Abstract
Aim:
To evaluate the effects of aldosterone with or without high sodium intake on blood pressure, myocardial structure and left ventricular function in rats, and to investigate the mechanisms underlying the effects.
Methods:
Eight-week-old male Sprague-Dawley rats were randomly divided into 3 groups: (1) control (CON) group fed a normal sodium diet, (2) aldosterone (ALD) group receiving aldosterone infusion and a normal sodium diet, and (3) high sodium plus aldosterone (HS-ALD) group receiving 1% NaCl diet in conjunction with aldosterone infusion. Aldosterone was administered through continuously subcutaneous infusion with osmotic minipump at the rate of 0.75 μg/h for 8 weeks. The myocardium structure was observed using transthoracic echocardiography and transmission electron microscopy. The collagen deposition in left ventricle was evaluated with Masson's trichrome staining. The expression of IL-18, p22phox, and p47phox proteins was examined using Western blot analysis.
Results:
The systolic blood pressure in the ALD and HS-ALD groups was significantly higher than that in the CON group after 2-week treatment. But the blood pressure showed no significant difference between the HS-ALD and ALD groups. The left ventricular hypertrophy, myocardial collagen deposition and oxidative stress were predominantly found in the HS-ALD and ALD group. Furthermore, the breakdown of myocardial structure and oxidative stress were more apparent in the HS-ALD group as compared with those in the ALD group.
Conclusion:
Long-term infusion of aldosterone results in hypertension and profibrotic cardiovascular responses in rats fed a normal sodium diet, which were mediated by oxidative stress. High-sodium intake could aggravate myocardial injuries induced by aldosterone.
Keywords: sodium intake, aldosterone, blood pressure, myocardial injury, left ventricular hypertrophy, oxidative stress
Introduction
A series of recent studies have revealed that patients with primary aldosteronism have higher incidence of cardiovascular complications compared with demographically and hemodynamically similar essential hypertension patients1. Furthermore, two clinical trials, the randomized aldactone evaluation study (RALES)2 and the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS)3, have shown that mineralocorticoid receptor (MR) antagonists reduce mortality in patients with heart failure.
Experimental studies have shown that the combined administration of aldosterone and high sodium in uninephrectomized rats induces cardiovascular injuries4, 5. However, it has remained unclear whether the cardiac injury was due to the direct action of aldosterone, the sodium loading or uninephrectomy. The contribution of dietary sodium intake is illustrated by the finding that the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME)/angiotensin II treated animals fed a low-salt diet do not develop vascular damage, even though plasma aldosterone levels were 10-fold higher than those of animals on a high-salt diet, suggesting that blood levels of aldosterone alone are insufficient to cause vascular injury on a low-salt diet6. In modern society, humans could easily increase sodium intake via food diversity7. Little information is available concerning the effect of excess aldosterone on cardiac injury of rats on a normal sodium diet. To clarify these issues, we performed experiments to evaluate the effects of chronic subcutaneous aldosterone infusion with or without the addition of 1% sodium chloride on blood pressure, myocardial structure and left ventricular (LV) function in normotensive Sprague-Dawley (SD) rats.
Materials and methods
Animals model
This study was approved by the Ethics Committee of Sun Yat-sen University (Guangzhou, China). All procedures were performed in accordance with “Institutional Guidelines for Animal Research of Sun Yat-sen University” and “Guide for the Care and Use of Laboratory Animals of National Institutes of Health”. Eight week-old male SD rats with an initial body weight of 260–280 g were purchased (Laboratory Animal Center of Sun Yat-sen University, Guangzhou, China) and used in this study. The rats were randomly divided into three groups (n=8 in each group) and assigned to one of the following protocols for 8 weeks: vehicle (CON group), excess aldosterone and a normal sodium diet (ALD group), or 1% (w/v) NaCl in conjunction with excess aldosterone (HS-ALD group). All of the rats were anesthetized, and implanted subcutaneously with an osmotic minipump (Alzet model 2004, DURECT Corp, Cupertino, CA, USA). The CON group received a continuously subcutaneous infusion of 5% (v/v) ethanol (vehicle, Sigma-Aldrich, St Louis, MO, USA). The ALD group and the HS-ALD group received continuously subcutaneous infusion of aldosterone (0.75 μg/h, Sigma-Aldrich, St Louis, MO, USA) dissolved in 5% ethanol. The mini-osmotic pumps were replaced every 4 weeks under anesthesia. In addition to standard rat chow [Na, 0.3% (w/v)], all of the animals had free access to tap water (the CON group and the ALD group) or 1% NaCl solution (the HS-ALD group). The animals were maintained in an environment with a constant temperature and 12 h light-dark cycles. Systolic blood pressure (SBP) was measured in conscious rats by the tail-cuff method (BP-98A; Softron Co, Tokyo, Japan) before treatment and at 1-week interval thereafter.
Echocardiographic assessment
Transthoracic echocardiographic studies were performed at week 8 using an echocardiographic system equipped with 13-MHz echocardiographic probe (Technos MPX, Biosound Esaote, Indianapolis, IN, USA). A single investigator unaware of the make-up of the experimental groups performed the task. The rats were anesthetized, and were held in the dorsal decubitus. M-mode tracings were recorded through the LV anterior and posterior walls (AW and PW, respectively) at the papillary muscle level to measure the AW thickness at end diastole, PW thickness at end diastole, LV end-diastolic dimension (LVEDD), fractional shortening (FS), and LV ejection fraction (EF). Pulse-wave Doppler spectra of mitral inflow were recorded from the apical 4-chamber view, with the sample volume placed near the tips of the mitral leaflets and adjusted to the position at which the velocity was maximal and the flow pattern laminar. Mitral inflow measurements of early and late filling velocities (Emax and Amax, respectively) were obtained. The ratio of Emax to Amax (E/A) was also recorded.
Tissue and blood collection and analysis
The rats were transferred to metabolic cages prior to the completion of the experiment. Body weight, food intake and urine volume of 24 h were recorded. The sodium and potassium in the urine and plasma were measured. The rats were then sacrificed at week 8. Blood samples from puncturing heart were collected into heparin tubes for the measurement of plasma sodium and potassium, into EDTA tubes for the measurement of plasma renin activity (PRA, RIA kit, DiaSorin, Saluggia, Italy) and into xeransis tubes for the measurement of serum aldosterone (RIA kit, Diagnostic System Laboratories, Webster, TX, USA) and 8-isoprostane (ELISA kit, Assay Designs 900-010, Assay Designs Inc, Ann Arbor, MI, USA). The hearts were divided into right ventricle (RV) and LV plus septum. Hearts was rapidly excised and weighed. The LV mass index (LVMI) was determined as the ratio of the LV mass to the body weight. Part of the left ventricle was fixed in glutaraldehyde and freshly prepared 4% (w/v) paraformaldehyde in phosphate buffer solution for morphometric studies. The remaining left ventricle was frozen in liquid nitrogen for later protein extraction.
Transmission electron microscopy examination for myocardial ultrastructure
Heart tissue was thinly sliced and placed in primary electron microscopy fixative. After secondary fixation, the specimens were placed on a rocker overnight, embedded, and polymerized at 60 °C for 24 h. The 85-nm thin sections were stained with 5% uranyl acetate and Sato's Triple lead stain, and then viewed by a transmission electron microscopy (TEM) (CM10, Philips, Amsterdam, Holland).
Masson's trichrome-stained method for interstitial and perivascular collagen
The middle of the LV was excised, fixed in 4% (w/v) paraformaldehyde, and embedded in paraffin. Sections, 5 μm thickness, were made and stained with Masson's trichrome-stained method. Collagen fibers were shown in blue, and muscles were shown in red. Ten fields in each LV section were recorded randomly by photograhy (T-B2.5XA, Nikon, Tokyo, Japan). The collagen volume fraction (CVF) was determined by measuring the area of stained blue tissue within a given field. The area stained blue was calculated as a percentage of the total area within a field. CVF examination excluded scars, artifacts, perivascular collagen areas and incomplete tissue. For each LV section, five cut cross-sectional intramyocardial coronary arteries were examined individually. The area of collagen immediately surrounding the blood vessels was calculated, and the perivascular collagen was determined as the ratio of the area of collagen surrounding the vessel wall to the total area of the vessel (perivascular collagen area/vessel luminal area, PVCA/VA). It was determined by quantitative morphometry with Image-pro plus 5.0 (Media Cybernetics, Bethesda, MD, USA). The operator was blinded to the experimental group during the analysis.
Immunohistochemical staining for ED1 expression
To evaluate the focal inflammatory infiltration of the LV myocardium, we evaluated changes in inflammatory markers. At sites of injury, infiltrating monocytes differentiate into macrophages and express ED-1. Therefore, an ED-1 monoclonal antibody (Millipore Biotechnology, Billerica, MA, working dilution 1:50) was used to identify the macrophages. For immunohistochemical staining, sections were labeled with primary antibody of mouse monoclonal antibody against ED1 after antigen retrieval. The binding of the primary antibodies was revealed by horseradish peroxidase-conjugated secondary antibodies (DAKO, Carpinteria, CA, USA) and detected with diaminobenzidine staining (DAKO, Carpinteria, CA, USA). Positive staining appeared as brown. Controls for immunospecificity were included in all experiments, and the primary antibody was replaced by phosphate buffered saline.
Western blot analysis for IL-18, p22phox, and p47phox protein expression
NADPH oxidase is a critical source of ROS production within the vascular wall and heart8. p22phox and p47phox subunits seem to be key molecules of NADPH oxidase9. In addition, IL-18 is a pleiotropic cytokine and several lines of evidence support a causal role for it in the pathogenesis of cardiovascular disease. Therefore, to demonstrate the relationship among IL-18, oxidative stress and aldosterone treatment, we analyzed the expression of IL-18, p22phox, and p47phox subunits in the LV. About 100 mg LV was homogenized in 50 mmol/l Tris buffer (pH 7.4), 150 mol/L NaCl, 1% (v/v) Triton X-100, 1% sodium(w/v) deoxycholate, 0.1% (w/v) SDS and some inhibitors with a homogenizer on the ice and then centrifuged at 12 000 r/min for 15 min at 4°C. The resulting supernatants were collected and either frozen at -80 °C or used immediately. Protein concentrations were determined using the BCA method. Equal amounts of protein (20 μg per sample) were analyzed by 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto polyvinylidene difluoride (PVDF) membranes for 1 h at 200 mA (Bio-Rad, Hercules, CA). The membranes were blocked in 5% nonfat milk (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at room temperature and then incubated in primary antibody against IL-18 (R&D Systems, Minneapolis, MN, working dilution 2 μg/mL) p22phox (Santa Cruz Biotechnology, Santa Cruz, CA, working dilution 1:500) or p47phox (Millipore Biotechnology, Billerica, MA, working dilution 1:1000) overnight at 4 °C. The binding of the primary antibodies was revealed by horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The proteins of the membranes were detected using an enhanced chemiluminescence immunoblotting detection system (Thermo Fisher Scientific, Rockford, IL). The results were quantified by densitometric analysis using Image-Quant software. Values were corrected by the absorbance of the internal control (GAPDH).
Statistical analysis
Data were expressed as mean±standard deviation. Statistical analysis was performed with software (SPSS version 13.0; SPSS, Chicago, IL) between two groups using two-tailed Student's t-test for unpaired values, and P<0.05 was considered statistically significant.
Results
Physiological characteristics and time-course of SBP in SD rats
The body weight and food intake did not differ between the CON, ALD, and HS-ALD groups. The serum aldosterone level was higher and the PRA was lower in the ALD group compared with those in the CON group (aldosterone level 1664.9±389.9 vs 623.1±80.1 pg/mL, P<0.01; PRA 4.17±0.25 vs 7.22±0.07 ng·mL−1·h−1, P<0.01). Urine output and urinary potassium excretion were increased, plasma sodium concentrations were higher and the plasma potassium levels were lower in the ALD group compared with those in the CON group. Serum aldosterone levels were not significantly different between the HS-ALD group and the ALD group. However, the HS-ALD group exhibited a lower PRA(1.04±0.89 ng·mL−1·h−1 in the HS-ALD group vs 4.17±0.25 ng·mL−1·h−1 in the ALD group, P<0.01), greater urine output, increased urinary sodium and potassium excretion, and a lower plasma potassium level (P<0.05) (Table 1).
Table 1. Physiologic and morphologic characteristics in Sprague-Dawley rats. Mean±SD. n=8. bP<0.05, cP<0.01 vs CON; eP<0.05, fP<0.01 vs ALD.
| CON | ALD | HS-ALD | |
|---|---|---|---|
| Body weight (g) | 438.7±14.7 | 429.2±28.7 | 416.7±24.6 |
| Food intake (g/100 g BW) | 5.85±0.81 | 5.67±0.34 | 5.89±0.84 |
| Urinary volume (mL/100 g BW) | 2.8±0.8 | 5.6±1.4c | 12.3±1.5f |
| Urinary sodium (mmol/24 h) | 1.06±0.42 | 1.39±0.41 | 6.80±1.15f |
| Urinary potassium (mmol/24 h) | 1.48±0.37 | 2.24±0.46b | 2.98±0.58e |
| Plasma sodium (mmol/L) | 141.5±2.4 | 148.7±1.1b | 149.5±0.8 |
| Plasma potassium (mmol/L) | 4.3±0.2 | 3.1±0.3b | 2.5±0.1e |
| Serum aldosterone (pg/mL) | 623.1±80.1 | 1664.9±389.9c | 1474.3±452.4 |
| PRA (ng·mL−1·h−1) | 7.22±0.07 | 4.17±0.25c | 1.04±0.89f |
| LVMI (mg/g) | 2.06±0.10 | 2.36±0.17b | 2.67±0.24e |
CON, control rats; ALD, aldosterone-infused rats; HS-ALD, 1% NaCl in conjunction with aldosterone in rats. BW, body weight; PRA, plasma renin activity; LVMI, left ventricular mass index.
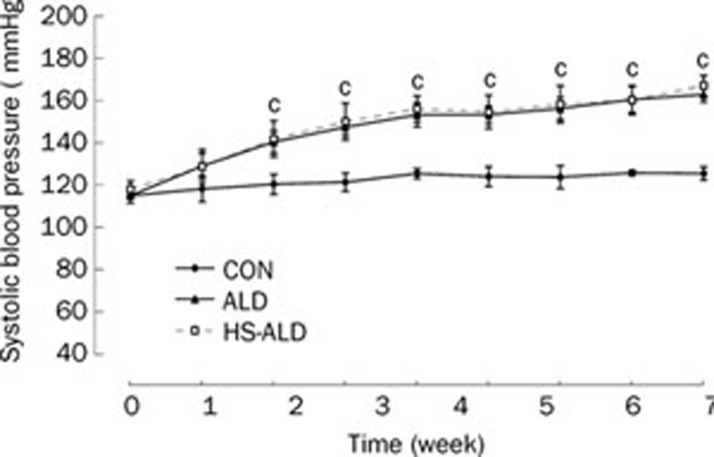
The time-course of SBP in the rats of the three groups was shown in Figure 1. At the first week of treatment, aldosterone infusion had not significantly increased the animals' blood pressure. However, following 2-week aldosterone treatment alone, the SBP of the ALD group was slightly but significantly higher than that of the CON group (140±5 vs 125±4 mmHg, P<0.01). Following 8-week aldosterone treatment, the SBP of the ALD group was moderately and significantly higher than that of the CON group (162±4 vs 127±3 mmHg, P<0.01). The SBP was not significantly different between the ALD group and the HS-ALD group.
Figure 1.
Systolic blood pressure of control rats (CON), aldosterone alone-infused rats (ALD) and 1% sodium chloride intake in conjunction with excess aldosterone (HS-ALD) at 0, 1, 2, 3, 4, 5, 6, 7, and 8 weeks. Mean±SD. n=8. cP<0.01 vs CON.
LV weight, echocardiographic analysis and ultrastructure
Cardiac structure and function were examined to determine the effects of aldosterone infusion with or without the addition of 1% sodium chloride on the heart. Cardiac hypertrophy, as suggested by LVMI, was observed. LVMI was significantly higher in the ALD group compared with that in the CON group (2.36±0.17 vs 2.06±0.10, P<0.05). The LVMI was also significantly higher in the HS-ALD group compared with that in the ALD group (2.67±0.24 vs 2.36±0.17, P<0.05) (Table 1).
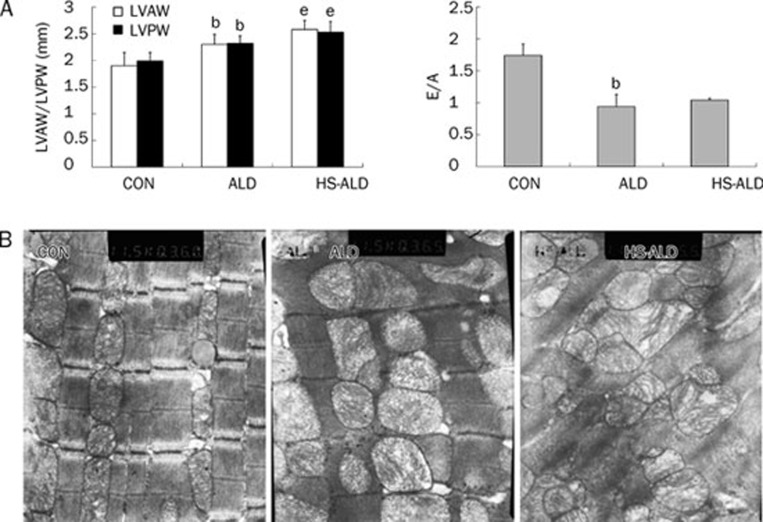
As shown by echocardiographic analysis in Figure 2A, the thickness of both the LVAW and the LVPW (2.30±0.19 mm and 2.32±0.21 mm, respectively) in the ALD group was significantly greater after 8 weeks compared with that in the CON group (1.90±0.25 mm and 1.99±0.16 mm, respectively; P<0.05). The E/A was significantly lower in the ALD group compared with that in the CON group (0.94±0.19 vs 1.74±0.18, P<0.05). Furthermore, the LVAW and LVPW thickness were significantly greater in the HS-ALD group (2.58±0.17 mm and 2.53±0.20 mm, respectively) compared with that in the ALD group (P<0.05). Other echocardiographic parameters, including HR (441±34, 435±36, and 428±36 bpm in the CON, ALD,and HS-ALD groups, respectively; P>0.05), LVEDD (5.52±0.69, 6.10±0.76, and 5.71±1.11 mm in the CON, ALD, and HS-ALD groups, respectively; P>0.05), FS (45.4±3.9, 54.1%±5.2%, and 45.8%±3.4% in the CON, ALD, and HS-ALD groups, respectively; P>0.05), and EF (81.8%±3.6%, 87.9%±6.1%, and 82.3%±3.2% in the CON, ALD, and HS-ALD groups, respectively; P>0.05) were not significantly changed by aldosterone infusion with or without the addition of 1% sodium chloride.
Figure 2.
Aldosterone infusion with or without additional 1% sodium chloride intake induce left ventricular (LV) hypertrophy by echocardiographic analysis and ultrastructural remodeling visible by TEM in rats. (A) aldosterone infusion with or without additional 1% sodium chloride intake induce LV hypertrophy and diastolic dysfunction. LVAW, LV anterior wall thickness at end diastole; LVPW, LV posterior wall thickness at end diastole; E/A, the ratio of mitral inflow measurements of early to late filling velocities; CON, control rats; ALD, excess aldosterone alone-infused rats; HS-ALD, 1% NaCl in conjunction with aldosterone in rats. Mean±SD. n=8. bP<0.05 vs CON; eP<0.05 vs ALD. (B) Representative image from CON demonstrating a line of sarcolemmal mitochondria just beneath the sarcomeres (the distance between two Z lines) of the myocardium. Representative remodeled mitochondria in ALD rats. This image represents marked increase of swollen and denatured mitochondria. The myocardial ultrastructure was disappeared in some spaces and mitochondria of myocardium were dissolved in HS-ALD group. Original magnifications: ×10 000.
To further evaluate the changes in cardiac morphology, the cardiac ultrastructure was observed using TEM. TEM images of the rat heart following aldosterone infusion with or without the addition of 1% sodium chloride revealed striking changes in the mitochondria and myofilaments. The myofilaments were sparser, and there was a marked increase in swollen and denatured mitochondria in the ALD group. Moreover, the myocardial ultrastructure was not visible in some spaces and mitochondria were seriously damaged in the HS-ALD group (Figure 2B).
LV collagen deposition
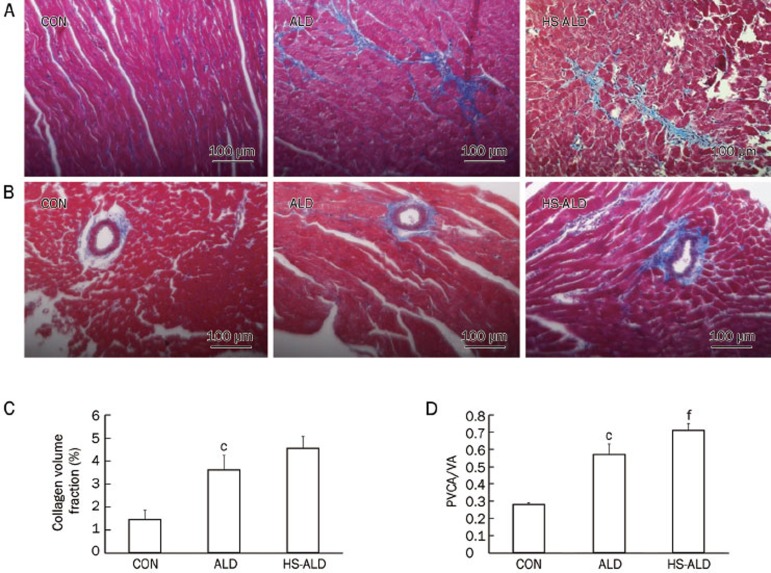
Cardiac fibrosis in rats following aldosterone infusion with or without the addition of 1% sodium chloride was shown in Figure 3. The CVF and PVCA/VA were higher in the ALD group (3.61%±0.63% and 0.57±0.062, respectively) than those in the CON group (1.44%±0.41% and 0.28±0.01, respectively; P<0.01). CVF was 4.55%±0.52% and PVCA/VA was 0.71±0.04 in the HS-ALD group. The CVF was not significantly different between the ALD group and the HS-ALD group. However, PVCA/VA was higher in the HS-ALD group compared with that in the ALD group (P<0.01). We found that aldosterone infusion alone induced a significant increase in cardiac fibrosis, in terms of collagen deposition, in the myocardial interstitium (Figure 3A and 3C) and the perivascular space of the intramyocardial coronary arteries (Figure 3B and 3D). Furthermore, high sodium in conjunction with aldosterone induced more obvious myocardial fibrosis, especially in the perivascular space of the intramyocardial coronary arteries (Figure 3).
Figure 3.
Effects of aldosterone infusion with or without additional 1% sodium chloride intake on cardiac fibrosis. Representative photomicrographs show collagen changes in midmyocardium (A, C) and perivascular space of intramyocardial coronary arteries (B, D) after aldosterone infusion with or without additional 1% sodium chloride intake by masson's trichrome-stained method. Original magnifications: ×200. Collagen fibers were shown in blue and muscles were shown in red. Collagen volume fraction was determined by measuring the area of collagen within a given field. PVCA/ VA, perivascular collagen area/vessel luminal area. CON, control rats; ALD, aldosterone alone-infused rats; HS-ALD, 1% NaCl in conjunction with aldosterone in rats. Mean±SD. n=8. cP<0.01 vs CON; fP<0.01 vs ALD.
LV inflammatory infiltration and oxidative stress
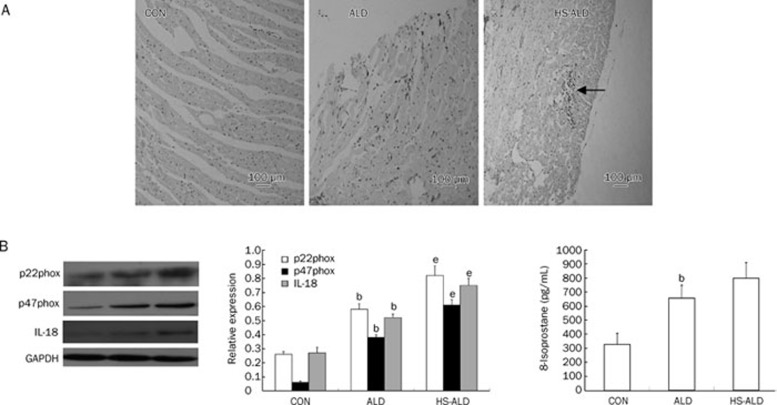
As shown in Figure 4A, LV inflammatory infiltration was not detected in the CON group. Focal inflammatory infiltration in LV characterized by ED-1-positive cells (macrophages) was observed in the ALD group. More obvious inflammatory infiltration was detected in the HS-ALD group.
Figure 4.
(A) Macrophage infiltration was presented at sites of myocardium induced by aldosterone infusion with or without additional 1% sodium chloride intake. Positive staining appeared as brown. Appearance of focal inflammatory infiltration in LV has been observed in ALD group. The focal inflammatory lesions were enlarged in HS-ALD group (the macrophage infiltration had been labeled). Original magnifications: ×100. (B) Effects of aldosterone infusion with or without additional 1% sodium chloride intake on the parameters about oxidative stress and IL-18 in LV myocardium. (C) Effects of aldosterone infusion with or without additional 1% sodium chloride intake on the serum 8-isoprostane levels. CON, control rats; ALD, aldosterone alone-infused rats; HS-ALD, 1% NaCl in conjunction with aldosterone in rats. Mean±SD. n=8. bP<0.05 vs CON; eP<0.05 vs ALD.
The expression of NADPH oxidase was determined by Western blot detection of the subunits p22phox and p47phox. In addition, we analyzed the expression of the IL-18 protein in three groups. Our results showed that the expressions of p22phox, p47phox, and IL-18 were up-regulated in the ALD group compared with those in the CON group (P<0.05). Furthermore, p22phox, p47phox, and IL-18 protein expressions in the HS-ALD group were up-regulated compared with those in the ALD group (P<0.05) (Figure 4B).
We found that serum 8-isoprostane levels, a marker for global oxidative stress, were significantly higher in the ALD group than those in the CON group (654.22±92.47 vs 326.96±79.65 pg/mL, P<0.05). There was also an increasing trend in serum 8-isoprostane levels in the HS-ALD group compared with those in the ALD group. However, serum 8-isoprostane levels were not significantly different between the HS-ALD group and the ALD group (797.50±111.33 pg/mL in the HS-ALD group, P>0.05) (Figure 4C).
Discussion
Aldosterone on a normal sodium diet could have a pivotal effect on cardiac injury. Some investigators have shown that aldosterone excess in the presence of salt loading and uninephrectomy is associated with cardiovascular remodeling4, 5. It has also been reported that other cofactors, such as low nitric oxide bioavailability, hypertension, or congestive heart failure must be present together with a high-sodium intake for damage to occur6. Due to the influence of high-sodium intake or other factors on myocardial injury, the actual effects of aldosterone on cardiac injury have remained poorly understood. Our study showed that chronic subcutaneous aldosterone infusion of 8 weeks' duration in the absence of sodium loading induced hypertension and LV hypertrophy, which was accompanied by an inflammatory response and collagen deposition in the myocardium.
Regarding the echocardiography observations, the present study showed that E/A was significantly lower in animals infused with aldosterone for 8 weeks compared with that in the CON group, indicating the impairment of diastolic function in the ALD group. Yoshida et al demonstrated that rats infused with aldosterone on a normal sodium diet for only 2 weeks showed cardiac hypertrophy. However, FS and EF were not significantly altered10. Unfortunately, they did not evaluate whether the LV diastolic function was altered. With prolonged infusion of aldosterone to 8 weeks, we observed that LVEDD, FS, and EF were not different compared with the control. Together, these studies indicated that more prolonged aldosterone exposure alone might lead to LV diastolic dysfunction and not influence LV systolic function. The possibility was also raised that diastolic dysfunction might occur earlier than systolic dysfunction in that condition.
Additional features of the myocardium following aldosterone alone treatment included cardiac interstitial fibrosis and perivascular fibrosis of the intramyocardial coronary arteries in ALD rats. LV hypertrophy was also observed following aldosterone infusion, as shown by increased LVMI and greater LVAW and LVPW thickness. Moreover, the marked increase in swollen and denatured mitochondria observed by TEM in the ALD group pointed to remodeling of the myocardial structure and insufficient myocardium energy. Previous work has shown that diffuse accumulation of fibrosis tissue in the cardiac spaces contributes to increase ventricular diastolic stiffness and leads to, in severe cases, electrical conduction defects11. The resultant heterogeneity in myocardial structure, created by a disproportionate accumulation of collagen, may serve to explain the important clinical observations that patients with high-renin essential hypertension have a greater incidence of adverse cardiovascular events and impaired ventricular function, respectively12, 13.
High-sodium intake could aggravate myocardial injuries induced by aldosterone, which was partly independent of blood pressure. The 8% sodium chloride alone or 8% sodium chloride administration in conjunction with exogenous aldosterone is usually used in experimental studies to detect the effects of high-sodium intake on the cardiac structure. In such situations, sodium intake levels are twenty times higher than that on a 0.3% normal sodium diet in rats. In our study urinary sodium of 24 h in the HS-ALD group was about six times higher than that in the CON group and the ALD group. We showed that SBP was not significantly different between the ALD group and the HS-ALD group. However, LV hypertrophy was more predominant in the HS-ALD group compared with that in the ALD group and was accompanied by significantly increased collagen deposition in the perivascular space of the coronary arteries and the breakdown of myocardial ultrastructure. This result suggested that moderate elevation of sodium chloride intake could aggravate myocardial injuries induced by aldosterone, which was partly independent of blood pressure. Regarding the synergistic action between aldosterone and sodium, fibroblast collagen synthesis may be involved in the regulation by aldosterone of Na+/K+-ATPase, and the expression of Na+/K+-ATPase is increased only in the presence of salt14. Moreover, a previous clinical study indicated that the association of serum aldosterone with both an increase in blood pressure and the later development of hypertension was seen only in the persons whose urine sodium index was at or above the median, but not in those whose urine sodium excretion was below the median15. Such an interaction between aldosterone and sodium is also supported by the observations made in Yanomamo Indians who consume a very low-salt diet. They exhibit markedly elevated serum aldosterone levels but little or no blood-pressure elevation16. In modern society, humans could easily increase sodium intake via food diversity. Therefore, lowering sodium intake can compensate for the risk of cardiac and vascular injury by aldosterone, especially in essential hypertension, primary and secondary aldosteronism.
The myocardial injury may be attributable to significantly up-regulated oxidative stress induced by aldosterone and high sodium intake. It is now established that the specificity of MR occupancy by aldosteone in epithelial tissues is determined by 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2)17, 18, but the amount of 11β-HSD2 in non-epithelial tissues, such as heart, is at a negligible level19. MR in non-epithelial tissues should be exclusively occupied by glucocorticoids with very limited accessible aldosterone. The glucocorticoid-MR complex is inactive under the steady-state condition. Funder et al recently proposed a novel and intriguing hypothesis for MR activation in non-epithelial tissues, suggesting that the inactive glucocorticoid-MR may be activated by the generation of ROS20. With the possible positive feedback system triggered by inflammation and increased oxidative stress, further activation of the glucocorticoid-MR complex could occur, thus accounting for the vicious cycle of the aldosterone-induced cardiovascular injury. This postulated mechanism could well explain our study results, with high-sodium intake potentially aggravating myocardial injuries induced by aldosterone through up-regulated oxidative stress. The hypothesis has been supported by previous studies, which showed that the development of hypertension and the regression of cardiovascular remodeling were attenuated in mineralocorticoid treated animals after the treatment of antioxidant drugs, such as the superoxide dismutase mimetic Tempol21, 22, the NADPH oxidase inhibitor apocynin23, 24 or N-acetylcysteine5.
Increased oxidative stress might also trigger and deteriorate inflammation. In the present study, noticeable inflammatory injury and significantly up-regulated IL-18 protein expression were observed in the ALD group compared with that in controls. Furthermore, the inflammatory injury was more serious and the IL-18 protein expression was up-regulated in the HS-ALD group compared with that in the ALD group. Previous experimental studies have indicated that aldosterone infusion alone for a shorter time may exclusively result in the infiltration of inflammation cells via oxidative stress10. Both of inflammation and increased oxidative stress may lead to the further activation of the glucocorticoid-MR complex, thus worsening myocardial inflammatory injuries induced by aldosterone21. It may be possible that aldosterone-induced oxidative stress stimulated a series of pro-inflammatory genes expression, such as IL-18, via a redox-sensitive mechanism, thereby leading to initiation of the cardiovascular inflammatory phenotype. Moreover, inflammation and, in particular, generation of free radicals may contribute to the activation of the fibrotic process and hypertrophy25. Cardiac fibrosis could be the reparative response to the inflammatory injury, although direct effects of aldosterone on fibrosis are possible26, 27. Furthermore, previous studies have indicated that adult cardiomyocytes express IL-18 and its receptors, and that proinflammatory cytokines and oxidative stress regulate their expression via activation of NF-κB28, 29, 30, 31. In the present study, we found that IL-18, which was a predictor of cardiovascular events, might be involved in the cardiovascular injuries induced by aldosterone.
In summary, the present work provided evidence that long-term infusion of aldosterone on a normal sodium diet could result in hypertension, persistent inflammatory infiltration, cardiac fibrosis, LV hypertrophy and LV diastolic dysfunction. Moderate high-sodium intake could aggravate myocardial injuries induced by aldosterone. These synergistic effects of sodium and aldosterone were mediated by oxidative stress via NADPH oxidase and IL-18. Our data could also underscore the potential benefits of lower sodium action in cardiac protection related to aldosterone. Further studies are needed to elucidate the detailed nature of the relationship between inflammation, oxidative stress, and activation of glucocorticoid/aldosterone-MR in the development of cardiovascular diseases.
Author contribution
Hua CHENG designed research; Jing-yi LI performed research; Shao-ling ZHANG and Meng REN analyzed data; Yan-ling WEN and Li YAN contributed new analytical tools and reagents; Jing-yi LI and Hua CHENG wrote the paper.
Acknowledgments
This work was supported by grants from the State “15” Science and Technology Research Projects (2004BA720A29).
References
- Rossi GP, Di Bello V, Ganzaroli C, Sacchetto A, Cesari M, Bertini A, et al. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension. 2002;40:23–7. doi: 10.1161/01.hyp.0000023182.68420.eb. [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. [PubMed] [Google Scholar]
- Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am J Pathol. 2002;161:1773–81. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, et al. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension. 2002;39:614–8. [PubMed] [Google Scholar]
- Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion BMJ 1988297319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–58. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–16. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Kim-Mitsuyama S, Wake R, Izumiya Y, Izumi Y, Yukimura T, et al. Excess aldosterone under normal salt diet induces cardiac hypertrophy and infiltration via oxidative stress. Hypertens Res. 2005;28:447–55. doi: 10.1291/hypres.28.447. [DOI] [PubMed] [Google Scholar]
- Weber KT, Sun Y, Tyagi SC, Cleutjens JP. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26:279–92. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, et al. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–9. doi: 10.1056/NEJM197203022860901. [DOI] [PubMed] [Google Scholar]
- Vensel LA, Devereux RB, Pickering TG, Herrold EM, Borer JS, Laragh JH. Cardiac structure and function in renovascular hypertension produced by unilateral and bilateral renal artery stenosis. Am J Cardiol. 1986;58:575–82. doi: 10.1016/0002-9149(86)90279-1. [DOI] [PubMed] [Google Scholar]
- Horisberger JD, Rossier BC. Aldosterone regulation of gene transcription leading to control of ion transport. Hypertension. 1992;19:221–7. doi: 10.1161/01.hyp.19.3.221. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation. 1975;52:146–51. doi: 10.1161/01.cir.52.1.146. [DOI] [PubMed] [Google Scholar]
- Funder JW. Mineralocorticoid receptors and hypertension. J Steroid Biochem Mol Biol. 1995;53:53–5. doi: 10.1016/0960-0760(95)00021-q. [DOI] [PubMed] [Google Scholar]
- White PC, Mune T, Agarwal AK. 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev. 1997;18:135–56. doi: 10.1210/edrv.18.1.0288. [DOI] [PubMed] [Google Scholar]
- Fuller PJ, Young MJ. Mechanisms of mineralocorticoid action. Hypertension. 2005;46:1227–35. doi: 10.1161/01.HYP.0000193502.77417.17. [DOI] [PubMed] [Google Scholar]
- Funder JW. Aldosterone, mineralocorticoid receptors and vascular inflammation. Mol Cell Endocrinol. 2004;217:263–9. doi: 10.1016/j.mce.2003.10.054. [DOI] [PubMed] [Google Scholar]
- Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–64. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- Hirono Y, Yoshimoto T, Suzuki N, Sugiyama T, Sakurada M, Takai S, et al. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology. 2007;148:1688–96. doi: 10.1210/en.2006-1157. [DOI] [PubMed] [Google Scholar]
- Park YM, Park MY, Suh YL, Park JB. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem Biophys Res Commun. 2004;313:812–7. doi: 10.1016/j.bbrc.2003.11.173. [DOI] [PubMed] [Google Scholar]
- Li L, Chu Y, Fink GD, Engelhardt JF, Heistad DD, Chen AF. Endothelin-1 stimulates arterial VCAM-1 expression via NADPH oxidase-derived superoxide in mineralocorticoid hypertension. Hypertension. 2003;42:997–1003. doi: 10.1161/01.HYP.0000095980.43859.59. [DOI] [PubMed] [Google Scholar]
- Nicoletti A, Michel JB. Cardiac fibrosis and inflammation: Interaction with hemodynamic and hormonal factors. Cardiovasc Res. 1999;41:532–43. doi: 10.1016/s0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- Young M, Head G, Funder J. Determinants of cardiac fibrosis in experimental hypermineralocorticoid states. Am J Physiol. 1995;269:E657–62. doi: 10.1152/ajpendo.1995.269.4.E657. [DOI] [PubMed] [Google Scholar]
- Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27:341–8. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- Doi T, Sakoda T, Akagami T, Naka T, Mori Y, Tsujino T, et al. Aldosterone induces interleukin-18 through endothelin-1, angiotensin II, Rho/Rho-kinase, and PPARs in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1279–87. doi: 10.1152/ajpheart.00148.2008. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Colston JT, de la Rosa SD, Rao PP, Freeman GL. TNF-alpha and H2O2 induce IL-18 and IL-18R beta expression in cardiomyocytes via NF-kappa B activation. Biochem Biophys Res Commun. 2003;303:1152–8. doi: 10.1016/s0006-291x(03)00496-0. [DOI] [PubMed] [Google Scholar]
- Rabkin SW. The role of interleukin 18 in the pathogenesis of hypertension-induced vascular disease. Nat Clin Pract Cardiovasc Med. 2009;6:192–9. doi: 10.1038/ncpcardio1453. [DOI] [PubMed] [Google Scholar]
- Wang M, Markel TA, Meldrum DR. Interleukin 18 in the heart. Shock. 2008;30:3–10. doi: 10.1097/SHK.0b013e318160f215. [DOI] [PubMed] [Google Scholar]