Abstract
β-adrenergic receptor (βAR) stimulation by the sympathetic nervous system or circulating catecholamines is broadly involved in peripheral blood circulation, metabolic regulation, muscle contraction, and central neural activities. In the heart, acute βAR stimulation serves as the most powerful means to regulate cardiac output in response to a fight-or-flight situation, whereas chronic βAR stimulation plays an important role in physiological and pathological cardiac remodeling.
There are three βAR subtypes, β1AR, β2AR and β3AR, in cardiac myocytes. Over the past two decades, we systematically investigated the molecular and cellular mechanisms underlying the different even opposite functional roles of β1AR and β2AR subtypes in regulating cardiac structure and function, with keen interest in the development of novel therapies based on our discoveries. We have made three major discoveries, including (1) dual coupling of β2AR to Gs and Gi proteins in cardiomyocytes, (2) cardioprotection by β2AR signaling in improving cardiac function and myocyte viability, and (3) PKA-independent, CaMKII-mediated β1AR apoptotic and maladaptive remodeling signaling in the heart. Based on these discoveries and salutary effects of β1AR blockade on patients with heart failure, we envision that activation of β2AR in combination with clinically used β1AR blockade should provide a safer and more effective therapy for the treatment of heart failure.
Keywords: β-adrenergic receptor, heart failure, signal transduction, cardiovascular system
Introduction
Heart failure (HF) is a syndrome characterized by the insufficient pumping of blood to meet the need of the body. It is a chronic and severely debilitating disease with people older than 65 composed more than 75% of all cases1. Regardless of the cause, the failing heart usually ends up in a viscous cycle of progressive functional decline. Owing to its high prevalence, morbidity, mortality and significant health-care costs, HF represents a major current health problem in China and its prevalence is in an upward trend as atherothrombotic diseases, which often lead to HF, will be the first cause of death in the world by 20202.
In congestive HF, both the activities of the sympathetic nervous system and the renin-angiotensin system (RAS) are increased3. Initially, the increased activity of these neurohormonal systems serves to compensate for the reduced blood pressure and cardiac output. But long term exposure to high levels of circulating catecholamines and angiotensin increases the workload of the heart, and causes maladaptive cardiac remodeling and myocyte death4, 5, 6. Many of these effects appear to be mediated by the signal transduction cascades of the receptors involved.
β-Adrenergic receptor (βAR) and angiotensin receptor belong to the superfamily of G protein-coupled receptors (GPCRs) or seven transmembrane receptors. GPCRs constitute the most ubiquitous of plasma membrane receptors. They are involved in the regulation of many important physiological functions and also serve as the most important drug targets7. Over the past 25 years, βAR antagonists (β-blockers), angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), alone or in combination, have been used to treat HF conditions. Their use ameliorates the deterioration of left ventricular function, improves symptoms and hemodynamics, and decreases the mortality rate and the need for hospitalization8, 9, 10, 11. However, these therapeutic agents have limited effectiveness in some patient populations and they also have some adverse effects. Therefore, there is a compelling need to develop new treatments that can improve clinical outcomes.
Subtype-specific βAR signaling in the heart
βARs exist as three subtypes, β1, β2, and β3, and the former two are important in the regulation of excitation-contraction coupling of myocardium. β1AR is the predominant receptor subtype expressed in the heart. Its stimulation results in the activation of the Gs-adenylyl cyclase (AC)-cAMP-protein kinase A (PKA) signaling cascade. In ventricular myocytes, the phosphorylation of PKA substrates including phospholamben, L-type calcium channel, ryanodine receptor, cardiac troponin I, and cardiac myosin-binding protein C results in the increase in calcium transient and contractility. In pacemaker cells, PKA-mediated phosphorylation of membrane ion channels and Ca2+ handling proteins increases Ca2+ cycling and pacing rate. Similarly, β2AR also has a functional role in cardiomyocyte contraction12. But unlike β1AR which couples only to Gs, β2AR also couples to pertussis toxin (PTX)-sensitive Gi proteins13. The β2AR-Gi signaling has negative effects on AC activity, cAMP synthesis, PKA activation, and the inotropic response mediated by Gs.
Importantly, persistent stimulation of β1AR and β2AR exhibits distinct outcomes under certain pathological circumstances such as HF. Specifically, persistent stimulation of β1AR triggers cardiomyocyte apoptosis by a Ca2+/calmodulin-dependent kinase II (CaMKII)-dependent, but PKA in-dependent mechanism14. Furthermore, the β1AR-activated CaMKII signaling, but not the PKA pathway, is involved in catecholamine-induced cardiomyocyte hypertrophy in vitro15 and maladaptive cardiac remodeling in vivo16, 17. In contrast to the cardiotoxic effects of persistent β1AR activation, persistent β2AR stimulation is cardioprotective. The cardioprotective effect of persistent β2AR signaling is largely mediated by β2AR-Gi coupling, which activates the Gβγ-phosphoinositol 3-kinase (PI3K)-Akt cell survival pathway18. Although beneficial in terms of cardiomyocyte viability, the protective effect of β2AR comes at the cost of compromised contractile support.
Heart failure-associated alterations in βAR signaling
During HF, β1AR is persistently downregulated at the mRNA and protein levels19, 20. Its density on the plasma membrane is reduced by 50%, while that of β2AR has no such change21. The resulting change in the ratio of β1/β2AR from an 80:20 distribution in the healthy heart to a ratio of 60:40 in the failing heart may indicate the prominent role of β2AR signaling in the disease condition. In the failing heart, the selective downregulation of β1AR is often associated with an upregulation of Gi and an enhanced β2AR-Gi signaling22, 23. Importantly, the β1AR-mediated contractile response is cross-inhibited by the enhanced β2AR-Gi signaling in the failing heart. Thus, the enhanced β2AR-Gi signaling contributes to the dysfunction of both β1AR- and β2AR-Gs signaling in the failing heart24, 25, 26, 27.
In addition, the signaling efficiency of β1AR is also markedly reduced in the failing heart as a result of desensitization28. This is attributed, in part, to a significant increase in the expression level of G protein coupled receptor kinase 2 (GRK2)29, the prototypical member of the GRK family. The process of βAR desensitization involves a series of events, including (a) the translocation of GRK2 to the plasma membrane facilitated by the free Gβγ subunits liberated from the activated heterotrimeric G proteins30, (b) the phosphorylation of the serine or threonine residues on the C-terminal tail of βARs by GRK2, (c) the recruitment of β-arrestins to the phosphorylated receptor, the physical displacement of Gsα from the β-arrestin-associated receptor, and (d) the β-arrestin-dependent internalization of the receptor (endocytosis)31. While β2AR stays at a similar level in the failing heart, its coupling efficiency to Gs is markedly reduced21. Desensitization of βARs leads to reduced Gs-mediated responses such as cAMP production and positive inotropic effect. Although receptor downregulation and desensitization are considered to be protective responses against excessive sympathetic stimulation during HF32, 33, the resultant abnormality in βAR signaling may lead to the activation of signaling pathways that are involved in cardiac remodeling, such as the PI3K cascades34.
Indeed, in humans or animal models with HF, chronic catecholamine elevation causes marked dysregulation of βARs, resulting in various molecular abnormalities, including the upregulation of GRK229, 35 and Gi proteins22, 23, 36. Upregulation of both of these proteins have been implicated as causal factors in the development of HF. In particular, GRK2 is the most abundant and best-characterized GRK in the heart37. GRK2 expression and activity are markedly elevated and play a central role in the HF-associated defect in βAR signaling38 and cardiac dysfunction39. Myocardial ischemia and hypertension in humans and animal models have also been associated with elevated GRK2 expression and activity40, 41. These previous studies have defined GRK2 upregulation as an early common event in cardiac maladaptive remodeling and HF.
Emerging evidence suggests that activation of GRK2 as well as PKA is essentially involved in the activation of the β2AR-coupled Gi signaling in mammalian cells. First, early work has shown that β2AR-induced activation of ERK1/2 in HEK293 cells is mediated by a Gi-dependent mechanism, and that phosphorylation of β2AR by PKA is a prerequisite for the switch of the receptor coupling from Gs to Gi42. Second, our recent studies43 have demonstrated that elevated β2AR phosphorylation by GRK2 acerbates the Gi signaling, whereas inhibition of GRK2 activity profoundly suppresses the β2AR-Gi coupling. Since GRK2 upregulation occurs prior to the onset of HF and contributes to the development of HF44, 45, enhanced GRK2 activation may play an important role in the exacerbated β2AR-coupled Gi signaling in the failing heart. Indeed, disruption of Gi signaling with PTX or inhibition of GRK2 with a peptide inhibitor, βARK-ct, can restore cardiac contractile response to βAR stimulation in multiple HF models46, 47, 48, 49.
Importantly, cardiac-specific transgenic overexpression of a mutant β2AR lacking PKA phosphorylation sites (PKA-TG), but not the wild type β2AR (WT TG) or a mutant β2AR lacking GRK sites (GRK-TG), led to exaggerated cardiac response to pressure overload, as manifested by markedly exacerbated cardiac maladaptive remodeling and failure, and early mortality43. Furthermore, inhibition of Gi signaling with PTX restores cardiac function in HF associated with increased β2AR to Gi coupling induced by removing PKA phosphorylation of the receptor and in GRK2 transgenic mice, indicating that enhanced phosphorylation of β2AR by GRK and resultant increase in Gi-biased β2AR signaling play an important role in the development of HF43. Altogether, our recent studies have demonstrated that enhanced β2AR phosphorylation by GRK leads the receptor to Gi-biased signaling which, in turn, contributes to the pathogenesis of HF, marking Gi-biased β2AR signaling as a primary event linking pathological upregulation of GRK to cardiac maladaptive remodeling, failure and cardiodepression. It is also noteworthy that, as is the case in the failing heart, enhanced β2AR-coupled Gi signaling is responsible for the defects of both β1AR and β2AR signaling in the GRK2 transgenic mice43, and that the previously reported beneficial effects of βARK-ct in improving the function of the failing heart38, 39, 50, 51, 52 is mediated, at least in part, by attenuating GRK-dependent Gi-biased β2AR signaling.
Carvedilol paradox
In clinical settings, long-term use of β-blockers improves clinical symptom of HF. Treatment with β-blockers improves left ventricular contractile function in the failing heart and reverses cardiac remodeling8, 9. In the molecular level, β-blockade may normalize βAR system through the upregulation of β1AR53 and the restoration of receptor sensitivity by decreasing the expression of GRK250. However, the effects of different β-blockers are not identical. The use of subtype non-selective β-blockers in early years has caused some major side effects including bronchial and blood vessel constriction54, 55. This is largely due to the inhibition of β2AR in non-cardiac tissues such as the respiratory system and blood vessels. These problems have been partially resolved with the introduction of selective β1AR antagonists, such as atenolol, metoprolol, bisoprolol and nebivolol. Recent clinical trials have indicated that only 3 out of 16 β-blockers are beneficial in terms of cardiovascular survival9, 56, 57, 58, with carvedilol emerging as the best59.
Apart from being a non-selective β-blocker, carvedilol also has several properties, such as α1-adrenergic blockade, antioxidant, anti-proliferative, anti-endothelin and anti-arrythmogenic effects60, 61, which may explain its higher efficacy. Interestingly, carvedilol has been found to be the only one among 16 blockers that activated ERK by a β2AR-mediated, G protein-independent, and β-arrestin-dependent mechanism62. Moreover, among 20 β-blockers tested, only atenolol and carvedilol could induce the β1AR-mediated transactivation of EGFR and this effect is also β-arrestin-dependent63. It has been implicated that this effect may contribute to the special therapeutic effect of carvedilol. In this regard, recent studies have shown that β-arrestin-dependent, G protein-independent activation of EGFR via β1AR confers cardioprotection in mice chronically stimulated with catecholamine64. These data suggest that a ligand can antagonize the G protein-dependent activity of a GPCR and at the same time stimulates signaling pathways in a G protein-independent β-arrestin-dependent fashion65. They are also of great relevance to our discussion in the next section about the application of this principle in the development of novel therapeutic agents.
Biased βAR signaling and drug discovery
In the classical paradigm of GPCR signaling, ligand binding leads to conformational change of the receptor from an in-active state R into a single activated state R* that results in the coupling of the receptor to heterotrimeric G proteins. Receptor coupling facilitates the exchange of the bound GDP with GTP in the α subunit of the G protein complex. This triggers dissociation of the complex into Gα and Gβγ subunits. They go on to activate their respective effectors such as AC, phospholipases and ion channels. These receptor mediated reactions often generate signaling molecules called second messengers which activate or inhibit other components of the cellular machinery. Thus, receptor stimulation produces a multitude of cellular responses via the activation of the signal transduction pathways downstream of G proteins. Agonist efficacy, a measure of the ability of an agonist to activate this cascade, quantitatively defines the agonist as partial or full. In this scheme, antagonist is defined as a ligand which binds to the receptor but produces no receptor activation and thus has the ability to block agonist-stimulated G protein activation. This unidirectional understanding of agonist efficacy is contradictory to the aforementioned findings that a ligand for a single GPCR can be an antagonist for the G protein-dependent signals and also an agonist for the β-arrestin-dependent signals62, 63.
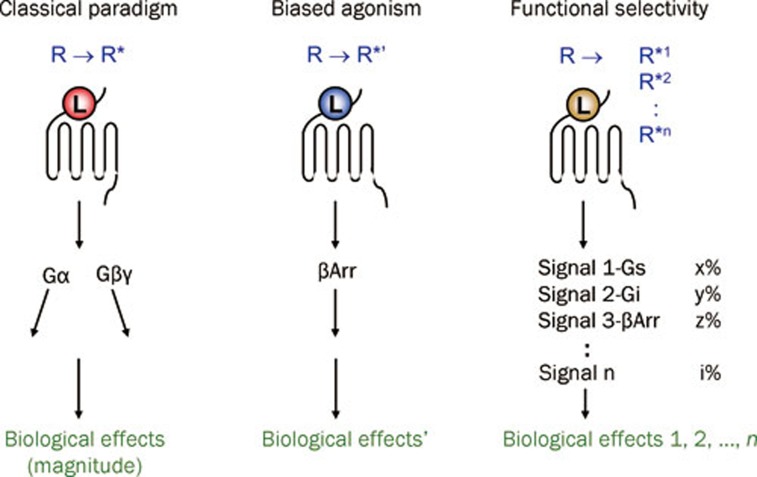
Over the past fifteen years, more and more evidence has accumulated indicating that a ligand for a given GPCR does not simply possess a single defined efficacy. Rather, a ligand possesses multiple efficacies, depending on the downstream signal transduction pathways analyzed. Moreover, GPCR can be differentially activated to target a specific subset of signal transduction pathways by the so-called “biased ligand”. In particular, research has revealed that GPCR can be stimulated to produce a β-arrestin-dependent but G protein-independent signal, which differs both spatially and temporally from the β-arrestin-mediated signal stemmed from receptor desensitization66. It is believed that the β-arrestin-biased ligand activates the alternative signaling pathway by stabilizing the receptor in a distinct active conformation R*'. Thus, in this new paradigm, GPCR may be stabilized by different ligands in distinct active conformations R*1-R*n each capable of activating a diverse array of signal transduction pathways and responses (Figure 1). This concept, described as functional selectivity, collateral/pluridimensional efficacy, or biased agonism, has major implications for pharmacological therapeutics65, 67, 68, 69, 70.
Figure 1.
Development of the receptor theory. In the classical paradigm, ligands have linear efficacies, referring to their abilities to stabilize the receptor into a single active state. The emerging concept of biased agonism suggests that a biased ligand may stabilize the receptor into a distinct active state that does not activate G proteins but activates β-arrestins. In the concept of functional selectivity, receptors may exist in multiple active conformations as stabilized by different ligands, and each of these conformations gives rise to different downstream signals and biological effects. βArr, β-arrestin; L, ligand; R, inactive conformation of GPCR; R*, active conformation of GPCR.
To add another layer of complexity to this scheme, the signal trafficked by a biased agonist is context-dependent, too. Not only does the selectivity of a ligand towards different signaling pathways change in different cell types, the change in the levels of cytosolic reactants of GPCR also has an impact on the functional selectivity of a ligand. For example, the specific β2AR antagonist ICI-118551 has been suggested to directly produce a negative inotropic effect by acting as an agonist for the Gi-coupled β2AR in myocytes from failing human heart71. This effect is not due to the blocking of the endogenous catecholamines and is also different in principle from an inverse agonistic effect also described for this ligand72. It is because this negative inotropic effect of ICI-118551 is PTX-sensitive, is observable at receptor levels with or without overexpression manipulation, and only becomes apparent under the conditions when the levels of Gi are raised.
In a recent study using a cardiomyocyte model49, we have screened a panel of β2AR agonists, including zinterol, salbutamol, and procaterol for their receptor-mediated contractility stimulatory activities and the sensitivities of these effects towards PTX. We have found that PTX augmented the contractile responses of most β2AR agonists but not that of fenoterol. These data indicates that while most β2AR agonists activate both Gs and Gi, fenoterol selectively activates Gs. This is the first evidence to show that different agonists can activate a receptor to couple to different G-proteins. It was further found that fenoterol fully reversed the diminished β2AR-mediated inotropic effect in cardiomyocytes isolated from failing spontaneous hypertensive rat hearts even in the absence of PTX. This study is particularly valuable in that fenoterol was identified to be a unique agonist capable of selectively stabilizing the coupled β2AR-Gs species in conditions that favor β2AR-Gi coupling. It also reveals the therapeutic potential of fenoterol in the treatment of HF.
The effectiveness of fenoterol in treating HF conditions has been demonstrated in a number of follow-up in vivo studies73, 74, 75, 76. Prolonged use of fenoterol not only improves cardiac function, but also retards cardiac maladaptive remodeling, and that the overall beneficial effects of fenoterol are greater than the salutary effects of β1AR blockade in a myocardial infarction induced rat model of dilated cardiomyopathy73. These studies suggest that selective activation of the β2-AR-coupled Gs signaling may provide a useful therapeutic target for the treatment of congestive HF. We envision that new Gs-biased β2AR agonists, such as fenoterol and its derivatives, may be developed into drugs to improve the structure and function of the failing heart.
Fenoterol contains two chiral centers and can exist as four stereoisomers. We have synthesized a cohort of fenoterol derivatives including the R,R-, R,S-, S,R-, and S,S-isomers77, 78. While the pharmaceutical preparation of fenoterol is a racemic mixture of its R,R- and S,S-enantiomers, our recent studies have shown that the R,R-enantiomer is the only active isomer in receptor binding and cardiomyocyte contraction assays77, 78. It has been known for a century that stereoisomers of catecholamines differ in their potency and efficacy. However, the molecular basis for the differences in the efficacies of GPCR ligand stereoisomers has remained poorly defined. We have, therefore, used some of these fenoterol derivatives to examine the hypothesis that the stereochemistry of an agonist determines functional selectivity of a given receptor coupling to different G protein(s) and resultant activation of subset(s) of downstream signaling pathways79. We found that while R,R-fenoterol failed to activate Gi signaling, as evidenced by the absence of PTX-sensitivity of its contractile response and its inability to activate Gi-dependent ERK1/2 signaling, S,R-fenoterol exhibited a robust PTX-sensitivity in these responses, suggesting that the S,R-isomer enables β2AR to activate both Gs and Gi. The same conclusion holds true for some fenoterol derivatives. For instance, S,R-methoxyfenoterol, but not R,R-methoxyfenoterol, activated β2AR-coupled Gi signaling in cardiomyocytes79. Thus, in addition to receptor subtype and phosphorylation status, the different stereoisomers of an agonist selectively activate distinct receptor-G protein interactions and downstream signaling events. This finding is important because it is the first account to show that even the subtle chemical differences within a ligand stereoisomer pair are sufficient to stabilize GPCR conformations with distinct G-protein coupling properties, highlighting how important it is to carefully examine both the “active” and the “inactive” stereoisomer to understand the exact mechanism of action and cellular effects of a GPCR ligand80.
This finding also has important clinical implications. In particular, it has been shown that long-term (1 year) treatment with racemic fenoterol enhances the beneficial effect of β1AR blockade with metoprolol in a rat model of dilated cardiomyopathy75, and the combined (fenoterol+metoprolol) therapy is as good as the clinical combination (metoprolol+ACEI) treatment with respect to mortality, and exceeds the latter with respect to cardiac remodeling and myocardial infarct expansion76. It will be interesting to study the effects of different fenoterol derivatives77, 78, 81 alone or in combination with β1AR blocker or RAS inhibitor in this model. Continued efforts on this research line may lead to the development of potential novel therapies with greater selectivity, efficacy and fewer side effects for human congestive HF. Topics related to the translation of this novel treatment regimen have been discussed extensively in another recent review82, which also contains a pathway map for βAR subtype signaling described in this article.
If suppression of β2AR-Gi signaling or enhancement of β2AR-Gs signaling is beneficial in HF, the next question is: what is the difference between β2AR-Gs signaling and β1AR-Gs signaling? In a recent elegant study83, Mangmool and co-authors have elucidated the molecular mechanism of CaMKII activation by β1AR. They found that stimulation of β1AR induces the formation of a β-arrestin-CaMKII-Epac1 complex, allowing its recruitment to the plasma membrane, and whereby promotes cAMP-dependent activation of CaMKII. Further studies using chimeric receptors with switched carboxyl-terminal tails of β1AR or β2AR suggested that β-arrestin binding to the carboxyl-terminal tail of β1AR promotes a conformational change within β-arrestin that allows CaMKII and Epac to remain in a stable complex with the receptor. These results demonstrate that only β1AR but not β2AR activates CaMKII significantly. As CaMKIIδ is a common intermediate of diverse death stimuli-induced apoptosis in cardiomyocytes84, is required for the transition from pressure overload-induced cardiac hypertrophy to HF85, and promotes life-threatening arrythmias in HF86, this explains why activation of β2AR-Gs signaling is usually not accompanied with the adverse effects observed in β1AR stimulation.
The molecular mechanism of the cardioprotective effect of β2AR-Gs signaling in HF is unclear. One possibility is the crosstalk of the Gs-AC-cAMP-PKA cascade to the tyrosine kinase receptor-mediated Akt phosphorylation87, 88, 89.
Concluding remark
In summary, recent studies have revealed opposing functional roles of β1AR and β2AR in regulating myocyte viability and myocardial remodeling with a cardiac protective effect of β2AR stimulation and a detrimental effect of β1AR stimulation. Unlike the sole Gs coupling of β1AR, β2AR couples to both Gs and Gi signaling pathways with the Gi coupling negating the Gs-mediated contractile support. In the failing heart, enhanced expression and activity of GRK2 and Gi proteins further promote Gi-biased β2AR signaling, thus blunting both β1AR- and β2AR-mediated cardiac reserve function, resulting in cardiac maladaptive remodeling and failure. These findings defined the β2AR-Gi signaling as an essential link between pathologic upregulation of GRK and the development of HF. Since GRK2 and resultant Gi-biased β2AR signaling are pathogenic factors of HF, Gs-biased β2AR agonists may present an important therapeutic strategy for the treatment of HF caused by various etiologies.
Acknowledgments
This work was supported by the National Basic Research Program of China (2012CB518000) and the National Major Scientific Research Program of China (2012CB910400).
References
- Rich MW. Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J Am Geriatr Soc. 1997;45:968–74. doi: 10.1111/j.1532-5415.1997.tb02968.x. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary: Heart disease and stroke statistics — 2010 update: A report from the American Heart Association. Circulation. 2010;121:948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- Levine TB, Francis GS, Goldsmith SR, Simon AB, Cohn JN. Activity of the sympathetic nervous system and renin-angiotensin system assessed by plasma hormone levels and their relation to hemodynamic abnormalities in congestive heart failure. Am J Cardiol. 1982;49:1659–66. doi: 10.1016/0002-9149(82)90243-0. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Mulieri LA, Allen PD, Just H, Alpert NR. Influence of isoproterenol and ouabain on excitation-contraction coupling, cross-bridge function, and energetics in failing human myocardium. Circulation. 1996;94:3155–60. doi: 10.1161/01.cir.94.12.3155. [DOI] [PubMed] [Google Scholar]
- Teerlink JR, Pfeffer JM, Pfeffer MA. Progressive ventricular remodeling in response to diffuse isoproterenol-induced myocardial necrosis in rats. Circ Res. 1994;75:105–13. doi: 10.1161/01.res.75.1.105. [DOI] [PubMed] [Google Scholar]
- Blaufarb IS, Sonnenblick EH. The renin-angiotensin system in left ventricular remodeling. Am J Cardiol. 1996;77:8C–16C. doi: 10.1016/s0002-9149(96)00183-x. [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there. Nat Rev Drug Disc. 2006;5:993–6. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–89. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- Waagstein F, Bristow MR, Swedberg K, Camerini F, Fowler MB, Silver MA, et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet. 1993;342:1441–6. doi: 10.1016/0140-6736(93)92930-r. [DOI] [PubMed] [Google Scholar]
- Beckwith C, Munger MA. Effect of angiotensin-converting enzyme inhibitors on ventricular remodeling and survival following myocardial infarction. Ann Pharmacother. 1993;27:755–66. doi: 10.1177/106002809302700617. [DOI] [PubMed] [Google Scholar]
- Lee VC, Rhew DC, Dylan M, Badamgarav E, Braunstein GD, Weingarten SR. Meta-analysis: angiotensin-receptor blockers in chronic heart failure and high-risk acute myocardial infarction. Ann Intern Med. 2004;141:693–704. doi: 10.7326/0003-4819-141-9-200411020-00011. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Hohl C, Altschuld R, Jones L, Livingston B, Ziman B, et al. β2-adrenergic receptor-stimulated increase in cAMP in rat heart cells is not coupled to changes in Ca2+ dynamics, contractility, or phospholamban phosphorylation. J Biol Chem. 1994;269:19151–6. [PubMed] [Google Scholar]
- Xiao RP, Ji X, Lakatta EG. Functional coupling of the β2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol. 1995;47:322–9. [PubMed] [Google Scholar]
- Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, et al. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–25. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucharov CC, Mariner PD, Nunley KR, Long C, Leinwand L, Bristow MR. A β1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am J Physiol Heart Circ Physiol. 2006;291:H1299–308. doi: 10.1152/ajpheart.00017.2006. [DOI] [PubMed] [Google Scholar]
- Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, et al. Myocardial-directed overexpression of the human β1-adrenergic receptor in transgenic mice. J Mol Cell Cardiol. 2000;32:817–30. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96:7059–64. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by β2-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98:1607–12. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow MR, Ginsburg R, Fowler M, Minobe W, Rassmussen R, Zera P, et al. β1 and β2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective β1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Minobe WA, Raynolds MV, Port JD, Rasmussen R, Ray PE, et al. Reduced β1 receptor messenger RNA abundance in the failing human heart. J Clin Invest. 1993;92:2737–45. doi: 10.1172/JCI116891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow MR, Hershberger RE, Port JD, Minobe W, Rasmussen R. β1- and β2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol Pharmacol. 1989;35:295–303. [PubMed] [Google Scholar]
- Bohm M, Eschenhagen T, Gierschik P, Larisch K, Lensche H, Mende U, et al. Radioimmunochemical quantification of Giα in right and left ventricles from patients with ischaemic and dilated cardiomyopathy and predominant left ventricular failure. J Mol Cell Cardiol. 1994;26:133–49. doi: 10.1006/jmcc.1994.1017. [DOI] [PubMed] [Google Scholar]
- Feldman AM, Cates AE, Veazey WB, Hershberger RE, Bristow MR, Baughman KL, et al. Increase of the 40 000-mol wt pertussis toxin substrate (G protein) in the failing human heart. J Clin Invest. 1988;82:189–97. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokuta AJ, Maertz NA, Meethal SV, Potter KT, Kamp TJ, Valdivia HH, et al. Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation. 2005;111:988–95. doi: 10.1161/01.CIR.0000156461.81529.D7. [DOI] [PubMed] [Google Scholar]
- Sato M, Gong H, Terracciano CM, Ranu H, Harding SE. Loss of β-adrenoceptor response in myocytes overexpressing the Na+/Ca2+-exchanger. J Mol Cell Cardiol. 2004;36:43–8. doi: 10.1016/j.yjmcc.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Balke CW. Na+/Ca2+ exchange linking β2-adrenergic Gi signaling to heart failure: associated defect of adrenergic contractile support. J Mol Cell Cardiol. 2004;36:7–11. doi: 10.1016/j.yjmcc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Zhu W, Zeng X, Zheng M, Xiao RP. The enigma of β2-adrenergic receptor Gi signaling in the heart: the good, the bad, and the ugly. Circ Res. 2005;97:507–9. doi: 10.1161/01.RES.0000184615.56822.bd. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, et al. Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–11. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–63. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, et al. Role of β γ subunits of G proteins in targeting the β-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–7. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–80. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Faulx MD, Ernsberger P, Vatner D, Hoffman RD, Lewis W, Strachan R, et al. Strain-dependent β-adrenergic receptor function influences myocardial responses to isoproterenol stimulation in mice. Am J Physiol Heart Circ Physiol. 2005;289:H30–6. doi: 10.1152/ajpheart.00636.2004. [DOI] [PubMed] [Google Scholar]
- Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, et al. A GRK5 polymorphism that inhibits β-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–7. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, et al. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–48. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerer M, Parruti G, Böhm M, Puzicha M, DeBlasi A, Erdmann E, et al. Expression of β-arrestins and β-adrenergic receptor kinases in the failing human heart. Circ Res. 1994;74:206–13. doi: 10.1161/01.res.74.2.206. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Avdonin P, Zhou YY, Cheng H, Akhter SA, Eschenhagen T, et al. Coupling of β2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- Hata JA, Koch WJ. Phosphorylation of G protein-coupled receptors: GPCR kinases in heart disease. Mol Interv. 2003;3:264–72. doi: 10.1124/mi.3.5.264. [DOI] [PubMed] [Google Scholar]
- Choi DJ, Koch WJ, Hunter JJ, Rockman HA. Mechanism of β-adrenergic receptor desensitization in cardiac hypertrophy is increased β-adrenergic receptor kinase. J Biol Chem. 1997;272:17223–9. doi: 10.1074/jbc.272.27.17223. [DOI] [PubMed] [Google Scholar]
- Perrino C, Naga Prasad SV, Schroder JN, Hata JA, Milano C, Rockman HA. Restoration of β-adrenergic receptor signaling and contractile function in heart failure by disruption of the βARK1/phosphoinositide 3-kinase complex. Circulation. 2005;111:2579–87. doi: 10.1161/CIRCULATIONAHA.104.508796. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Kessebohm K, Kronsbein K, Lohse MJ, Richardt G. Activation of β-adrenergic receptor kinase during myocardial ischemia. Circ Res. 1996;79:455–60. doi: 10.1161/01.res.79.3.455. [DOI] [PubMed] [Google Scholar]
- Gros R, Benovic JL, Tan CM, Feldman RD. G-protein-coupled receptor kinase activity is increased in hypertension. J Clin Invest. 1997;99:2087–93. doi: 10.1172/JCI119381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- Zhu W, Petrashevskaya N, Ren S, Zhao A, Chakir K, Gao E, et al. Gi-biased β2AR signaling links GRK2 upregulation to heart failure Circ Res 2011. doi: 10.1161/CIRCRESAHA.111.253260 [DOI] [PMC free article] [PubMed]
- Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr, et al. Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci U S A. 1998;95:7000–5. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G, Gao E, Ebert SN, Dorn GW, 2nd, Koch WJ. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J Biol Chem. 2010;285:16378–86. doi: 10.1074/jbc.M109.077859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakir K, Daya SK, Aiba T, Tunin RS, Dimaano VL, Abraham TP, et al. Mechanisms of enhanced β-adrenergic reserve from cardiac resynchronization therapy. Circulation. 2009;119:1231–40. doi: 10.1161/CIRCULATIONAHA.108.774752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, et al. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a βARK inhibitor. Science. 1995;268:1350–3. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- Tachibana H, Naga Prasad SV, Lefkowitz RJ, Koch WJ, Rockman HA. Level of β-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure overload-induced heart failure. Circulation. 2005;111:591–7. doi: 10.1161/01.CIR.0000142291.70954.DF. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Zhang SJ, Chakir K, Avdonin P, Zhu W, Bond RA, et al. Enhanced Gi signaling selectively negates β2-AR- but not β1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation. 2003;108:1633–9. doi: 10.1161/01.CIR.0000087595.17277.73. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by β-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–9. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial β-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A. 2000;97:5428–33. doi: 10.1073/pnas.090091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac βARK1 inhibition prolongs survival and augments β blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci U S A. 2001;98:5809–14. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund M, Jakob H, Becker H, Hanrath P, Schumacher C, Eschenhagen T, et al. Effects of metoprolol on myocardial β-adrenoceptors and Giα-proteins in patients with congestive heart failure. Eur J Clin Pharmacol. 1996;51:127–32. doi: 10.1007/s002280050172. [DOI] [PubMed] [Google Scholar]
- Terpstra GK, Raaijmakers JA, Wassink GA. Propranolol-induced bronchoconstriction: a non-specific side-effect of β-adrenergic blocking therapy. Eur J Pharmacol. 1981;73:107–8. doi: 10.1016/0014-2999(81)90154-0. [DOI] [PubMed] [Google Scholar]
- Eliasson K, Lins LE, Sundqvist K. Vasospastic phenomena in patients treated with β-adrenoceptor blocking agents. Acta Med Scand Suppl. 1979;628:39–46. doi: 10.1111/j.0954-6820.1979.tb00771.x. [DOI] [PubMed] [Google Scholar]
- CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial Lancet 19993539–13. [PubMed] [Google Scholar]
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF) Lancet 19993532001–7. [PubMed] [Google Scholar]
- Packer M, Coats AJS, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- Metra M, Cas LD, Di Lenarda A, Poole-Wilson P. β-blockers in heart failure: Are pharmacological differences clinically important. Heart Fail Rev. 2005;9:123–30. doi: 10.1023/B:HREV.0000046367.99002.a4. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–9. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, et al. A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci U S A. 2007;104:16657–62. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, et al. β-blockers alprenolol and carvedilol stimulate β-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci U S A. 2008;105:14555–60. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, et al. β-arrestin-mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–58. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–22. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–25. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Principles: Receptor theory in pharmacology. Trends Pharmacol Sci. 2004;25:186–92. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, Zastrow MV, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. Pharmacology. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–6. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci. 2007;28:407–15. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Gong H, Sun H, Koch WJ, Rau T, Eschenhagen T, Ravens U, et al. Specific β2AR blocker ICI 118,551 actively decreases contraction through a Gi-coupled form of the β2AR in myocytes from failing heart. Circulation. 2002;105:2497–503. doi: 10.1161/01.cir.0000017187.61348.95. [DOI] [PubMed] [Google Scholar]
- Bond RA, Leff P, Johnson TD, Milano CA, Rockman HA, McMinn TR. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the β2-adrenoceptor. Nature. 1995;374:272–6. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of β-adrenoceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–90. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Lakatta EG, Talan M. Pharmacological stimulation of β2-adrenergic receptors (β2AR) enhances therapeutic effectiveness of β1AR blockade in rodent dilated ischemic cardiomyopathy. Heart Fail Rev. 2005;10:289–96. doi: 10.1007/s10741-005-7543-3. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Krawczyk M, Zhu W, Woo AY, Morrell C, Poosala S, et al. Cardioprotective and survival benefits of long-term combined therapy with β2AR agonist and β1AR blocker in dilated cardiomyopathy post-myocardial infarction. J Pharmacol Exp Ther. 2008;325:491–9. doi: 10.1124/jpet.107.135335. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Morrell C, Lakatta EG, Talan MI. Therapeutic efficacy of a combination of a β1-adrenoreceptor (AR) blocker and β2-AR agonist in a rat model of postmyocardial infarction dilated heart failure exceeds that of a β1-AR blocker plus angiotensin-converting enzyme inhibitor. J Pharmacol Exp Ther. 2009;331:178–85. doi: 10.1124/jpet.109.157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigi F, Bertucci C, Zhu W, Chakir K, Wainer IW, Xiao RP, et al. Enantioselective separation and online affinity chromatographic characterization of R,R- and S,S-fenoterol. Chirality. 2006;18:822–7. doi: 10.1002/chir.20317. [DOI] [PubMed] [Google Scholar]
- Jozwiak K, Khalid C, Tanga MJ, Berzetei-Gurske I, Jimenez L, Kozocas JA, et al. Comparative molecular field analysis of the binding of the stereoisomers of fenoterol and fenoterol derivatives to the β2 adrenergic receptor. J Med Chem. 2007;50:2903–15. doi: 10.1021/jm070030d. [DOI] [PubMed] [Google Scholar]
- Woo AY, Wang TB, Zeng X, Zhu W, Abernethy DR, Wainer IW, et al. Stereochemistry of an agonist determines coupling preference of β2-adrenoceptor to different G proteins in cardiomyocytes. Mol Pharmacol. 2009;75:158–65. doi: 10.1124/mol.108.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Dove S. Functional selectivity of GPCR ligand stereoisomers: new pharmacological opportunities. Mol Pharmacol. 2009;75:13–8. doi: 10.1124/mol.108.052944. [DOI] [PubMed] [Google Scholar]
- Jozwiak K, Woo AY, Tanga MJ, Toll L, Jimenez L, Kozocas JA, et al. Comparative molecular field analysis of fenoterol derivatives: A platform towards highly selective and effective β2-adrenergic receptor agonists. Bioorg Med Chem. 2010;18:728–36. doi: 10.1016/j.bmc.2009.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talan MI, Ahmet I, Xiao RP, Lakatta EG. β2AR in the treatment of congestive heart failure: long path to translation. J Mol Cell Cardiol. 2011;51:529–33. doi: 10.1016/j.yjmcc.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangmool S, Shukla AK, Rockman HA. β-Arrestin-dependent activation of Ca2+/calmodulin kinase II after β1-adrenergic receptor stimulation. J Cell Biol. 2010;189:573–87. doi: 10.1083/jcb.200911047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Woo AY, Yang D, Cheng H, Crow MT, Xiao RP. Activation of CaMKIIδC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J Biol Chem. 2007;282:10833–9. doi: 10.1074/jbc.M611507200. [DOI] [PubMed] [Google Scholar]
- Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, et al. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–40. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–79. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ma N, Xia J, Liu J, Xu Z.β2-adrenergic receptor-induced transactivation of EGFR and PDGFR via SRC kinase promotes rat cardiomyocytes survival Cell Biol Int 2011. doi: 10.1042/CBI20110162 [DOI] [PubMed]
- Stuenaes JT, Bolling A, Ingvaldsen A, Rommundstad C, Sudar E, Lin FC, et al. β2-Adrenoceptor stimulation potentiates insulin-stimulated PKB phosphorylation in rat cardiomyocytes via cAMP and PKA. Br J Pharmacol. 2010;160:116–29. doi: 10.1111/j.1476-5381.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisco C, Condorelli G, Trimarco V, Bellis A, Marrone C, Condorelli G, et al. Akt mediates the cross-talk between β-adrenergic and insulin receptors in neonatal cardiomyocytes. Circ Res. 2005;96:180–8. doi: 10.1161/01.RES.0000152968.71868.c3. [DOI] [PubMed] [Google Scholar]