Abstract
We report on posttransplant relapsed pediatric patients with B-precursor acute lymphoblastic leukemia with no further standard of care therapy who were treated with the T-cell engaging CD19/CD3-bispecific single-chain antibody construct blinatumomab on a compassionate use basis. Blast load was assessed prior to, during and after blinatumomab cycle using flow cytometry to detect minimal residual disease, quantitative polymerase chain reaction for rearrangements of the immunoglobulin or T-cell receptor genes, and bcr/abl mutation detection in one patient with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blinatumomab was administered as a 4-week continuous intravenous infusion at a dosage of 5 or 15 μg/m2/day. Nine patients received a total of 18 cycles. Four patients achieved complete remission after the first cycle of treatment; 2 patients showed a complete remission from the second cycle after previous reduction of blast load by chemotherapy. Three patients did not respond, of whom one patient proceeded to a second cycle without additional chemotherapy and again did not respond. Four patients were successfully retransplanted in molecular remission from haploidentical donors. After a median follow up of 398 days, the probability of hematologic event-free survival is 30%. Major toxicities were grade 3 seizures in one patient and grade 3 cytokine release syndrome in 2 patients. Blinatumomab can induce molecular remission in pediatric patients with posttransplant relapsed B-precursor acute lymphoblastic leukemia and facilitate subsequent allogeneic hematopoietic stem cell transplantation from haploidentical donor with subsequent long-term leukemia-free survival.
Introduction
Patients with relapsed B-lineage acute lymphoblastic leukemia (ALL) after allogeneic stem cell transplantation remain a therapeutical challenge.1 For these patients, subsequent hematopoietic stem cell transplantation (HSCT) offers the only curative treatment.1,2 Despite several advances over the last decade which led to significantly reduced transplant-related mortality, relapse rates are still high and represent the major cause of death in these patient cohorts.3–6
Level of pre-transplant minimal residual disease (MRD) has been shown to be an important adverse prognostic parameter for estimating the risk of relapse; MRD, therefore, influences long-term event-free survival after allogeneic stem cell transplantation.7–12 In particular, patients who relapsed posttransplant have an extremely poor prognosis and need to achieve another hematologic remission or an even more advantageous very low or negative MRD level before proceeding to a subsequent SCT.6 Additional chemotherapy will often be of limited benefit and may be associated with high toxicity.7,8 Moreover, with duration and progression of relapsed ALL, treatment faces multi-drug resistance which constitutes an unsolved problem.13–15 Therefore, new therapeutics with known but also alternative mode of action and lower toxicity profile are necessary.16–19 The novel class of bispecific single-chain antibody construct (BiTE) blinatumomab is currently under investigation. Blinatumomab recruits and activates T cells through CD3 of the T-cell receptor (TCR) complex for redirected lysis of malignant cells and non-malignant cells expressing CD19. Thus, it leads to close targeted cell-cell contact enabling the formation of cytolytic synapses which is the prerequisite for leukemic cell lysis.20–22 Pediatric B-precursor ALL blasts show a stable and sufficient expression of CD19 to make it a good target for immunotherapeutic approaches.23–28 Furthermore, an excellent standard of detection of MRD levels can be achieved with high specificity and sensitivity. Multicolor flow cytometry enables complex patient-specific aberrant immunophenotyping of the blasts with a regular detection level of 1×10−4. Established MRD monitoring by real-time quantitative polymerase chain reaction (PCR) or reverse transcriptase PCR reaches an even lower detection threshold.11,29 In a phase I study in patients with relapsed non-Hodgkin lymphoma, continuous infusion of blinatumomab showed dosage-dependent induction of remission,30 and in a recent phase II trial in adult B-lineage ALL, blinatumomab was able to induce and consolidate hematologic remission in 80% of patients with chemorefractory MRD.31 Given these results, we used this agent in pediatric patients with refractory B-lineage ALL at extremely high risk. All patients had had a first or subsequent posttransplant relapse and had not demonstrated a sufficient response to previous applied chemotherapy. Therefore, blinatumomab was administered on a compassionate use basis in order to induce another remission and facilitate a subsequent HSCT from a haploidentical family donor.
Methods
Pediatric patients with posttransplant relapsed B-precursor acute lymphoblastic leukemia who consistently expressed CD19 were considered for blinatumomab treatment. Relapse was defined as more than 5% blasts in bone marrow. Multiparametric flow cytometry and quantitative PCR were used to detect and assess MRD.
The treatment approach with blinatumomab in the reported patient cohort was not a clinical study but a treatment for compassionate use. Data concerning Patients 2 and 3 have been previously published by Handgretinger et al.32 All reported patients were treated at our institution between August 2008 and December 2011, and were registered with the local authorities of the University Children’s Hospital, Tuebingen, Germany. The treatment for compassionate use in all 9 reported patients was conducted according to German ethical codes and regulations with the involvement of the Independent Ethics Committee of the University Children’s Hospital, Tuebingen, Germany, and in accordance with the provisions of the Declaration of Helsinki. All patients and their representative in law gave informed consent before blinatumomab was started.
None of the patients in the study received antileukemic therapeutics during blinatumomab administration.
Patients received blinatumomab as continuous intravenous infusion (central venous catheter) at an initial dose of 5 or 15 μg/m2/day for 28 days followed by a treatment-free interval of 7–14 days, adapted from an adult schedule.30,31 This was defined as 1 cycle of treatment. In case of non-response, a dose escalation with increase of the initial dose to 15 μg/m2/day was carried out or another chemotherapy cycle was added with or without subsequent stem cell boost before blinatumomab treatment was continued. Responders were to receive at least one additional cycle to consolidate remission status. Patients who achieved molecular remission proceeded to a subsequent transplantation from a haploidentical stem cell donor. Side-effects were assessed according to common terminology criteria for adverse events (CTCAE) v.4.
Monitoring of bone marrow and MRD detection
Microscopic examination of bone marrow aspirates and peripheral blood was assessed by Zeiss Axiolab microscope. Aberrant immunophenotype detected by multiparameter flow cytometry BD FACS LSR II,33 as well as individual clonal immunoglobulin and T-cell receptor gene rearrangements12,34 and bcr/abl translocation fusion genes measured by real-time quantitative PCR were used as MRD markers. MRD levels of all patients were assessed prior to, during and after blinatumomab administration. Flow cytometry-based MRD detection was performed as previously published.11 MRD levels below 1×10−4 were defined as negative.
Flow cytometry
Flow cytometry monitoring was performed with regard to blast count and T-cell count (CD3+CD4+ and CD3+CD8+), as well as NK-cell count (CD56+CD16+CD3−).
Statistical methods
GraphPad PRISM software v.5.04 was used for statistical analysis. Kaplan-Meier estimates for event-free survival probability and overall survival probability were calculated from the date of first blinatumomab administration to the date of relapse or the date of last follow up in continuous complete remission. In cases of non-response, the number of days until relapse is “0”.
Details about clinical monitoring, acquisition of laboratory parameters and additional statistical analyses are available in the Online Supplementary Appendix.
Results
Outcome
Patients’ characteristics are shown in Table 1. Six patients had received one previous allogeneic HSCT and 3 patients had had 2 or more previous allogeneic HSCTs; donors were matched unrelated, matched related and mismatched haploidentical related. Four patients had had no prior chemotherapy before blinatumomab was initiated. Five patients had received chemotherapy and one patient additional central nervous system (CNS) irradiation prior to the first blinatumomab cycle of treatment.
Table 1.
Patients’ characteristics.
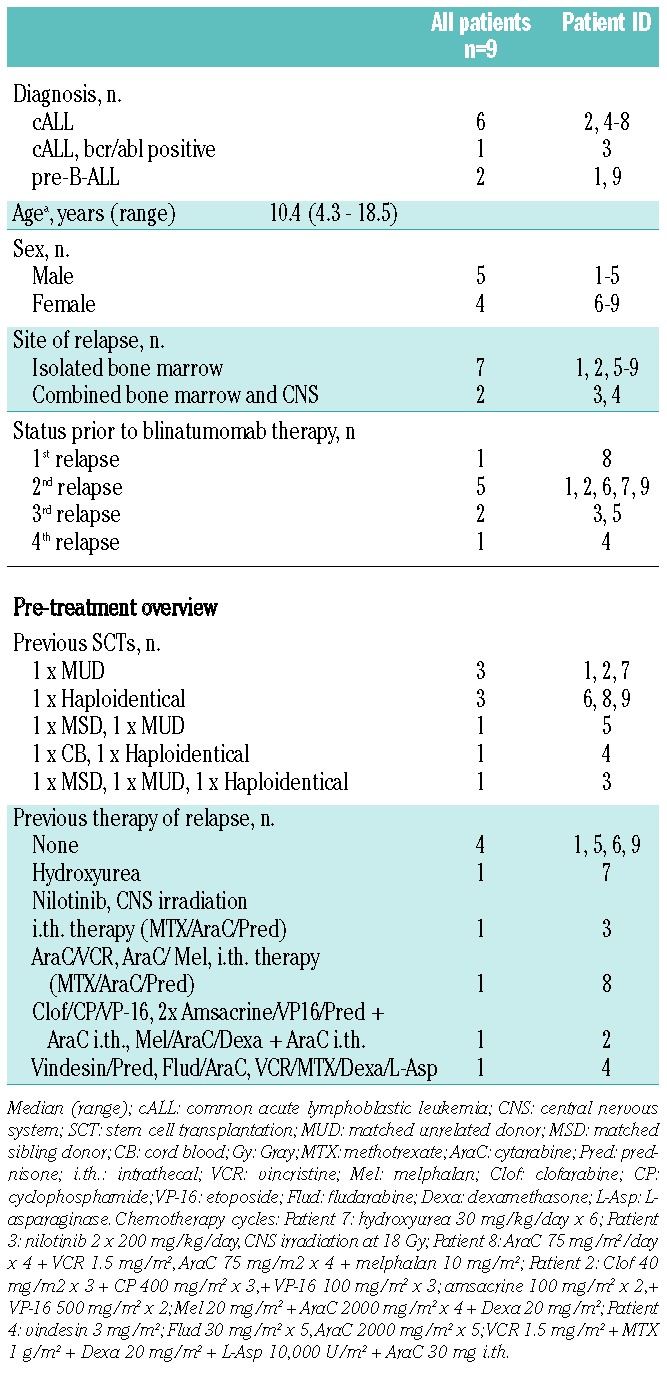
Between August 2008 and December 2011, 9 patients (median age 10 years) with posttransplant relapsed CD19 positive precursor ALL of the B-lineage, were treated with blinatumomab with a total of 18 cycles on a compassionate use basis (Table 2). The patient cohort includes 2 patients (Patients 2 and 3) who have already been reported by us in 2010.32 Four of 9 patients (Patients 1, 2, 3 and 4) showed a remission within the first cycle of blinatumomab. Three of these responders (Patients 1, 2 and 4) had at least an M2 marrow prior to treatment. One patient (Patient 3) had a level of MRD 0.4×10−2. In 2 patients (Patients 5 and 6), who were refractory to the first cycle of blinatumomab at a dose of 5 μg/m2/day, chemotherapy was administered after blinatumomab treatment to stop leukemia progression. Remission was finally achieved by the second cycle of blinatumomab, which was administered at a higher dose of 15 μg/m2/day (Online Supplementary Figure S1). Patient 5 received melphalan (20 mg/m2) and cytarabine (1000 mg/m2) on two consecutive days followed by the second blinatumomab cycle at 15 μg/m2/day, which led to low MRD below quantitative range (PCR MRD load 1×10−6). For consolidation he received two additional courses of blinatumomab which reduced MRD below the qualitative detection level (<1×10−6). After the fourth cycle, the patient experienced a bilateral extramedullary leukemic relapse in his mastoids while retaining molecular remission in the bone marrow. Thus he received a fractionated irradiation of the viscerocranium at 18 Gray and the neurocranium at 12 Gray for local leukemia control before a reduced-intensity conditioning regimen was initiated with ATG-Fresenius rabbit (3 × 5 mg/kg BW), fludarabine (4 × 40 mg/m2), thiotepa (10 mg/kg BW) and melphalan (2 × 70 mg/m2). Patient 6 received vincristine (1 mg/m2), clofarabine (3 × 40 mg/m2), cyclophosphamide (3 × 400 mg/m2) and etoposide (3 × 100 mg/m2) with stem cell rescue (5×106/kg BW CD34+ cells, T-cell depleted graft) from his previous parental haploidentical donor and then a second blinatumomab cycle at increased dose of 15 μg/m2/day. Thereafter, this patient reached MRD negativity and was retransplanted from the other parental haploidentical donor. The conditioning regimen included ATG-Fresenius rabbit (3 × 5 mg/kg BW), clofarabine (4 × 50 mg/m2), thiotepa (10 mg/kg BW) and melphalan (2 × 70 mg/m2).
Table 2.
Overview and outcome.
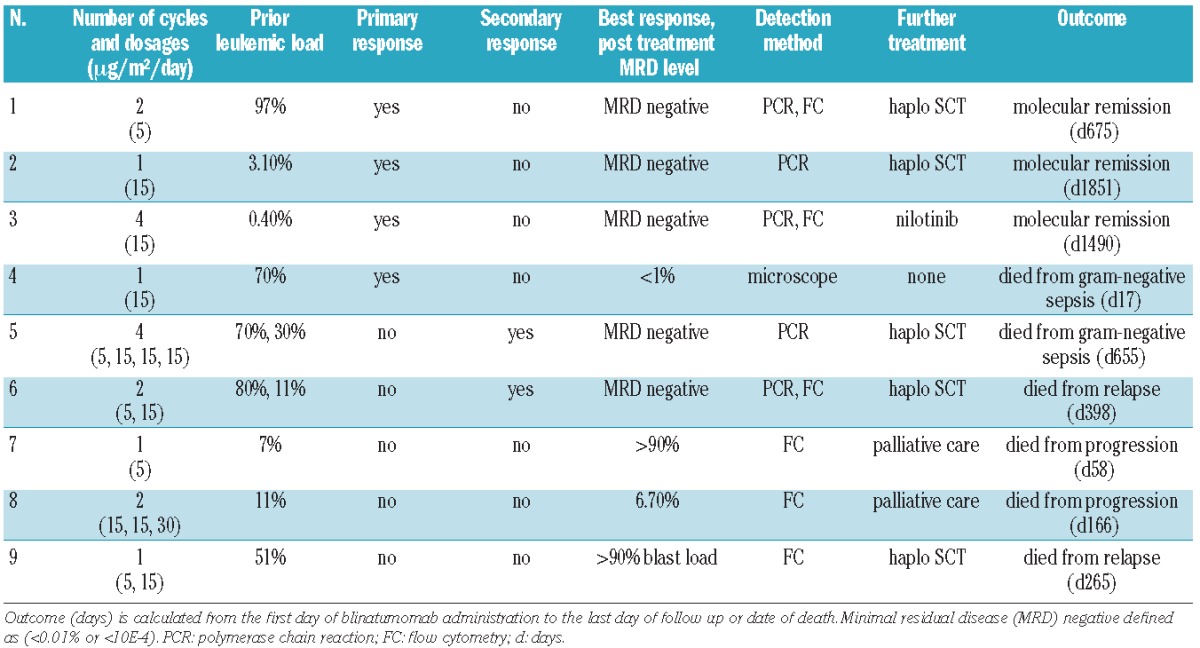
Three patients (Patients 7, 8 and 9) did not show any relevant response to blinatumomab and died from leukemia progression between Days 58 to 166 after blinatumomab had been initiated. Patient 8 received a second blinatumomab cycle at increased dose 15 μg/m2/day, with a dose escalation schedule up to 30 μg/m2/day for one single day without previous additional chemotherapy, and did not show any relevant clinical response. Two patients (Patients 7 and 9) were not able to receive a second cycle due to infectious complications and worsening general condition (Patient 7) or showed rapid leukemia progression during blinatumomab infusion (Patient 9). Patient 9 proceeded to a second haploidentical HSCT despite leukemia progression (> 90% blasts in BM). She received a conditioning regimen including fludarabine (3 × 40 mg/m2), thiotepa (10 mg/kg BW), melphalan (2 × 70 mg/m2) and TNI (7 Gray).
Six patients (Patients 1, 2, 3, 4, 5 and 6) achieved an M1 bone marrow. In 5 out of the 6 responders (Patients 1, 2, 3, 5 and 6), MRD conversion from positive to negative could be detected. Patient 4 had already been treated with a combination of appropriate antibiotics prior to initial blinatumomab treatment due to long-lasting infestation of multi-drug resistant Pseudomonas as well as ongoing local soft tissue infection with scrotal abscess formation because of multi-drug resistant Enterococcus faecalis. Despite a pre-existing septic complication and the obviously worsening general condition of the patient, blinatumomab treatment was started. Patient 4 achieved at least hematologic CR. There was no further investigation of MRD level in this patient because the patient died from gram-negative sepsis in aplasia during the first cycle of treatment. Four of 5 patients (Patients 1, 2, 5 and 6) with sustained molecular remission were retransplanted from another haploidentical donor after 2–4 blinatumomab cycles. Two (Patients 1 and 2) of the 4 patients retransplanted in molecular remission are in posttransplant sustained molecular remission of 675 days and 1851 days; one patient (Patient 6) died due to a subsequent (third) relapse 155 days posttransplant and 243 days post the first blinatumomab administration. One of 5 patients (Patient 3), with sustained molecular remission and with bcr/abl positive ALL, was subsequently treated with nilotinib and has been in remission for four years (1490 days).
Taken together, 3 of 9 patients are alive in enduring molecular remission with a median follow up of 1490 days (range 675–1851 days). The Kaplan-Meier survival estimation of event-free survival probability for all of our patients is 30% (Figure 1), while for responders this is 44%. Six of 9 patients died. Four patients died from leukemia progression (Patients 7 and 8) or relapse after rehaploidentical HSCT (Patients 6 and 9). Two patients (Patients 4 and 5) died from gram-negative sepsis. Patient 4 died from pre-existing infectious complication and Patient 5 died from late posttransplant infectious complication 655 days after blinatumomab had been initiated, i.e. 428 days post third allogeneic HSCT with no association to blinatumomab treatment.
Figure 1.
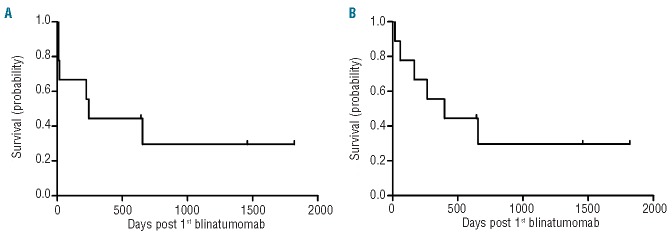
Probability of event-free survival (A) and overall-survival (B) (in days) after the first day of blinatumomab application in all 9 patients. Median follow up for overall survival 398 days; event-free survival 243 days (range for both 17 to 1819 days).
Four patients (Patients 1, 5, 6 and 9) received additional prophylactic immunotherapy using an FC-optimized CD19 antibody after blinatumomab treatment had been finished.35
Immune response and recovery
We grouped the patients according to their response to blinatumomab treatment. The first group comprised patients who responded directly when blinatumomab was started, achieving complete remission. We defined these patients as primary responding patients or primary responders (Patients 1, 2, 3 and 4). The second group comprised patients who were originally refractory to blinatumomab, but who responded to a modified regimen including chemotherapy followed by blinatumomab (Patients 5 and 6). These patients were defined as secondary responding patients or secondary responders. Patients who did not respond were defined as non-responding patients or non-responders (Patients 7, 8 and 9).
Non-responders (Patients 7, 8 and 9) and secondary responders (Patients 5 and 6) in the non-responding first cycle did not display any significant changes in the absolute counts and composition of mononuclear cells (e.g. CD3+, CD19+ counts) during blinatumomab administration. On the contrary, in the responding cycles, mainly the first responding cycle of the patients (Patients 1, 2, 3, 4, 5 and 6) led to significant changes in the absolute counts and composition of mononuclear cells, in particular T cells and leukemic blasts. The most evident changes occurred within the first few days of the first responding cycle with a substantial increase in T-cell count and in a parallel fall in the number of CD19+ blasts (Figure 2). The subsequent cycles showed stable counts of CD3+ T cells (CD4+ and CD8+) as well as other cells of the immune compartment with further depletion and efficacious suppression of all CD19+ cells, including blasts and non-malignant B cells.
Figure 2.
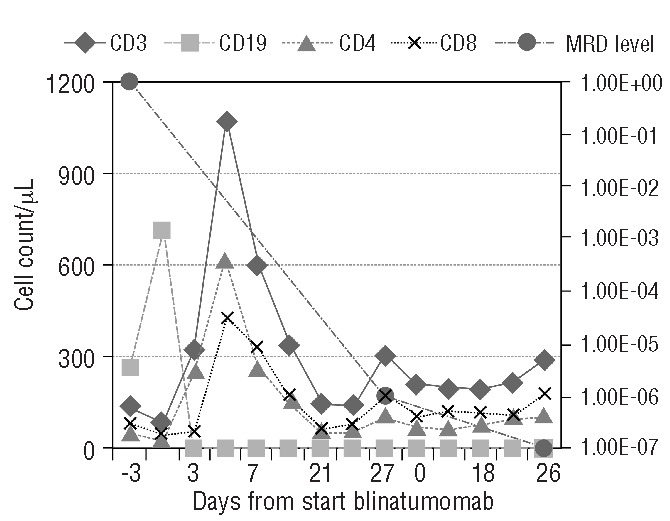
Pharmacodynamics of blinatumomab on peripheral blood mononuclear cells. The course of B and T cells in the peripheral blood of patient (Patient 1). When blinatumomab was started the blast load was 97% in the bone marrow. After the 1st cycle of treatment, the patient reached the MRD level of 1×106 and after the 2nd cycle there was no longer any detectable MRD (<1×106). The figure shows the effect of blinatumomab on the absolute counts of CD3+, CD4+, CD8+ and CD19+ cells as well as the course of the MRD level (PCR) displayed on the secondary axis.
We grouped the cycles of treatment with blinatumomab into non-responding, responding and maintenance cycles. Non-responding cycles were cycles with no reduction or increase in blast load in the bone marrow. Responding cycles were defined as reduction of blast load of less than 5% in the bone marrow and a minimum 2 log-fold reduction in blasts. Maintenance cycles were defined as blinatumomab cycles that preserved the patient’s current MRD status.
The comparison of absolute T-cell count in the peripheral blood (n=12; P=0.69) in non-responding cycles (median CD3+ 179/ml) versus responding cycles (median CD3+ 194/ml) and percentage as well as absolute blast count in the bone marrow in non-responding cycles (median blast load 31%; median absolute blast count 2150/μl) versus responding cycles (median blast load 21.5%; median absolute blast count 1240/μl) prior to the start of blinatumomab revealed no big differences (Online Supplementary Figure S2).
Regarding Patients 5 and 6 who showed secondary response, we observed no change in the T-cell count comparing the first non-responding and the second responding cycle in Patient 6 but a nearly 1-log-fold decrease in T-cell count in Patient 5 in the responding cycle. Furthermore, there was an approximate 1-log-fold reduction in blasts in Patient 6 but a mere 50% reduction of blasts in Patient 5 achieved by the additional chemotherapy administered between the non-responding and the responding cycle. Both Patients 5 and 6 showed an obviously higher T-cell-to-blast ratio in the responding cycle.
The time interval from HSCT to the first day of blinatumomab treatment had no impact on response, when comparing the time interval in days of non-responders (median 260 days) and responders (median 282 days) (Online Supplementary Figure S3). Neither did time off immunosuppression have any impact on response, when comparing non-responders (median 22 days) and responders (median 23 days) to the first day of blinatumomab administration (Online Supplementary Figure S4).
Toxicity
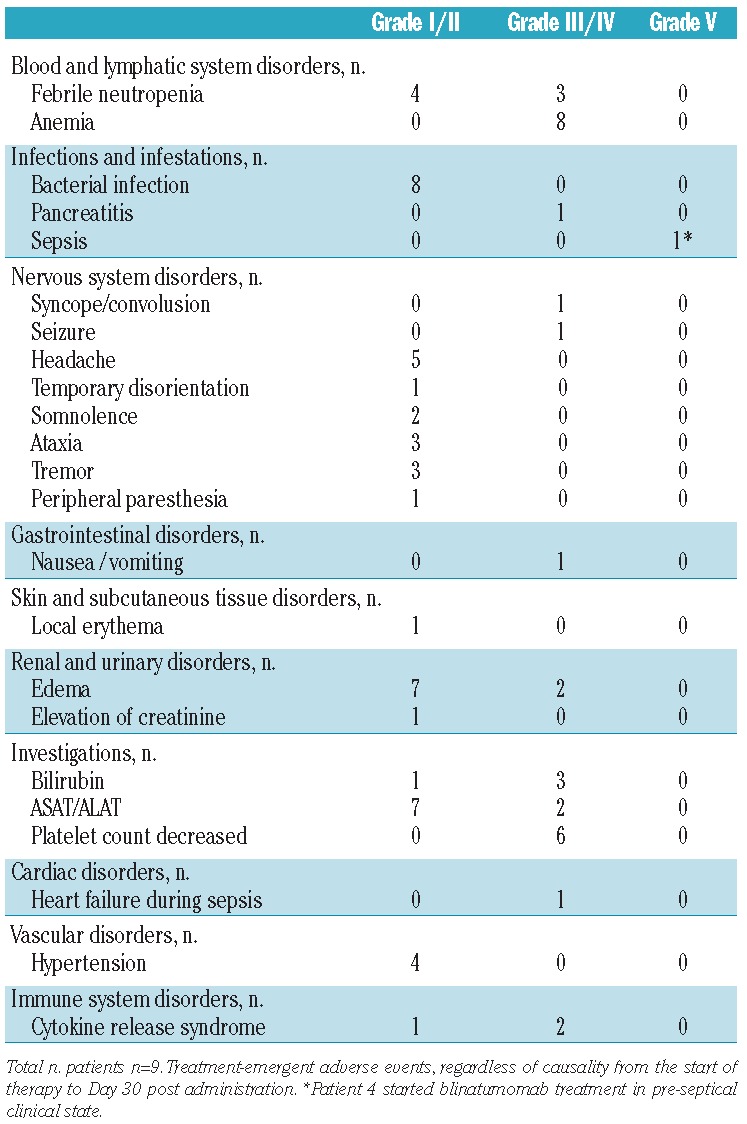
The most common toxicities independent of cause were decreased neutrophil and lymphocyte count, anemia and low platelet count, as well as increase of acute-phase proteins and pyrexia (Table 3). Three patients were temporarily affected by central ataxia, tremor and somnolence (Patients 2, 5 and 8) that resolved completely within days without any specific treatment under continuous blinatumomab administration. Grade 3/4 toxicities were elevation of bilirubin in 3 patients (Patients 4, 5 and 8) and two seizures, one with apnea and consecutive syncope in Patient 5. The first generalized cerebral seizure occurred with apnea (minimal oxygen saturation level 80%) on Day 7 of the second blinatumomab cycle, which was the first responding cycle in this patient. Intravenous lorazepam terminated the seizure within two minutes. Antiepileptic prophylaxis with levetiracetam was started at 40 mg/kg BW and dexamethasone 15 mg/m2 was applied for one week. Three days later, on Day 10 of the second cycle, a second generalized seizure occurred (minimal oxygen saturation level 88%) and was resolved, again with lorazepam. Levetiracetam was increased to 70 mg/kgBW. No further seizures were observed. Anticonvulsive treatment successfully ensured continuation of blinatumomab administration. Elevation of transaminases and bilirubin was not accompanied by any impairment of liver function in terms of protein synthesis or detoxification. The pharmacodynamic effect of blinatumomab was accompanied by clinical features of cytokine release such as fever, low blood pressure and fatigue. At the beginning of the first cycle, in the presence of T cells and blasts, all patients experienced transient fever except for Patient 3 who had low blast load (MRD level) and Patient 7 who showed no response to blinatumomab treatment. Patient 1 developed only mild hypotension and was not treated with either additional fluid substitution or with any medication. Fluid substitution due to hypotension caused by blinatumomab treatment was necessary only in Patient 5 for two days when the second blinatumomab cycle was started, which was the first responding cycle of this patient. There was no need for catecholamine treatment or transfer to the intensive care unit. A grade 5 treatment-emergent adverse event occurred in Patient 4 who died from pre-existing Pseudomonas sepsis with hypotension and need for catecholamines during blinatumomab administration. Patient 8 suffered from pre-existing pancreatitis during blinatumomab administration that resolved during blinatumomab treatment. There were no deaths due to graft-versus-host disease (GvHD) or related to blinatumomab.
Table 3.
Adverse events independent from cause.
Discussion
The current report intends to demonstrate that, in addition to conventional treatment in refractory and post-HSCT relapsed pediatric ALL, an immunotherapeutical approach with blinatumomab is feasible and might increase long-term leukemia-free survival.
In adult B-precursor ALL, Topp et al. demonstrated that blinatumomab is very potent at inducing molecular remission in chemorefractory patients.31 The last follow up of this patient cohort with refractory disease, and therefore the very poor prognosis, indicate significantly increased long-term relapse-free survival after induction and consolidation of remission by blinatumomab used as a single agent with or without subsequent HSCT.36
The salvage treatment of posttransplant relapsed B-precursor ALL using blinatumomab as a single agent in our cohort of pediatric patients was administered on a compassionate use basis in accordance with previous treatment in adult CD19+ malignancies, including non-Hodgkin lymphomas and B-ALL.30,31,36 In the first year posttransplant there is little tolerance for chemotherapy, and hardly any chance for cure without proceeding to another transplant, which is only beneficial in a low or preferably negative MRD state.7,8,10,12,37 We have shown that blinatumomab administration was feasible and had impressive antileukemic activity against highly chemorefractory disease in our pediatric patients, as has already been shown for adult B-ALL.31,36 Six of 9 patients achieved complete remission after blinatumomab treatment irrespective of leukemia burden prior to the start of therapy. There was good primary response with the induction of complete MRD remission by blinatumomab. It is remarkable that in 2 patients with primary non-response, additional chemotherapy treatment after initial blinatumomab courses resulted in a secondary response. The synergic effect of conventional chemotherapy to reduce blast load and then reattempt blinatumomab treatment might be a reasonable therapeutical strategy in non-responders. In patients with poor bone marrow function, the use of stem cell boosts from the previous donor might enable the patient to receive such additional chemotherapy and may contribute to relevant T-cell response.
In 2 patients of our cohort (Patients 5 and 6) we observed, via flow cytometric analyses of T cells in the peripheral blood and CD19+ blasts in the bone marrow as well as in the peripheral blood, that effector-to-target ratio could play a more crucial role in treatment success than absolute T-cell count (T cells/μl) in the peripheral blood and absolute blast count in the bone marrow. In general, in responding cycles, absolute CD3+ cell count in the peripheral blood was no higher than in non-responding cycles (P=0.7) (Online Supplementary Figure S5). Yet in the responding cycles of Patients 5 and 6, the T-cell-to-blast ratio was remarkably shifted in favor of T cells by the additional chemotherapy given after the first non-responding cycle. The T-cell-to-blast ratio in the non-responding cycle was below 0.2, whereas in the responding cycle it was over 10 (Patient 6) and over 100 (Patient 5). All blinatumomab cycles with a T-cell-(PB)-to-blast(BM) ratio over 7 either showed a complete MRD response or maintenance of complete remission (Online Supplementary Figure S5).
Based on this observation, it might be helpful to achieve a favorable T-cell-to-blast ratio by any means of action in order to increase the response rate in refractory patients. Further investigation of peripheral blood and bone marrow is warranted to find out if T-cell count, T-cell-to-blast ratio and functional T-cell analyses will help to predict probability of response.
Patient 3 with Philadelphia chromosome-positive ALL, who was previously reported by Handgretinger et al.,32 is an example of the successful treatment of relapse (combined CNS and BM) post third HSCT with long-term leukemia-free survival, without having proceeded to another HSCT due to high pre-treatment organ toxicity. Molecular remission was induced by blinatumomab and then was consolidated and maintained by the tyrosine kinase inhibitor nilotinib. However, pediatric posttransplant relapsed patients who again achieve low MRD or remission through blinatumomab treatment are in need of a second HSCT to facilitate long-term survival. Second allogeneic HSCT can cure approximately 35% of patients who achieve complete remission. Therefore, blinatumomab can facilitate long-term survival by inducing complete remission for subsequent HSCT.6 At a median observation time of 398 days, the event-free survival in our cohort of patients is 30%. Three of 9 patients remain in ongoing complete remission. Thus, the combination of chemotherapy- and blinatumomab-induced molecular remission, followed by subsequent HSCT may be considered a new treatment strategy that can induce long-term leukemia-free survival in posttransplant relapsed B-ALL in childhood.
In adult patients, blinatumomab administration was well tolerated.36 In our pediatric patient cohort treated with blinatumomab at a dose of 5 and 15 μg/m2/day, predominant dysfunction of hematopoiesis and infectious complications were pre-existent and based on the primary disease. Side-effects including seizure and hypotension could be managed satisfactorily in all of our patients and consequently blinatumomab therapy was not terminated because of treatment-emergent adverse events in any of them. Cytokine release syndrome was not a major issue in our patients. It has been shown in one case of severe clinical cytokine release syndrome that tocilizumab treatment can ameliorate symptoms and clinical signs rapidly after discontinuation of blinatumomab.38
In conclusion, blinatumomab is a novel and efficacious treatment for pediatric patients who suffer from posttransplant relapsed B-lineage precursor ALL. The experience of this treatment for compassionate use is in line with the convincing data acquired in adult patients.31,36 For posttransplant relapsed and primary refractory CD19-expressing childhood precursor ALL of the B-lineage in general, blinatumomab might come to the forefront. Systematic data for pediatric patients will be provided by the ongoing blinatumomab trial.39
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB685), from the BMBF and from the Reinhold Beitlich-Stiftung to PL.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Moricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dordelmann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111(9):4477–89 [DOI] [PubMed] [Google Scholar]
- 2.Borgmann A, von Stackelberg A, Hartmann R, Ebell W, Klingebiel T, Peters C, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003;101(10):3835–9 [DOI] [PubMed] [Google Scholar]
- 3.Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109(3):896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gassas A, Sung L, Saunders EF, Doyle J. Graft-versus-leukemia effect in hematopoietic stem cell transplantation for pediatric acute lymphoblastic leukemia: significantly lower relapse rate in unrelated transplantations. Bone Marrow Transplant. 2007;40(10):951–5 [DOI] [PubMed] [Google Scholar]
- 5.Locatelli F, Zecca M, Messina C, Rondelli R, Lanino E, Sacchi N, et al. Improvement over time in outcome for children with acute lymphoblastic leukemia in second remission given hematopoietic stem cell transplantation from unrelated donors. Leukemia. 2002;16(11):2228–37 [DOI] [PubMed] [Google Scholar]
- 6.Kato M, Horikoshi Y, Okamoto Y, Takahashi Y, Hasegawa D, Koh K, et al. Second allogeneic hematopoietic SCT for relapsed ALL in children. Bone Marrow Transplant. 2012;47(10):1307–11 [DOI] [PubMed] [Google Scholar]
- 7.Attarbaschi A, Mann G, Panzer-Grumayer R, Rottgers S, Steiner M, Konig M, et al. Minimal residual disease values discriminate between low and high relapse risk in children with B-cell precursor acute lymphoblastic leukemia and an intrachromosomal amplification of chromosome 21: the Austrian and German acute lymphoblastic leukemia Berlin-Frankfurt-Munster (ALL-BFM) trials. J Clin Oncol. 2008;26(18):3046–50 [DOI] [PubMed] [Google Scholar]
- 8.Sramkova L, Muzikova K, Fronkova E, Krejci O, Sedlacek P, Formankova R, et al. Detectable minimal residual disease before allogeneic hematopoietic stem cell transplantation predicts extremely poor prognosis in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;48(1):93–100 [DOI] [PubMed] [Google Scholar]
- 9.Lankester AC, Bierings MB, van Wering ER, Wijkhuijs AJ, de Weger RA, Wijnen JT, et al. Preemptive alloimmune intervention in high-risk pediatric acute lymphoblastic leukemia patients guided by minimal residual disease level before stem cell transplantation. Leukemia. 2010;24(8):1462–9 [DOI] [PubMed] [Google Scholar]
- 10.Klingebiel T, Handgretinger R, Lang P, Bader P, Niethammer D. Haploidentical transplantation for acute lymphoblastic leukemia in childhood. Blood Rev. 2004;18(3):181–92 [DOI] [PubMed] [Google Scholar]
- 11.Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:7–12 [DOI] [PubMed] [Google Scholar]
- 12.Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377–84 [DOI] [PubMed] [Google Scholar]
- 13.Hijiya N, Gaynon P, Barry E, Silverman L, Thomson B, Chu R, et al. A multi-center phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia. 2009;23(12):2259–64 [DOI] [PubMed] [Google Scholar]
- 14.Hijiya N, Thomson B, Isakoff MS, Silverman LB, Steinherz PG, Borowitz MJ, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118(23):6043–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeha S, Razzouk B, Rytting M, Rheingold S, Albano E, Kadota R, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute myeloid leukemia. J Clin Oncol. 2009;27(26):4392–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat Rev Drug Discov. 2007;6(2):149–65 [DOI] [PubMed] [Google Scholar]
- 17.Orentas RJ, Lee DW, Mackall C. Immunotherapy targets in pediatric cancer. Front Oncol. 2012;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254–61 [DOI] [PubMed] [Google Scholar]
- 19.Quintas-Cardama A, Wierda W, O’Brien S. Investigational immunotherapeutics for B-cell malignancies. J Clin Oncol. 2010;28(5):884–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mack M, Riethmuller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci USA. 1995;92(15):7021–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loffler A, Kufer P, Lutterbuse R, Zettl F, Daniel PT, Schwenkenbecher JM, et al. A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95(6):2098–103 [PubMed] [Google Scholar]
- 22.Rader C. DARTs take aim at BiTEs. Blood. 2011;117(17):4403–4 [DOI] [PubMed] [Google Scholar]
- 23.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2(12):945–56 [DOI] [PubMed] [Google Scholar]
- 24.Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9(3):195–205 [DOI] [PubMed] [Google Scholar]
- 25.Tedder TF. CD19: a promising B cell target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):572–7 [DOI] [PubMed] [Google Scholar]
- 26.Hoelzer D. Novel antibody-based therapies for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:243–9 [DOI] [PubMed] [Google Scholar]
- 27.Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984;63(6):1424–33 [PubMed] [Google Scholar]
- 28.Lang P, Barbin K, Feuchtinger T, Greil J, Peipp M, Zunino SJ, et al. Chimeric CD19 antibody mediates cytotoxic activity against leukemic blasts with effector cells from pediatric patients who received T-cell-depleted allografts. Blood. 2004;103(10):3982–5 [DOI] [PubMed] [Google Scholar]
- 29.Zhao XS, Liu YR, Zhu HH, Xu LP, Liu DH, Liu KY, et al. Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2012;91(2):183–92 [DOI] [PubMed] [Google Scholar]
- 30.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–7 [DOI] [PubMed] [Google Scholar]
- 31.Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–8 [DOI] [PubMed] [Google Scholar]
- 32.Handgretinger R, Zugmaier G, Henze G, Kreyenberg H, Lang P, von Stackelberg A. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2010;25(1):181–4 [DOI] [PubMed] [Google Scholar]
- 33.Kerst G, Kreyenberg H, Roth C, Well C, Dietz K, Coustan-Smith E, et al. Concurrent detection of minimal residual disease (MRD) in childhood acute lymphoblastic leukaemia by flow cytometry and real-time PCR. Br J Haematol. 2005;128(6):774–82 [DOI] [PubMed] [Google Scholar]
- 34.Bader P, Hancock J, Kreyenberg H, Goulden NJ, Niethammer D, Oakhill A, et al. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16(9):1668–72 [DOI] [PubMed] [Google Scholar]
- 35.Lang P, Seidel U, Schlegel P, Grosse-Hovest L, Ebinger M, Feuchtinger T, et al. Prophylactic and therapeutic treatment of children with B-lineage acute lymphoblastic leukaemia with an Fc-optimized CD19 antibody: results of a pilot study. Presented at the 39th Meeting of the European Group for Blood and Marrow Transplantation, London, UK, April 7–10, 2013 (P859) [Google Scholar]
- 36.Topp MS, Gokbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–7 [DOI] [PubMed] [Google Scholar]
- 37.Lankester AC, Bierings MB, van Wering ER, Wijkhuijs AJ, de Weger RA, Wijnen JT, et al. Preemptive alloimmune intervention in high-risk pediatric acute lymphoblastic leukemia patients guided by minimal residual disease level before stem cell transplantation. Leukemia. 2010;24(8):1462–9 [DOI] [PubMed] [Google Scholar]
- 38.Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121(26):5154–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gore L, Zugmaier G, Handgretinger R, Locatelli F, Trippett TM, Rheingold SR, et al. Cytological and molecular remissions with blinatumomab treatment in second or later bone marrow relapse in pediatric acute lymphoblastic leukemia (ALL). J Clin Oncol. 2013;(Abstract 10007) [Google Scholar]


