Abstract
Aim:
To identify a novel coumarin analogue with the highest anticancer activity and to further investigate its anticancer mechanisms.
Methods:
The viability of cancer cells was investigated using the MTT assay. The cell cycle progression was evaluated using both flow cytometric and Western blotting analysis. Microtubule depolymerization was observed with immunocytochemistry in vivo and a tubulin depolymerization assay in vitro. Apoptosis was demonstrated using Annexin V/Propidium Iodide (PI) double-staining and sub-G1 analysis.
Results:
Among 36 analogues of coumarin, 6-chloro-4-(methoxyphenyl) coumarin showed the best anticancer activity (IC50 value about 200 nmol/L) in HCT116 cells. The compound had a broad spectrum of anticancer activity against 9 cancer cell lines derived from colon cancer, breast cancer, liver cancer, cervical cancer, leukemia, epidermoid cancer with IC50 value of 75 nmol/L–1.57 μmol/L but with low cytotocitity against WI-38 human lung fibroblasts (IC50 value of 12.128 μmol/L). The compound (0.04–10 μmol/L) induced G2-M phase arrest in HeLa cells in a dose-dependent manner, which was reversible after the compound was removed. The compound (10–300 μmol/L) induced the depolymerization of purified porcine tubulin in vitro. Finally, the compound (0.04–2.5 μmol/L) induced apoptosis of HeLa cells in dose- and time-dependent manners.
Conclusion:
6-Chloro-4-(methoxyphenyl) coumarin is a novel microtubule-targeting agent that induces G2–M arrest and apoptosis in HeLa cells.
Keywords: anticancer drug, 6-chloro-4-(methoxyphenyl) coumarin, cell cycle arrest, microtubule depolymerization, apoptosis
Introduction
Coumarins are natural products widely abundant in natural sources, especially green plants. Coumarins have multiple biological activities1, including anticoagulant2, 3, 4, anti-inflammatory5, 6, antimicrobial7, 8, 9, 10, 11, 12, antioxidant13, 14, 15, 16, antiallergic17, 18, 19, 20, anti-HIV21, anticancer22, 23, 24, 25, 26, 27, 28 and antiviral activities29, 30, 31, 32. It has been suggested that alterations in the chemical structure of coumarins could change their cytotoxic properties. It has also been known for many years that coumarins have significant therapeutic potential33, 34, 35 and are present in many natural therapeutic products36, 37, 38, 39, 40. Due to their attractive properties and potential clinical utility, we synthesized a series of coumarin analogues and evaluated their anticancer properties to find a novel coumarin analogue with good anticancer activity.
In the present study, we show that 6-chloro-4-(methoxyphenyl) coumarin (CMC) has the best anticancer activity among 36 different coumarin analogues. CMC had broad-spectrum anticancer activities in 9 cancer cell lines derived from 6 different tissues. Further analysis showed that CMC caused G2-M arrest and apoptosis in HeLa cells via microtubule depolymerization.
Materials and methods
Cell culture
The colon cancer cell line LS-174t was cultured in MEM medium with 10% fetal bovine serum (Hyclone, Thermo Scientific, Logan, UT, USA). The colon cancer cell line HCT-116 was cultured in McCoy's 5A modified medium with 10% fetal bovine serum. The colon cancer cell lines Colo-205 and HCT-15, the breast cancer cell line MDA-MB-435S, and the leukemia cell line HL-60 were cultured in RPMI-1640 with 10% fetal bovine serum. The liver cancer cell line HepG2, the epidermoid cancer cell line A431 and the cervical cancer cell line HeLa were cultured in DMEM (high glucose) with 10% fetal bovine serum. All cell lines mentioned above were purchased from the Cell Bank (Chinese Academy of Sciences, Shanghai, China). A human fetal lung fibroblast cell line (WI-38) was kindly provided by Dr Mei-yu GENG (Shanghai Institute of Materia Medica) and was cultured in MEM with 10% fetal bovine serum (Gibco, Invitrogen, USA). All cells were kept in a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
Reagents
Coumarin analogues were synthesized and provided by Prof Jie WU from Fudan University. The analogues were dissolved in 100% DMSO with a 5 g/L stock solution. The working solution was prepared by dilution of the stock solution with the culture medium.
An anti-phospho-Ser/Thr-Pro MPM-2 (Cat #05-368) antibody was purchased from Millipore Corporation (Boston, MA, USA). Anti-CDC25C (Cat #4688) antibody was from Cell Signal Technology (Boston, MA, USA). Peroxidase-affiniPure goat anti-rabbit IgG (Code: 112-035-175) and goat anti-mouse IgG (Code: 115-035-174) were purchased from Jackson ImmunoResearch Laboratories, Inc (Baltimore, MD, USA). Hoechst 33342 dye (Cat #H3570) and Alexa Fluor 488 dye-labeled donkey anti-mouse IgG (Cat #A-21202) were purchased from Invitrogen Corporation (Carlsbad, CA, USA).
Cell proliferation assay
The inhibitory effects of synthesized coumarin analogues on the growth of cancer cell lines were evaluated using the MTT viability assay as described previously41. Briefly, cells (3000 cells/well) were seeded onto plastic 96-well cell culture plates and cultured at 37 °C. After 24 h, compounds with doses ranging from 10 μmol/L to 10 nmol/L at a dilution ratio of 1:4 were added, and the cells were further incubated for 72 h. MTT was then added to each well at a final concentration of 1 g/L. After a 3 h incubation at 37 °C, the medium was gently discarded and DMSO (100 μL/well) was added to dissolve the formazan product. The optical density was determined at 550 nm/690 nm using a VersaMax Microplate Reader (Molecular Devices). Experiments were performed in four replicates. IC50 values were derived from a nonlinear regression model (curvefit) based on a sigmoidal dose response curve (variable slope) and computed using Graphpad Software (Graphpad Prism version 5.02). Data were expressed as the mean±SEM.
Cell cycle analysis
As described previously42, both synchronized and asynchronized HeLa cells were treated with either DMSO (negative control), nocodazole (positive control), or CMC at 37 °C for the indicated time. The cells were then digested with 0.5 g/L trypsin, collected, washed twice with cold 1×PBS and fixed in 1 mL of 70% ethanol at 4 °C overnight. The next day, cells were washed twice with cold 1×PBS and incubated with 20 μg/mL RNase at 37 °C for 15 min. Cells were then stained with 20 mg/L PI for 30 min at 4 °C. Cell cycle distribution was determined using a BD FACSCalibur Flow Cytometer.
Cell synchronization
HeLa cells were synchronized using a double thymidine block as described previously43. Briefly, 1.5×105 cells were seeded in each well of a 6-well cell culture plate. The next day, a double thymidine block was performed with an initial block for 17 h and a 10 h release and was followed by a second block for 16 h. The final concentration of thymidine used in the block medium was 2 mmol/L. Following release from the second block, synchronized cells were treated with either DMSO (negative control), nocodazole (positive control) or CMC for the indicated times, and samples were collected for flow cytometry analysis.
Western analysis of G2-M regulatory proteins
HeLa cells were treated with varying doses of CMC for 24 h. Cells were then lysed with cell lysis buffer [1% NP-40, 150 mmol/L NaCl, 20 mmol/L Tris-HCl, 1 mmol/L EDTA, 1 mmol/L EGTA and complete protease inhibitor cocktail (Cat #11697498001, Roche)]. Equal amounts of protein were resolved by SDS-polyacrylamide gel electrophoresis, transferred onto Hybond-C nitrocellulose membranes (GE Life Sciences) and immunoblotted as described previously44. Immunoreactive bands were detected with the enhanced chemiluminescence (ECL) system (GE Life Sciences).
Immunocytochemistry assay
Microtubules were observed using an immunocytochemistry assay45, 46. Briefly, HeLa cells were grown on glass coverslips for 24 h and then treated with varying doses of CMC for 8 h. Cells were then fixed with cold methanol (4 °C) for 5 min, blocked for 1 h with 5% BSA in 1×PBS at room temperature and incubated with monoclonal β-tubulin antibody (T-4026; Sigma, St Louis, MO, USA) overnight at 4 °C. Cells were then washed three times with 1×PBS and incubated with Alexa Fluor 488-labeled donkey anti-mouse IgG (Invitrogen) at room temperature for 1 h. The coverslips were washed, stained with 5 mg/L Hoechst 33342 dye (Molecular Probes, Invitrogen) and photographed using an Olympus confocal microscope (Olympus, Tokyo, Japan).
In vitro tubulin polymerization assay
An in vitro fluorescence-based tubulin polymerization assay kit (BK011, Cytoskeleton, Inc) was used according to the manufacturer's protocol for monitoring the time-dependent polymerization of tubulin to microtubules. The reaction mixture had a final volume of 50 μL in PEM buffer (80 mmol/L PIPES, 0.5 mmol/L EGTA, 2 mmol/L MgCl2, pH 6.9) and contained 2 g/L bovine brain tubulin, 10 μmol/L fluorescent reporter and 1 mmol/L GTP in either the presence or absence of test compounds at 37 °C. Tubulin polymerization was followed by monitoring fluorescence enhancement due to the incorporation of a fluorescent reporter into microtubules as polymerization proceeded. Fluorescence emission at 450 nm (excitation wavelength of 360 nm) was measured for 1 h at 0.5 min intervals in a FlexStation 3 Microplate Reader (Molecular Devices). Nocodazole was used as positive control.
Apoptosis detection assay
The quantitative assessment of apoptosis was determined using Annexin V-FITC and PI double staining. Annexin V binds to phosphatidylserine (PS) and other negatively charged phospholipids, thereby producing fluorescence primarily indicative of PS translocation from the inner to the outer cell membrane leaflet. This change reflects aminophospholipid translocase activity in apoptotic cells47. PI is a nucleic acid dye that penetrates the nuclear envelope of necrotic cells and was used here as a counterstain to differentiate between live, apoptotic, late-stage apoptotic/early stage necrotic and necrotic cells. Briefly, HeLa cells were treated with varying doses of either CMC or 1 μmol/L stauporine for the indicated times and were then stained with an Annexin V-FITC/PI double staining kit (KGA108, Kaiji Bio Co, Nanjing, China). After washing twice with cold 1×PBS, 5×105 cells were collected, resuspended in 500 μL binding buffer with 0.1 g/L Annexin V-FITC and 0.05 g/L PI, and then incubated for 15 min in the dark at room temperature. Finally, the percent of apoptotic cells was immediately measured with a BD FACS Calibur Flow Cytometer and analyzed with CellQuest software (BD Biosciences).
Results
CMC (compound 8) showed the best anticancer activity in vitro among the synthesized coumarin analogues
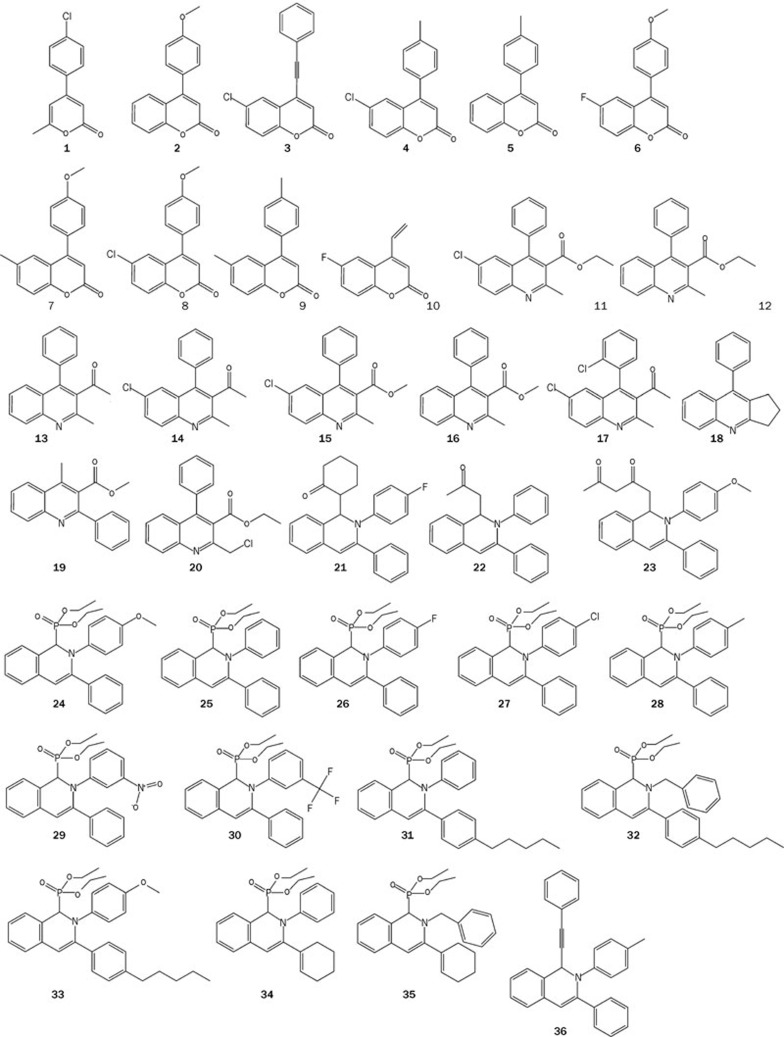
The anticancer activities of different synthesized coumarin analogues were evaluated in HCT116 colon cancer cells using the MTT viability assay. The corresponding chemical structures are shown in Figure 1, and the anticancer activities against HCT116 cells are shown in Table 1. Among the coumarin analogues, CMC (compound 8) had the best anticancer activity with an IC50 value of approximately 200 nmol/L and was selected for further mechanistic study.
Figure 1.
The chemical structures of synthesized coumarin analogues.
Table 1. The in vitro anti-proliferation activities of 36 coumarin analogues in HCT116 colorectal carcinoma cells.
| Compound ID | IC50 (μmol/L)* |
|---|---|
| 1 | 28.153±2.130 |
| 2 | 1.237±0.159 |
| 3 | 7.809±0.492 |
| 4 | 21.307±1.736 |
| 5 | 33.893±2.764 |
| 6 | 1.661±0.266 |
| 7 | 0.248±0.049 |
| 8 | 0.202±0.038 |
| 9 | 16.297±1.087 |
| 10 | 23.211±1.236 |
| 11 | 28.678±3.109 |
| 12 | 29.303±2.622 |
| 13 | 10.133±1.041 |
| 14 | 34.203±3.131 |
| 15 | 31.893±3.503 |
| 16 | 14.806±1.500 |
| 17 | 10.130±1.120 |
| 18 | 27.552±2.772 |
| 19 | 34.145±3.894 |
| 20 | 3.177±0.200 |
| 21 | 40.520±6.534 |
| 22 | 31.859±3.403 |
| 23 | 16.720±1.653 |
| 24 | 13.440±1.112 |
| 25 | 17.468±0.753 |
| 26 | 13.892±0.576 |
| 27 | 13.897±0.623 |
| 28 | 12.273±0.713 |
| 29 | 12.535±0.631 |
| 30 | 11.548±0.909 |
| 31 | 14.906±0.592 |
| 32 | 9.579±0.441 |
| 33 | 6.896±0.416 |
| 34 | 21.314±0.779 |
| 35 | 22.095±1.536 |
| 36 | 8.183±0.704 |
| doxorubicin | 0.061±0.006 |
*Cell proliferation assay was done according to the method mentioned in the Materials and methods section. The IC50 values represent the mean±SEM of quadruplicate determinations.
CMC exhibited very potent anticancer activity against different cancer cell lines
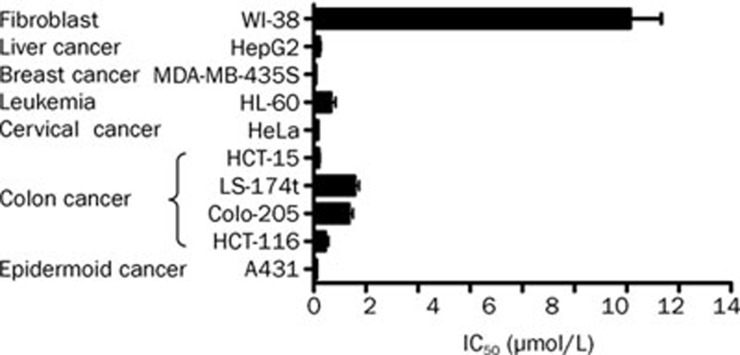
The effect of CMC on the viability of 9 human cancer cell lines derived from 6 different tissues was evaluated using an MTT assay. As shown in Figure 2, CMC exhibited very potent anticancer activity. The IC50 values for CMC ranged from 75 nmol/L to 1.57 μmol/L, and the average IC50 value was approximately 0.53 μmol/L. Then the selective cytotoxicity of CMC was further evaluated using human normal fetal fibroblast cell line WI-38. CMC exerted markedly weaker cytotoxicity against WI-38 cells with an IC50 value of approximately 12.128 μmol/L than against other 9 cancer cell lines.
Figure 2.
CMC had good anticancer activity in 9 different cancer cell lines. The viability of 9 cancer cell lines and 1 human fetal lung fibroblast cell line was assessed by MTT assay after 72 h of treatment with CMC. All results are expressed as the mean±SEM of four independent experiments.
CMC specifically and reversibly induced G2-M phase arrest in HeLa cells
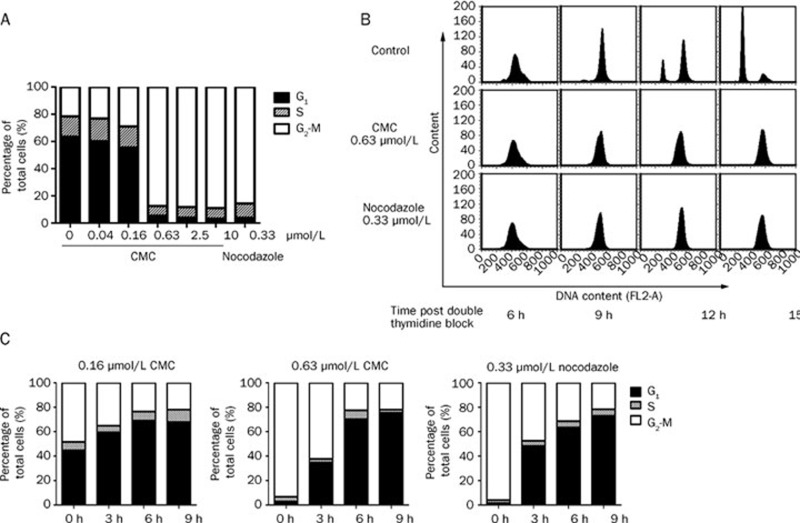
Using brightfield microscopy, we found that treatment with CMC caused detachment of adherent cancer cells. The cells became round (data not shown), a phenomenon that occurs during mitosis. To test the possibility that CMC affects mitosis, the effect of CMC on cell cycle progression in HeLa cells was examined. First, HeLa cells were treated with CMC at different concentrations for 24 h. As shown in Figure 3A, CMC treatment resulted in a dose-dependent accumulation of HeLa cells in G2-M phase with concomitant losses from G0-G1 phase. No change in S-phase was observed.
Figure 3.
CMC-treated HeLa cells specifically and reversibly arrested in G2-M phase. (A) HeLa cells arrested in G2-M phase in a dose-dependent manner. HeLa cells were treated with CMC at doses ranging from 10 μmol/L to 0.04 μmol/L for 24 h. 0.33 μmol/L nocodazole was used as a positive control. The samples were fixed, stained with PI, and analyzed using flow cytometry. (B) HeLa cells were specifically arrested in G2-M phase. To evaluate if CMC only induced G2-M arrest, HeLa cells were synchronized at the G1/S border using a thymidine-thymidine block. The cells were then released and treated with 0.63 μmol/L CMC. Samples were collected at 6 h, 9 h, 12 h, and 15 h and then subjected to flow cytometry analysis. (C) HeLa cells could re-enter the cell cycle following deprivation of CMC. HeLa cells were treated with either CMC or nocodazole (positive control) for 12 h. The medium containing CMC was then removed and fresh medium was added. Samples were collected at 0 h, 3 h, 6 h, and 9 h after deprivation of CMC and then subjected to flow cytometry analysis. All of the data shown are representative of three independent experiments with similar results.
To examine the specificity of the CMC-elicited mitotic arrest, HeLa cells were synchronized at the G1/S boundary by double thymidine block and were then treated with either 0.63 μmol/L CMC or 0.33 μmol/L nocodazole (positive control) immediately following their release from the block. Flow cytometry analysis was conducted to examine cell cycle progression of CMC-treated cells. Within 6 h and 9 h post-release, CMC-treated cells entered S phase and G2 phase, respectively, just as did the control cells. However, at the 12 h time point after release, CMC-treated cells were arrested at mitosis in striking contrast to the entrance of control cells into the next cell cycle (Figure 3B). These data indicated that CMC induced an accumulation of cells specifically at G2-M phase without affecting other cell cycle phases.
Finally, the reversibility of CMC-induced mitotic arrest was assessed by withdrawing CMC immediately after 12 h of treatment. Following CMC withdrawal, arrested cells began to exit from mitosis within 3 h, and 6 h later, most cells entered the next G1 phase. This result indicated that CMC-induced mitotic arrest is reversible (Figure 3C).
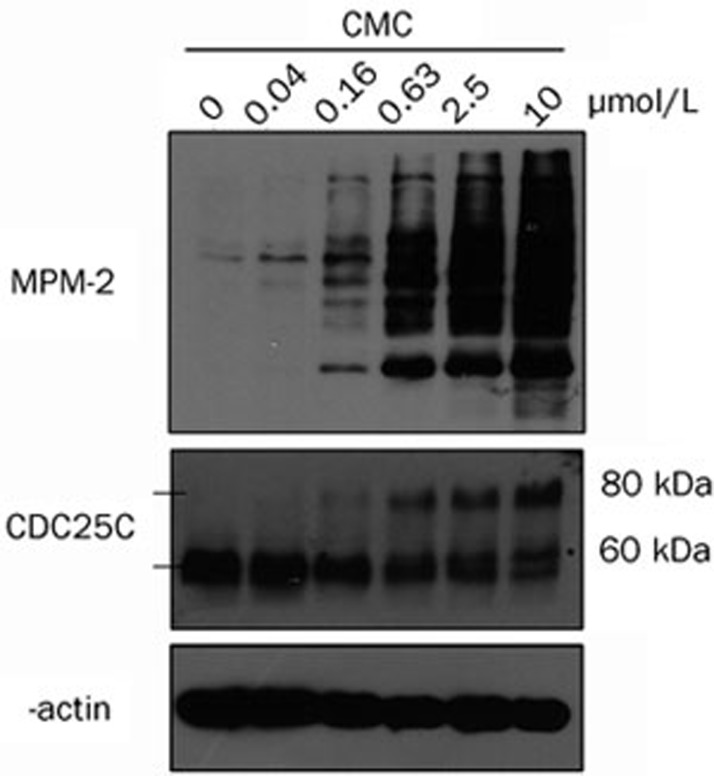
CMC changed the phosphorylation state of G2-M regulators in HeLa cells
As expected from the previous results, alterations in mitosis-specific protein expression were also detected. Briefly, HeLa cells were treated with CMC at different concentrations for 24 h and then samples were prepared. The levels of MPM-2, CDC25C and β-actin were measured using Western blot analysis. MPM-2 commonly reflects the phosphorylation level of mitosis-specific proteins. As shown in Figure 4, MPM-2 was slightly increased when cells were treated with 0.16 μmol/L CMC but was significantly increased when cells were treated with 0.63 μmol/L CMC. Consistent with this result, there was a shift to a slower migrating form of CDC25C that increased in a dose-dependent manner, which is indicative of changes in the phosphorylation state of the protein. These changes in protein phosphorylation are consistent with cell cycle arrest in mitosis as has been shown previously48.
Figure 4.
CMC changed the phosphorylation state of G2-M regulators. HeLa cells were treated with CMC at doses ranging from 10 μmol/L to 0.04 μmol/L for 24 h. 0.1% DMSO was used as a negative control. Phosphorylation of a G2-M-specific protein (MPM-2) and CDC25C were detected using western blot analysis. β-actin was used as an internal control. MPM-2 and CDC25C antibodies were diluted at 1:1000 in 1×TBST. β-actin antibody was diluted at 1:10000 in 1×TBST. A non-specific band that cross-reacted with the CDC25C antibody is marked with an asterisk.
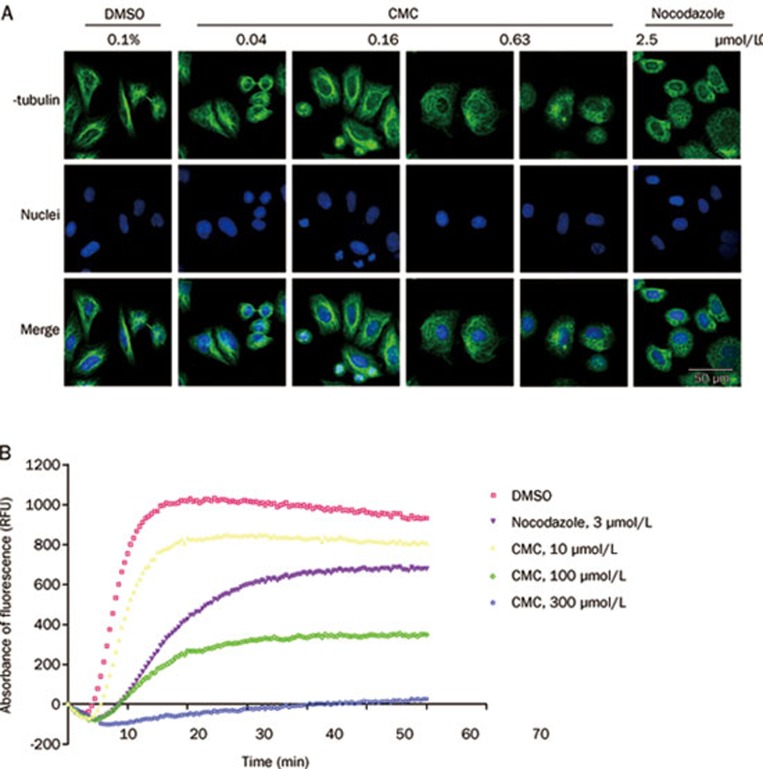
CMC induces G2-M arrest through the depolymerization of microtubules in a direct manner
Cellular microtubules are important components of spindles, which play an important role in mitosis. After sister chromatids are pulled apart by spindles, a single mitotic cell can divide into two cells. To investigate whether CMC affected tubulin polymerization, the microtubule status of CMC-treated HeLa cells was detected by immunocytochemistry. Briefly, cells were exposed to either CMC or a reference drug (nocodazole) for 8 h, fixed and then incubated with β-tubulin antibody at 4 °C overnight. The next day, cells were incubated with Alexa Fluor® 488-labeled donkey anti-mouse IgG, stained with Hoechst 33342 and observed with confocal microscopy.
CMC depolymerized microtubules in a dose-dependent manner (Figure 5A). When treated with 0.16 μmol/L CMC, the polymerization status of microtubules was only slightly changed; however, when cells were treated with 0.63 μmol/L CMC, almost all microtubules were depolymerized compared with the control group. This phenomenon was consistent with the aforementioned results of the cell cycle and Western blot analyses.
Figure 5.
CMC inhibited the polymerization of microtubules. (A) CMC depolymerized microtubules in vivo. HeLa cells were treated with CMC at doses ranging from 2.5 μmol/L to 0.04 μmol/L for 8 h. 0.1% DMSO was used as a negative control, and 0.33 μmol/L nocodazole was used as a positive control. Samples were then prepared as mentioned in the “Materials and methods” section, and the status of microtubules was observed using an Olympus confocal microscope (Olym pus, Tokyo, Japan). (B) CMC depolymerized purified tubulin in vitro. CMC was added to fluorescently labeled bovine tubulin at 37 °C for 1 h, and its effect on tubulin polymerization was detected with a FlexStation 3 Microplate Reader (Molecular Devices). Nocodazole (3 μmol/L) was used as a positive control, and DMSO was used as a negative control.
To deduce the mode of CMC-mediated microtubule depolymerization, we used a fluorescence-based tubulin polymerization assay. Nocodazole was used as the positive control. As shown in Figure 5B, CMC inhibited tubulin polymerization in a dose-dependent manner, thereby indicating that CMC inhibited the polymerization of tubulin in a direct manner.
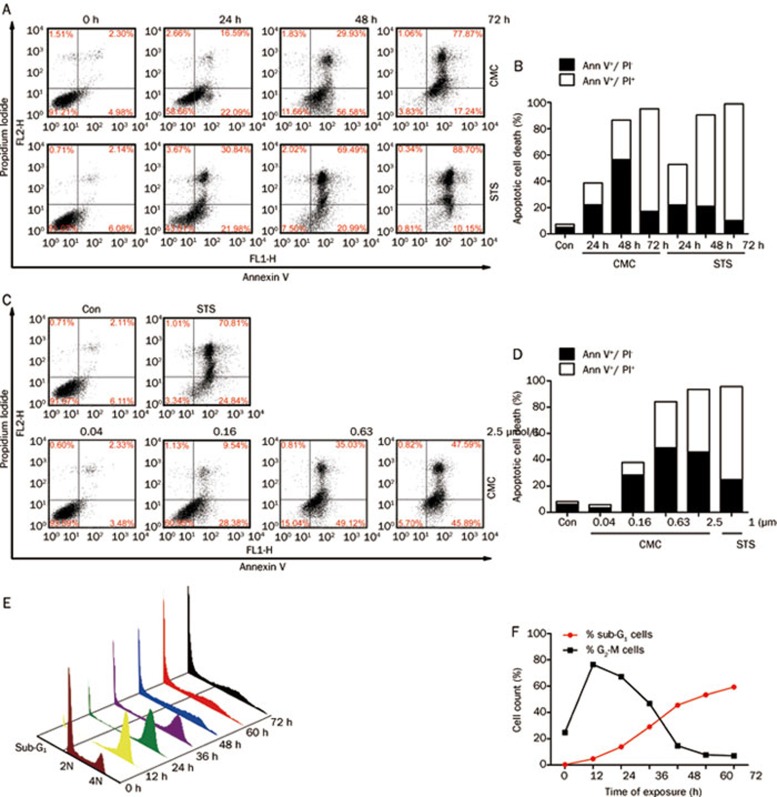
CMC induced apoptosis in a time- and dose-dependent manner
It has been reported that G2-M arrest caused by microtubule depolymerization is followed by apoptosis49, 50, 51; therefore, we chose to further investigate the apoptosis induced by CMC.
As shown in Figures 6A and 6B, persistent treatment with CMC led to a progressive increase in apoptosis in a time-dependent manner. Apoptosis of CMC-treated cells increased within 24 h (58.66% viable, 22.09% in early apoptosis and 16.59% cells in late apoptosis). Most cells were apoptotic at 48 h (56.58% in early apoptosis and 29.93% in late apoptosis). At 72 h, most cells were in late apoptosis (17.24% in early apoptosis and 77.87% in late apoptosis).
Figure 6.
CMC induced apoptosis in a time- and dose-dependent manner. (A) (B) HeLa cells were treated with 0.63 μmol/L CMC. Samples were then collected at 24 h, 48 h, and 72 h followed by staining with Annexin V-FITC/PI. The level of apoptosis was detected using flow cytometry. (C) (D) HeLa cells were treated with CMC at doses ranging from 2.5 μmol/L to 0.04 μmol/L for 48 h. Samples were then stained with Annexin V-FITC/PI. Apoptosis was detected using flow cytometry. (E) (F) HeLa cells were treated with 0.63 μmol/L CMC for 12 h-72 h, and the percentage of sub-G1 cells and G2-M cells was analyzed using the ModFit software provided with the FACSCalibur flow cytometer.
HeLa cells were then treated with varying doses of CMC for 48 h. As shown in Figures 6C and 6D, the levels of apoptosis in cells treated with 0.04 μmol/L CMC (a dose without induction of G2-M arrest as shown in Figure 3A) was the same as the negative control. The levels of apoptosis increased in a dose-dependent manner when the cells were treated with G2-M-arrest-inducing doses (doses greater than 0.16 μmol/L as shown in Figure 3A).
The three-dimensional profile of cell cycle progression versus time of CMC treatment shown in Figure 6E demonstrated that G2-M arrest was maximal (∼76.43%) at 12 h of treatment. After this point, the G2-M population disappeared concomitant with the emergence of a characteristic hypodiploid (<2N DNA) sub-G1 peak, which indicates apoptotic cells (Figures 6E and 6F). This apoptotic population peaked at 72 h (∼59.33%) posttreatment. The in vitro findings strongly therefore indicate that CMC-treated cells arrest in G2-M phase before beginning to apoptose.
Discussion
Coumarins are a hot topic of research due to their diverse pharmaceutical activities and wide distribution in nature. To find a coumarin analogue with good anticancer activity, we synthesized a series of coumarin analogues (Figure 1) and evaluated their effects on the viability of HCT116 cells (Table 1). CMC had the best anticancer activity and was thus selected for further mechanistic study. Nine cancer cell lines derived from 6 different tissues and the WI-38 cell line derived from normal embryonic (3 month gestation) lung tissue were used to evaluate the anticancer effects of CMC. We found that CMC had a high level of anticancer activity in vitro, with an IC50 value ranging from 75 nmol/L to 1.57 μmol/L. The cytotoxic effect of CMC on WI-38 cells was less potent, with an IC50 value of 12.128 μmol/L (Figure 2), which implies that CMC has relative selectivity for cancer cells versus normal cells. CMC also had the best anticancer activity and similar IC50 values against the HeLa, MDA-MB-435S, HCT-15, and A431 cell lines. The HeLa cell line was subsequently used for further anticancer mechanism study.
During the above experiments, it came to our attention that CMC caused the evident detachment of HeLa cells that became round (data not shown), a phenomenon frequently observed during the mitotic process. The effect of CMC on cell cycle progression was therefore evaluated to see if CMC affected cellular mitosis. After 24 h of treatment, CMC induced G2-M arrest in a dose-dependent manner. The minimal dose that caused nearly complete arrest in G2-M phase was approximately 0.63 μmol/L (Figure 3A); importantly, no concurrent change in S-phase was observed. To determine whether CMC only induced G2-M arrest, we treated synchronized HeLa cells and found that CMC only influenced G2-M phase without affecting other cell cycle phases (Figure 3B). G2-M arrest caused by CMC could be reversed by deprivation of CMC (Figure 3C). Western blot analysis showed that increasing doses of CMC induced increased levels of phosphorylation of G2-M phase-specific proteins, which provided proof of G2-M arrest (Figure 4).
When cells were treated with CMC, the cell shape became round with an increased disorder of M-phase-condensed chromosome alignment in a dose-dependent manner (data not shown), which is reported to be induced by alterations to the microtubular cytoskeleton52. The microtubule state of CMC-treated cells was therefore tested by ICC. ICC analysis showed that CMC induced microtubule depolymerization after an eight-hour treatment (Figure 5A). Furthermore, CMC effects on tubulin polymerization were tested. These results showed that CMC could inhibit tubulin polymerization directly (Figure 5B). Many articles have reported that G2-M arrest induced by tubulin-targeting agents is caused by their microtubule depolymerization effects53, 54, 55, 56, 57, 58. The results shown in Figures 3,4,5 indicate that CMC caused G2-M arrest by directly mediating depolymerization of microtubules.
Replicated chromosomes must be accurately segregated into each daughter cell during mitosis, and the spindle checkpoint is a surveillance mechanism that delays anaphase onset until all chromosomes are correctly attached in a bipolar fashion to the mitotic spindle59. Chemical inhibition of spindle dynamics, which relieves tension but does not destroy kinetochore-microtubule attachments, activates the spindle checkpoint60, 61. From the results shown in Figures 3 and 5, CMC depolymerized microtubules and induced G2-M arrest in a dose-dependent manner. The dose of CMC needed to depolymerize microtubules is the same as the dose of CMC needed to induce G2-M arrest, implying that the G2-M arrest induced by CMC is via microtubule depolymerization.
It has been reported that microtubule-targeting agents can induce apoptosis via activation of the spindle checkpoint62; thus, apoptosis induced by CMC was evaluated using Annexin V/PI double staining. As shown in Figures 6A and 6B, 0.63 μmol/L CMC triggered apoptosis at 24 h and induced apoptosis in a time-dependent manner (Figures 6A and 6B). CMC also caused a significant increase in apoptosis at 48 h in a dose-dependent manner (Figures 6C and 6D), and G2-M arrest induced by CMC occurs before the commencement of apoptosis (Figures 6E and 6F).
As mentioned, the importance of microtubules in mitosis makes them a superb target for a group of highly successful, chemically diverse anticancer drugs63, 64, 65. In view of the success of this class of drugs, it has been argued that microtubules represent the best cancer target to be identified so far, and it seems likely that drugs of this class will continue to be important chemotherapeutic agents even as more selective approaches are developed63, 64, 65. Relatively weak microtubule-targeting coumarins could also be used as adjuvants in chemotherapy to attain increased efficacy with decreased toxicity63, 64, 65. The maintenance of low concentrations of microtubule-targeted drugs in tumor tissue for long durations could be more efficacious in killing tumor cells than the rapidly rising and falling drug concentrations associated with bolus administration at maximum tolerated doses63, 64, 65. These advantages make coumarins a hot area for further study.
Still elusive is the fact that different anticancer coumarins with different substitutions can have different mechanisms. It is reported that coumarin can reduce the expression of Ras and Myc, and it can also induce G0/G1 arrest and apoptosis via ROS66. Another coumarin analogue, decursin, inhibits the proliferation of the advanced human prostate carcinoma cell lines DU145, PC-3 and LNCaP by causing G1 arrest via an induction of Cip1/p21 and Kip1/p2722. Ferulenol and dicoumarol stimulate tubulin assembly67, 68, and geiparvarin is able to inhibit GTP-induced polymerization69. Here, we report on a novel microtubule-targeting coumarin analogue with high anticancer activity. Our results provide clues for structure-activity relationship studies and for further structural design of novel microtubule-targeting coumarin analogues.
Author contribution
Yi-ming MA, Yu-bo ZHOU and Jia LI designed the research; Yi-ming MA, Chuan-ming XIE, and Dong-mei CHEN performed the research; Yi-ming MA, Yu-bo ZHOU, and Jia LI analyzed the data; and Yi-ming MA and Yu-bo ZHOU wrote the paper.
Acknowledgments
The authors thank Prof Jie WU from Fudan University for the synthesis of coumarin analogues and Prof Yi CHEN from the Shanghai Institute of Materia Medica for her helpful comments on this work. This work was supported by grants from the National Natural Science Foundation of China (No 30801405 and 81072667).
References
- Kumar V, Tomar S, Patel R, Yousaf A, Parmar VS, Malhotra SV. FeCl3- catalyzed Pechmann synthesis of coumarins in lonic liquids. Synthetic Commun. 2008;38:2646–54. [Google Scholar]
- Mousa SA. Anticoagulants in thrombosis and cancer: the missing link. Expert Rev Anticancer Ther. 2002;2:227–33. doi: 10.1586/14737140.2.2.227. [DOI] [PubMed] [Google Scholar]
- Lowenthal J, Birnbaum H. Vitamin K and coumarin anticoagulants: dependence of anticoagulant effect on inhibition of vitamin K transport. Science. 1969;164:181–3. doi: 10.1126/science.164.3876.181. [DOI] [PubMed] [Google Scholar]
- Bobek V, Boubelik M, Kovarik J, Taltynov O. Inhibition of adhesion breast cancer cells by anticoagulant drugs and cimetidine. Neoplasma. 2003;50:148–51. [PubMed] [Google Scholar]
- Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/ antioxidant activities. Curr Pharm Design. 2004;10:3813–33. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- Hadjipavlou-Litina DJ, Litinas KE, Kontogiorgis C. The anti-inflammatory effect of coumarin and its derivatives. Curr Med Chem - Anti-Inflam Anti-Aller Agents. 2007;6:293–306. [Google Scholar]
- Ouahouo BM, Azebaze AG, Meyer M, Bodo B, Fomum ZT, Nkengfack AE. Cytotoxic and antimicrobial coumarins from Mammea africana. Ann Trop Med Parasit. 2004;98:733–9. doi: 10.1179/000349804X3126. [DOI] [PubMed] [Google Scholar]
- Creaven BS, Egan DA, Kavanagh K, McCann M, Noble A, Thati B, et al. Synthesis, characterization and antimicrobial activity of a series of substituted coumarin-3-carboxylatosilver(I) complexes. Inorg Chim Acta. 2006;359:3976–84. [Google Scholar]
- Widelski J, Popova M, Graikou K, Glowniak K, Chinou I. Coumarins from Angelica lucida L--antibacterial activities. Molecules. 2009;14:2729–34. doi: 10.3390/molecules14082729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyasa KB, Nimavat KS, Jani GR, Hathi MV. Synthesis and antimicrobial activity of coumarin derivatives metal complexes: an in vitro evaluation. Orbital. 2009;1:183–92. [Google Scholar]
- Hishmat OH, Miky JAA, Farrag AA, Fadl-Allah EM. Synthesis of some coumarin derivatives and their antimicrobial activity. Arch Pharm Res. 1989;12:181–5. [Google Scholar]
- Ojala T, Remes S, Haansuu P, Vuorela H, Hiltunen R, Haahtela K, et al. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J Ethnopharmacol. 2000;73:299–305. doi: 10.1016/s0378-8741(00)00279-8. [DOI] [PubMed] [Google Scholar]
- Orlov YE. Chemical structure and antioxidant activity among natural coumarins. Chem Nat Comp. 1986;22:340. [Google Scholar]
- Thuong PT, Hung TM, Ngoc TM, Ha DT, Min BS, Kwack SJ, et al. Antioxidant activities of coumarins from Korean medicinal plants and their structure-activity relationships. Phytother Res. 2010;24:101–6. doi: 10.1002/ptr.2890. [DOI] [PubMed] [Google Scholar]
- Torres R, Faini F, Modak B, Urbina F, Labbe C, Guerrero J. Antioxidant activity of coumarins and flavonols from the resinous exudate of Haplopappus multifolius. Phytochemistry. 2006;67:984–7. doi: 10.1016/j.phytochem.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Yu W, Liu ZQ, Liu ZL. Antioxidant effect of coumarin derivatives on free radical initiated and photosensitized peroxidation of linoleic acid in micelles. J Chem Soc, Perkin Trans. 1999;2:969–74. [Google Scholar]
- Simonyan AV, Vlasenko SP, Dimoglo AS. Electron topological study of the link between antiallergic activity and structure for chalcone, coumarin and cinnamic acid derivatives. Pharm Chem J. 1993;27:490–4. [Google Scholar]
- Nugroho AE, Riyanto S, Sukari MA, Maeyama K. Anti-allergic effects of Marmin, a coumarine isolated from Aegle marmelos Correa: In vitro study. Int J Phytomed. 2011;3:84–97. [Google Scholar]
- Gonsior E, Schultze-Werninghaus G, Wuthrich B. Protective antiallergic effects of a new coumarin compound (BM 15.100) in experimental asthma. Int J Clin Pharmacol Biopharm. 1979;17:283–9. [PubMed] [Google Scholar]
- Matsuda H, Tomohiro N, Ido Y, Kubo M. Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol Pharmaceut Bull. 2002;25:809–12. doi: 10.1248/bpb.25.809. [DOI] [PubMed] [Google Scholar]
- Buckle DR, Outred DJ, Smith H, Spicer BA. N-benzylpiperazino derivatives of 3-nitro-4-hydroxycoumarin with H1 antihistamine and mast cell stabilizing properties. J Med Chem. 1984;27:1452–7. doi: 10.1021/jm00377a013. [DOI] [PubMed] [Google Scholar]
- Yim D, Singh RP, Agarwal C, Lee S, Chi H, Agarwal R. A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res. 2005;65:1035–44. [PubMed] [Google Scholar]
- Belluti F, Fontana G, Dal Bo L, Carenini N, Giommarelli C, Zunino F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg Med Chem. 2010;18:3543–50. doi: 10.1016/j.bmc.2010.03.069. [DOI] [PubMed] [Google Scholar]
- Kamal A, Adil SF, Tamboli, Jaki R, Siddardha B, Murthy USN. Synthesis of coumarin linked naphthalimide conjugates as potential anticancer and antimicrobial agents. Lett Drug Design Disc. 2009;6:201–9. [Google Scholar]
- Kawase M, Sakagami H, Motohashi N, Hauer H, Chatterjee SS, Spengler G, et al. Coumarin derivatives with tumor-specific cytotoxicity and multidrug resistance reversal activity. In Vivo. 2005;19:705–11. [PubMed] [Google Scholar]
- Reddy NS, Mallireddigari MR, Cosenza S, Gumireddy K, Bell SC, Reddy EP, et al. Synthesis of new coumarin 3-(N-aryl) sulfonamides and their anticancer activity. Bioorg Med Chem Lett. 2004;14:4093–7. doi: 10.1016/j.bmcl.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SS, Paul S, Mandal SK, Banerjee A, Boujedaini N, Khuda-Bukhsh AR. A synthetic coumarin (4-methyl-7 hydroxy coumarin) has anti-cancer potentials against DMBA-induced skin cancer in mice. Eur J Pharm. 2009;614:128–36. doi: 10.1016/j.ejphar.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Devji T, Reddy C, Woo C, Awale S, Kadota S, Carrico-Moniz D. Pancreatic anticancer activity of a novel geranylgeranylated coumarin derivative. Bioorg Med Chem Lett. 2011;21:5770–3. doi: 10.1016/j.bmcl.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kotake K, Ishii H. Synthesis of toddacoumaquinone, a coumarin-naphthoquinone dimer, and its antiviral activities. Chem Pharm Bull. 1995;43:1039–41. doi: 10.1248/cpb.43.1039. [DOI] [PubMed] [Google Scholar]
- Hwu JR, Singha R, Hong SC, Chang YH, Das AR, Vliegen I, et al. Synthesis of new benzimidazole-coumarin conjugates as anti-hepatitis C virus agents. Antiviral Res. 2008;77:157–62. doi: 10.1016/j.antiviral.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Yang Z, Xiao H, Liu Y, Liu J, Wen L. Antiviral effect of coumarin analogue against respiratory syncytical virus infection in vitro and in vivo. Antiviral Res. 1995;26:350–350. [Google Scholar]
- Curini M, Epifano F, Maltese F, Marcotullio MC, Gonzales SP, Rodriguez JC. Synthesis of collinin, an antiviral coumarin. Aust J Chem. 2003;56:59–60. [Google Scholar]
- Hoult JR, Payá M. Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacol. 1996;27:713–22. doi: 10.1016/0306-3623(95)02112-4. [DOI] [PubMed] [Google Scholar]
- Oldenburg J, Seidel H, Potzsch B, Watzka M. New insight in therapeutic anticoagulation by Coumarin derivatives. Hamostaseologie. 2008;28:44–50. [PubMed] [Google Scholar]
- Riveiro ME, De Kimpe N, Moglioni A, Vázquez R, Monczor F, Shayo C, et al. Coumarins: old compounds with novel promising therapeutic perspectives. Curr Med Chem. 2010;17:1325–38. doi: 10.2174/092986710790936284. [DOI] [PubMed] [Google Scholar]
- Enderle C, Müller W, Grass U. Drug interaction: omeprazole and phenprocoumon. BMC Gastroenterol. 2001;1:2. doi: 10.1186/1471-230X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095–106. doi: 10.1001/archinte.165.10.1095. [DOI] [PubMed] [Google Scholar]
- Irena K. Studying plant-derived coumarins for their pharmacological and therapeutic properties as potential anticancer drugs. Expert Opin Drug Disc. 2007;2:1605–18. doi: 10.1517/17460441.2.12.1605. [DOI] [PubMed] [Google Scholar]
- Montes R, Ruiz de Gaona E, Martinez-González MA, Alberca I, Hermida J. The c.-1639G > A polymorphism of the VKORC1 gene is a major determinant of the response to acenocoumarol in anticoagulated patients. Br J Haematol. 2006;133:183–7. doi: 10.1111/j.1365-2141.2006.06007.x. [DOI] [PubMed] [Google Scholar]
- Thornes RD, Lynch G, Sheehan MV. Cimetidine and coumarin therapy of melanoma. Lancet. 1982;2:328. doi: 10.1016/s0140-6736(82)90295-1. [DOI] [PubMed] [Google Scholar]
- Zang Y, Yu LF, Pang T, Fang LP, Feng X, Wen TQ, et al. AICAR induces astroglial differentiation of neural stem cells via activating the JAK/STAT3 pathway independently of AMP-activated protein kinase. J Biol Chem. 2008;283:6201–8. doi: 10.1074/jbc.M708619200. [DOI] [PubMed] [Google Scholar]
- Zang Y, Yu LF, Nan FJ, Feng LY, Li J. AMP-activated protein kinase is involved in neural stem cell growth suppression and cell cycle arrest by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside and glucose deprivation by down-regulating phospho-retinoblastoma protein and cyclin D. J Biol Chem. 2009;284:6175–84. doi: 10.1074/jbc.M806887200. [DOI] [PubMed] [Google Scholar]
- Bengoechea-Alonso MT, Punga T, Ericsson J. Hyperphosphorylation regulates the activity of SREBP1 during mitosis. Proc natl Acad Sci U S A. 2005;102:11681–6. doi: 10.1073/pnas.0501494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YB, Feng X, Wang LN, Du JQ, Zhou YY, Yu HP, et al. LGH00031, a novel ortho-quinonoid inhibitor of cell division cycle 25B, inhibits human cancer cells via ROS generation. Acta Pharmacol Sin. 2009;30:1359–68. doi: 10.1038/aps.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooberry SL, Tien G, Hernandez AH, Plubrukarn A, Davidson BS. Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res. 1999;59:653–60. [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem. 1997;272:26159–65. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- Scatena CD, Stewart ZA, Mays D, Tang LJ, Keefer CJ, Leach SD, et al. Mitotic phosphorylation of Bcl-2 during normal cell cycle progression and Taxol-induced growth arrest. J Biol Chem. 1998;273:30777–84. doi: 10.1074/jbc.273.46.30777. [DOI] [PubMed] [Google Scholar]
- Aneja R, Liu M, Yates C, Gao J, Dong X, Zhou B, et al. Multidrug resistance-associated protein-overexpressing teniposide-resistant human lymphomas undergo apoptosis by a tubulin-binding agent. Cancer Res. 2008;68:1495–503. doi: 10.1158/0008-5472.CAN-07-1874. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Takagi M, Murakami H, Sekido Y, Shin-ya K. Induction of tubulin polymerization and apoptosis in malignant mesothelioma cells by a new compound JBIR-23. Cancer Lett. 2011;300:189–96. doi: 10.1016/j.canlet.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S, Das A, Bhattacharya A, Chakrabarti G. Theaflavins depolymerize microtubule network through tubulin binding and cause apoptosis of cervical carcinoma HeLa cells. J Agr Food Chem. 2011;59:2040–8. doi: 10.1021/jf104231b. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Chandley AC, Gosden RG. Alterations to the microtubular cytoskeleton and increased disorder of chromosome alignment in spontaneously ovulated mouse oocytes aged in vivo: an immunofluorescence study. Chromosoma. 1986;94:337–45. doi: 10.1007/BF00328633. [DOI] [PubMed] [Google Scholar]
- Loganzo F, Discafani CM, Annable T, Beyer C, Musto S, Hari M, et al. HTI-286, a synthetic analogue of the tripeptide hemiasterlin, is a potent antimicrotubule agent that circumvents P-glycoprotein-mediated resistance in vitro and in vivo. Cancer Res. 2003;63:1838–45. [PubMed] [Google Scholar]
- Bacher G, Nickel B, Emig P, Vanhoefer U, Seeber S, Shandra A, et al. D-24851, a novel synthetic microtubule inhibitor, exerts curative antitumoral activity in vivo, shows efficacy toward multidrug-resistant tumor cells, and lacks neurotoxicity. Cancer Res. 2001;61:392–9. [PubMed] [Google Scholar]
- Zhang LH, Wu L, Raymon HK, Chen RS, Corral L, Shirley MA, et al. The synthetic compound CC-5079 is a potent inhibitor of tubulin polymerization and tumor necrosis factor-alpha production with antitumor activity. Cancer Res. 2006;66:951–9. doi: 10.1158/0008-5472.CAN-05-2083. [DOI] [PubMed] [Google Scholar]
- Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–21. [PubMed] [Google Scholar]
- Kasibhatla S, Baichwal V, Cai SX, Roth B, Skvortsova I, Skvortsov S, et al. MPC-6827: a small-molecule inhibitor of microtubule formation that is not a substrate for multidrug resistance pumps. Cancer Res. 2007;67:5865–71. doi: 10.1158/0008-5472.CAN-07-0127. [DOI] [PubMed] [Google Scholar]
- Tahir SK, Han EK, Credo B, Jae HS, Pietenpol JA, Scatena CD, et al. A-204197, a new tubulin-binding agent with antimitotic activity in tumor cell lines resistant to known microtubule inhibitors. Cancer Res. 2001;61:5480–5. [PubMed] [Google Scholar]
- May KM, Hardwick KG. The spindle checkpoint. J Cell Sci. 2006;119:4139–42. doi: 10.1242/jcs.03165. [DOI] [PubMed] [Google Scholar]
- Clute P, Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat Cell Biol. 1999;1:82–7. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- Nicklas RB, Waters JC, Salmon ED, Ward SC. Checkpoint signals in grasshopper meiosis are sensitive to microtubule attachment, but tension is still essential. J Cell Sci. 2001;114:4173–83. doi: 10.1242/jcs.114.23.4173. [DOI] [PubMed] [Google Scholar]
- Carré M, Braguer D.Microtubule damaging agents and apoptosisIn: Tito Fojo Editor. The role of microtubules in cell biology, neurobiology and oncology. Humana Press; 2008p 479–518.
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature Reviews. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Hadfield JA, Ducki S, Hirst N, McGown AT. Tubulin and microtubules as targets for anticancer drugs. Prog Cell Cycle Res. 2003;5:309–25. [PubMed] [Google Scholar]
- Jordan MA, Kamath K. How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Tar. 2007;7:730–42. doi: 10.2174/156800907783220417. [DOI] [PubMed] [Google Scholar]
- Chuang JY, Huang YF, Lu HF, Ho HC, Yang JS, Li TM, et al. Coumarin induces cell cycle arrest and apoptosis in human cervical cancer HeLa cells through a mitochondria- and caspase-3 dependent mechanism and NF-kappaB down-regulation. In Vivo. 2007;21:1003–9. [PubMed] [Google Scholar]
- Bocca C, Gabriel L, Bozzo F, Miglietta A. Microtubule-interacting activity and cytotoxicity of the prenylated coumarin ferulenol. Planta Med. 2002;68:1135–7. doi: 10.1055/s-2002-36342. [DOI] [PubMed] [Google Scholar]
- Madari H, Panda D, Wilson L, Jacobs RS. Dicoumarol: a unique microtubule stabilizing natural product that is synergistic with Taxol. Cancer Res. 2003;63:1214–20. [PubMed] [Google Scholar]
- Miglietta A, Bocca C, Gabriel L, Rampa A, Bisi A, Valenti P. Antimicrotubular and cytotoxic activity of geiparvarin analogues, alone and in combination with paclitaxel. Cell Biochem Funct. 2001;19:181–9. doi: 10.1002/cbf.919. [DOI] [PubMed] [Google Scholar]