Abstract
Somatic insertions/deletions in the calreticulin gene have recently been discovered to be causative alterations in myeloproliferative neoplasms. A combination of qualitative and quantitative allele-specific polymerase chain reaction, fragment-sizing, high resolution melting and Sanger-sequencing was applied for the detection of three driver mutations (in Janus kinase 2, calreticulin and myeloproliferative leukemia virus oncogene genes) in 289 cases of essential thrombocythemia and 99 cases of primary myelofibrosis. In essential thrombocythemia, 154 (53%) Janus kinase 2 V617F, 96 (33%) calreticulin, 9 (3%) myeloproliferative leukemia virus oncogene gene mutation-positive and 30 triple-negative (11%) cases were identified, while in primary myelofibrosis 56 (57%) Janus kinase 2 V617F, 25 (25%) calreticulin, 7 (7%) myeloproliferative leukemia virus oncogene gene mutation-positive and 11 (11%) triple-negative cases were identified. Patients positive for the calreticulin mutation were younger and had higher platelet counts compared to Janus kinase 2 mutation-positive counterparts. Calreticulin mutation-positive patients with essential thrombocythemia showed a lower risk of developing venous thrombosis, but no difference in overall survival. Calreticulin mutation-positive patients with primary myelofibrosis had a better overall survival compared to that of the Janus kinase 2 mutation-positive (P=0.04) or triple-negative cases (P=0.01). Type 2 calreticulin mutation occurred more frequently in essential thrombocythemia than in primary myelofibrosis (P=0.049). In essential thrombocythemia, the calreticulin mutational load was higher than the Janus kinase 2 mutational load (P<0.001), and increased gradually in advanced stages. Calreticulin mutational load influenced blood counts even at the time point of diagnosis in essential thrombocythemia. We confirm that calreticulin mutation is associated with distinct clinical characteristics and explored relationships between mutation type, load and clinical outcome.
Introduction
The characterization of the genetic background of BCR-ABL1-negative chronic myeloproliferative neoplasms (MPN) was greatly advanced by the discovery that the V617F mutation of Janus kinase 2 (JAK2) is very common in the three classic MPN, occurring in 90–95% of cases of polycythemia vera (PV), and in 40–60% of cases of essential thrombocythemia (ET) and primary myelofibrosis (PMF).1–4 This led to the inclusion of V617F mutation screening in the diagnostic criteria for MPN.5 JAK2 exon 12 mutations occur in rare cases of V617F-negative PV allowing a close to perfect coverage by specific genetic alterations in PV.6,7 On the other hand, clinicians continue to face challenges during the diagnosis of JAK2 mutation (JAK2mut)-negative ET and PMF. Moving the field very close to full coverage, in two parallel seminal discoveries, Klampfl et al.8 and Nangalia et al.9 recently described somatic, recurrent insertions/deletions exclusively affecting exon 9 of the calreticulin (CALR) gene. Affecting the same driver pathway, CALR mutations were mutually exclusive with JAK2 V617F or myeloproliferative leukemia virus oncogene gene (MPL) mutations. In a plethora of subsequent studies published within 3 months, the initial findings were confirmed and extended focusing on the clinical correlates.10–13 Several additional studies described low frequencies of CALR mutations in different MPN-related diseases, but not in other hematologic diseases.8,9,14–18
As a result of the discovery of CALR mutations, definite molecular diagnostics have become available for 75–90% of clonal MPN. However, for the introduction of CALR mutation screening into routine clinical practice, it is essential to characterize the potential effects of CALR mutations on disease phenotype and progression in several independent cohorts. The aim of our study was to apply a complex array of molecular techniques to identify driver mutations. We aimed to confirm previous associations between the presence of acquired genetic alterations and clinical characteristics in a large, independent cohort of patients with MPN. An additional purpose was to systematically analyze the roles of particular CALR mutation types and load.
Methods
Subjects
Our study population consisted of 603 patients with BCR-ABL1-negative MPN (260 males, 343 females; median age: 60, range: 10–94 years) diagnosed between 1974 and 2013, as an extension of those published earlier.19,20 According to World Health Organization 2008 criteria, 215 patients had JAK2 V617F positive PV, 289 had ET and 99 had PMF. Laboratory parameters and clinical features at the time of diagnosis were collected retrospectively. Coagulation complications and myelofibrotic or acute leukemic transformation were recorded if they were present at diagnosis or occurred during the follow-up. Coagulation complications included venous thrombotic events (deep vein thrombosis, pulmonary embolism, splanchnic or cerebral venous thrombosis), arterial thrombosis (transient ischemic attack, ischemic stroke, acute myocardial infarction, or peripheral arterial vascular complications) and hemorrhagic problems (gastrointestinal bleeding, hemorrhagic stroke, hematuria, severe bleeding during surgery or dental procedures). The median follow-up was 5.7 years (range, 0–40 years). All participants signed informed consent. The study was approved by the Hungarian National Ethics Committee.
Detection of driver somatic mutations
All analyses were performed using genomic DNA isolated from peripheral blood or bone marrow. In a subset of patients, sampling and diagnostic ascertainment occurred within 1 year. In some analyses, these cases were considered separately as the ones best reflecting the patients’ condition at diagnosis.
All MPN patients were screened for JAK2 V617F (c.1849G>T) by allele-specific polymerase chain reaction (PCR).1 In JAK2mut cases, real-time quantitative PCR was performed to determine the V617F load.21 The JAK2mut load was calculated as follows: JAK2mut/(JAK2mut+JAK2wild-type).
JAK2mut-negative ET and PMF patients were screened for CALR mutations by high resolution sizing of fluorescence-labeled PCR products by capillary electrophoresis (fragment analysis).8 In CALRmut cases, the precise mutation was identified by Sanger-sequencing.8 To determine the mutant load, the ratio of peak heights was calculated using an analogous formula: CALRmut/(CALRmut+CALRwild-type). Since fragment analysis was a semi-quantitative approach and the final amounts of the PCR products were influenced by the preferential amplification of shorter amplicons, we calculated the load after PCR with 25 cycles, which was a reduced cycling condition compared to the screening condition (35 cycles).
In JAK2mut and CALRmut negative ET and PMF patients, screening for MPL S505N and W515 codon mutations was performed by high resolution melting analysis.22 In cases that were positive in this screening analysis, the exact type of MPL mutation was determined by allele-specific PCR and sequencing.23
Statistics
Continuous variables are presented as medians with 25th and 75th percentiles. Mann-Whitney or Kruskal-Wallis tests were used to compare continuous variables, while χ2 or Fisher exact tests were used for dichotomous variables. A log-rank test was performed to compare overall survival between subgroups according to driver mutations. In the case of hematopoietic stem cell transplantation (n=13), the follow-up period was terminated at the date of the transplant. For multivariate analysis, age was considered in a Cox proportional hazards model beside driver mutations. Hazard ratios (HR) and 95% confidence interval (CI) values were computed. The analyses were conducted using the SPSS (version 20.0) software package.
Results
Distribution of different types of driver mutations and comparisons of clinical parameters in subgroups according to driver mutation status
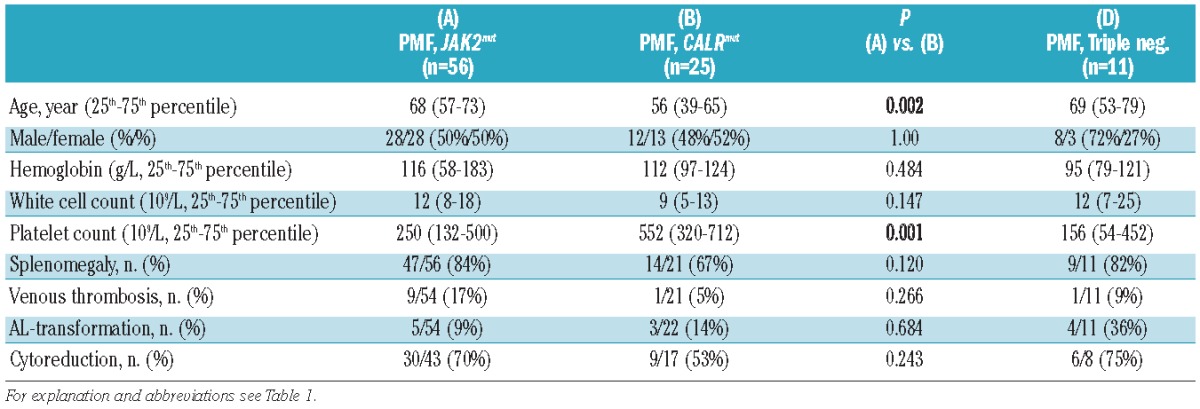
Only JAK2 V617F positive (JAK2mut) PV patients (n=215) were included in this study. In patients with PV, qualitative and quantitative JAK2 V617F tests, but no CALR and MPL molecular tests, were performed. For ET, the distribution of driver mutations was as follows: JAK2mut: 154/289 (53%), CALRmut: 96/289 (33%), MPLmut: 9/289 (3%), triple-negative: 30/289 (11%) cases (Table 1). For PMF, the corresponding figures were: JAK2mut: 56/99 (57%), CALRmut: 25/99 (25%), MPLmut: 7/99 (7%), triple-negative: 11/99 (11%) cases (Table 2).
Table 1.
Clinical and laboratory characteristics of PV and ET patients according to the presence of a driver somatic mutation.
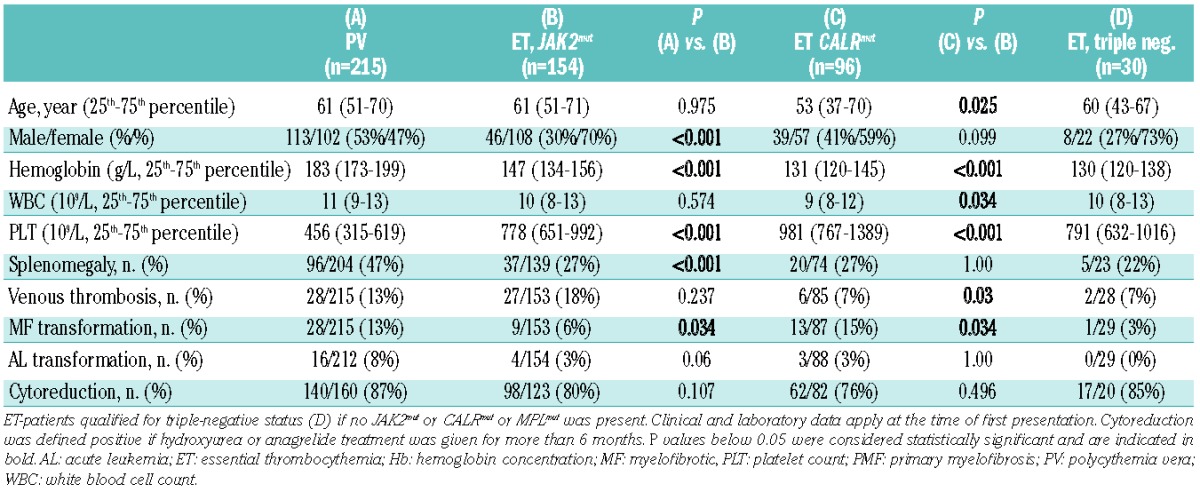
Table 2.
Clinical and laboratory characteristics of PMF patients according to the presence of a driver somatic mutation.
The patients’ clinical and laboratory parameters were systematically compared according to diagnosis and driver mutation. Given the low number of cases, patients with MPL mutation were omitted from all subsequent analyses. Compared to the JAK2mut PV cohort, the JAK2mut ET cohort contained more females (P<0.001), had lower hemoglobin levels (P<0.001), higher platelet counts (P<0.001), less frequent splenomegaly (P<0.001) and less frequent myelofibrotic transformation (P=0.034) (Table 1). Compared to the JAK2mut ET cohort, in the CALRmut ET patients we found a tendency toward less pronounced female predominance (P=0.099), younger age at diagnosis (P=0.025), lower hemoglobin levels (P<0.001), lower white blood cell counts (P=0.034) and higher platelet counts (P<0.001). Coagulation complications (venous and arterial thromboses, plus hemorrhages, taken together) were more frequent in JAK2mut ET patients (36%, 55/153) than in CALRmut ET patients (18%, 15/85; P=0.003). Venous thrombosis was more frequent in JAK2mut ET than in CALRmut ET (P=0.03). Arterial thrombosis occurred in 14% (22/153) of JAK2mut and in 9% (8/85) of CALRmut ET (P=0.3), and hemorrhage in 9% (14/153) of JAK2mut and in 5% (4/85) of CALRmut ET (P=0.3). Myelofibrotic transformation occurred more frequently in the CALRmut cohort (P=0.03). We did not find any significant differences comparing triple-negative ET patients to other ET subgroups: the sample size (n=30) was, however, small.
Similar analyses in the PMF cohort (Table 2) showed younger age at presentation (P=0.002) and higher platelet counts (P=0.001) in the CALRmut subgroup than in the JAK2mut PMF subgroup. Other variables were not different, nor were any of the characteristics of the triple-negative PMF patients except for an increased rate of acute leukemic transformation in triple-negative cases (36% versus 9% in JAK2mut, P=0.038 or versus 14% in CALRmut, P=0.19).
Regarding outcome parameters, overall survival was analyzed, initially by a univariate Kaplan-Meier approach, in patients with ET (Figure 1A) and PMF (Figure 1B) stratified according to different driver mutations. Out of the 289 ET patients, 261 cases had appropriate follow-up information [JAK2mut (n=150), CALRmut (n=85) and triple-negative (n=26) subgroups]. As shown in Figure 1A, no differences were detected in overall survival by univariate analyses (P=0.846).
Figure 1.
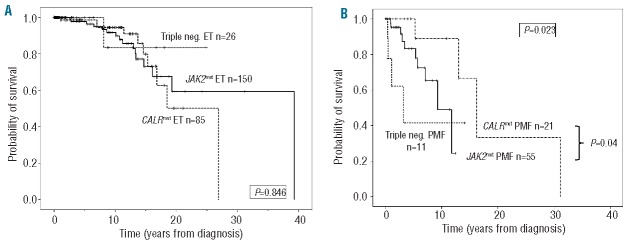
Kaplan-Meier analysis of overall survival in patients with ET (panel A, n=261) and PMF (panel B, n=87) according to the presence of different driver mutations. Patients qualified for triple-negative status if JAK2mut and CALRmut and MPLmut were all absent. For a subset of patients (ET: n=2, PMF: n=11) treated by hematopoietic stem cell transplantation, the follow-up was censored at the date of this intervention. In ET, univariate analyses resulted in an overall P value of 0.846 (A). In PMF, the same comparison gave a P value of 0.023 (B). Upon pairwise univariate comparisons, the CALRmut subgroup showed significantly better survival compared to JAK2mut (P=0.04) and triple-negative (P=0.01) PMF patients while JAK2mut patients showed only a tendency towards better overall survival compared to triple-negative patients (P=0.076). ET: essential thrombocythemia; PMF: primary myelofibrosis.
Among PMF patients (n=87), the subgroups were as follows: JAK2mut (n=55), CALRmut (n=21) and triple-negative (n=11). In contrast to ET, the overall comparison resulted in P=0.023 (Figure 1B) with the best overall survival in the CALRmut subgroup and the worst in the triple-negative subgroup. This was further confirmed by pairwise univariate comparisons, namely CALRmut versus JAK2mut (P=0.04) and CALRmut versus triple-negative (P=0.01). The JAK2mut PMF patients showed only a tendency towards having a better overall survival than the triple-negative patients (P=0.076). To further analyze the potential effect of driver mutations on survival, we utilized a Cox proportional hazard model choosing the triple-negative PMF subgroup as the reference. In this model, age was a factor that significantly affected survival (HR 1.082, 95% CI: 1.023–1.144; P=0.006), while the presence of a driver mutation showed an overall tendency (P=0.059). Performing pairwise comparisons, the CALRmut subgroup was characterized by a HR=0.131 (95% CI: 0.023–0.739; P=0.021) indicating a significantly better survival than that of triple-negative patients. Comparing the CALRmut patients to the JAK2mut subgroup, only a tendency towards better survival was observed in the presence of CALR exon 9 mutation (HR=0.159, 95% CI: 0.018–1.391; P=0.097).
Subgroup analyses in patients with CALRmut according to type of mutation
Following fluorescence PCR and fragment analyses by capillary electrophoresis, the presence of CALR exon 9 mutation was confirmed by Sanger-sequencing. In the entire cohort of 121 CALR-positive cases, we found 64 (53%) with a type 1 mutation (52 base pair deletion, c.1092_1143del), 34 (28%) with a type 2 mutation (5 base pair insertion, c.1154_1155insTTGTC) and 23 (19%) with other types of mutations. These other mutations comprised the following types: 3 (c.1095_1140del), 4 (c.1102_1135del), 5 (c.1091_1142del), 19 (c.1110_1140del), 22 (c.1120_1123del), 24 (c.1120_1138del), 29 (c.1135_1152delinsCCTCCTCTTTGTCT), 33 (c.1154_1155insATGTC), 34 (c.1154_delins CTTGTC), 35 (c.1154delinsTTTGTC) and potentially novel variants (n=8). The occurrence of these variants according to diagnosis was as follows: ET: type 1: 48 (50%), type 2: 31 (32%) and other: 17 (18%); PMF: type 1: 16 (64%), type 2: 3 (12%) and other: 6 (24%). Comparing patients with ET and PMF and only considering types 1 and 2, we found a tendency to a different distribution with 48 type 1 (61%) and 31 type 2 (39%) mutations in patients with EF versus 16 type 1 (84%) and 3 type 2 (16%) in patients with PMF (P=0.064). An increased frequency of type 2 mutations (versus non-type 2 mutations) was observed in ET (32%) compared to PMF (12%, P=0.049, Online Supplementary Table S1).
Next, we compared demographic and clinical parameters within patients with CALRmut ET according to mutation types. We found a tendency towards older age among type 2 carriers compared to type 1 carriers (median age at diagnosis: 59 years versus 51 years, P=0.06) or compared to non-type 2 carriers (median age at diagnosis: 59 years versus 51 years, P=0.09). Similarly, platelet count at diagnosis tended to be higher in the subgroup of type 2 mutation carriers than in patients with the type 1 mutation: 1237×109/L (25th–75th percentile: 884–1472) versus 946 ×109/L (25th–75th percentile: 764–1280) (P=0.081); or compared to those with non-type 2 mutation: 865×109/L (25th–75th percentile: 755–1218) (P=0.041). The frequency of cytoreductive therapy was higher among type 2 versus type 1 ET patients (89% versus 67% P=0.05). No further differences in any demographic or clinical characteristics (including overall survival) were found upon comparing the two ET subgroups with different types of mutations (Online Supplementary Table S2). Similar analyses were not feasible for the PMF cohort because of the low number (n=3) of patients with the type 2 CALR mutation.
Subgroup analyses in patients with a positive driver mutation (JAK2mut or CALRmut) according to mutational load
A systematic comparison was performed between MPN subgroups in relation to the quantity of driver mutation (Figure 2). The JAK2 V617F load was determined by real-time quantitative allele-specific PCR using the TaqMan detection system,21 while the semi-quantitative estimation of the CALR mutation was achieved by high resolution sizing of fluorescence-labeled PCR products.8 The mutational load in JAK2 V617F and CALR mutation-positive patients was calculated with a similar formula i.e. the quantity of mutant was expressed as a percentage fraction of total gene copies allowing the comparison of loads across MPN subgroups with different mutations. Performing pairwise comparisons, we made the following observations (Figure 2). (i) Neither JAK2mut, nor CALRmut ET patients without myelofibrotic transformation at the sampling time showed significantly different driver mutational loads comparing the subgroup of patients whose samples were taken within 1 year of diagnosis and the subgroup of patients whose samples were taken at a later time point. On the other hand, the two PV-subgroups (44 patients with sampling within 1 year of diagnosis and 140 patients with sampling at later time points but without myelofibrotic transformation at this later time point) showed a tendency toward increasing allele burden (P=0.066). (ii) JAK2 loads increased gradually in parallel with the appearance of more advanced stages of MPN (JAK2mut ET versus PV: P<0.001, PV versus post-PV myelofibrosis: P<0.001). (iii) Within patients with ET, the CALR load was significantly higher than the JAK2 load (P<0.001). (iv) CALR load showed a steady, significant, but less steep increase corresponding to the appearance of more advanced MPN stages (CALRmut ET versus CALRmut post-ET MF: P=0.01; CALRmut ET versus CALRmut PMF: P<0.001). (v) In contrast to JAK2mut load, the CALRmut load only rarely exceeded 50% (in 11 cases between 51–75% and in 1 case above 75%).
Figure 2.
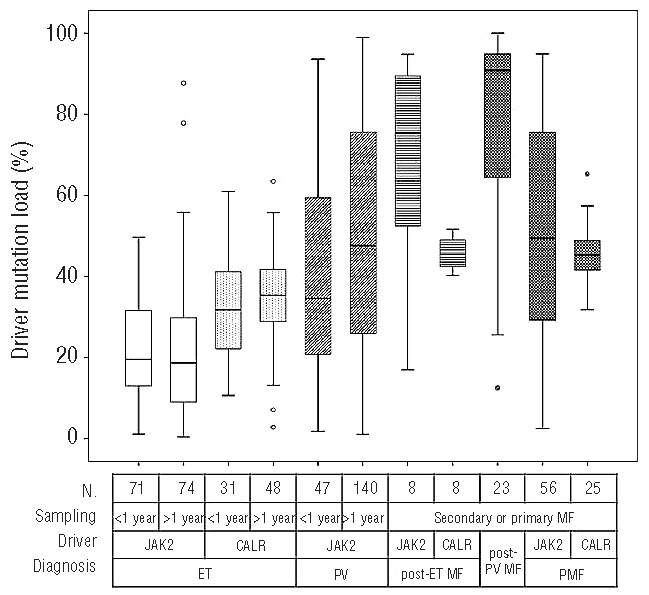
Comparisons of relative quantities of driver mutations (JAK2 V617F or CALR) in different MPN subgroups according to diagnosis and the relation of time of sampling and time of diagnosis. The first row under the x axis shows the number of patients (n) in the respective subgroups. If no primary or secondary myelofibrosis was present at the time of sampling, sampling within 1 year of diagnosis qualified, as seen in the second lines, as “<1 year”; all other situations were allocated into the “>1 year” subgroup. If primary or secondary myelofibrosis was present at the time of sampling, no such subgrouping was performed (“secondary or primary MF”). Quantification of the respective driver mutation was performed by real-time quantitative allele-specific PCR (JAK2 V617F) or fragment analysis (CALR exon 9). Pairwise comparisons were performed with the Mann-Whitney test. ET: essential thrombocythemia; PMF: primary myelofibrosis; MF: myelofibrosis; PV: polycythemia vera.
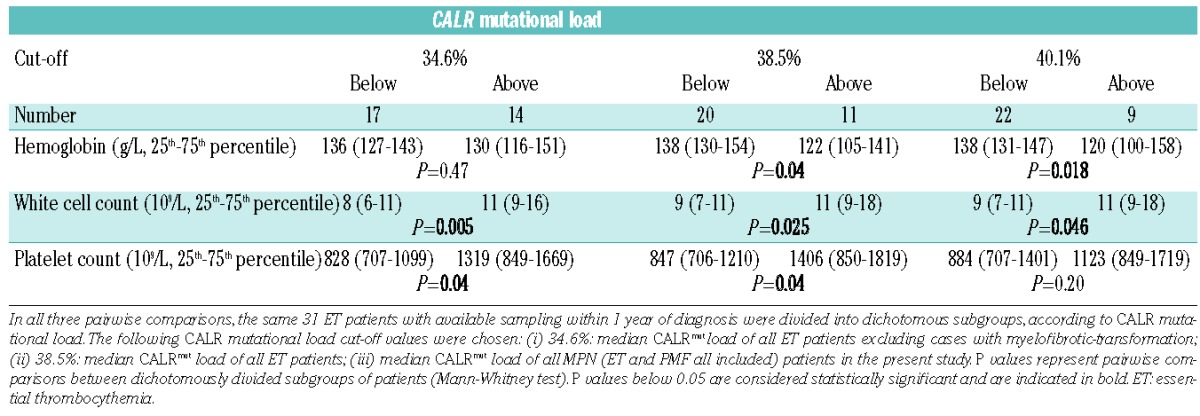
To investigate the potential effects of CALR mutational load, we divided CALRmut ET patients with samples available within 1 year after diagnosis (n=31) into two subgroups according to CALRmut load, with the cut-off value being 38.5%, which was the median CALR mutational load of all patients with ET in the study (n=20 below the cut off and n=11 above the cut off; Table 3). White blood cell counts (9 versus 11×109/L, P=0.025) and platelet counts (848 versus 1406×109/L, P=0.04) were lower among patients with low CALRmut load, while hemoglobin concentration was higher in the same comparison (138 versus 122 g/dL, P=0.04). Applying different cut-off values to discriminate between groups with lower and higher CALRmut burden, white blood cell counts were always lower in subgroups with lower CALRmut burden, although the differences shrank between the lower and the higher CALRmut burden subgroups applying higher cut-offs. While the differences in hemoglobin levels between patients with lower and higher CALRmut load were only detectable above the cut-off value of 38.5% or higher, the differences in platelet counts diminished above this cut-off. We observed higher CALRmut burden in type 1 (n=14, 41%) compared to type 2 ET (n=15, 27%, P=0.023). However, this difference may have been influenced by the preferential amplification of the shorter fragment in the presence of the type 1 mutant (52 base pair deletion) during PCR, thus this observation needs to be confirmed with an alternative technique of quantification.
Table 3.
Laboratory characteristics of ET patients according to the CALR mutational load.
Discussion
We set up sequential application of different molecular techniques to identify the driver mutations in a large cohort of MPN patients, noting that JAK2 V617F, CALR and MPL mutations were described as mutually exclusive driver mutations in the first studies.8,9 Concurrent JAK2 and CALR mutations were reported in only two individuals in subsequent studies.13,14 Confirming earlier observations in different MPN cohorts,8–10,13 we found a similar frequency of CALR mutations in an independent group of MPN patients, with the frequency of these mutations being high (121/162, 75%) among JAK2 V617F and MPL-negative ET patients, thus leaving only a small cohort of triple-negative patients lacking a disease-causing genetic marker.
In our previous study comparing the clinical characteristics of JAK2mut and JAK2neg MPN patients (n=328),19 we already noted a female predominance, older age at presentation, higher hemoglobin values and a higher incidence of coagulation complications (thrombotic and hemorrhagic) in JAK2 V617F-positive MPN patients versus V617F-negative counterparts (including CALRmut patients). The identification of the CALR mutation allowed a more straight forward comparison of MPN patients with a homogeneous genetic background. Our present data, showing distinct clinical characteristics of CALRmut ET subgroups in comparison with JAK2mut cohorts, confirmed previous observations regarding younger age, sex distribution with less prominent female abundance (only in the ET subgroup), lower hemoglobin concentration, lower white blood cell counts (not significant in our PMF cohort), and higher platelet counts.8–12
Considering venous thrombosis and other coagulation complications, our observations confirmed previous findings describing a lower risk of thrombosis in patients with CALRmut ET than in those with JAK2mut ET.8 In their extended cohort, Rumi et al.12 found that the rates of thrombosis at diagnosis in the CALRmut and JAK2mut groups were 2.8% and 7.1%, respectively (P=0.059), and observed a reduced cumulative incidence of thrombosis (25 versus 10 events per 1000 person-years) in CALRmut patients (P=0.001). In an independent series of patients with ET, Rotunno et al. found that the incidence of thrombosis (in the 2 years preceding diagnosis and during follow-up, combined) was 13.5% in their CALRmut cohort compared to 30.1% among JAK2mut patients (P=0.011).
With respect to transformation of ET to myelofibrosis, we observed a more than 2-fold higher transformation rate among CALRmut patients (15%) compared to JAK2mut patients (6%, P=0.034), the rate in the former being similar to that in the cohort of patients with PV (13%). Nangalia et al. also found an increased risk of transformation to myelofibrosis (19% in CALRmut versus 2% in JAK2mut patients; P=0.03) in a smaller cohort of patients with ET.9 In a large study, the incidence of myelofibrotic transformation was 7% (95% CI 3–13) in CALRmut versus 5% (95% CI 3–8) in JAK2mut ET versus 8% (95% CI 5–12) in JAK2mut PV, while the 15-year cumulative incidence of myelofibrotic transformation was 13.4% (CI 95% 5.4–25.2%) in CALRmut versus 8.4% (CI 95% 3.9–15.3%) in JAK2mut ET versus 13.6% (CI 95% 7.3–21.9%) in JAK2mut PV (P=not significant).12 In our cohort, the median follow-up tended to be longer in the CALRmut ET group (8 years in CALRmut versus 4 years in JAK2mut; P=0.09), which might have resulted in a higher frequency of myelofibrotic transformation.
Among patients with ET, we did not find differences in overall survival according to the presence of a specific driver mutation. In contrast, Klampfl et al. observed better survival in patients with CALRmut than in those with JAK2mut (P=0.04).8 Interestingly, analyzing a larger ET cohort with substantial overlap with that of Klampfl et al., in univariate analysis, Rumi et al. found only a trend (P=0.085) towards a better overall survival at 15 years for CALRmut patients compared to JAK2mut patients, and the trend disappeared when age was considered as a covariate.12 Similarly, Rotunno et al. did not find any difference in overall survival among patients with ET.11
As for patients with ET and in previous reports,8,13 younger age at disease onset and elevated platelet counts were found to characterize our CALRmut PMF cohort. Our observations in PMF were in good agreement with previous reports,8,13 indicating a significant beneficial effect of the presence of CALRmut, compared to JAK2mut, on overall survival. In our dataset, the differences were further substantiated by pairwise comparisons in a multivariate (with age added as a covariate) Cox model indicating a trend towards better survival in CALRmut patients compared to JAK2mut ones and a significantly better survival between CALRmut and triple-negative PMF patients. In addition, the decreased leukemia-free survival in triple-negative PMF patients reported by Tefferi et al.13 was in line with the increased rate of acute leukemic transformation in our triple-negative PMF cases (Table 2). Recently, a set of gene mutations were described as detrimental in PMF. The presence of any “high molecular risk” mutations affecting ASXL1, EZH2, SRSF2, and IDH1/2 genes predicted shorter overall survival and increased risk of leukemic transformation. A CALR mutation proved to be an independent protective factor for survival in PMF.24 ASXL1 mutations, as the most frequent detrimental mutations, were reported to occur with similar frequency in JAK2mut, CALRmut and triple-negative PMF patients;13 thus, in our PMF-cohort, the survival benefit observed in the group of CALRmut patients was unlikely to result from an uneven distribution of ASXL1 mutations. The presence of CALRmut in PMF may affect prognostication and should be incorporated in the scoring system, as recently suggested.25,26
Our data regarding the frequencies of type 1 and type 2 CALRmut in ET and in PMF were in good agreement with those of previous cohorts of patients with ET8–10,12 and PMF.8,13 The asymmetric distribution of different types of CALRmut in ET and PMF, i.e. the relatively lower frequency of type 2 CALRmut in PMF, was in line with data from Klampfl et al.8 They also observed a lower (21/105, 20%) relative proportion of type 2 mutant cases among PMF patients than among those with ET (74/195, 38%). The profound differences in the CALR protein structure may explain this asymmetric distribution and may imply slight differences in the pathogenic effects of the respective variants. The observed asymmetric distribution prompted us to systematically analyze clinical and outcome differences in ET patients according to CALRmut type, with the key findings being older age at diagnosis, higher platelet counts and more frequent use of cytoreductive therapy in patients with type 2 CALRmut compared to type 1 or non-type 2 CALRmut. To our knowledge, similar data in ET have not been published yet. In line with our data in ET, very recently Tefferi et al.27 observed distinct characteristics in PMF in the context of CALR type 2 mutations and EZH2, IDH mutations, leukocytosis, and peripheral blast percentage. Univariate survival analysis suggested a less favorable outcome in the presence of type 2 CALR mutations.27 These differences may indicate a distinct pathomechanism related to insertion or deletion type CALR mutations.
The systematic analyses of CALRmut load in several MPN subgroups indicated higher CALRmut load compared to JAK2mut load in ET. In contrast to the JAK2mut load, the CALRmut load rarely exceeded 50%, indicating a decreased tendency of the CALRmut locus to undergo mitotic recombination. Uniparental disomy was reported to be less frequent at the CALR locus (19p13.3–p13.2) than at the JAK2 locus (9p24). Using an alternative quantitative PCR technique, we are in the process of addressing the observation that CALRmut load may be higher at diagnosis in type 1 CALRmut carriers than in type 2 carriers. Our results indicate that the mutational load of CALRmut resembled that of JAK2mut in that there was a clear trend towards increased load in parallel with the appearance of more severe phenotypes of MPN. Campbell et al.28 suggested that JAK2 V617F mutated ET, PV and post-ET/PV-myelofibrosis or PMF form a biological continuum, in which the different MPN phenotypes are determined by JAK2 V617F allele burden modified by other environmental and inherited factors. The increasing JAK2 V617F allele burden through ET-PV-myelofibrosis phenotypes was later confirmed in several studies,29–31 and also in a transgenic mouse model.32 Our study shows, for the first time, that hematologic laboratory parameters (white blood cell count, hemoglobin concentration, platelet count) were directly influenced by CALR mutational burden in ET patients with samples available within 1 year of diagnosis. Similarly to JAK2mut MPN, CALRmut ET and PMF also show overlapping clinical features, suggesting that the common molecular basis represents a biological continuum between CALRmut ET and CALRmut myelofibrosis, in which the clinical phenotype is modified by the actual CALR mutational load.12
In summary, analyzing a relatively large, independent cohort of MPN patients, we confirmed recent observations that the presence of CALR mutations is responsible for the development of a distinct clinical phenotype in both ET and PMF. These distinctive features manifest as different clinical characteristics at diagnosis, different patterns of complications as well as significant differences in overall survival. Our data supplement and extend previous observations regarding the distribution of various types of CALRmut and associations with different quantities of CALRmut. Our study shows, for the first time, that hematologic laboratory parameters are directly influenced by CALR mutational burden. The recent discovery of somatic CALR mutations not only improves the precision of noninvasive diagnostics of patients with MPN, but may influence prognosis and therapy.
Acknowledgments
The authors thank Csehné Bánhidi Klára, Haluska Brigitta, Horváth Csongorné, Mezibroczky Martina, Pfundt Antalné, and Petró Péterné for their technical assistance. This work was supported by grants from OTKA (K104903). HA is a recipient of the Janos Bolyai Research Scholarship from the Hungarian Academy of Sciences, and benefited from a scholarship for the Third Training School of MPN&MPNr-EuroNet (COST Action BM0902) in May 2012. HA and AT are members of MPN&MPNr-EuroNet.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61 [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8 [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90 [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97 [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A, Vainchenker W. Myeloproliferative neoplasms: molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol. 2011;29(5):573–82 [DOI] [PubMed] [Google Scholar]
- 6.Scott LM. The JAK2 exon 12 mutations: a comprehensive review. Am J Hematol. 2011;86(8):668–76 [DOI] [PubMed] [Google Scholar]
- 7.Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356(5):459–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–90 [DOI] [PubMed] [Google Scholar]
- 9.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi J, Nicolaou KA, Nicolaidou V, Koumas L, Mitsidou A, Pierides C, et al. Calreticulin gene exon 9 frameshift mutations in patients with thrombocytosis. Leukemia. 2014;28(5):1152–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123(10):1552–5 [DOI] [PubMed] [Google Scholar]
- 12.Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123(10):1544–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014. January 9. 10.1038/leu.2014.3 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Broseus J, Lippert E, Harutyunyan AS, Jeromin S, Zipperer E, Florensa L, et al. Low rate of calreticulin mutations in refractory anaemia with ring sideroblasts and marked thrombocytosis. Leukemia. 2014;28(6):1374–6 [DOI] [PubMed] [Google Scholar]
- 15.Hou HA, Kuo YY, Chou WC, Chen PH, Tien HF. Calreticulin mutation was rarely detected in patients with myelodysplastic syndrome. Leukemia. 2014. February 17. 10.1038/leu.2014.71 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Lasho TL, Elliott MA, Pardanani A, Tefferi A. CALR mutation studies in chronic neutrophilic leukemia. Am J Hematol. 2014;89(4):450. [DOI] [PubMed] [Google Scholar]
- 17.Maffioli M, Genoni A, Caramazza D, Mora B, Bussini A, Merli M, et al. Looking for CALR mutations in familial myeloproliferative neoplasms. Leukemia. 2014;28(6):1357–60 [DOI] [PubMed] [Google Scholar]
- 18.Patnaik MM, Belachew A, Finke C, Lasho TL, Hanson CA, Tefferi A. CALR mutations are infrequent in WHO-defined refractory anemia with ring sideroblasts. Leukemia. 2014;28(6):1370–1 [DOI] [PubMed] [Google Scholar]
- 19.Andrikovics H, Meggyesi N, Szilvasi A, Tamaska J, Halm G, Lueff S, et al. HFE C282Y mutation as a genetic modifier influencing disease susceptibility for chronic myeloproliferative disease. Cancer Epidemiol Biomarkers Prev. 2009;18(3):929–34 [DOI] [PubMed] [Google Scholar]
- 20.Andrikovics H, Nahajevszky S, Koszarska M, Meggyesi N, Bors A, Halm G, et al. JAK2 46/1 haplotype analysis in myeloproliferative neoplasms and acute myeloid leukemia. Leukemia. 2010;24(10):1809–13 [DOI] [PubMed] [Google Scholar]
- 21.Larsen TS, Christensen JH, Hasselbalch HC, Pallisgaard N. The JAK2 V617F mutation involves B- and T-lymphocyte lineages in a subgroup of patients with Philadelphia-chromosome negative chronic myeloproliferative disorders. Br J Haematol. 2007;136(5):745–51 [DOI] [PubMed] [Google Scholar]
- 22.Boyd EM, Bench AJ, Goday-Fernandez A, Anand S, Vaghela KJ, Beer P, et al. Clinical utility of routine MPL exon 10 analysis in the diagnosis of essential thrombocythaemia and primary myelofibrosis. Br J Haematol. 2010;149(2):250–7 [DOI] [PubMed] [Google Scholar]
- 23.Bergamaschi GM, Primignani M, Barosi G, Fabris FM, Villani L, Reati R, et al. MPL and JAK2 exon 12 mutations in patients with the Budd-Chiari syndrome or extrahepatic portal vein obstruction. Blood. 2008;111(8):4418. [DOI] [PubMed] [Google Scholar]
- 24.Guglielmelli P, Lasho TL, Rotunno G, Score J, Mannarelli C, Pancrazzi A, et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: an international study of 797 patients. Leukemia. 2014. February 19. 10.1038/leu.2014.76 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Guglielmelli P, Nangalia J, Green AR, Vannucchi AM. CALR mutations in myeloproliferative neoplasms: hidden behind the reticulum. Am J Hematol. 2014;89(5):453–6 [DOI] [PubMed] [Google Scholar]
- 26.Tefferi A, Guglielmelli P, Lasho TL, Rotunno G, Finke C, Mannarelli C, et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014;89(5):453–6 [DOI] [PubMed] [Google Scholar]
- 27.Tefferi A, Lasho TL, Finke C, Belachew AA, Wassie EA, Ketterling RP, et al. Type 1 vs type 2 calreticulin mutations in primary myelofibrosis: differences in phenotype and prognostic impact. Leukemia. 2014. February 26. 10.1038/leu.2014.83 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366(9501):1945–53 [DOI] [PubMed] [Google Scholar]
- 29.Antonioli E, Guglielmelli P, Poli G, Bogani C, Pancrazzi A, Longo G, et al. Influence of JAK2V617F allele burden on phenotype in essential thrombocythemia. Haematologica. 2008;93(1):41–8 [DOI] [PubMed] [Google Scholar]
- 30.Passamonti F, Rumi E, Pietra D, Della Porta MG, Boveri E, Pascutto C, et al. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107(9):3676–82 [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Xu Z, Liu L, Gale RP, Cross NC, Jones AV, et al. JAK2V617F allele burden, JAK2 46/1 haplotype and clinical features of Chinese with myeloproliferative neoplasms. Leukemia. 2014;27(8):1763–7 [DOI] [PubMed] [Google Scholar]
- 32.Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111(8):3931–40 [DOI] [PubMed] [Google Scholar]


