Abstract
The benefit of intrathecal therapy and systemic rituximab on the outcome of diffuse large B-cell lymphoma at risk of central nervous system disease is controversial. Furthermore, the effect of intrathecal treatment and rituximab in diffuse large B-cell and Burkitt lymphoma with occult leptomeningeal disease detected by flow cytometry at diagnosis is unknown. Untreated diffuse large B-cell (n=246) and Burkitt (n=80) lymphoma at clinical risk of central nervous system disease and having had pre-treatment cerebrospinal fluid were analyzed by flow cytometry and cytology. Spinal fluid involvement was detected by flow cytometry alone (occult) in 33 (13%) diffuse large B-cell and 9 (11%) Burkitt lymphoma patients, and detected by cytology in 11 (4.5%) and 5 (6%) patients, respectively. Diffuse large B-cell lymphoma with occult spinal fluid involvement had poorer survival (P=0.0001) and freedom from central nervous system relapse (P<0.0001) compared to negative cases. Burkitt lymphoma with occult spinal fluid involvement had an inferior freedom from central nervous system relapse (P=0.026) but not survival. The amount of intrathecal chemotherapy was quantitatively associated with survival in diffuse large B-cell lymphoma with (P=0.02) and without (P=0.001) occult spinal fluid involvement. However, progression of systemic disease and not control of central nervous system disease was the principal cause of treatment failure. In diffuse large B-cell lymphoma, systemic rituximab was associated with improved freedom from central nervous system relapse (P=0.003) but not with survival. Our results suggest that patients at risk of central nervous system disease should be evaluated by flow cytometry and that intrathecal prophylaxis/therapy is beneficial.
Introduction
Involvement of the central nervous system (CNS) is an infrequent but often fatal complication of aggressive B-cell lymphomas.1 It mostly presents as leptomeningeal disease with involvement of the cerebrospinal fluid (CSF). Although prophylactic treatment likely reduces the incidence of CNS relapse, it increases the toxicity of systemic chemotherapy and is unnecessary in most patients.2,3 This has led to the development of clinical risk paradigms to identify patients for CNS prophylaxis, but only a few patients will potentially benefit.1,4
Among patients who develop CNS disease, only a small fraction have cytologically detectable malignant cells in the CSF on initial staging.4 This observation raised the question of whether cytology was unable to detect low volume disease or if leptomeningeal spread was a late event. Cytology of the CSF, the diagnostic gold standard, however, has a low sensitivity with a reported false negative rate of 20–60%, suggesting pre-treatment leptomeningeal involvement is greater than initially reported.5,6 Several studies now show that CSF flow cytometry (FCM), which is significantly more sensitive than cytology, detects a relatively high rate of occult disease.4,7,8
The ability to detect occult leptomeningeal involvement early in the disease raises uncertainties regarding optimal treatment. FCM provides an opportunity to ’prospectively’ identify patients with leptomeningeal disease, thereby enabling the assessment of intrathecal chemotherapy on CNS recurrence and survival, which is an area of controversy. Indeed, multiple retrospective studies have examined the effect of systemic rituximab and intrathecal ‘prophylaxis’ on the risk of CNS recurrence in DLBCL and have reported inconsistent results.9–13 To help address these questions, we conducted an analysis of untreated patients with DLBCL or BL at clinical risk of CNS disease who underwent FCM and cytology of the CSF at initial staging. We provide new information on the effect of intrathecal chemotherapy and systemic rituximab on the incidence of CNS recurrence and survival in patients at risk of CNS disease who did and did not have evidence of occult CSF involvement at initial diagnosis.
Methods
Eligibility
This is an analysis of 326 patients with previously untreated DLBCL and BL at clinical risk of CNS disease who had CSF analysis by FCM and cytology at initial staging. Patients were retrospectively identified between March 1999 and September 2010 from the National Cancer Institute (one center); Spanish Group for the Study of CNS Disease in non-Hodgkin lymphoma (NHL) (26 centers with the majority of cases from 4 centers); and Daniel den Hoed Cancer Center (one center), Rotterdam, The Netherlands. Patient eligibility included untreated disease, de novo DLBCL or BL histology, and CSF FCM and cytology at initial diagnosis. HIV-positive patients have a similar outcome to HIV-negative patients in the present era and these were included.14,15 DLBCL patients were considered at risk of CNS disease as defined by the presence of at least one extranodal site and elevated lactate dehydrogenase.1,4 In addition, patients with BL, HIV infection and/or patients with neurological symptoms were considered at risk of CNS disease. Tumor histology was confirmed by each institution according to the World Health Organization classification of lymphoid tissue.16
Study design
The primary objectives were: 1) to determine the incidence of leptomeningeal lymphoma by CSF FCM and cytology at initial diagnosis; 2) to assess freedom from CNS recurrence (FFCR) and survival in patients with occult (FCM+/cytology−) CSF involvement; 3) to assess the effect of intrathecal treatment and systemic rituximab on FFCR and survival; and 4) to identify features associated with CSF involvement.
An Excel database was created to capture the following data elements: patients’ characteristics, diagnosis, HIV status, dates of treatment and response, systemic chemotherapy, amount and type of intrathecal chemotherapy, CSF chemistry, FCM and cytology, CNS response to treatment, and dates of CNS recurrence, systemic recurrence, and/or death, and contributing institution. The study was approved by the Institutional Review Board, exempted from informed consent and the data were coded. Statistics are described in the Online Supplementary Appendix.
Flow cytometry
Flow cytometry analysis complied with the International Working Group on Flow Cytometric Immunophenotyping of the CSF.17 The protocol is designed for 3–8 colors, depending on the number of parameters available on the flow cytometer used. The method can be applied to freshly obtained or stabilized samples. Samples were processed within 1 h after lumbar puncture or stabilized using TransFix® (Cytomark, UK) or RPMI transport medium to avoid in vitro cell deterioration. The protocol describes the simultaneous assessment of surface and cytoplasmic antigens, and a method to calculate absolute numbers of both normal and pathological cell subpopulations by adding counting beads to the assay. In most cases, clonal neoplastic cells were detected by the presence of light chain restriction concordant with the primary tumor, whereas malignant cells that did not express light chains were detected by an abnormal B-cell phenotype concordant with the primary tumor.4
Treatment
Patients with DLBCL received standard doxorubicin-based regimens as described in the Online Supplementary Appendix.18,19 Patients with Burkitt lymphoma received Burkitt regimens as described in the Online Supplementary Appendix.20–22 Central nervous system active and prophylactic treatments are described in the Online Supplementary Appendix.
Results
Patients’ characteristics
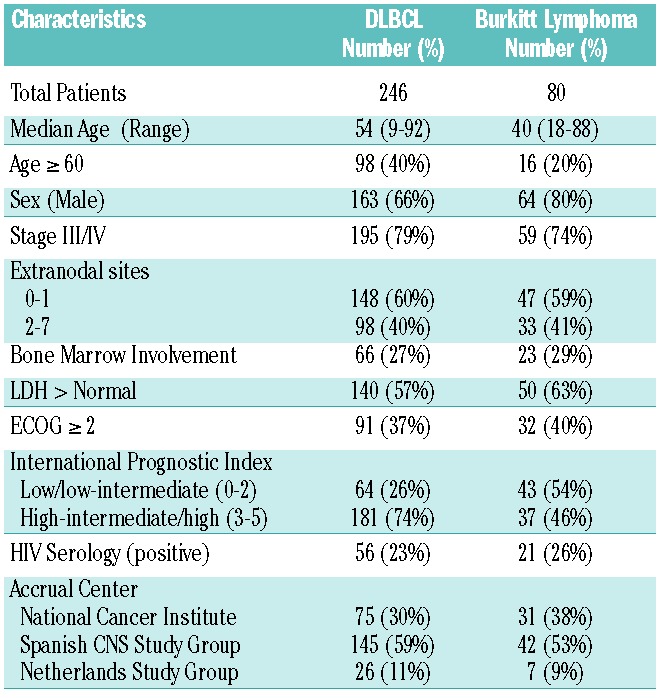
The study included 246 patients with DLBCL and 80 patients with BL who were at clinical risk of CNS disease. Patients with DLBCL had a median age of 54 years (range 9−92 years) and 66% were male (Table 1). Clinical characteristics associated with an increased risk of CNS disease in DLBCL included 2 or more extranodal sites of disease in 40%, elevated LDH in 57%, and high-intermediate/high international prognostic index score in 74%. BL patients had a median age of 40 years (range 18−88 years) and 74% had advanced stage disease, which is associated with a risk of CNS disease.
Table 1.
Patients’ characteristics at diagnosis.
Cerebrospinal fluid analysis
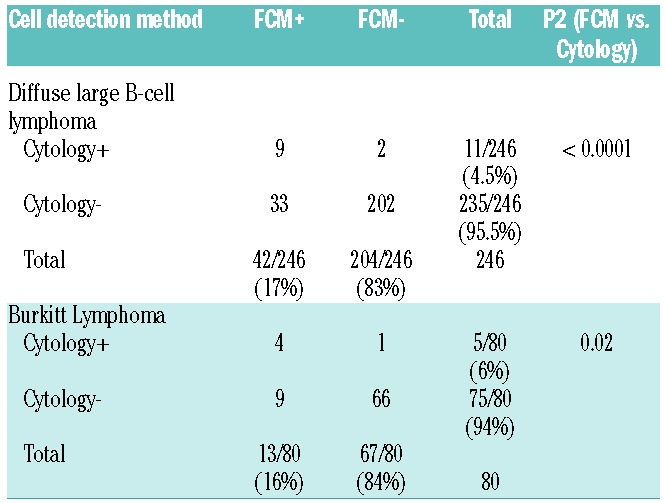
Lymphoma was detected in the CSF by FCM in 42 (17%) patients and by cytology in 11 (4.5%) patients with DLBCL (P<0.0001) (Table 2). All but 2 cases detected by cytology were also detected by FCM, whereas cytology only detected 26% of FCM-positive cases. Among patients with BL, lymphoma was detected by FCM in 13 (16%) patents and by cytology in 5 (6%) patients (P=0.02). Similarly, cytology only detected 31% of FCM-positive cases in BL. HIV infection was not associated with an increased incidence of leptomeningeal disease.
Table 2.
Cerebrospinal spinal fluid malignant cell analysis.
We examined the association between brain parenchymal disease and CSF involvement pretreatment. Ten (4%) patients with DLBCL and 1 (1%) patient with BL had radiological evidence of parenchymal involvement. Of these cases, 4 (2%) patients, all with DLBCL, also had involvement of the CSF, which was detected by FCM in 3 and by cytology in 2 patients.
To determine if the presence of occult disease (FCM+/cytology−) in the CSF was associated with increased CSF protein or cell counts, we compared patients with occult disease to patients without CSF involvement. There was no significant difference in the median (range) cell count (1.2 (0–26.9) and 0.9 (0–42); P=0.054) or total protein (44 (9–109) and 33 (1–608); P=0.077) in patients with or without occult CSF involvement, respectively, consistent with the low tumor burden of occult disease.
Outcome of cerebrospinal fluid involvement
Patients with DLBCL had an overall survival of 65% at three years, and a median follow up of 50 months (Figure 1A). Within the DLBCL patients, occult disease was associated with lower survival (38% vs. 69% at 3 years; P=0.0001) and freedom from CNS relapse (73% vs. 94% at 3 years; P<0.0001) compared to patients without CSF involvement (Figure 1B and C). The findings were statistically similar when confined to the patients who received systemic rituximab. There was no difference in survival (P=0.54) or freedom from CNS relapse (P=0.72) among patients with a positive compared to negative cytology.
Figure 1.
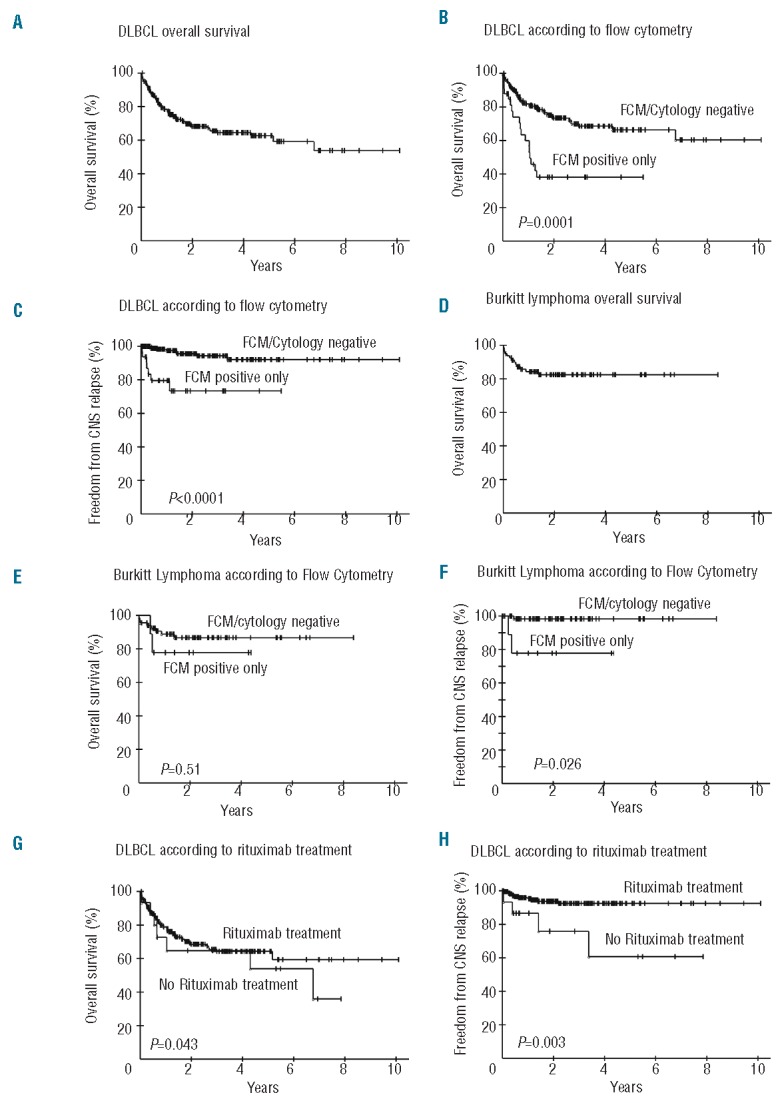
Kaplan-Meier plots of OS and FFCR of DLBCL and BL according to FCM and cytology CSF status. (A) OS of 246 patients with DLBCL. (B) OS of 203 DLBCL patients without CSF involvement (FCM and/or cytology negative) and 33 DLBCL patients with occult CSF involvement (FCM positive only). (C) FFCR of 203 DLBCL patients without CSF involvement and 33 DLBCL patients with occult CSF involvement. (D) OS of 80 BL patients. (E) OS of 66 BL patients without CSF involvement and 9 BL patients with occult CSF involvement (Exact log rank test). (F) FFCR of 66 BL patients without CSF involvement and 9 BL patients with occult CSF involvement.
Patients with BL had an overall survival of 83% at three-years and a median follow up of 43 months (Figure 1D). In BL, occult CSF involvement was associated with a lower freedom from CNS relapse (78% vs. 98% at 3 years; P=0.026) but not survival (Figure 1E and F). In contrast, patients with a positive CSF cytology had a lower survival compared to patients with a negative cytology (87% vs. not reached at three years; P=0.005) but no difference in freedom from CNS relapse (data not shown).
Effect of intrathecal chemotherapy and systemic rituximab
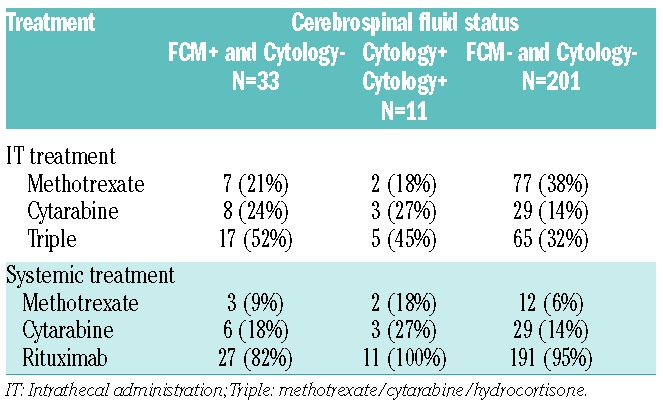
To assess the effect of intrathecal chemotherapy on the outcome of patients at risk of CNS involvement, we divided the patients into three risk groups; 1) occult CSF involvement (FCM+/Cytology−); 2) cytology-positive CSF (Cytology+/FCM +/−) and; 3) no CSF involvement (FCM−/Cytology−) (Table 3). Within the group with occult CSF involvement, all but 1 patient received active treatment with intrathecal methotrexate (21%), cytarabine (24%) or a combination of methotrexate, cytarabine and hydrocortisone (Triple) (52%). Prophylactic intrathecal chemotherapy was administered in 85% of patients who had no evidence of CSF involvement. A minority of patients in both groups received systemic methotrexate and/or cytarabine as part of their systemic treatment (Table 3).
Table 3.
DLBCL central nervous system treatment.
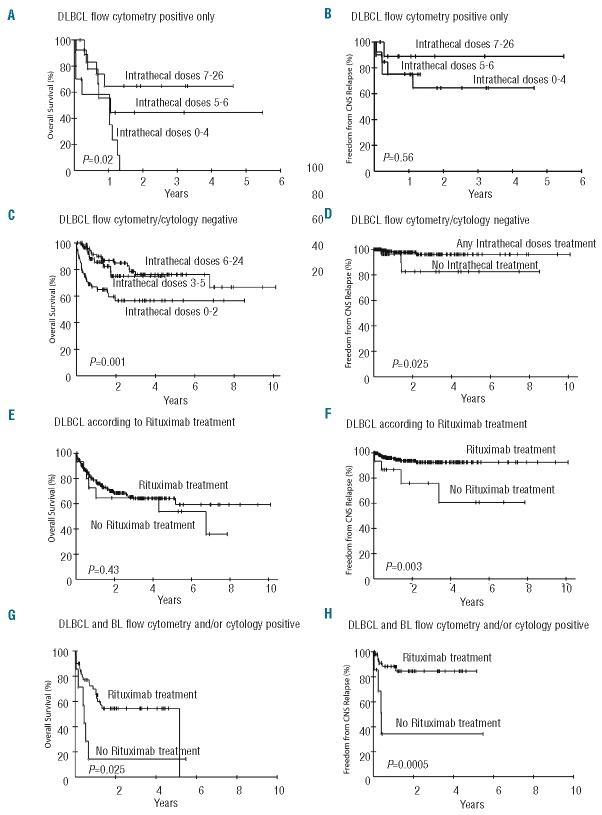
To investigate quantitative effect of intrathecal therapy on outcome, we divided each group into tertiles based on the number of intrathecal doses (Table 4). Within the 33 patients with occult CSF disease, we observed an association between the number of intrathecal doses and survival (0% vs. 44% vs. 65% at three-years for 0–4, 5–6 and 7–26 doses, respectively) (P=0.02) but not with freedom from CNS relapse (P=0.56) (Figure 2A and 2B). Seventy percent of patients in the 0–4 dose had systemic disease failure compared to only 23% and 36% of patients in the 5–6 and 7–26 dose tertile, respectively (Table 4). In contrast, the CSF of most patients became flow negative including 63% of patients in the first tertile who only received 0–4 doses. Furthermore, the frequency of CSF and brain parenchymal relapse was similar among tertiles. These results suggest that among patients with occult CSF involvement, the principal cause of treatment failure is systemic disease and not CNS recurrence. Among 11 patients with cytology positive CSF, all cleared their CSF and only 18% developed systemic disease (Table 4).
Table 4.
Outcome of intrathecal treatment in DLBCL.
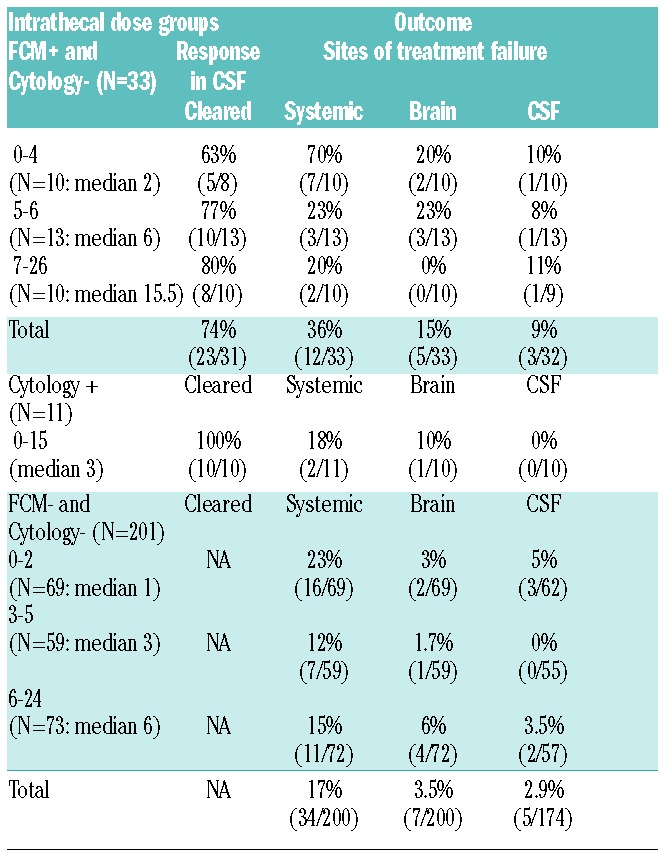
Figure 2.
Kaplan-Meier plots of OS and FFCR of DLBCL according to the amount of intrathecal chemotherapy doses and systemic rituximab. (A) OS of 33 DLBCL patients with occult CSF involvement (FCM positive only) according to the number of intrathecal treatments divided into tertiles. (B) FFCR of 33 DLBCL patients with occult CSF involvement (FCM positive only) according to the number of intrathecal doses divided into tertiles. (C) OS of 201 DLBCL patients without CSF involvement according to the number of intrathecal treatments divided into tertiles. (D) FFCR of 201 DLBCL patients without CSF involvement according to according to the number of intrathecal treatments divided into tertiles. (E) OS of DLBCL patients according to whether they did (n=231) or did not (n=15) receive systemic rituximab. (F) FFCR of DLBC patients according to whether they did (n=231) or did not (n=15) receive systemic rituximab. (G) OS of 44 DLBCL and 13 BL patients with CSF involvement according to whether they did (n=50) or did not (n=7) receive systemic rituximab. (H) FFCR of 44 DLBCL and 13 BL patients with CSF involvement according to whether they did (n=50) or did not (n=7) receive systemic rituximab.
We also looked at the 201 patients at risk of CNS disease and without evidence of CSF involvement by FCM and/or cytology; 171 (85%) received at least 1 dose of intrathecal chemotherapy (Table 3). In these patients, the number of intrathecal doses was also significantly associated with improved survival (76% vs. 75% vs. 57% at 3 years for 6–24, 3–5 and 0–2 doses, respectively) (Table 4 and Figure 2C) but not with freedom from CNS relapse (data not shown). However, patients who received any intrathecal chemotherapy had a significantly better freedom from CNS relapse compared to patients who received no intrathecal doses (Figure 2D). There was no significant difference in the incidence of systemic or CNS recurrence among the three tertiles (Table 4). All three groups received similar systemic methotrexate, cytarabine and/or rituximab.
We analyzed the survival and freedom from CNS relapse of DLBCL patients who did or did not receive systemic rituximab. Rituximab administration did not affect overall survival, but significantly improved the freedom from CNS relapse (93% vs. 76% at 4 years) (Figure 2E and F). Notably, there was no difference in the frequency of intrathecal chemotherapy in the patients who did or did not receive rituximab (P=1.00). We also looked at the subgroup of patients who had CSF involvement pre treatment to assess if rituximab improved the outcome of patients with established CSF disease. For this analysis, we included both DLBCL and BL patients with CSF involvement. This analysis showed a significant association between systemic rituximab and improved survival (54% vs. 14%; P=0.025) and freedom from CNS relapse (85% vs. 34%; P=0.0005) (Figure 2G and H).
Risk factors for CSF involvement by lymphoma
In a univariate analysis in DLBCL patients, extranodal disease (P<0.0001), performance status (P=0.009), stage (P=0.002) and international prognostic index (P<0.0001) were associated with a positive CSF. However, multivariate analysis only showed extranodal disease (P<0.0001) and age (P=0.02) were independent predictors. In BL, only disease stage (P=0.03) was associated with CSF involvement in a univariate analysis.
Discussion
The present analysis validates our previous findings that FCM is significantly more sensitive than cytology for the detection of CSF involvement by aggressive B-cell lymphoma, and indicates the presence of CSF involvement in a high proportion of untreated patients at clinical risk for CNS disease.4,7 The high sensitivity of FCM raises the question of whether occult CSF involvement is clinically meaningful. To address this question, we analyzed the survival and freedom from CNS relapse of patients with occult CSF involvement and compared them to patients without evidence of CSF involvement. In DLBCL, patients with occult CSF involvement had lower survival and freedom from CNS relapse compared to those without any involvement. Occult CSF involvement in BL was also associated with a lower freedom from CNS relapse, but not survival. However, cytology was associated with a worse survival in BL patients. Several conclusions can be drawn from these data. In DLBCL, the majority of patients who developed CNS recurrence appear to have occult CSF involvement at initial diagnosis based on the reported frequency of CNS recurrence.9,12,24 Furthermore, occult CSF involvement is associated with a worse outcome, confirming it is a clinically meaningful and actionable finding. The findings were somewhat different in BL, reflecting differences in disease biology and treatment. In BL, cytology but not FCM was significantly associated with overall survival. This may reflect the known ability of treatment to control or sterilize leptomeningeal disease in BL, which would be more likely in patients with occult levels of involvement.
Our study shows for the first time that occult leptomeningeal disease is associated with an adverse outcome in DLBCL and BL. These results are consistent with several reports on the outcome of FCM+ CSF involvement in pooled analyses of aggressive B-cell lymphomas.4,25,26 One small retrospective study showed an association between occult CSF involvement and risk of CNS relapse, and a second prospective study showed an adverse association between FCM+ CSF and survival and risk of CNS progression.25,26
The benefit of intrathecal therapy is controversial in DLBCL.9,13,24,27 The reasons for this controversy are multifactorial. To help address this question, we first looked at patients with pre-treatment occult CSF involvement who in the absence of FCM analysis would have been clinically considered for prophylactic treatment. As there is no standard for the number of intrathecal doses in prophylactic or active treatment, we took a quantitative approach by analyzing the clinical outcome according to the number of intrathecal doses divided into tertiles. We observed a quantitative association between the amount of intrathecal chemotherapy and survival with 0-4 doses having a worst outcome than at least 5–6 doses. Notably, FCM showed a complete response in the CSF of most patients, including 63% of patients who only received 0–4 doses. Progression of systemic disease, which occurred in 70% of patients who received 0–4 doses, was the principal cause of treatment failure. Overall, 74% of patients with occult involvement achieved a complete remission by FCM in their CSF, and only 9% relapsed in the CSF. We also looked at patients without evidence of CSF involvement and found a similar association between the amount of intrathecal chemotherapy and survival. Like patients with occult CSF disease, systemic progression and not CNS progression was the principal cause of treatment failure. These results show that intrathecal therapy controls CNS disease in most patients and in this regard confers benefit. Our results also suggest that the biology associated with CNS disease leads to a higher risk of systemic disease failure in some patients.
The effect of systemic rituximab on the risk of CNS relapse is also controversial.9–13 To assess this, we looked at the effect of rituximab on survival and freedom from CNS relapse. Within all patients with DLBCL, systemic rituximab was associated with a significant reduction in CNS relapse suggesting it may treat established CNS disease. Alternatively, systemic rituximab may reduce the secondary spread of lymphoma to the CNS and not directly affect established CNS disease due to its poor CNS penetration.28 To address this issue, we assessed the effect of systemic rituximab on the outcome of patients with pretreatment CSF involvement. In this analysis, which included DLBCL and BL, we observed a significant association between systemic rituximab and improved survival and freedom from CNS relapse, suggesting that systemic rituximab has therapeutic effects on established CSF disease. It is important to note, however, that most patients also received intrathecal chemotherapy, precluding any conclusion that rituximab alone can eradicate CNS disease.
The low incidence of CNS disease in DLBCL, clinically observed in 5% of unselected patients and 8% of high-risk patients, has made it difficult to study the benefit of CNS prophylaxis.1,2,29 In the pre-rituximab era, a randomized study of CHOP versus ACVBP (doxorubicin; cyclophosphamide; vindesine; bleomycin; prednisone; and intrathecal treatment) in poor prognosis aggressive lymphomas showed a significantly lower incidence of CNS recurrence on the ACVBP arm where CNS-directed therapy was used, suggesting benefit.2 However, several retrospective rituximab-era studies in DLBCL did not find a benefit of intrathecal prophylaxis.9,10,13,27 Two studies from the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) reported that CNS prophylaxis was of no significant benefit in high-risk young patients or elderly patients with aggressive B-cell lymphoma.13,27 The conclusions are confounded by two methodological problems. First, patients were designated to receive intrathecal prophylaxis based on clinical criteria that were inconsistent with the authors’ own optimal risk model for CNS disease. Second, the authors compared the patients in this ‘high-risk’ group who received intrathecal prophylaxis with those who did not (due to a protocol violation). Hence, patients at high risk of CNS disease were not optimally identified and the control group was biased with patients who the treating physicians felt did not need prophylaxis. Unfortunately, another large negative database study had related methodological issues.9
Our results provide the first evidence that occult CSF involvement by DLBCL or BL is clinically meaningful and associated with an adverse outcome. Intrathecal therapy was associated with excellent control of CNS disease and systemic progression was the main cause of treatment failure. Finally, our results suggest that systemic rituximab has therapeutic activity against established CSF disease and reduces CNS relapse. These results indicate that patients at risk of CNS disease will benefit from intrathecal chemotherapy, although the optimal therapy remains to be determined. Based on these results, we suggest that patients at risk of CNS disease undergo evaluation of the CSF by FCM, and that patients receive prophylactic or therapeutic CNS-directed chemotherapy, as indicated by the absence or presence of leptomeningeal disease, respectively.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
The work was partially supported by the following grants RD06/0020/0035 and RD12/0036/0048 from RETICS-FEDER (Instituto de Salud Carlos III, Ministerio de Economia y Competitividad, Madrid, Spain) and an unrestricted grant from Mundipharma Pharmaceuticals, SL (Madrid, Spain).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.van Besien K, Ha CS, Murphy S, McLaughlin P, Rodriguez A, Amin K, et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood. 1998;91(4):1178–84 [PubMed] [Google Scholar]
- 2.Tilly H, Lepage E, Coiffier B, Blanc M, Herbrecht R, Bosly A, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102(13):4284–9 [DOI] [PubMed] [Google Scholar]
- 3.Young RC, Howser DM, Anderson T, Fisher RI, Jaffe E, DeVita VT., Jr Central nervous system complications of non-Hodgkin’s lymphoma. The potential role for prophylactic therapy. Am J Med. 1979;66(3):435–43 [DOI] [PubMed] [Google Scholar]
- 4.Hegde U, Filie A, Little RF, Janik JE, Grant N, Steinberg SM, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105(2):496–502 [DOI] [PubMed] [Google Scholar]
- 5.Schiff D, Feske SK, Wen PY. Deceptively normal ventricular fluid in lymphomatous meningitis. Arch Intern Med. 1993;153(3):389–90 [PubMed] [Google Scholar]
- 6.Glass JP, Melamed M, Chernik NL, Posner JB. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979;29(10):1369–75 [DOI] [PubMed] [Google Scholar]
- 7.Quijano S, Lopez A, Manuel Sancho J, Panizo C, Deben G, Castilla C, et al. Identification of leptomeningeal disease in aggressive B-cell non-Hodgkin’s lymphoma: improved sensitivity of flow cytometry. J Clin Oncol. 2009;27(9):1462–9 [DOI] [PubMed] [Google Scholar]
- 8.Bromberg JE, Breems DA, Kraan J, Bikker G, van der Holt B, Smitt PS, et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology. 2007;68(20):1674–9 [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Vanderplas A, LaCasce AS, Rodriguez MA, Crosby AL, Lepisto E, et al. Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era: findings from a large national database. Cancer. 2012;118(11):2944–51 [DOI] [PubMed] [Google Scholar]
- 10.Tai WM, Chung J, Tang PL, Koo YX, Hou X, Tay KW, et al. Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): pre- and post-rituximab. Ann Hematol. 2011;90(7):809–18 [DOI] [PubMed] [Google Scholar]
- 11.Shimazu Y, Notohara K, Ueda Y. Diffuse large B-cell lymphoma with central nervous system relapse: prognosis and risk factors according to retrospective analysis from a single-center experience. Int J Hematol. 2009;89(5):577–83 [DOI] [PubMed] [Google Scholar]
- 12.Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21(5):1046–52 [DOI] [PubMed] [Google Scholar]
- 13.Schmitz N, Zeynalova S, Glass B, Kaiser U, Cavallin-Stahl E, Wolf M, et al. CNS disease in younger patients with aggressive B-cell lymphoma: an analysis of patients treated on the Mabthera International Trial and trials of the German High-Grade Non-Hodgkin Lymphoma Study Group. Ann Oncol. 2012;23(5):1267–73 [DOI] [PubMed] [Google Scholar]
- 14.Wilson WH, Grossbard ML, Pittaluga S, Cole D, Pearson D, Drbohlav N, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002;99(8):2685–93 [DOI] [PubMed] [Google Scholar]
- 15.Wilson WH, Dunleavy K, Pittaluga S, Hegde U, Grant N, Steinberg SM, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26(16):2717–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swerdlow SH, Campo E, Harris NL, Campo E, Harris NL, Jaffe ES, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed Lyon: International Agency for Research on Cancer; 2008 [Google Scholar]
- 17.Kraan J, Gratama JW, Haioun C, Orfao A, Plonquet A, Porwit A, et al. Flow cytometric immunophenotyping of cerebrospinal fluid. Curr Protoc Cytom. 2008;Chapter 6:Unit 6 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glass B, Kloess M, Bentz M, Schlimok G, Berdel WE, Feller A, et al. Dose-escalated CHOP plus etoposide (MegaCHOEP) followed by repeated stem cell transplantation for primary treatment of aggressive high-risk non-Hodgkin lymphoma. Blood. 2006;107(8):3058–64 [DOI] [PubMed] [Google Scholar]
- 19.Wilson WH, Jung SH, Porcu P, Hurd D, Johnson J, Martin SE, et al. A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. 2012;97(5):758–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magrath I, Adde M, Shad A, Venzon D, Seibel N, Gootenberg J, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14(3):925–34 [DOI] [PubMed] [Google Scholar]
- 21.Thomas DA, Cortes J, O’Brien S, Pierce S, Faderl S, Albitar M, et al. Hyper-CVAD program in Burkitt’s-type adult acute lymphoblastic leukemia. J Clin Oncol. 1999;17(8):2461–70 [DOI] [PubMed] [Google Scholar]
- 22.Hoelzer D, Ludwig WD, Thiel E, Gassmann W, Loffler H, Fonatsch C, et al. Improved outcome in adult B-cell acute lymphoblastic leukemia. Blood. 1996;87(2):495–508 [PubMed] [Google Scholar]
- 23.Agresti A. Categorical data analysis. New York: John Wiley and Sons, Inc., 1990 [Google Scholar]
- 24.Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 – the Southwest Oncology Group. J Clin Oncol. 2009;27(1):114–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sancho JM, Orfao A, Quijano S, Garcia O, Panizo C, Perez-Ceballos E, et al. Clinical significance of occult cerebrospinal fluid involvement assessed by flow cytometry in non-Hodgkin’s lymphoma patients at high risk of central nervous system disease in the rituximab era. Eur J Haematol. 2010;85(4):321–8 [DOI] [PubMed] [Google Scholar]
- 26.Benevolo G, Stacchini A, Spina M, Ferreri AJ, Arras M, Bellio L, et al. Final results of a multicenter trial addressing role of CSF flow cytometric analysis in NHL patients at high risk for CNS dissemination. Blood. 2012;120(16):3222–8 [DOI] [PubMed] [Google Scholar]
- 27.Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German HighGrade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113(17):3896–902 [DOI] [PubMed] [Google Scholar]
- 28.Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101(2):466–8 [DOI] [PubMed] [Google Scholar]
- 29.Feugier P, Virion JM, Tilly H, Haioun C, Marit G, Macro M, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol. 2004;15(1):129–33 [DOI] [PubMed] [Google Scholar]