Abstract
After allogeneic stem cell transplantation patients are at risk of invasive aspergillosis, especially during the period of neutropenia. Recent data suggest that impaired T-cell immune reconstitution after transplantation plays an important role in this increased risk. In this study we investigated whether Aspergillus-specific T cells are involved in the recovery from invasive aspergillosis by analyzing the Aspergillus-specific T-cell response in patients with invasive aspergillosis. In nine patients whose Aspergillus infection improved, we identified Crf1- or Catalase1-specific T cells on the basis of CD154 expression and interferon-γ production following stimulation with overlapping peptides of the A. fumigatus proteins Crf1 and Catalase1. These Aspergillus-specific T cells were induced at the moment of regression of the aspergillus lesions. Crf1- and Catalase1-specific T cells, sorted on the basis of CD154 expression at the peak of the immune response, had a T helper-1 phenotype and recognized a variety of T-cell epitopes. In contrast, in two patients with progressive invasive aspergillosis, no Crf1- or Catalase1-specific T cells were identified. These data indicate that the presence of Aspergillus-specific T cells with a T helper-1 phenotype correlates with the clearance of aspergillus infection.
Introduction
Invasive aspergillosis is a common, life-threatening complication in recipients of allogeneic stem cell transplants.1 Patients are most at risk in the neutropenic phase, although a substantial number of patients are diagnosed with invasive aspergillosis in a later phase after the transplant at a time when neutrophil granulocyte counts have recovered to normal. It is hypothesized that impaired T-cell mediated immunity after stem cell transplantation plays a role in the increased risk of aspergillus infection in these patients.
In murine studies, vaccination with Aspergillus recombinant proteins or adoptive transfer of Aspergillus-pulsed dendritic cells or Aspergillus-specific CD4+ splenocytes decreased fungal burden and improved survival after experimental aspergillus infection.2–5 In humans, it was shown that aspergillus infections after haploidentical stem cell transplantation were cleared more often in patients who were treated with T-cell lines generated by stimulation with Aspergillus crude extracts than in patients who did not receive adoptive immunotherapy.6
In healthy individuals the presence of Aspergillus-specific T cells in peripheral blood was demonstrated by stimulating peripheral blood mononuclear cells (PBMC) with Aspergillus crude extracts or conidia, A. fumigatus recombinant proteins or overlapping peptides of A. fumigatus proteins.5,7–13 We have previously identified Crf1- and Catalase1-specific T cells in healthy individuals using overlapping peptides. Crf1- and Catalase1-specific T cells recognizing a broad variety of T-cell epitopes were identified in the majority of healthy individuals and Aspergillus reactivity was shown by stimulating the T cells with Aspergillus protein extract or recombinant protein.14 This method of studying Aspergillus-specific T-cell responses, which has been demonstrated to be efficient and reliable, can also be used to study the T-cell-mediated immune response in patients with invasive aspergillosis. Previously, the Aspergillus-specific immune response in patients with aspergillosis was studied by stimulating PBMC with Aspergillus crude extracts. A lymphoproliferative response was identified in patients whose aspergillus lesions regressed; however, the kinetics of Aspergillus-specific T-cell responses were not studied in detail.5 When Aspergillus-specific T cells are important in the prevention or clearance of aspergillosis, there could be a role for adoptive immunotherapy in managing this invasive infection.
In this study in patients with invasive aspergillosis, we demonstrate the induction of Crf1- or Catalase1-specific CD4+ T cells coinciding with the regression of aspergillus lesions. These data support the hypothesis that Aspergillus-specific T cells with a T helper 1 phenotype play an important role in the prevention and/or clearance of aspergillus infection.
Methods
Aspergillus antigens and synthetic peptides
Overlapping peptides of Aspergillus proteins Crf1 and Catalase1 were synthesized by JPT Peptide Technologies (Berlin, Germany). To confirm the specificity of the T-cell clones, synthetic peptides were made at the Leiden University Medical Center (LUMC, Leiden, The Netherlands). For the production of Catalase1 recombinant protein, three Catalase1 fragments were generated with a 12-amino acid overlap. We used the A. fumigatus strain CBS144.89 for the in-house preparation of Aspergillus crude extract. Commercially available crude extracts of strain CBS192.65 (HAL Allergy, Leiden, The Netherlands) and strain CBS545.65 (Allergon, Ängelholm, Sweden) were also used (see the Online Supplementary Methods for details).
Flowcytometry
All studies were conducted with the approval of the institutional review board of the LUMC and after obtaining informed consent from the patients.
Peripheral blood samples were obtained from patients before and at regular intervals after allogeneic stem cell transplantation and cryopreserved until further use. PBMC (0.5×106) were stimulated with overlapping peptide pools (10−6M) in 96-well plates, cultured for 7 days in 150 μL T-cell medium consisting of Iscove’s Modified Dulbecco’s Medium (IMDM, Lonza, Breda, The Netherlands), supplemented with 5% fetal calf serum (Gibco, Invitrogen, Bleiswijk, The Netherlands), 5% human serum and 100 IU/mL interleukin (IL)-2 (Novartis, Emeryville, CA, USA), and restimulated with non-loaded or peptide-pulsed autologous PBMC (0.5×106). One hour after restimulation, 10 μg/mL brefeldin A (Sigma-Aldrich, Zwijndrecht, The Netherlands) was added to promote intracellular accumulation of cytokines. Five hours after restimulation, cells were stained with peridinin chlorophyll-labeled anti-CD4 (BD/Pharmingen, Breda, The Netherlands), fixed with paraformaldehyde 1% (pharmacy LUMC) and permeabilized with saponin 0.1% (Sigma-Aldrich). Phycoerythrin-labeled anti-CD154 (Beckman Coulter, Woerden, The Netherlands) and allophycocyanin-labeled anti-interferon (IFN)γ (BD/Pharmingen) were added for intracellular staining of IFNγ production and the activation marker CD154. Cells were collected and analyzed on a Calibur II (BD, Breda, The Netherlands).
Generation of T-cell clones
PBMC (0.5×106) were stimulated with overlapping peptide pools (10−6 M) in a 96-well plate, cultured for 7 days in 150 μL T-cell medium and restimulated with peptide-pulsed autologous PBMC (0.5×106). Anti-CD40 antibody (1 μg/mL) was added and 48 h after restimulation Aspergillus-specific T cells were selected on the basis of CD154 expression, by sorting one cell per well by fluorescence activated cell sorting (FACS) after staining with phycoerythrin-labeled anti-CD154 (Beckman Coulter) and fluorescein isothiocyanate-labeled anti-CD4 (BD/Pharmingen).14
Epitope identification and determination of Aspergillus reactivity and HLA-restriction
For epitope identification, T-cell clones were stimulated with an autologous Epstein-Barr virus-transformed B lymphoblastoid cell line (EBV-LCL, responder:stimulator ratio 1:4) loaded with subpools (10−6M) of overlapping peptides. To confirm the identified epitopes, clones were tested against single peptides.
To determine Aspergillus reactivity, peptide-specific clones were stimulated with an autologous EBV-LCL (responder:stimulator ratio 1:4) loaded with recombinant protein (100 μg/mL) or with autologous monocyte-derived dendritic cells preloaded with Aspergillus crude extract (responder:stimulator ratio 1:4) (see the Online Supplementary Methods for details). To determine the HLA-restriction, we used HLA-blocking antibodies, an HLA-typed EBV-LCL panel and HLA-class II-negative Hela cells transduced with relevant HLA-DR, -DQ or -DP molecules14 (see the Online Supplementary Methods for details).
Results
Clinical characteristics of the patients
For inclusion in this study, we screened 33 patients who were diagnosed with probable or proven invasive aspergillosis after allogeneic stem cell transplantation, according to revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group (EORTC/MSG) consensus group.15 Twenty-two patients could not be included in the study. The median survival period of these 22 patients was 4 weeks (range, 1 week to 3 months). Due to the short survival period no peripheral blood samples could be collected from 13 of these patients. Nine patients had no T cells in this short follow-up period after invasive aspergillosis and analysis of the presence of Aspergillus-specific T cells was not, therefore, possible. Of the patients not included in the study, 15 had progressive invasive aspergillosis, six had stable aspergillus infection and in one patient the aspergillus infection was improving.
For the remaining 11 patients blood samples with sufficient T cells for analysis were available after aspergillosis and these patients were included in this study. The median survival period of the patients included was 9 months (range, 4 months to more than 6 years). The characteristics of the 11 patients analyzed are summarized in Table 1. The microbiological diagnosis was made on the basis of A. fumigatus culture and Galactomannan in serum or broncho-alveolar lavage fluid; A. fumigatus polymerase chain reaction analysis and information on β-Glucan were not available. One patient (FBV) underwent a lung biopsy and was diagnosed with proven invasive aspergillosis. Nine patients with a probable invasive aspergillosis had a positive test for Galactomannan in serum, broncho-alveolar lavage fluid or both, and one patient (ESF) was diagnosed with probable invasive aspergillosis on the basis of repeated positive sputum cultures for A. fumigatus. All patients had typical computed tomography (CT) findings. Aspergillus infection was diagnosed 2 weeks to 10 months after allogeneic stem cell transplantation and antifungal treatment was started with voriconazole in eight patients, liposomal amphotericin B in two patients, and caspofungin in one patient. In the case of lack of improvement of aspergillus infection, azole-resistance was tested, voriconazole serum levels were measured and antifungal treatment was optimized by increasing the dose or changing the antifungal medication (Table 1).
Table 1.
Characteristics of the patients with invasive aspergillosis.
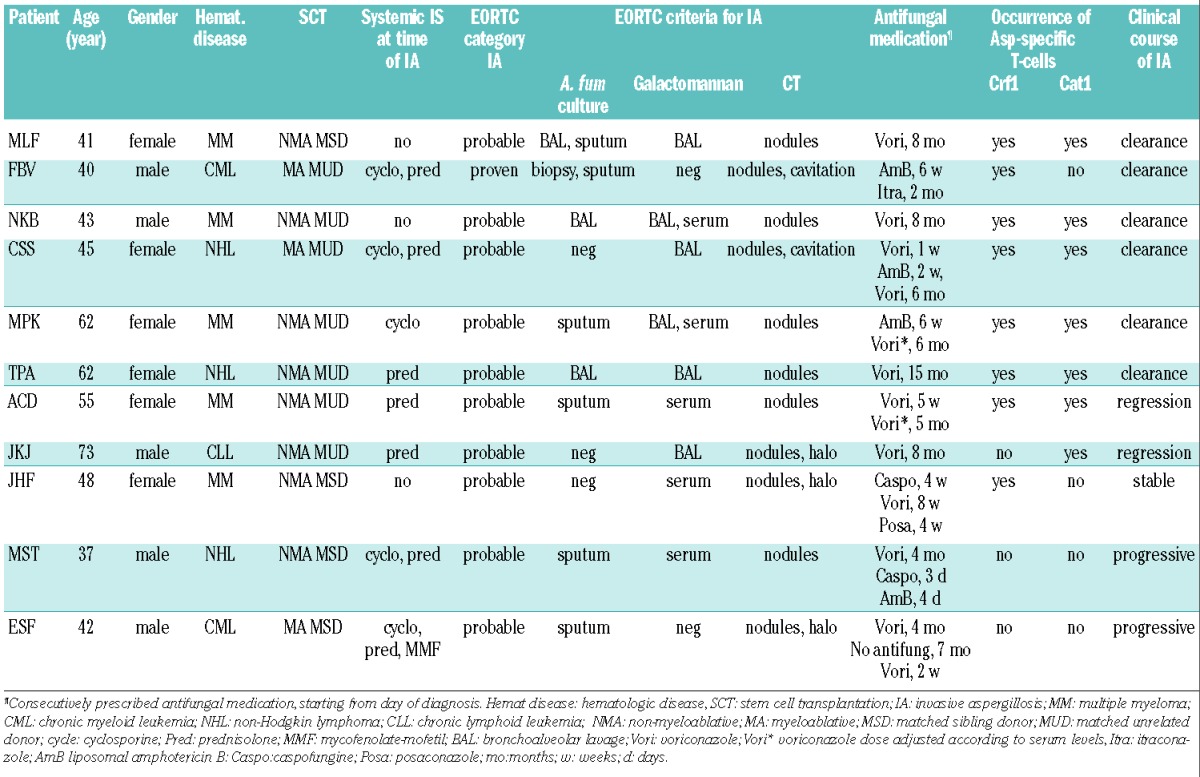
All patients had received a T-cell depleted allogeneic stem cell transplant because of a hematologic malignancy. In four cases the graft was from a matched sibling donor, whereas in seven cases it was from a matched unrelated donor. The myeloablative conditioning regimen consisted of total body irradiation (9 Gy), whereas non-myeloablative conditioning therapy consisted of fludarabine (150 mg/m2), busulfan (6.4 mg/kg body weight) and either antithymocyte globulin (20 mg/kg body weight) or alemtuzumab (30 mg). Nine patients needed systemic immunosuppression for post-transplantation graft-versus-host disease (GvHD) or auto-immune phenomena (Table 1).
Crf1- and Catalase1-specific T cells develop in patients with invasive aspergillosis, coinciding with the regression of aspergillus lesions
The frequencies of Aspergillus-specific T cells at several time points before and after the diagnosis of invasive aspergillosis were analyzed by flow cytometry. IFNγ production and CD154 expression in CD4+ T cells were measured after stimulating and restimulating PBMC with Crf1 or Catalase1 overlapping peptides.
FACS-plots, imaging studies and graphs summarizing the kinetics of Aspergillus-specific T cells from two representative patients with improvement of invasive aspergillosis are shown in Figure 1. In patient MLF a steep increase was seen in the frequencies of Crf1- and Catalase1-specific T cells, within 3 weeks after the diagnosis of invasive aspergillosis (Figure 1A). The frequencies of Crf1- and Catalase1-specific T cells peaked at the moment of regression of the aspergillus lesion on the CT scan (Figure 1D) and, after clearance of the infection, decreased to undetectable levels (Figure 1A–C). The rise and decline of Crf1- and Catalase1-specific T cells showed a similar pattern, when analyzed on the basis of CD154 expression (Figure 1B) or IFNγ production (Figure 1C). During the course of her aspergillus infection patient MLF used topical immunosuppressive medication because of GvHD, and did not experience leukopenia or granulocytopenia (Figure 1B,C).
Figure 1.
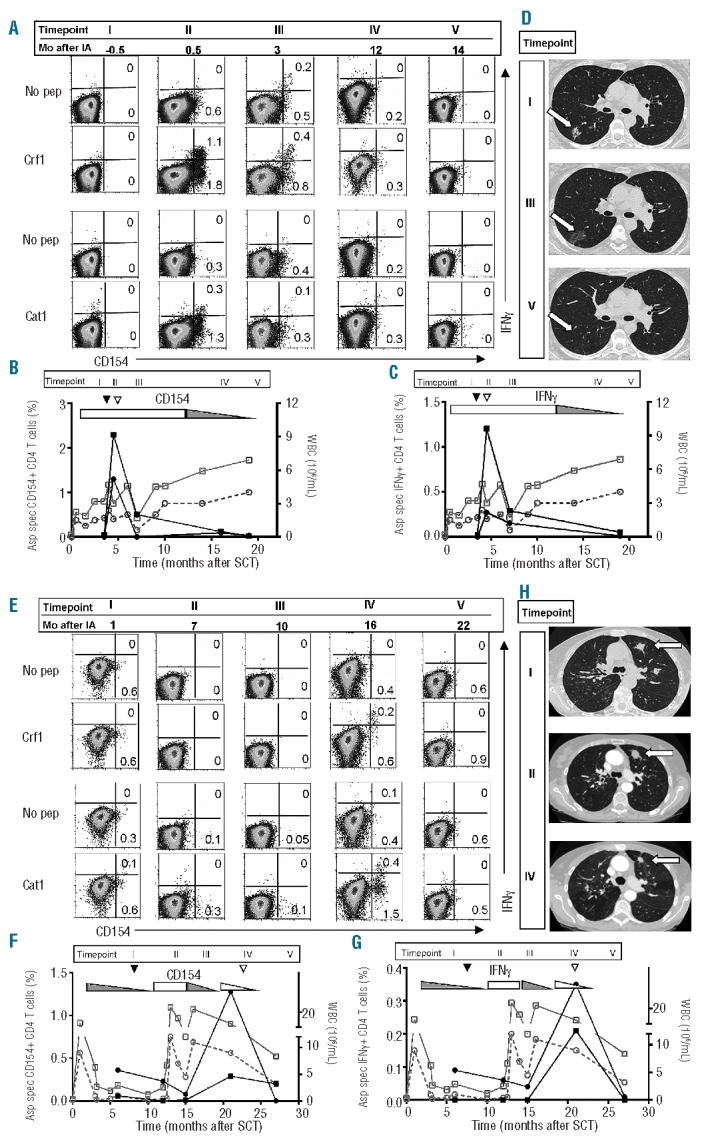
Aspergillus-specific T-cell responses and imaging studies in patients with improvement of invasive aspergillosis. FACS-analyses imaging studies and kinetics of the Aspergillus-specific T-cell responses of patient MLF (A–D) and patient TPA (E–H). (A and E) FACS analyses were performed on PBMC that were stimulated with Crf1 or Catalase1, and restimulated with Crf1- or Catalase1-pulsed PBMC or with unpulsed PBMC (no pep). T cells were analyzed 5 h after restimulation and FACS-plots are gated on CD4+ T cells. The kinetics of the percentages of Crf1- (■) and Catalase1-specific (•) CD154+ CD4+ T-cells (left y-axis, B and F) and IFNγ+ CD4+ T cells (left y-axis, C and G) and absolute leukocyte (□) and granulocyte counts (○) (right y-axis) are shown before and after diagnosis (▼) and improvement (▽) of aspergillus infection. Use of topical (▭) and systemic (▬) immunosuppression and lowering of the dose (◺,◣) during the study period are depicted in the graphs. Percentages of Aspergillus-specific CD154+ CD4+ T cells were calculated as % CD154+ of CD4+ T cells after restimulation with peptide-pulsed PBMC minus % CD154+ of CD4+ T cells after restimulation with unpulsed PBMC. The same method of calculation was used for IFNγ+ CD4+ T cells. Pulmonary CT scans of patient MLF (D) and patient TPA (H) at different time points after diagnosis show the changes in the size of aspergillus lesions during the course of infection Aspergillus lesions on the CT scans are indicated with an arrow. The timepoints indicated by Roman numerals in the top of the graphs in (B) and (C) and in (F) and (G) correspond to the time points of FACS analyses and CT scans in respectively (A) and (D), and (E) and (H). In figure (A) and (E) the corresponding period in months after invasive aspergillosis (Mo after IA) is given for every timepoint. SCT: stem cell transplantation; WBC: white blood cells.
Patient TPA was treated for invasive aspergillosis for more than a year because of a prolonged period of leukopenia and granulocytopenia (Figure 1F,G). During this period she was treated alternately with systemic and topical immunosuppression for GvHD (figure 1F,G). More than 1 year after the diagnosis of invasive aspergillosis, 8 months after recovery of leukocyte and granulocyte counts, peaks in both Crf1- and Catalase1-specific T cells were identified on the basis of CD154 expression (Figure 1E,F) and IFNγ production (Figure 1E,G), coinciding with regression of the aspergillus lesions (Figure 1H).
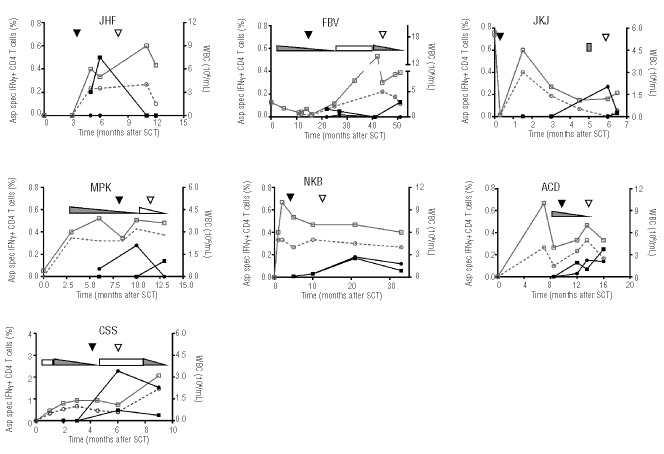
Aspergillus-specific T cells were also identified in the seven other analyzed patients with improvement of aspergillus infection (Figure 2). The majority received systemic immunosuppression during the course of their aspergillus infection. In two patients (JHF and FBV) only Crf1-specific T cells were present, one patient (JKJ) had only Catalase1-specific T cells, and in four patients (NKB, MPK, ACD and CSS) both Crf1- and Catalase1-specific T cells were identified (Table 1). Peak frequencies of Crf1-and Catalase1-specific T cells occurred around the time of regression of the aspergillus lesions, and varied between 0.1% and 2.3% of CD4+ T cells on the basis of IFNγ production.
Figure 2.
Kinetics of Aspergillus-specific T cells in patients with improvement of aspergillus infection. Percentages of Crf1- (■) and Catalase1-specific (•) IFNγ+ CD4+ T cells (left y-axis) and absolute leukocyte (□) and granulocyte counts (○) (right y-axis) in seven patients before and after diagnosis (▼) and improvement (▽) of aspergillus infection. Use of topical (▭) and systemic (▬) immunosuppression and lowering of the dose (◺,◣) during the study period are depicted in the graphs. Percentages of Aspergillus-specific IFNγ+ CD4+ T cells were calculated as % IFNγ+ of CD4+ T cells after restimulation with peptide-pulsed PBMC minus % IFNγ+ of CD4+ T cells after restimulation with unpulsed PBMC
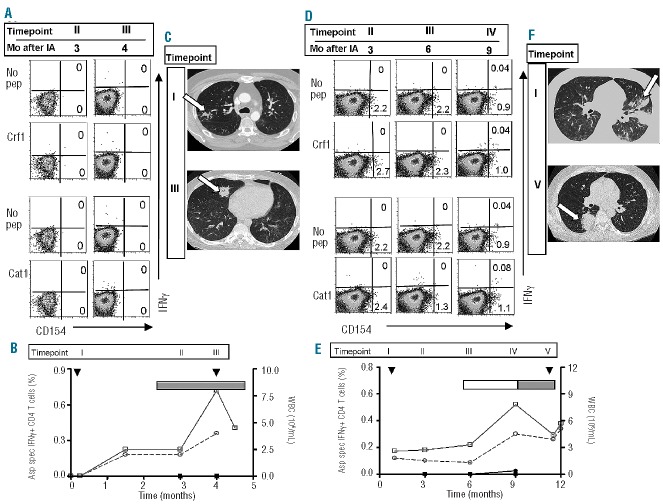
Two patients had progressive aspergillus infection. Patient MST was diagnosed with a probable invasive aspergillosis 1 week before allogeneic stem cell transplantation and was treated with antifungal medication. At that moment sputum cultures were positive for A. fumigatus, but serum Galactomannan was negative. Despite recovery of leukocyte and granulocyte counts (Figure 3B), the patient developed new nodular lesions on the CT scan 4 months later, with positive serum Galactomannan (Figure 3C). A. fumigatus and Rhizomucor were grown from a sputum culture. At this moment the patient had GvHD of the skin, liver and colon for which he was treated with prednisolone and cyclosporine. The patient died of respiratory failure and refractory shock due to progressive fungal infection and suspected bacterial superinfection. No Crf1-or Catalase1-specific T cells could be identified on the basis of CD154 expression or IFNγ production (Figure 3A,B). In patient ESF possible invasive aspergillosis was diagnosed 2 weeks after allogeneic stem cell transplantation. This patient was treated for 4 months until chest X-rays normalized. Seven months later, he had a progressive, probable aspergillosis with positive A. fumigatus sputum cultures and new lesions on a CT scan (Figure 3F), despite normal leukocyte and granulocyte counts (Figure 3E). The patient had been treated with topical and systemic immunosuppression because of skin GvHD, which eventually worsened with development of GvHD of the liver. Shortly after, he deteriorated and died of brain herniation due to an intracerebral bleed. Retrospectively, very low frequencies of Catalase1-specific T cells were identified, 0.04% of CD4+ T cells, on the basis of IFNγ production, 3 months before his death (Figure 3D,E). No samples were available from the last period of his life. To exclude that the absence of Aspergillus-specific T cells was caused by the limited number of antigens used for stimulation, we stimulated PBMC with peptide pools of four different Aspergillus proteins. After stimulation and restimulation with Aspf1, Aspf2, Aspf3 and Aspf4 no Aspergillus-specific T cells were identified in patients MST and ESF (Online Supplementary Figure S1).
Figure 3.
Aspergillus-specific T-cell responses and imaging studies in patients with progressive invasive aspergillosis. FACS analyses, imaging studies and kinetics of the Aspergillus-specific T-cell responses of patient MST (A–C) and ESF (D–F). (A and D) FACS analyses were performed on PBMC after restimulation with Crf1- or Catalase1-pulsed PBMC or with unpulsed PBMC (no pep). T cells were analyzed 5 h after restimulation and FACS-plots are gated on CD4+ T cells. (B and E) Percentages of Crf1- (■) and Catalase1-specific (•) IFNγ+ CD4+ T cells (left y-axis) and absolute leukocyte (□) and granulocyte counts (○) (right y-axis) after diagnosis (▼) of aspergillus infection. Use of topical (▭) and systemic (▬) immunosuppression during the study period is depicted in the graphs. Percentages of Aspergillus-specific IFNγ+ CD4+ T cells were calculated as % IFNγ+ of CD4+ T cells after restimulation with peptide-pulsed PBMC minus % IFNγ+ of CD4+ T cells after restimulation with unpulsed PBMC. Pulmonary CT scans of patient MST (C) and ESF (F) show progressive disease with new nodular lesions at later time points. Aspergillus lesions on the CT scans are indicated with an arrow. The time points indicated by Roman numerals in the top of the graphs in (B) and (E) correspond to the time points of FACS analyses and CT scans in, respectively, (A) and (C), and (D) and (F). In (A) and (D) the corresponding period in months after invasive aspergillosis (Mo after IA) is given for every time point.
The mean percentage of Crf1-specific T cells of all analyzed patients around diagnosis was 0.03% (range, 0–0.2%), while that of Catalase1-specific T cells was 0.02% (range, 0–0.09%). The percentages of Crf1- and Catalase1-specific T cells were not changed at the first time point with normal leukocyte counts. However, around the moment of improvement of aspergillus infection, the mean percentage of Crf1-specific T cells was significantly higher (0.35%; range, 0.03–1.2%) (P<0.01). For Catalase1-specific T cells a trend towards an increase (P=0.07) was seen, with the mean percentage being 0.42% (range, 0–2.28%) (Figure 4).
Figure 4.
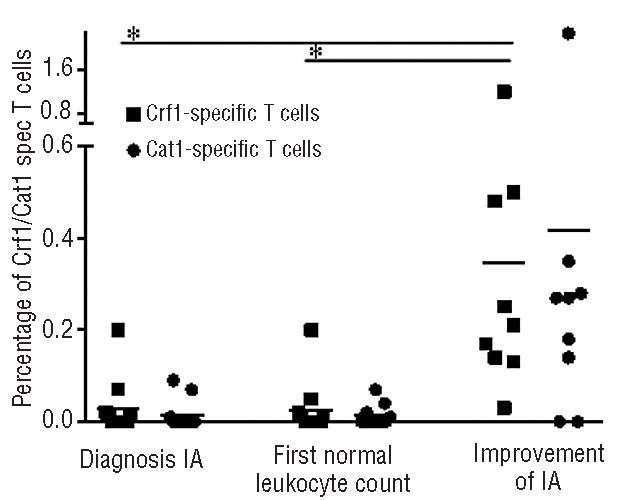
Increases of Crf1- and Catalase1-specific T cells at the time of improvement of aspergillus infection. Percentages of Crf1- (■) and Catalase1-specific (•) IFNγ+ CD4+ T cells around diagnosis of aspergillus infection (11 patients), at the first time point with normal leukocyte counts (11 patients) and at the time of improvement of aspergillus infection (9 patients). *Significant difference between compared groups (P<0.01, two-tailed Student T test) Horizontal lines indicate the mean percentage of Crf1- or Catalase1-specific T cells among all analyzed patients at this time point.
Overall, in nine of 11 analyzed patients who had an improvement of their aspergillus infection, we identified Aspergillus-specific T cells on the basis of CD154 expression and IFNγ production, with peak frequencies occurring around the time of regression of the aspergillus lesions. In two patients with progressive disease no or very low frequencies (below 0.05%) of Aspergillus-specific T cells were detected.
Aspergillus-specific T-cell clones from patients with invasive aspergillosis mainly have a T helper 1 phenotype
It has been suggested in the past that Aspergillus-specific T helper 1 cells are beneficial in invasive aspergillosis, whereas T helper 2 cells could be detrimental.2–5,16 To analyze the cytokine profiles of Aspergillus-specific T cells in patients with improvement of their aspergillus infection, we sorted single CD4+ T cells from six patients at the peak of the immune response on the basis of CD154 expression, after stimulation and restimulation with overlapping peptide pools of Crf1 and Catalase1.
From the patients we isolated 49 T-cell clones specific for either Crf1 or Catalase1, of which 19 were unique clones based on Vβ usage and specificity (Online Supplementary Table S1). To analyze the cytokine profiles of the clones, the T-cell clones were stimulated with overlapping peptide pools and production of IFNγ, IL-4, IL-10 and IL-17 was measured using standard enzyme-linked immmunosorbent assays. Of the 19 T-cell clones, 16 produced large amounts of IFNγ and a minority of the IFNγ-producing T-cell clones also produced small amounts of IL-4, IL-10 or IL-17. Two clones only produced IL-17, and one only produced IL-4 (Online Supplementary Table S1). T-cell clones with different cytokine profiles can be present in one patient; for example, in patient ACD we identified four IFNγ-producing T-cell clones and one IL-17-producing T-cell clone and in another patient (FBV) we identified two IFNγ-producing T-cell clones and one IL-4-producing clone. Epitope specificity did not correlate with cytokine profile, since we identified two T-cell clones directed against the same peptide with a different cytokine profile (amino acids 57–67, presented in HLA-DQB1*0301) (Online Supplementary Table S1).
To exclude that the T helper 1 phenotype that we found was due to the type of antigens used, we performed an additional analysis of patient TPA as well as some healthy controls, using intracellular staining for IFNγ, IL-4 and IL-5. After stimulation and restimulation with four other A. fumigatus proteins in addition to Crf1 and Catalase1, we found a T helper 1 response against these proteins in some of the healthy controls. In patient TPA we again observed a T helper 1 response against Crf1 and Catalase1, whereas no response against Aspf1, Aspf2, Aspf3 or Aspf4 was observed (data not shown).
Aspergillus-specific T cells that develop in patients recovering from invasive aspergillosis recognize a diverse repertoire of Crf1 and Catalase1 epitopes
Previously, we showed that the immune response against A. fumigatus in healthy individuals is directed against a broad variety of T-cell epitopes.14 To study the diversity of the Aspergillus-specific immune response in patients in more detail, we determined the epitope-specificity of the T-cell clones by stimulating the T cells with EBV-LCL loaded with subpools of Crf1 or Catalase1 overlapping peptides, which have been composed in a matrix, as described previously.14 As every peptide is only present in two subpools, we could identify the epitopes recognized directly from the subpool analysis. A representative example is shown in Online Supplementary Figure S2A. T-cell clone ACD3 recognized subpools 1G and 11, and therefore recognized peptide 1G11 (amino acids 333–347, DPTKIVPEEFVPITK). The T-cell epitopes were confirmed by stimulating the T-cell clones with newly synthesized peptides: an example is shown in Online Supplementary Figure S2B.
HLA restrictions of the majority of the T-cell clones were determined by using HLA-class II monoclonal antibodies and an HLA-typed EBV-LCL panel. In the case of unclear results, HLA restriction was established using HLA-transduced Hela cells. An example is shown in Online Supplementary Figure S2C. On the basis of HLA-class II monoclonal antibodies and the HLA-typed EBV-LCL panel the HLA restriction of clone ACD3 was determined to be HLA-DPB1*0401 and HLA-DPB1*0402 (data not shown). This was confirmed by stimulating the T-cell clone with peptide-loaded Hela cells transduced with different HLA-DR-, DP- or DQ-molecules expressed by individual ACD. IFNγ production was seen after stimulation with the peptide-loaded HLA-DPB1*0401 and HLA-DPB1*0402-transduced Hela cells (Online Supplementary Figure S2C). T-cell clones directed against several different epitopes of Crf1 or Catalase1 protein were identified in the majority of patients. Eight of 16 epitopes identified in these patients had been previously identified in healthy individuals (Online Supplementary Table S1).
To confirm that the Crf1- and Catalase1-specific T cells were Aspergillus-specific and developed in the immune response against A. fumigatus, we tested the Aspergillus reactivity of the T-cell clones. All Crf1-specific T-cell clones were reactive to Aspergillus crude extract (Online Supplementary Figure S2D). Five of the seven Catalase1-specific T-cell clones recognized Catalase1 recombinant protein, and three of these also recognized Aspergillus protein extract (Online Supplementary Figure S2E).
Discussion
In this study we demonstrated the induction of Crf1-and Catalase1-specific T cells in nine patients with invasive aspergillosis. The appearance of these Aspergillus-specific T cells coincided with regression of the aspergillus lesions, indicating that Aspergillus-specific T cells are important in the clearance of aspergillus infection. In two patients with progressive aspergillus infection, we were not able to identify Crf1- or Catalase1-specific T cells. In patients recovering from invasive aspergillosis, Aspergillus-specific T cells were directed against various different epitopes derived from Crf1 or Catalase1 and preferentially exerted a T helper 1 phenotype.
In previous studies low frequencies of Aspergillus-specific T cells were identified in the peripheral blood of healthy individuals stimulated with conidia of A. fumigatus,5,7,10 A. fumigatus crude extract,5,8,10 A. fumigatus recombinant proteins5,7,8 or overlapping peptides of A. fumigatus proteins.9,11–14 Aspergillus-specific T-cell responses were equivalent when stimulated with Catalase1 or Crf1 recombinant protein compared to conidia7 or crude extracts,5 and we have previously demonstrated that T cells directed against A. fumigatus can be identified by using overlapping peptides of Crf1 and Catalase1.14 It was not, however, investigated whether these T cells play a role in the defense against A. fumigatus.
In this study we were able to follow, over time, 11 patients who were diagnosed with invasive aspergillosis after allogeneic stem cell transplantation. In the nine patients recovering from invasive aspergillosis we were able to show the induction of CD4+ T cells directed against the A. fumigatus proteins Crf1 and Catalase1 at the time of regression of the aspergillus lesions. There were blood samples with sufficient T cells for analysis from only two patients with progressive aspergillus infection. No Crf1- or Catalase1-specific T cells were identified in these two patients. The small size of the group of patients with progressive aspergillosis who could be analyzed for the presence of Aspergillus-specific T cells precluded a statistical analysis of the comparison between patients whose infection was progressing or recovering. However, among nine patients with lymphopenia who were screened for this study, but not included in it, eight also had progressive invasive aspergillosis, suggesting that in these patients the total absence of T cells may have played a role. Based on the amount and size of aspergillus lesions we expect no difference in the load of A. fumigatus between patients whose infection cleared and those in whom the infection progressed. Thus, the presence or absence of Aspergillus-specific T cells cannot be explained by a difference in the amount of A. fumigatus antigen present in these patients. However, in the patients with progressive infection, as well as in five of the nine patients recovering from invasive aspergillosis, levels of systemic immunosuppression were high during aspergillus infection, whereas at the moment of recovery of invasive aspergillosis doses of systemic immunosuppression were lowered. Thus, in these patients the high levels of immunosuppression probably led to the absence of Aspergillus-specific T cells, contributing to the increased risk of invasive aspergillosis. Furthermore, when comparing the frequencies of Crf1- and Catalase1-specific T cells at the moment of regression of aspergillus lesions and the frequencies at the moment of diagnosis or recovery of leukocyte count, we found a significant difference in frequencies between these time points, further pointing to a role for Aspergillus-specific T cells in the recovery from invasive aspergillosis.
Previously, Hebart et al. studied the T-cell response to Aspergillus crude extracts in patients with invasive aspergillosis.5 For the majority of patients in that study, only one time point after stem cell transplantation was analyzed, and therefore no correlation between the induction of Aspergillus-specific T cells and the regression of aspergillus lesions could be demonstrated. Furthermore, many patients in that cohort were diagnosed as having possible invasive aspergillosis, so the diagnosis was more open to doubt. Finally, the T-cell immune responses identified by Hebart et al. may have been overestimated due to reactivity to other components than A. fumigatus proteins in the crude extract.
In previous studies, Aspergillus-specific T cells from patients with a favorable response had a high IFNγ/IL-10 ratio,5 whereas high levels of IL-10 production were documented in patients with invasive aspergillosis before adoptive immunotherapy with Aspergillus-specific T cells,6 and in patients with progressive disease.5 In this study, similarly to what was demonstrated in healthy individuals,14 the majority of T-cell clones from patients recovering from invasive aspergillosis were directed against a broad diversity of Crf1 and Catalase1 epitopes and had a T helper 1 phenotype, producing large amounts of IFNγ. A selection of clones also produced IL-4, IL-10 or IL-17, but only three T-cell clones produced only IL-4 or IL-17, indicating that T regulatory cells and T helper 2 cells probably did not play a major role in these patients. Although this could be a consequence of the culture procedure or the selection method based on CD154 expression, it fits with previous data indicating that T helper 1 cells are associated with improvement of aspergillus infection,2–5,7,17 whereas T helper 2 and T helper 17 cells are associated with increased fungal growth.5,6,16,17 We were not able to select T-cell clones from the patients with progressive infection because of the lack of CD154+ T cells after stimulation with the overlapping peptides. This suggests the absence of Aspergillus-specific T cells in these patients rather than a T helper 2-skewed cytokine profile, because we could not detect an increase in CD154-expressing, non-IFNγ-producing T cells by flow cytometry. Furthermore, an additional analysis of a subset of patients using intracellular cytokine staining after stimulation with four other A. fumigatus proteins did not detect any IL-4- or IL-5-producing T cells, either in patients recovering from invasive aspergillosis or in patients with progressive disease.
Although the peaks of Aspergillus-specific T cells occurred around the time of regression of the aspergillus lesions, the sequence of events could not be established. No validated, sensitive, non-invasive diagnostic method is available to diagnose invasive aspergillosis, in contrast to, for example, cytomegalovirus infection, which can be confirmed reliably and quantitatively by determining the plasma viral load.18 In cytomegalovirus infection it has been demonstrated that the clearance of the viral infection is preceded by the occurrence of CMV-specific T cells in the peripheral blood.19 Invasive aspergillosis is diagnosed when a patient fulfills the EORTC criteria for a possible, probable or proven diagnosis of aspergillosis,15 based on clinical, microbiological and radiological data. The exact time of improvement of the aspergillus infection is hard to establish. Non-culture-based methods can be used, but are not sensitive, such as serum Galactomannan, or not validated, like Aspergillus polymerase chain reaction analysis. Radiological improvement can often only be observed on a CT scan, a diagnostic test that is not performed on a regular basis. However, it seems likely that the induction of Aspergillus-specific T cells preceded the improvement of the aspergillus infection, indicating a role for Aspergillus-specific CD4+ T cells in the clearance of invasive aspergillosis. This is also in line with the positive results obtained in several murine studies2–4,7 and a human study6 on adoptive immunotherapy for invasive aspergillosis, which demonstrated a beneficial effect of the administration of Aspergillus-specific T cells.
In summary, we analyzed the natural course of Aspergillus-specific T-cell immunity in 11 patients with invasive aspergillosis. In patients recovering from invasive aspergillosis, IFNγ-producing Crf1- or Catalase1-specific CD4+ T cells, directed against several T-cell epitopes, were identified at the time of regression of aspergillus lesions, whereas no Aspergillus-specific T cells were present in patients with progressive aspergillus infection. These data indicate that CD4+ T cells specific for A. fumigatus proteins play an important role in the recovery from invasive aspergillosis in patients with normal neutrophil granulocyte counts, and suggest that adoptive immunotherapy might be a valuable additional tool in the management of invasive aspergillosis after allogeneic stem cell transplantation.
Acknowledgments
The authors would like to thank Guido de Roo, Menno van der Hoorn and Erwin de Haas for expert technical assistance, and Arend Mulder for providing the monoclonal antibodies used for the HLA-blocking experiments.
This work was supported by “Doelfonds Leukemie of the Bontius Stichting of the Leiden University Medical Center”.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Pagano L, Caira M, Nosari A, Van Lint MT, Candoni A, Offidani M, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study – Sorveglianza epidemiologica infezioni fungine nelle emopatie maligne. Clin Inf Dis. 2007;45(9):1161–70 [DOI] [PubMed] [Google Scholar]
- 2.Bozza S, Gaziano R, Lipford GB, Montagnoli C, Bacci A, di Francesco P, et al. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 2002;4(13):1281–90 [DOI] [PubMed] [Google Scholar]
- 3.Bozza S, Perruccio K, Montagnoli C, Gaziano R, Bellocchio S, Burchielli E, et al. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood. 2003;102(10):3807–14 [DOI] [PubMed] [Google Scholar]
- 4.Cenci E, Mencacci A, Bacci A, Bistoni F, Kurup VP, Romani L. T cell vaccination in mice with invasive pulmonary aspergillosis. J Immunol. 2000;165(1):381–8 [DOI] [PubMed] [Google Scholar]
- 5.Hebart H, Bollinger C, Fisch P, Sarfati J, Meisner C, Baur M, et al. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood. 2002;100(13):4521–8 [DOI] [PubMed] [Google Scholar]
- 6.Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106(13):4397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozza S, Clavaud C, Giovannini G, Fontaine T, Beauvais A, Sarfati J, et al. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J Immunol. 2009;183(4):2407–14 [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary N, Staab JF, Marr KA. Healthy human T-cell responses to Aspergillus fumigatus antigens. PLoS One. 2010;5(2):e9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna N, Stuehler C, Conrad B, Lurati S, Krappmann S, Einsele H, et al. Generation of a multipathogen-specific T-cell product for adoptive immunotherapy based on activation-dependent expression of CD154. Blood. 2011;118(4):1121–31 [DOI] [PubMed] [Google Scholar]
- 10.Ramadan G, Konings S, Kurup VP, Keever-Taylor CA. Generation of Aspergillus- and CMV-specific T-cell responses using autologous fast DC. Cytotherapy. 2004;6(3):223–34 [DOI] [PubMed] [Google Scholar]
- 11.Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of Th1 T cell responses directed to a HLA class II restricted epitope from the Aspergillus f16 allergen. Clin Exp Immunol. 2005;139(2):257–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuehler C, Khanna N, Bozza S, Zelante T, Moretti S, Kruhm M, et al. Cross-protective TH1 immunity against Aspergillus fumigatus and Candida albicans. Blood. 2011;117(22):5881–91 [DOI] [PubMed] [Google Scholar]
- 13.Zhu F, Ramadan G, Davies B, Margolis DA, Keever-Taylor CA. Stimulation by means of dendritic cells followed by Epstein-Barr virus-transformed B cells as antigen-presenting cells is more efficient than dendritic cells alone in inducing Aspergillus f16-specific cytotoxic T cell responses. Clin Exp Immunol. 2008;151(2):284–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jolink H, Meijssen IC, Hagedoorn RS, Arentshorst M, Drijfhout JW, Mulder A, et al. Characterization of the T-cell-mediated immune response against the Aspergillus fumigatus proteins Crf1 and Catalase 1 in healthy individuals. J Infect Dis. 2013;208(5):847–56 [DOI] [PubMed] [Google Scholar]
- 15.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cenci E, Mencacci A, Del Sero G, Bacci A, Montagnoli C, d’Ostiani CF, et al. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J Infect Dis. 1999;180(6):1957–68 [DOI] [PubMed] [Google Scholar]
- 17.Chai LY, van d V, Marijnissen RJ, Cheng SC, Khoo AL, Hectors M, et al. Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology. 2010;130(1):46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boeckh M, Huang M, Ferrenberg J, Stevens-Ayers T, Stensland L, Nichols WG, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by realtime PCR. J Clin Microbiol. 2004;42(3):1142–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tormo N, Solano C, Benet I, Nieto J, de la CR, Garcia-Noblejas A, et al. Kinetics of cytomegalovirus (CMV) pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells during episodes of viral DNAemia in allogeneic stem cell transplant recipients: potential implications for the management of active CMV infection. J Med Virol. 2010;82(7):1208–15 [DOI] [PubMed] [Google Scholar]