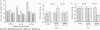Abstract
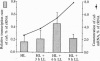
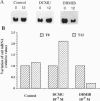
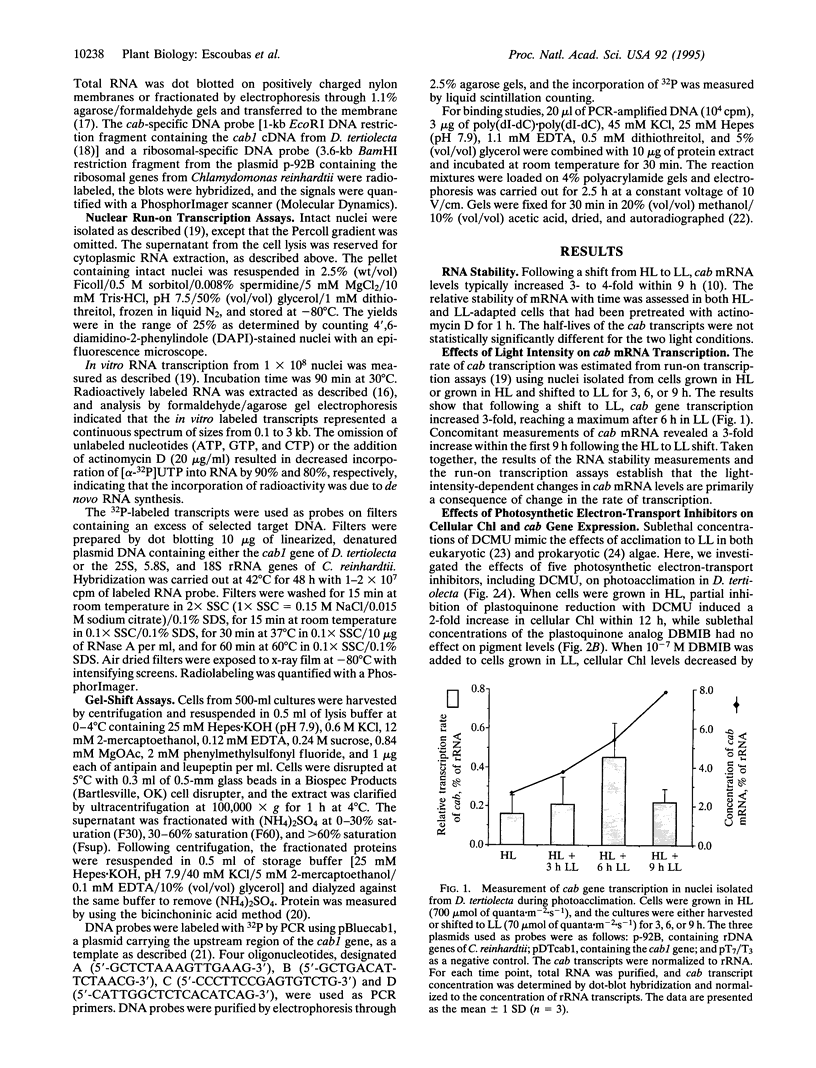
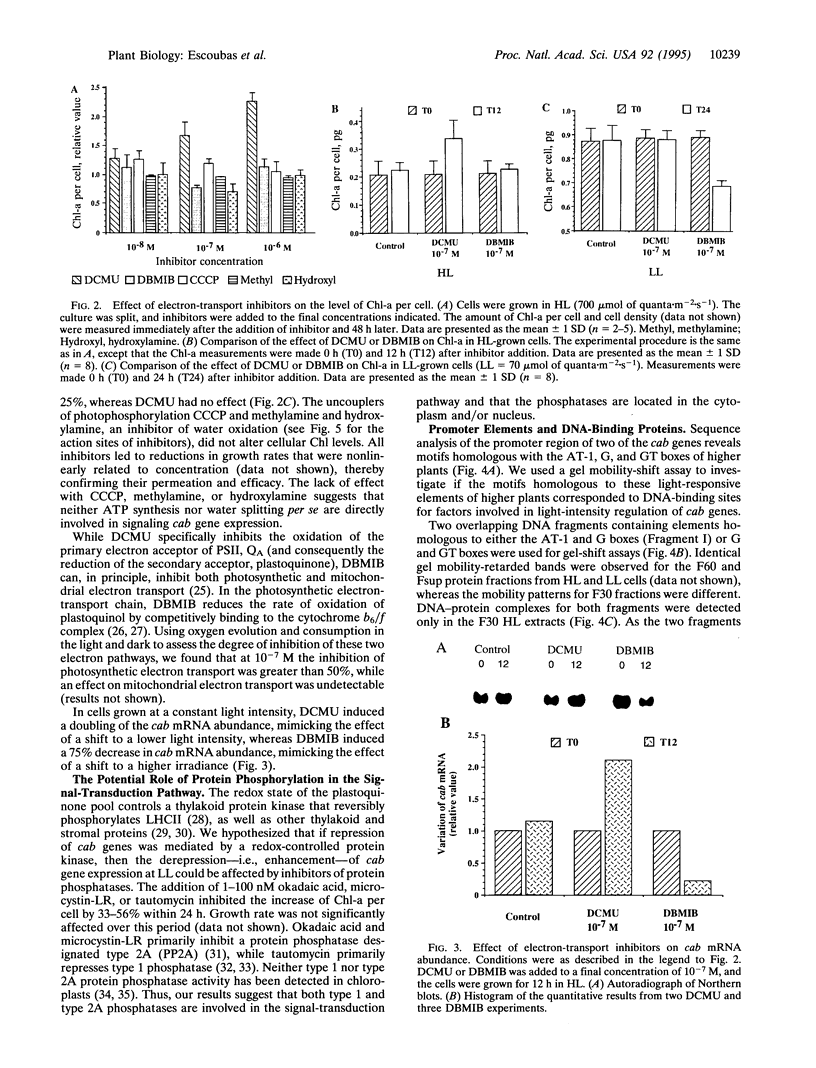
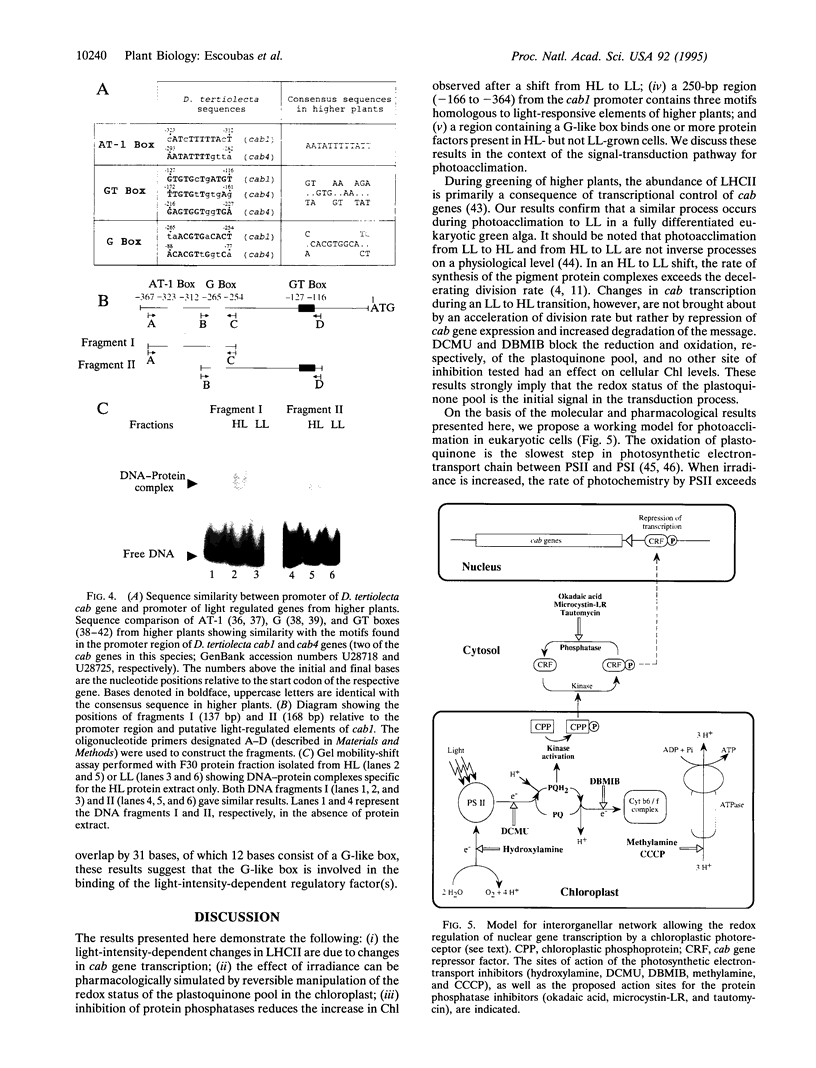
The eukaryotic green alga Dunaliella tertiolecta acclimates to decreased growth irradiance by increasing cellular levels of light-harvesting chlorophyll protein complex apoproteins associated with photosystem II (LHCIIs), whereas increased growth irradiance elicits the opposite response. Nuclear run-on transcription assays and measurements of cab mRNA stability established that light intensity-dependent changes in LHCII are controlled at the level of transcription. cab gene transcription in high-intensity light was partially enhanced by reducing plastoquinone with 3-(3,4-dichlorophenyl)-1,1-dimethyl urea (DCMU), whereas it was repressed in low-intensity light by partially inhibiting the oxidation of plastoquinol with 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB). Uncouplers of photosynthetic electron transport and inhibition of water splitting had no effect on LHCII levels. These results strongly implicate the redox state of the plastoquinone pool in the chloroplast as a photon-sensing system that is coupled to the light-intensity regulation of nuclear-encoded cab gene transcription. The accumulation of cellular chlorophyll at low-intensity light can be blocked with cytoplasmically directed phosphatase inhibitors, such as okadaic acid, microcystin L-R, and tautomycin. Gel mobility-shift assays revealed that cells grown in high-intensity light contained proteins that bind to the promoter region of a cab gene carrying sequences homologous to higher plant light-responsive elements. On the basis of these experimental results, we propose a model for a light intensity signaling system where cab gene expression is reversibly repressed by a phosphorylated factor coupled to the redox status of plastoquinone through a chloroplast protein kinase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. F. Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta. 1992 Jan 22;1098(3):275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- Beale S. I., Appleman D. Chlorophyll synthesis in chlorella: regulation by degree of light limitation of growth. Plant Physiol. 1971 Feb;47(2):230–235. doi: 10.1104/pp.47.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla P., Bennett J. Chloroplast phosphoproteins: phosphorylation of a 12-kDa stromal protein by the redox-controlled kinase of thylakoid membranes. Arch Biochem Biophys. 1987 Jan;252(1):97–104. doi: 10.1016/0003-9861(87)90012-9. [DOI] [PubMed] [Google Scholar]
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crane F. L. Hydroquinone dehydrogenases. Annu Rev Biochem. 1977;46:439–469. doi: 10.1146/annurev.bi.46.070177.002255. [DOI] [PubMed] [Google Scholar]
- Danon A., Mayfield S. P. Light-regulated translation of chloroplast messenger RNAs through redox potential. Science. 1994 Dec 9;266(5191):1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- Datta N., Cashmore A. R. Binding of a pea nuclear protein to promoters of certain photoregulated genes is modulated by phosphorylation. Plant Cell. 1989 Nov;1(11):1069–1077. doi: 10.1105/tpc.1.11.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald R. G., Schindler U., Batschauer A., Cashmore A. R. The plant G box promoter sequence activates transcription in Saccharomyces cerevisiae and is bound in vitro by a yeast activity similar to GBF, the plant G box binding factor. EMBO J. 1990 Jun;9(6):1727–1735. doi: 10.1002/j.1460-2075.1990.tb08296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Gilmartin P. M., Sarokin L., Memelink J., Chua N. H. Molecular light switches for plant genes. Plant Cell. 1990 May;2(5):369–378. doi: 10.1105/tpc.2.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G., Pichersky E., Malik V. S., Timko M. P., Scolnik P. A., Cashmore A. R. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7089–7093. doi: 10.1073/pnas.85.19.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter K., Kircher S., Frohnmeyer H., Krenz M., Nagy F., Schäfer E. Light-regulated modification and nuclear translocation of cytosolic G-box binding factors in parsley. Plant Cell. 1994 Apr;6(4):545–559. doi: 10.1105/tpc.6.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper F., Quednau B., Kortenjann M., Johanningmeier U. Control of cab gene expression in synchronized Chlamydomonas reinhardtii cells. J Photochem Photobiol B. 1991 Nov;11(2):139–150. doi: 10.1016/1011-1344(91)80256-h. [DOI] [PubMed] [Google Scholar]
- King J., Khanna V. A Nitrate Reductase-less Variant Isolated from Suspension Cultures of Datura innoxia (Mill.). Plant Physiol. 1980 Oct;66(4):632–636. doi: 10.1104/pp.66.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRoche J., Bennett J., Falkowski P. G. Characterization of a cDNA encoding for the 28.5-kDa LHCII apoprotein from the unicellular marine chlorophyte, Dunaliella tertiolecta. Gene. 1990 Nov 15;95(2):165–171. doi: 10.1016/0378-1119(90)90358-x. [DOI] [PubMed] [Google Scholar]
- Lam E., Chua N. H. GT-1 binding site confers light responsive expression in transgenic tobacco. Science. 1990 Apr 27;248(4954):471–474. doi: 10.1126/science.2330508. [DOI] [PubMed] [Google Scholar]
- Laroche J., Mortain-Bertrand A., Falkowski P. G. Light Intensity-Induced Changes in cab mRNA and Light Harvesting Complex II Apoprotein Levels in the Unicellular Chlorophyte Dunaliella tertiolecta. Plant Physiol. 1991 Sep;97(1):147–153. doi: 10.1104/pp.97.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. J., Whitmarsh J. Photosynthetic apparatus of pea thylakoid membranes : response to growth light intensity. Plant Physiol. 1989 Mar;89(3):932–940. doi: 10.1104/pp.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Siedow J. N. Regulation by redox poise in chloroplasts. Science. 1995 May 5;268(5211):695–696. doi: 10.1126/science.268.5211.695. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Beattie K. A., Klumpp S., Cohen P., Codd G. A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990 May 21;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Coggins J., Cohen P. Plant protein phosphatases. Subcellular distribution, detection of protein phosphatase 2C and identification of protein phosphatase 2A as the major quinate dehydrogenase phosphatase. Biochem J. 1991 Feb 1;273(Pt 3):733–738. doi: 10.1042/bj2730733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C., Klumpp S. Tautomycin from the bacterium Streptomyces verticillatus. Another potent and specific inhibitor of protein phosphatases 1 and 2A. FEBS Lett. 1990 Dec 17;277(1-2):137–140. doi: 10.1016/0014-5793(90)80828-7. [DOI] [PubMed] [Google Scholar]
- Manzara T., Carrasco P., Gruissem W. Developmental and organ-specific changes in promoter DNA-protein interactions in the tomato rbcS gene family. Plant Cell. 1991 Dec;3(12):1305–1316. doi: 10.1105/tpc.3.12.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell D. P., Falk S., Trick C. G., Huner NPA. Growth at Low Temperature Mimics High-Light Acclimation in Chlorella vulgaris. Plant Physiol. 1994 Jun;105(2):535–543. doi: 10.1104/pp.105.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortain-Bertrand A., Bennett J., Falkowski P. G. Photoregulation of the Light-Harvesting Chlorophyll Protein Complex Associated with Photosystem II in Dunaliella tertiolecta: Evidence that Apoprotein Abundance but Not Stability Requires Chlorophyll Synthesis. Plant Physiol. 1990 Sep;94(1):304–311. doi: 10.1104/pp.94.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sukenik A., Bennett J., Mortain-Bertrand A., Falkowski P. G. Adaptation of the Photosynthetic Apparatus to Irradiance in Dunaliella tertiolecta: A Kinetic Study. Plant Physiol. 1990 Apr;92(4):891–898. doi: 10.1104/pp.92.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G., Markwell J. Lack of Types 1 and 2A Protein Serine(P)/Threonine(P) Phosphatase Activities in Chloroplasts. Plant Physiol. 1992 Oct;100(2):620–624. doi: 10.1104/pp.100.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Mumby M. Protein serine/threonine phosphatases and cell transformation. Biochim Biophys Acta. 1993 Aug 23;1155(2):207–226. doi: 10.1016/0304-419x(93)90005-w. [DOI] [PubMed] [Google Scholar]
- Zerbib D., Prentki P., Gamas P., Freund E., Galas D. J., Chandler M. Functional organization of the ends of IS1: specific binding site for an IS 1-encoded protein. Mol Microbiol. 1990 Sep;4(9):1477–1486. [PubMed] [Google Scholar]