOka) virus, a Japanese clinical isolate that was attenuated empirically by passage in human and guinea pig embryo fibroblasts, yielding vaccine Oka (vOka) (Gershon 2001). This virus stock is used to make varicella vaccines and the high potency live attenuated vaccine for zoster prevention. We used the SCID mouse model to compare the virulence of vOka in skin xenografts with pOka, another low-passage clinical isolate, and the Ellen strain, which is a highly passaged laboratory virus (Moffat et al. 1998a, b). Although replication of these viruses was not different in cultured cells, pOka and the other clinical isolate showed the most extensive lesion formation and produced the highest titers of infectious virus in skin xenografts. These experiments provided the first evidence of a genetic basis for vOka attenuation and suggested that it results from the accumulation of mutations resulting from tissue culture passage (Arvin 2001b). Of interest, the highly passaged Ellen strain was avirulent in skin xenografts, indicating that prolonged adaptation in vitro can completely abrogate the pathogenic potential of VZV.
The genetic basis of vOka attenuation in skin xenografts was analyzed further by making chimeras from five fragment pOka and vOka cosmids (Zerboni et al. 2005a, b). The virulence of these pOka/vOka chimeras was variable in skin, suggesting that vOka attenuation is due to mutations in various VZV genes that are located in different regions of the genome. Extensive sequencing of the vaccine Oka stock has demonstrated that it represents a mixture of genomes with different mutations, as described in the chapters on VZV and vaccine genomics. Importantly, the comparative evaluation of pOka and vOka replication in T cell xenografts showed no attenuation of T cell tropism in vivo, which is consistent with the capacity of the vaccine virus to cause viremia and a varicella-like illness in immunocompromised children (Gershon 2001). These experiments suggest that functions required for T cell infection are not affected by attenuation achieved through passage in fibroblasts.
4 Conclusion
VZV remains a medically important pathogen despite the availability of live attenuated vaccines and antiviral drugs. Information about the determinants of VZV tropism for T cells and skin has the potential to yield new approaches to a “genetically engineered” vaccine and new targets for antiviral drugs. In particular, a vaccine based on a VZV recombinant virus that has impaired infectivity for T cells should be less likely to disseminate in high risk patients or to establish latency in individuals.
Acknowledgments
This summary describes the accomplishments of the graduate students, postdoctoral fellows, and research staff whose investigations of VZV molecular virology and pathogenesis are reported in detail in their published work. These studies were supported by NIH grants AI053846, AI20459, and CA49605.
Contributor Information
Ann M. Arvin, Email: aarvin@stanford.edu, Department of Pediatrics and Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA.
Jennifer Moffat, Department of Microbiology, University of New York, Syracuse, NY, USA.
Marvin Sommer, Department of Pediatrics and Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA.
Stefan Oliver, Department of Pediatrics and Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA.
Xibing Che, Department of Pediatrics and Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA.
Susan Vleck, Department of Pediatrics and Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA.
Leigh Zerboni, Department of Pediatrics and Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA.
Chia-Chi Ku, The Graduate Institute of Immunology, College of Medicine, National Taiwan University, Taipei, Taiwan.
References
- Arvin AM. Varicella-zoster virus. In: Knipe DM, Howley P, editors. Fields’ virology. 4th edn. Philadelphia: Lippincott-Williams & Wilkins; 2001a. pp. 2731–2768. [Google Scholar]
- Arvin AM. Varicella vaccine: genesis, attenuation and efficacy. Virology. 2001b;284:153–158. doi: 10.1006/viro.2001.0918. [DOI] [PubMed] [Google Scholar]
- Arvin AM, Schaap AC, Ku C-C, Jones JO, Sommer M, Zerboni Z. Investigations of the molecular mechanisms of varicella-zoster virus pathogenesis. In: Sandri-Golden R, editor. The alphaherpesviruses. Horizon Press Inc: UK: 2006. [Google Scholar]
- Baiker A, Bagowski C, Ito H, Sommer M, Zerboni L, Fabell K, Hay J, Ruyechan W, Arvin AM. The immediate early 63 protein of varicella-zoster virus: analysis of functional domains required for replication in vitro and for T cell and skin tropism in the SCID hu model in vivo. J Virol. 2004;78:1181–1194. doi: 10.1128/JVI.78.3.1181-1194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser J, Sommer MH, Zerboni L, Bagowski C, Ito H, Moffat J, Ku C-C, Arvin AM. Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse. J Virol. 2003;77:5964–5974. doi: 10.1128/JVI.77.10.5964-5974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser J, Ikoma M, Fabel K, Sommer MH, Zerboni L, Grose C, Arvin AM. Differential requirement for cell fusion and virion formation in varicella-zoster virus infection of skin and T cells. J Virol. 2004;78:13293–13305. doi: 10.1128/JVI.78.23.13293-13305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X, Zerboni L, Sommer MH, Arvin AM. Varicella-zoster virus open reading frame 10 is a virulence determinant in skin cells but not in T-cells in vivo. J Virol. 2006;7:3238–3248. doi: 10.1128/JVI.80.7.3238-3248.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X, Berarducci B, Sommer M, Ruyechan WT, Arvin AM. The ubiquitous cellular transcriptional factor USF targets the varicella-zoster virus open reading frame 10 promoter and determines virulence in human skin xenografts in SCIDhu mice in vivo. J Virol. 2007;81:3229–3239. doi: 10.1128/JVI.02537-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X, Reichelt M, Sommer MH, Rajamani J, Zerboni L, Arvin AM. Functions of the ORF9-to-ORF12 gene cluster in varicella-zoster virus replication and in the pathogenesis of skin infection. J Virol. 2008;82:5825–5834. doi: 10.1128/JVI.00303-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Seidel KE. Generation of VZV and viral mutants from cosmid DNAs: VZV thymidine kinase is not essential for replication in vitro. Proc Natl Acad Sci USA. 1993;90:7376–7380. doi: 10.1073/pnas.90.15.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Krogmann T, Bontems S, Sadzot-Delvaux C, Pesnicak L. Regions of the varicella-zoster virus open reading frame 63 latency-associated protein important for replication in vitro are also critical for efficient establishment of latency. J Virol. 2005;79:5069–5077. doi: 10.1128/JVI.79.8.5069-5077.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Straus S, Arvin A. Varicella-zoster virus. In: Knipe DM, Howley P, editors. Fields’ virology. 5th edn. Philadelphia: Lippincott-Williams & Wilkins; 2007. pp. 2547–2586. [Google Scholar]
- Gershon AA. Live attenuated varicella vaccine. Infect Dis Clin N Am. 2001;15 doi: 10.1016/s0891-5520(05)70268-3. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- Jones JO, Arvin AM. Microarray analysis of host cell gene transcription in response to varicella-zoster virus infection of human T cells and fibroblasts in vitro and SCID hu skin xenografts in vivo. J Virol. 2003;77:1268–1280. doi: 10.1128/JVI.77.2.1268-1280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JO, Arvin AM. Inhibition of the NF-kB pathway by varicella-zoster virus in vitro and in human epidermal cells in vivo. J Virol. 2006;80:5113–5124. doi: 10.1128/JVI.01956-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble GW, Annunziato P, Lungu O, Winter RE, Cha TA, Silverstein SJ, Spaete RR. Open reading frame S/L of varicella- zoster virus encodes a cytoplasmic protein expressed in infected cells. J Virol. 2000;74:11311–11321. doi: 10.1128/jvi.74.23.11311-11321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropchak CM, Solem S, Diaz PS, Arvin AM. Investigation of varicella-zoster virus infection of lymphocytes by in situ hybridization. J Virol. 1989;63:2392–2395. doi: 10.1128/jvi.63.5.2392-2395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C-C, Padilla J, Grose C, Butcher EC, Arvin AM. Tropism of varicella-zoster virus for human tonsillar CD4+ T lymphocytes that express activation, memory and skin homing markers. J Virol. 2002;76:11425–11433. doi: 10.1128/JVI.76.22.11425-11433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C-C, Zerboni L, Ito H, Wallace M, Graham B, Arvin AM. Transport of varicella-zoster virus to skin by infected CD4 T cells and modulation of viral replication by epidermal cell interferon-a. J Exp Med. 2004;200:917–925. doi: 10.1084/jem.20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C-C, Besser J, Abendroth A, Grose C, Arvin AM. Varicella-zoster virus pathogenesis and immunobiology: New concepts emerging from investigations in the SCIDhu mouse model. J Virol. 2005;79(5):2651–2658. doi: 10.1128/JVI.79.5.2651-2658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory S, Sommer M, Arvin AM. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein conformation and trafficking. J Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat JF, Stein MD, Kaneshima H, Arvin AM. Tropism of varicella-zoster virus for human CD4+ and CD8+ T-lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–5242. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat J, Zerboni L, Stein M, Grose C, Kaneshima H, Arvin A. The attenuation of the vaccine Oka strain of varicella-zoster virus and the role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J Virol. 1998a;72:965–974. doi: 10.1128/jvi.72.2.965-974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat JF, Zerboni L, Sommer MH, Heineman TC, Cohen JI, Kaneshima H, Arvin AM. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc Natl Acad Sci USA. 1998b;95:11969–11974. doi: 10.1073/pnas.95.20.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizuma T, Sommer MH, Ito H, Hinchliffe S, Zerboni L, Arvin AM. Mutational analysis of varicella-zoster virus ORF65 protein and its role in infection of human skin and T cell xenografts in the SCIDhu mouse model. J Virol. 2003;77:6062–6065. doi: 10.1128/JVI.77.10.6062-6065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver SL, Sommer M, Zerboni L, Rajamani J, Grose C, Arvin AM. Mutagenesis of varicella zoster virus glycoprotein B: putative fusion loop residues are essential for viral replication, and the furin cleavage motif contributes to pathogenesis in skin tissue in vivo. J Virol. 2009;83:7495–7506. doi: 10.1128/JVI.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato B, Ito H, Hinchliffe S, Sommer MH, Zerboni L, Arvin AM. Effects of mutations in open reading frames 62 and 71, encoding the immediate early transactivating protein, IE62, of varicella-zoster virus on replication in vitro and in skin xenografts in the SCID-hu mouse. J Virol. 2003;77:5607–5620. doi: 10.1128/JVI.77.10.5607-5620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap A, Fortin J-F, Sommer M, Zerboni L, Stamatis S, Ku C-C, Arvin AM. T cell tropism and the role of ORF66 protein in the pathogenesis of varicella-zoster virus infection. J Virol. 2005;79:12921–12933. doi: 10.1128/JVI.79.20.12921-12933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap-Nutt A, Sommer M, Che X, Zerboni L, Arvin AM. ORF66 protein kinase function is required for T cell tropism of varicella-zoster virus in vivo. J Virol. 2006;80:11806–11816. doi: 10.1128/JVI.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong W, Schultz JC, Patera AC, Sommer MH, Cohen JI. Infection of human T lymphocytes with varicella-zoster virus: an analysis with viral mutants and clinical isolates. J Virol. 2000;74:1864–1870. doi: 10.1128/jvi.74.4.1864-1870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer BK, Kaufer BB, Sommer M, Wussow F, Arvin AM, Osterrieder N. A self-excisable infectious bacterial artificial chromosome clone of varicella-zoster virus allows analysis of the essential tegument protein encoded by ORF9. J Virol. 2007;81:13200–13208. doi: 10.1128/JVI.01148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleck SE, Oliver SL, Reichelt M, Rajamani J, Zerboni L, Jones C, Zehnder J, Grose C, Arvin AM. Anti-glycoprotein H antibody impairs the pathogenicity of varicella-zoster virus in skin xenografts in the SCID Mouse Model. J Virol. 2010;84:141–152. doi: 10.1128/JVI.01338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerboni L, Arvin AM. The pathogenesis of varicella zoster virus neurotropism and infection. In: Carol R, editor. Neurotropic viral infections. New York: Cambridge Press; 2008. pp. 225–250. [Google Scholar]
- Zerboni L, Hinchliffe S, Sommer MH, Ito H, Besser J, Stamatis S, Cheng J, DiStefano D, Kraiouchkine N, Shaw A, Arvin AM. Analysis of varicella-zoster virus attenuation by evaluation of chimeric parent Oka/vaccine Oka recombinant viruses in skin xenografts in the SCIDhu mouse model. Virology. 2005a;332:337–346. doi: 10.1016/j.virol.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Zerboni L, Ku C-C, Jones C, Zehnder J, Arvin AM. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci USA. 2005b;102:6490–6495. doi: 10.1073/pnas.0501045102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Rowe J, Wang W, Sommer M, Arvin A, Moffat J, Zhu H. Genetic analysis of varicella zoster virus ORF0 to 4 using a novel luciferase bacterial artificial chromosome system. J Virol. 2007;81:9024–9033. doi: 10.1128/JVI.02666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]