Abstract
The last decade has seen an exponential growth in the number of exosome-related publications. Although many of these studies have used exosomes from biological fluids (blood, and ascites or pleural effusions) the vast majority employed vesicles isolated from large volumes of tissue culture supernatants. While several techniques are available for their isolation, all require a significant reduction in volume to obtain sufficient concentrations for study. One approach is to concentrate the medium before proceeding with their isolation, however, these procedures are very time consuming and require specialized laboratory equipment. Here we provide a new and effective method for the isolation of tumor-derived exosomes based on “charge neutralization” with acetate. We show that titration of tissue culture supernatants with 0.1M acetate to pH 4.75 results in immediate precipitation of virtually all the exosomes. The precipitated exosomes can be washed to remove residual media and are readily “resolubilized” upon resuspension in acetate-free buffer at neutral pH. This simple cost effective method significantly increases the yield of exosomes from an unlimited quantity of culture supernatants. Exosomes isolated by this technique are indistinguishable from exosomes recovered by direct ultracentrifugation.
Keywords: Tumors, exosomes, tissue culture, precipitation
1. Introduction
Exosomes are nanometer-sized membrane-derived vesicles that are actively secreted by cells in vivo and in vitro. They are generated from the late endosomes by the inward budding and scission of the endosomal membrane creating multivesicular bodies (MVBs) that contain intraluminal vesicles. These exosomes are released to the extracellular space upon fusion of the MVB with the plasma membrane. Because they originate from the cell’s plasma membrane and are formed by invagination of the endosomal membrane, secreted exosomes possess plasma membrane and endosome proteins that encapsulate a cytosol-derived aqueous space.
Exosomes exert a broad array of important physiologic functions by acting as molecular messengers that traffic information between different cell types. They deliver proteins, lipids and soluble factors including RNA and microRNAs (1) that, depending on their source, participate in signaling pathways that can influence apoptosis (2–4), metastasis (5), angiogenesis (4,6), tumor progression (7,8) thrombosis (9,10) and immunity by directing T cells towards immune activation (11,12) or immune suppression (13–15).
Several techniques have been described for the isolation and purification of relatively homogenous exosome populations from malignant effusions and the peripheral blood of cancer patients and from supernatants of in vitro cultivated cell lines. These methods include differential centrifugation (16), affinity chromatography (17) polyetheleneglycol-mediated precipitation (18,19) and capture on defined pore-size membranes (20,21). These techniques, however, can be time-consuming, cumbersome and/or costly, and limited by the amounts of material to be processed.
Based on studies showing that exosomes express the negatively-charged phospholipid phosphatidylserine (PS) (7,18,20) we investigated the possibility of precipitating them from solution by neutralizing their surface charge with acetate. We show that titration of culture supernatants with 0.1M acetate to pH 4.75 results in immediate precipitation of virtually all the exosomes. The precipitated exosomes can be washed to remove residual media and are readily “resolubilized” upon resuspension in acetate-free buffer at neutral pH. Exosomes isolated by this technique are indistinguishable from those purified by ultacentrifugation.
2. Materials and methods
2.1 Tissue culture
K1735P melanoma cells (provided by I.J. Fidler, M. D. Anderson Cancer Center, Houston, TX) were cultured in minimal essential media (MEM) supplemented with L-glutamine (2 mM), Na pyruvate (1 mM), penicillin (100 U/mL), streptomycin (100 µg/mL), nonessential amino acids and fetal bovine serum (10%). Cells (~25 × 106 in 15 mL media) were seeded into the lower chamber of CELLine AD 1000 flasks (Integra Biosciences AG) that contained 250 mL media in the upper chamber (22). Conditioned media (~15 mL) was collected from the lower chamber weekly. The compartment was washed once with 15 mL of phosphate-buffered saline (PBS) and combined with the conditioned media. Fresh media was then added to the lower chamber. The upper chamber was replenished weekly by replacing ~100 mL spent media with fresh media. Weekly collections typically yielded 75 – 125 µg of purified exosomes/mL of conditioned media.
2.2 Isolation of tumor-derived exosomes
Ultracentrifugation – Cell conditioned media was cleared of cells, cell debris and large membrane vesicles by sequential centrifugation at 500g for 30 min followed by 12,000g for an additional 30 min. Exosomes were collected from the cleared supernatants after centrifugation at 100,000g for 1 hr. The pellets were resuspended in ~2 mL HEPES-saline (HBS; NaCl 150 mM, HEPES 20 mM, EGTA 2mM, pH 7.6). Exosome quantity was estimated by BCA protein assay.
Standard precipitation protocol – Cell conditioned media was cleared of cells, cell debris and large membrane vesicles by sequential centrifugations as described above. 1/10th volume of Na acetate buffer (1.0 M; pH 4.75) was mixed with the cleared supernatants and left on ice for 30–60 min and then transferred to 37°C for an additional 5 min. The turbid suspension was centrifuged for 10 min at 5000g and the resulting pellet was washed once with 0.1M Na acetate buffer. The suspension was again centrifuged and the pellet “solubilized” in HBS. If necessary, further purification was achieved by an additional round of precipitation. The purified exosomes were stored at 4°C.
2.3 Flow cytometry
Tumor exosomes (10 µg protein) in 0.5 mL PBS were mixed overnight at 4°C with 5 µL of 4 µM aldehyde-activate latex beads (4% w/v) (Invitrogen). The beads were then blocked by adding 0.5 mL of 1% bovine serum albumin (BSA) for 1 hr followed by 0.1 mL of 100 mM glycine for an additional hour. The beads were then washed and resuspended in PBS. Antibodies included rabbit anti-alix (sc-99010; Santa Cruz) and rabbit anti-tubulin (sc-5546; Santa Cruz) as an isotype control followed by CY3-labeled donkey anti-rabbit Ig. The primary antibody was incubated with the beads for 1 hr on ice, washed twice, followed by an additional 1 hr with the labeled secondary. The beads were again washed and analyzed. FITC-annexin 5 (BD Biosciences) was used as described by the manufacturer. Samples included BSA-blocked beads and exosome-beads incubated in the presence and absence of Ca2+ (1 mM).
2.4 Electron microscopy
Isolated exosomes were prepared for examination by transmission electron microscopy using a procedure slightly modified from that described by Thery et., al (16). Briefly, 25 µl of exosome suspension was place on parafilm and carbon-coated grids were suspended, face down, on the suspension for 1 min. The grids were then washed by three sequential passages for 1 min each on water. The grids were then stained by placing them on a 25 µl droplet of 2% uranyl acetate for 1 min and again washed in water as described above. Excess water was removed by blotting on filter paper. The grids were then air dried for several min. Samples were examined with a JEOL 1200 EX electron microscope.
2.5 Gel electrophoresis and western blotting
Exosomes were solubilized at 95°C for 5 min in an equal volume of Laemmli sample buffer (Bio-Rad) containing 5% α-mercaptoethanol. Aliquots (20 µg protein) were subjected to electrophoresis through duplicate “Ready Cast” 10% acrylamide gels (Bio-Rad). One gel was stained with Coomassie blue and the duplicate was transferred to PVDF membranes overnight at 4°C. The membranes were blocked with 5% non-fat milk in TRIS-buffered saline (TBS) containing 1% Tween-20. The indicated primary antibodies (anti-alix, anti-hsp70; Santa Cruz), were added for 1 hr followed by three washes with TBS. Antibody binding was assessed with appropriate HRP-conjugated secondary antibodies and visualized by enhanced chemoluminescence.
3.0 Results
3.1 “Salting-out” method for the isolation of tumor-derived exosomes
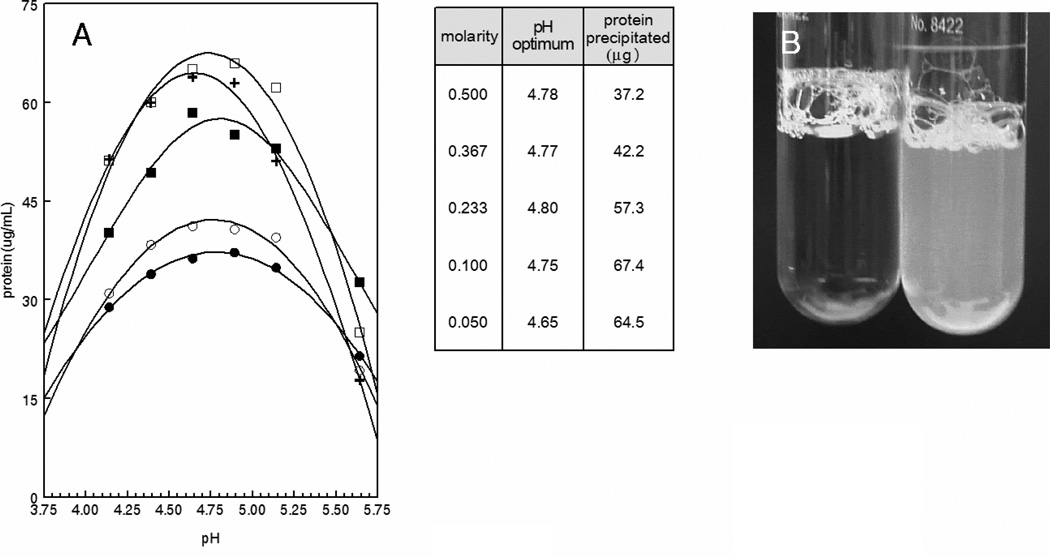
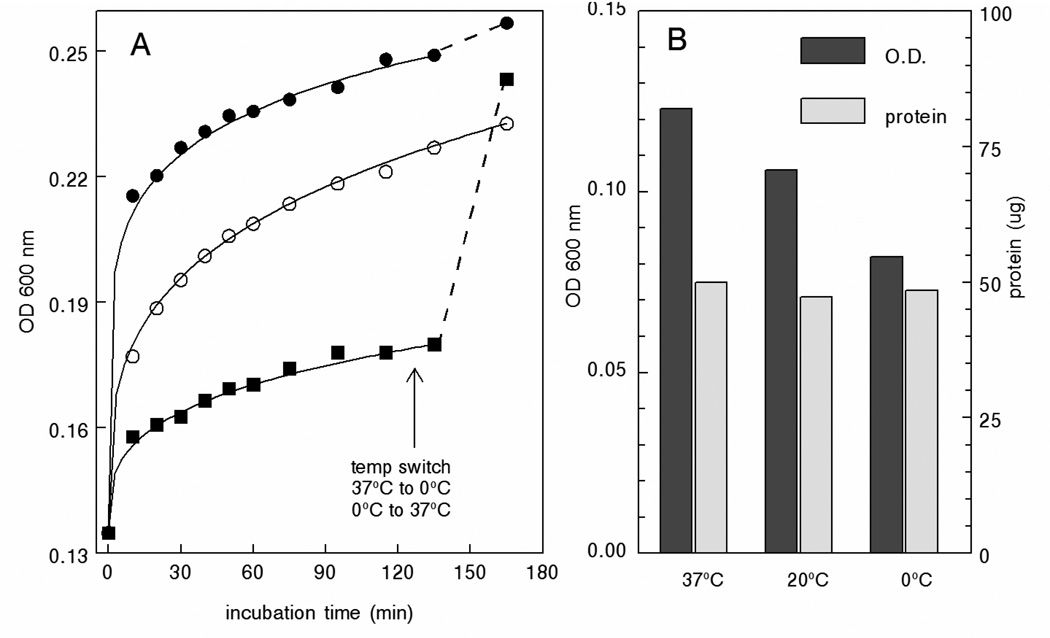
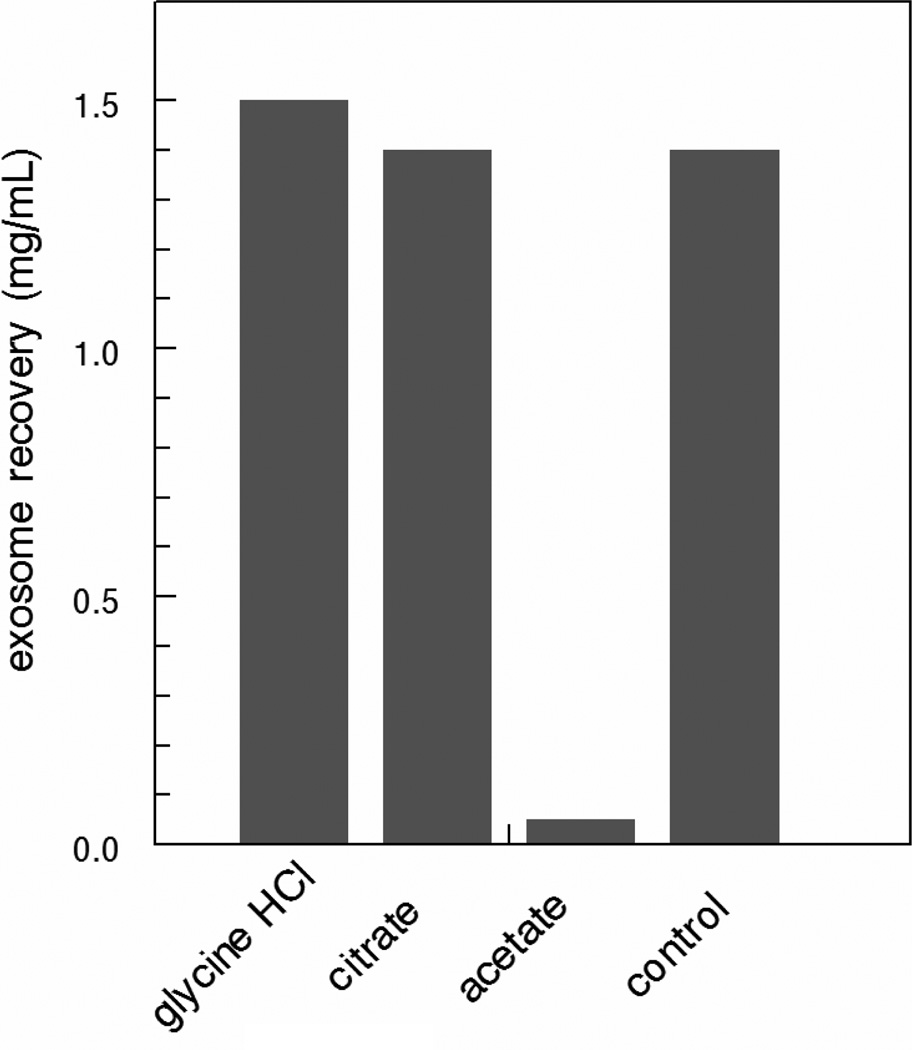
Tumor cell cultures were established in the highly efficient CELLline AD tissue culture system (22). Supernatants were retrieved from the lower cell-containing chamber and intact cells and cellular debris were removed by centrifugation at 500g and 10,000g, respectively. The clear supernatants were then mixed with 1/10th volume of increasing 10× acetate concentrations titrated to the indicated pH with acetic acid. Figure 1A shows that precipitation was dependent on both pH and salt concentration with maximal precipitation occurring at pH ~4.75 with 0.1M acetate. Under these conditions, precipitation was essentially instantaneous and clearly visible (Fig 1B). Analysis of the effect of temperature showed that the development of turbidity was temperature-dependent. An immediate temperature-dependent increase in turbidity occurred upon the addition of acetate which then began to level off. Continued incubation showed a modest ~2-fold increase in rate between 0°C and 20°C. However, no significant difference in rate was observed upon increasing the temperature from 20°C to 37°C (Fig. 2A). Interestingly, once the reaction plateaued at 0°C, increasing the temperature to 37°C resulted in an immediate increase in turbidity to levels that approached that of samples incubated for the entire period at 37°C. Conversely, decreasing the temperature of samples incubated at 37°C was without effect (Fig. 2A). Irrespective of turbidity, the amounts of protein recovered in the pelleted precipitates were identical indicating that exosome precipitation is essentially temperature-independent with larger aggregates being formed at higher temperatures (Fig. 2B). Precipitation was dependent on the presence of acetate since precipitation did not occur with supernatants acidified with HCl or citrate (Fig. 3).
Fig. 1.
Salt and pH dependence of exosome precipitation. A) 4.5 mL aliquots of pre-cleared K1735 supernatants were mixed with 1/10th volume (0.5 mL) of the indicated 10× concentrated buffer solutions. The suspensions where incubated on ice for 60 min and centrifuged at 5,000g for 10 min. The pellets were then solubilized and brought back to their initial volume and protein was assessed by Bradford assay. B) K1735 supernatant (right) or control media collected from the upper chamber of the CELLine flask (left) was mixed with 1/10th volume of 1.0 M acetate pH 4.75 and photographed after 5 min. incubation on ice. ●, 0.5M; ○, 0.367M; ■, 0.233M; □, 0.1M; +, 0.05M
Fig. 2.
Temperature dependence of exosome precipitation. A) 0.1 mL of 1M Na acetate was rapidly mixed with 0.9 mL of pre-cleared K1735 supernatants and incubated at the indicated temperature while simultaneously monitoring turbidity. The arrow shows when the samples incubated at 0°C and 37°C were transferred to 37°C and 0°C, respectively. B) Acetate/exosome mixtures were prepared as in (A). Aliquots were diluted 2-fold for measurement of OD and the remaining suspension was centrifuged for 10 min at 5,000g. Precipitated protein was quantified by Bradford assay. ■, 0°C; ○, 20°C; ●, 37°C.
Fig. 3.
Acetate is required for exosome precipitation. Cleared tissue culture supernatants were mixed with the indicated buffers (1/10th volume of 1.0 M solutions) for 1 hr at 4°C. The suspensions were then centrifuged at 5000g for 15 min. The supernatants were collected, centrifuged at 100,000g for 1 hr and the concentration of protein in the pellets were determined by Bradford assay.
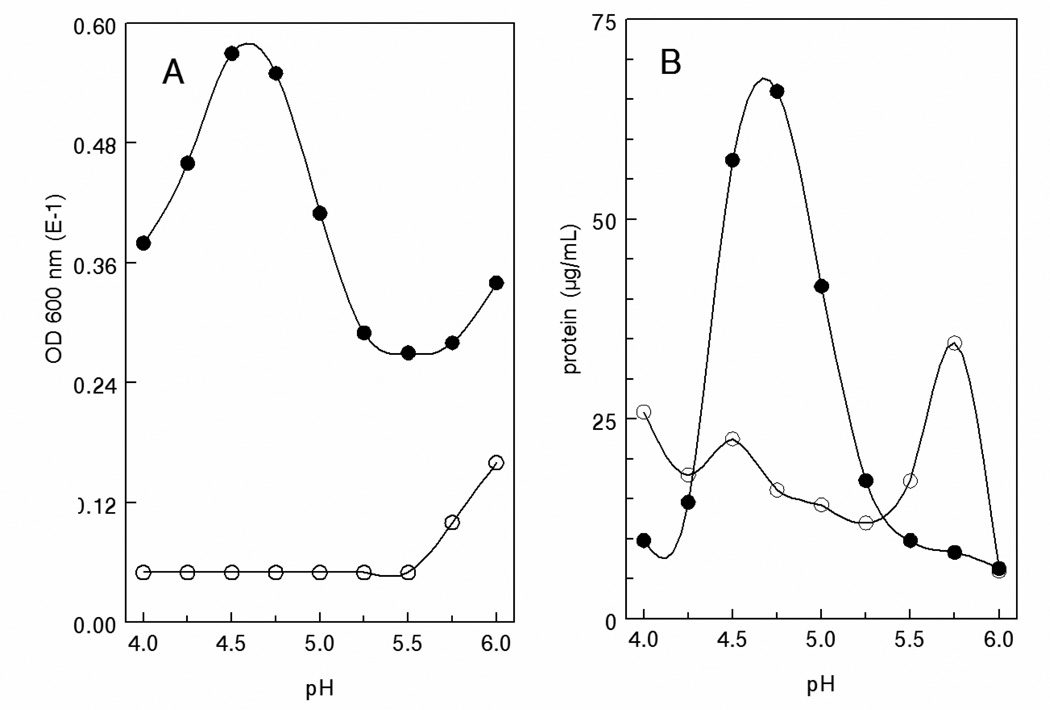
The pH dependence of precipitation is shown in Fig. 4. Spent media and cleared cell supernatants from the same Integra flasks were incubated for 1 hr in 0.1 M Na acetate buffers. Turbidity was assessed at 600 nm and precipitated protein (exosomes) was assessed by Bradford assay. Maximum turbidity and precipitated protein were obtained at pH 4.75 with the supernatants whereas no turbidity or precipitate was observed with the control media.
Fig. 4.
Differential precipitation from cell supernatants and spent media. Aliquots from the CELLine flasks lower (●, cells) and upper (○, media) chambers were pre-cleared and mixed with 1/10th volume of 1.0M acetate at the indicated pH. After incubation for 1 hr on ice, turbidity was assessed at 600 nm and protein in re-solubilized centrifuged (5,000g; 10 min) pellets. A) turbidity, B) protein.
3.2 Comparative yield of “salting-out” vs. ultracentrifugation
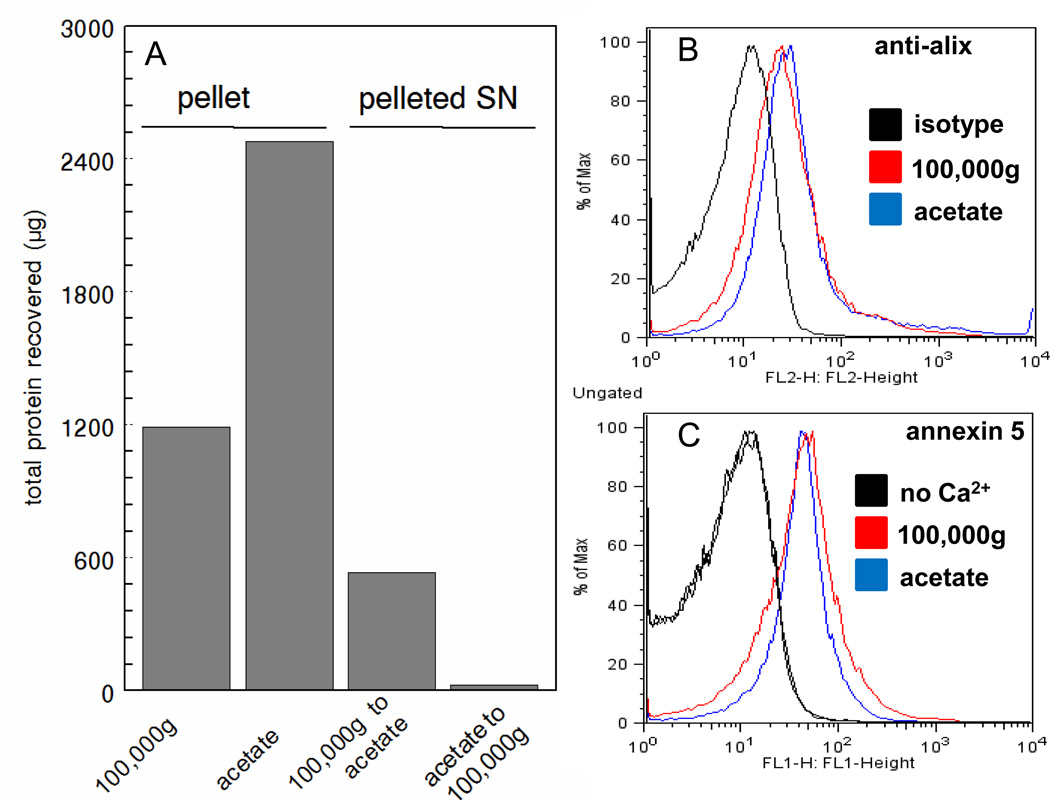
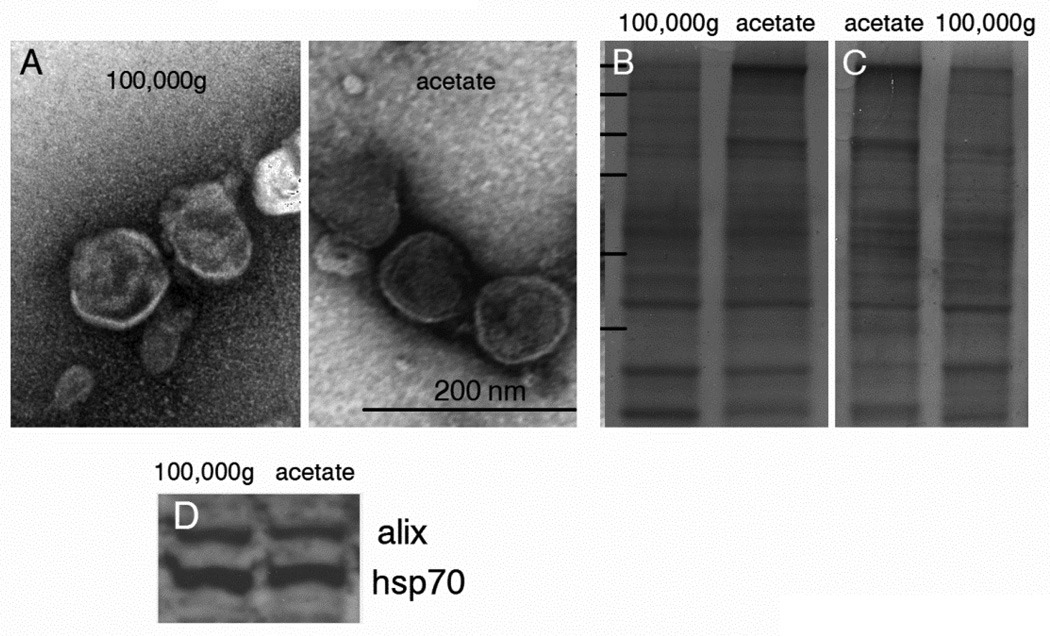
To directly compare the yield of exosomes obtained with acetate and with conventional 100,000g ultracentrifugation, identical aliquots of K1735 supernatants were isolated by both methods. In addition, the supernatants from the 100,000g ultracentrifugation and acetate protocols were subjected to an additional round of isolation by acetate pH 4.75 and ultracentrifugation (after bringing the solution to pH 7.5), respectively. Fig. 5 shows ~2.0-fold increase in protein yield with the acetate protocol over 100,000g (167.0 µg/ml vs 88.1 µg/ml) (Fig. 5a). Additional protein could not be recovered after ultracentrifugation of neutralized acetate supernatants suggesting that virtually all the exosomes were precipitated with acetate. On the other hand, acetate treatment of the 100,000g supernatants recovered an additional 533 µg of protein (39.5 µg/ml) suggesting that the acetate non-specifically precipitated non-exosomal proteins. SDS-PAGE of exosomes recovered from acetate precipitation and 100,000g precipitation showed similar protein patterns with the exception of a heavily stained band at the top of the acetate lane. Mass spectroscopy analysis indicated that the relatively heavily stained upper band (~160 kDa) in the acetate sample was α2-macroglobulin, a protein known to be secreted by some melanoma cells (23). The high concentration of α2-macroglobulin in the acetate sample is likely the result of acid-dependent co-precipitation unrelated to the precipitation of exosomes. To confirm this, neutralized acetate precipitated exosomes were washed once at 100,000g. SDS-PAGE of the washed exosomes revealed that most of the α2-macroglobulin was removed confirming that its presence in the original acetate precipitate was due to unrelated co-precipitation (Fig. 6C). Because of the error introduced by the assessment of yield based on protein, we also quantified relative yield by assessing alix and PS in both populations. Flow cytometry analysis of alix with Cy3-labeled alix antibodies (Fig. 5B) and PS with FITC-labeled annexin 5 (Fig. 5C) suggested that both methods yielded similar amounts of exosomes.
Fig. 5.
Comparative protein yields of exosomes isolated with acetate vs. 100,000g ultracentrifugation. A) 50 mL of pre-cleared cell supernatant was centrifuged at 100,000g for 1 hr or at 5,000g for 10 min. after 60 min incubation with acetate. The pellets were resuspended in HBS and protein was quantified. A second round of centrifugation and acetate treatment was carried out with the entire supernatant from the acetate treatment and ultracentrifugation, respectively. Flow analysis of exosomes stained with (B), alix antibodies and (C) FITC-annexin 5.
Fig. 6.
Characterization of exosomes. A) Negatively-stained melanoma-derived exosomes purified by 100,000g ultracentrifugation or with acetate were visualized by transmission electron microscopy. B) Coomassie stained SDS-PAGE gels. Note the relatively heavy α2-macroglobulin band at the top of the acetate lane. C) The acetate exosomes (left) were resuspended in HBS and centrifuged at 100,000g for 1 hr. Note that most of the α2-macroglobulin washed off. D) Western blotting for alix and hsp70.
Exosomes purified by traditional ultracentrifugation and with acetate were analyzed by electron microscopy, and by western blotting for characteristic exosome-associated markers. Exosomes recovered with acetate were morphologically indistinguishable from vesicles collected by ultracentrifugation. Both populations contained vesicles of identical size and had typical bilayer membranes enclosing a luminal space (Fig. 6A). Western blot analysis confirmed that both preparations contained the exosome markers alix and hsp70 (Fig. 6D).
4. Discussion
The number of studies focusing on cell-derived exosomes has increased exponentially in recent years. These studies range from methods for exosome isolation to their utility in cancer diagnosis and ability to mediate immune responses. Exosomes incorporate a wide range of cytosolic and membrane components that reflect the properties of the parent cell. For example, the lumen of exosomes contain various components entrapped from the cell cytosol including RNA and miRNA that express data signatures of disease that can be deciphered for the detection of neoplasia and identification of a specific tumor type (17,18,24,25). Exosome membranes contain MHC class I and II (6), heat shock protein 70 (26) that upregulates Th1-mediated immune responses and many cell surface components including tumor antigens from the plasma membrane of the parent cells. These observations suggest that tumor exosomes could be used as immunotherapeutics for the treatment of cancer. Indeed, several recent clinical trials have indicated that “immunization” with tumor exosomes have minimal side effects, are well tolerated, and elicit specific cytotoxic T cell responses (27,28).
The originally described and most widely used method for the purification of exosomes involves escalating centrifugation steps that remove cells and cellular debris, followed by a 100,000g step for pelleting exosomes (16). Other techniques include trapping on antibody-coated beads (29) and ELISA plates (30), filtration through defined pore-sized membranes (20) and polymer-based precipitation. With the exception of coated plates, all these methods can, in principle, accommodate large volumes of material. These methods, however, are laborious and require specialized equipment. The rapid and efficient “salting-out” procedure described here yields exosomes that are indistinguishable from those obtained by ultracentrifugation. Moreover, the method easily accommodates very large volumes of material and exosome purification can be accomplished without specialized equipment at minimal cost.
The mechanism responsible for exosome precipitation is unclear. It does not appear to be due to precipitation at the isoelectric point since exosomes failed to precipitate when the solution was acidified with HCl or citrate. Interestingly, observations made over 40 years ago showed that precipitation of vesicles formed by recombination of red cell apoproteins was dependent on the presence of negatively-charged phosphatidic acid or PS (31). Taken together this suggests that the principle mechanism responsible for the precipitation is acetate-mediated removal of the exosome hydration layer that promotes hydrophobic interactions resulting in increasing aggregation and concomitant precipitation. The decrease in precipitation on either side of pH 4.75 is likely due to increased positive or negative surface charge that reinforces the hydration layer thereby necessitating decreasing salt to affect the same degree of precipitation.
Comparison of the acidified versus ultracentrifuged population by flow cytometry, EM, SDS-PAGE and western blotting with alix and hsp70 antibodies indicated that both populations were indistinguishable from one another. It should be noted that there was a relatively heavy band at ~160 kDa in the acid precipitated population that was identified by mass spectroscopic analysis to be α2-macroglobulin. Since the protein was removed from the acetate precipitated exosomes by ultracentrifugation, it is likely that the protein co-precipitated independently of the exosomes in the acetate buffer. Extraneous protein precipitation is most likely dependent on the type of source cells. α2-macroglobulin is known to be produced by some melanoma and sarcoma cells (23,32). Indeed, it was not detectable in exosomes derived from B16 melanoma or TRAMP prostate carcinoma cells (not shown). While some extraneous protein precipitation will not interfere with nucleic acid-based exosome diagnostic assays, they should be removed for immunotherapeutics. In this case, once large volumes of culture supernatants are reduced to manageable volumes with acetate, 100,000g centrifugation of the resolubilized precipitate should remove any contaminating proteins.
Preliminary acetate precipitation experiments with whole human blood doped with known amounts of purified exosomes recovered ~50% and ~100% of the added exosomes from clotted serum samples and EGTA-plasma, respectively. Although somewhat variable, acetate treated control (no added exosomes) serum and plasma precipitated ~400 µg/mL and 1530 µg/mL protein, respectively. We suspected the large difference in the amount of protein recovered was due to precipitation of fibrinogen in the plasma samples. Indeed, removal of the fibrinogen by pre-incubation at 56°C for 3 min (33,34) reduced the levels of extraneous protein in the acetate precipitated samples to levels comparable to those obtained for the serum samples with essentially no loss of exosomes. As discussed above, extraneous proteins in the exosome preparation should not pose any problems for transcriptomic analysis. For use as immunogens, non-exosomal proteins can be easily removed by ultracentrifugation once volumes have been reduced to manageable volumes with acetate.
Acknowledgements
This work was supported by the Simmons Cancer Center Support Grant (5P30 CA142543-03) and by a sponsored research agreement with Peregrine Pharmaceuticals Inc. (Tustin, CA.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thery C, et al. Membrane vesicles as conveyors of immune responses. Nat.Rev.Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 2.Andreola G, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J.Exp.Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huber V, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 4.Kim JW, et al. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin.Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 5.Parolini I, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J.Biol.Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iero M, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death.Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 7.Keller S, et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009;278:73–81. doi: 10.1016/j.canlet.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Thery C, et al. Exosomes: composition, biogenesis and function. Nat.Rev.Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 9.Aharon A, Brenner B. Microparticles, thrombosis and cancer. Best Practice & Research Clinical Haematology. 2009;22:61–69. doi: 10.1016/j.beha.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Al Nedawi K, et al. Mast cell-derived exosomes activate endothelial cells to secrete plasminogen activator inhibitor type 1. Arterioscler.Thromb.Vasc.Biol. 2005;25:1744–1749. doi: 10.1161/01.ATV.0000172007.86541.76. [DOI] [PubMed] [Google Scholar]
- 11.Andre F, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J.Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 12.Chaput N, et al. The potential of exosomes in immunotherapy. Expert.Opin.Biol.Ther. 2005;5:737–747. doi: 10.1517/14712598.5.6.737. [DOI] [PubMed] [Google Scholar]
- 13.Szajnik M, et al. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS.One. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenti R, et al. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 15.Wieckowski EU, et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J.Immunol. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thery C, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr.Protoc.Cell Biol. 2006;Chapter 3(Unit) doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 17.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol.Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Taylor DD, et al. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol.Biol. 2011;728:235–246. doi: 10.1007/978-1-61779-068-3_15. [DOI] [PubMed] [Google Scholar]
- 19. www.systembio.com/exoquick; (US patent application 2013/0337440); www.lifetechnologies.com (US patent application 2013/0273544). [Google Scholar]
- 20.Grant R, et al. A filtration-based protocol to isolate human Plasma Membranederived Vesicles and exosomes from blood plasma. J.Immunol.Methods. 2011 doi: 10.1016/j.jim.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 21. www.biooscientific.com (US patent application 2013/0052647). [Google Scholar]
- 22.Mitchell JP, et al. Increased exosome production from tumour cell cultures using the Integra CELLine Culture System. J.Immunol.Methods. 2008;335:98–105. doi: 10.1016/j.jim.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Morgan AC., Jr alpha 2-macroglobulin production by cultured human melanoma cells. J.Natl.Cancer Inst. 1984;72:557–562. [PubMed] [Google Scholar]
- 24.Rabinowits G, et al. Exosomal microRNA: a diagnostic marker for lung cancer. Clin.Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 25.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat.Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JA, et al. MHC independent anti-tumor immune responses induced by Hsp70- enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275:256–265. doi: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Escudier B, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J.Transl.Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai S, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol.Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clayton A, et al. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J.Immunol.Methods. 2001;247:163–174. doi: 10.1016/s0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- 30.Logozzi M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS.One. 2009;4:e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zwaal RF, van Deenen LL. Interactions between proteins and lipids from human red cell membranes. Chem.Phys.Lipids. 1970;4:311–322. doi: 10.1016/0009-3084(70)90031-9. [DOI] [PubMed] [Google Scholar]
- 32.Bizik J, et al. Human tumor cells synthesize and secrete alpha-2-macroglobulin in vitro. Int.J.Cancer. 1986;37:81–88. doi: 10.1002/ijc.2910370114. [DOI] [PubMed] [Google Scholar]
- 33.Millar HR, et al. An evaluation of the heat precipitation method for plasma fibrinogen estimation. J.Clin.Pathol. 1971;24:827–830. doi: 10.1136/jcp.24.9.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marx G, et al. Heat denaturation of fibrinogen to develop a biomedical matrix. J.Biomed.Mater.Res.B Appl.Biomater. 2008;84:49–57. doi: 10.1002/jbm.b.30842. [DOI] [PubMed] [Google Scholar]