LETTER TO THE EDITOR,
Like other cancers, chronic lymphocytic leukemia (CLL) is initiated and/or progresses as a consequence of concurrent chromosomal abnormalities and recurrent somatic mutations. Using next-generation sequencing, a short list of recurrent mutations in various genes has been identified in CLL.1,2 One of these genes is SF3B1 that encodes a key component of the mRNA splicing machinery. SF3B1 mutations were initially discovered in myelodysplastic syndromes (MDS) and in some other solid tumors suggesting it is playing an important role in cancer biology. In CLL, SF3B1 mutations are associated with disease subtype,1 progression,3,4 chemotherapy resistance,4,5 and overall patient survival.6 However, the biological consequences of SF3B1 mutations in CLL pathogenesis are largely unknown. Here we find that acquisition of mutations in SF3B1 eventually leads to the loss of the wild-type copy of this gene suggesting the mutant SF3B1 gene plays a dominant role in clonal evolution. We also provide evidence that SF3B1 mutations are potentially oncogenic, supporting the possibility that mutant SF3B1 is an attractive druggable therapeutic target.
Wang et al reported that SF3B1 mutations are more prevalent in CLL patients with 11q deletion.1 We randomly picked 73 cryopreserved PBMC samples with 11q deletion from our CLL patient cohort, and screened for SF3B1 mutations. We identified 8 patients with various missense mutations including 5 patients with K700E (2098A>G), 1 with K649E (1945A>G), 1 with K622E (1866G>T), and 1 with K666E (1996A>G). These mutations have been observed by others in CLL, MDS, and other cancers.1,2,7 We also found that SF3B1 mutations are only present in a sub-allelic-fraction (ranging from 10% to 45%) of bulk DNA samples (Figure 1A).
Figure 1. SF3B1 genotyping in bulk and in single CLL cells.
A. Sanger DNA sequencing chromatograms of SF3B1 sequences amplified from four representative CLL patient samples. Purple and yellow color filled peaks show allelic burdens of mutant and wild-type nucleotide sequences, respectively. B. Schematic flowchart of single CLL cell preparation for SF3B1 mutation analysis. CD19+/CD5+ leukemic cells are sorted from patient PBMCs, and plated by limiting dilution in 96-well PCR plates for two rounds of PCR amplification for SF3B1 sequences. C. Representative chromatograms of Sanger DNA sequencing of SF3B1 sequences amplified from single CLL cells from patient 35. Purple and yellow color filled peaks are mutant and wild-type nucleotide sequences, respectively. D. Summary table of all four patient samples analyzed using DNA or RNA at the single cell level using a limiting dilution approach.
Cancer progression is typically characterized by the emergence and outgrowth of newly evolved subclones. By analyzing the allelic burden of SF3B1 mutations in CLL using Sanger sequencing in serial patient samples, Schwaederle et al3 showed that the weight of mutant SF3B1 increases as the disease progresses. However, the size of SF3B1 DNA allelic fractions does not necessarily reflect the size of the subclone, since it remains unknown if the observed mutant SF3B1 allelic increase at the bulk cell population level reflects a change in size of the mutant SF3B1 subclone or instead if there is a change in zygosity of SF3B1 mutations of the subclone. In fact, it has been postulated that SF3B1 mutations are heterozygous in MDS and CLL7-9 largely based on the observation that allelic burdens of mutant SF3B1 are typically <50%. To ascertain the zygosity of SF3B1 mutations in CLL, we analyzed SF3B1 mutations at the single cell level by DNA-based PCR (Figure 1B). As expected, many single cells exhibited either wild-type only (wt/wt), or wild-type plus mutant SF3B1 sequences (heterozygous, wt/mu). To our surprise owing to previous predictions, in all 4 CLL samples we detected multiple single cells possessing solely SF3B1 mutant sequences resembling “homozygous” genotypes (mu/mu-like). This observation suggests that a prominent CLL subclone in these patients exclusively carries mutant SF3B1. Figures 1C and 1D depict representative Sanger sequencing data of single cells from patient 35 and the summary of all 4 patients, respectively. Similar data were also obtained when single cells were collected directly into 96 well PCR plates using FACS (results not shown).
It is known that allelic drop out (ADO) is an artifact that can occur in DNA-based single cell PCR due to the fact that only one copy of DNA from each allele is present for testing. However, potential ADO can be largely overcome by analyzing 8 or more single cells.10,11 Because we performed single cell PCR analysis on 11 to 43 single cells from each patient, we believe it is unlikely that ADO underlies our observations that some CLL cells express only mutant SF3B1. In an added measure to rule out possible ADO in our DNA-based single cell PCR, we also performed RNA-based single cell RT-PCR on 18 single CLL cells from patient 35. Thus, the rate of ADO using this method is vastly reduced by the presence of many more copies of SF3B1 mRNA transcripts (wildtype or mutant) in a single cell as compared to DNA. Indeed, we also observed that a similar subset of CLL cells carry solely mutant SF3B1 transcripts (Figure 1C and D), confirming the reliability of our DNA-based single cell PCR.
Our results support a subclonal evolutionary pathway of SF3B1 mutations in CLL proceeding from wt/wt→wt/mu→mu/mu-like. The true SF3B1 genotype of the mutant SF3B1-only subclone is unknown, but it should fall into one of the following three possibilities: 1) bona fide homozygous SF3B1 mutation with an identical mutation on both alleles; 2) SF3B1 mutation on one allele with simultaneous loss of the wild-type copy on the other allele, i.e., loss of heterozygosity (LOH); or 3) copy-neutral LOH or uniparental disomy, where cells have gained a duplicated mutant copy of SF3B1 but lost the wild-type copy of the gene. Accurate identification of the precise genotype of cells with mutant SF3B1 at the single cell level, however, requires techniques that are yet to be developed. The emergence of mu/mu-like SF3B1 mutant subclones suggests they have a selection advantage over their heterozygous and wild-type precursor subclones. However, it is also conceivable that patients with a similar bulk SF3B1 mutation weight but different sizes and genotypes of the subclones may exhibit differences in clinical outcome. We believe that our single cell analysis approach will enable us to distinguish the two when analyzing serial patient samples (studies in progress). In addition, our approach also provides a proof-of-concept means to analyze true clonal and subclonal mutations in other cancer genes.
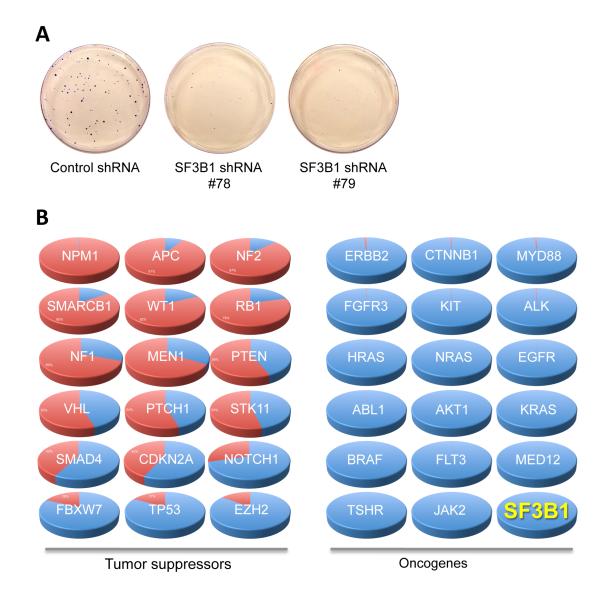
To address the biological functions of SF3B1, Isono, et al demonstrated that SF3B1 knockout in mice led to an early embryonic lethality.12 SF3B1 null embryos die around 2 days after conception (16-32 cell stage of development), the time point at which parental supplies of SF3B1 protein and mRNA are about to be exhausted. This observation suggests SF3B1 is an essential gene for cell survival, at least in mouse embryonic cells. To further validate this finding in human cells, we performed shRNA knockdown of SF3B1 in HEK293T cells and observed that knockdown of SF3B1 severely inhibits the formation of cell clones (Figure 2A), consistent with the mouse knockout data that SF3B1 is absolutely necessary for cell survival.
Figure 2. In silico profiling of SF3B1 mutations in the COSMIC database.
A. Clonogenic assay of HEK293 cells infected with control or SF3B1 shRNAs. B. Systemic profiling of mutations of the top 35 cancer genes in the COSMIC database. The portions in red are the percentages of protein truncating (frameshifting and nonsense) mutations while the blue coloring reflects the percentages of non-protein truncating mutations. The mutation patterns of mutated SF3B1 reported to date predict SF3B1 is a proto-oncogene.
The association of SF3B1 mutations with CLL progression3,4 suggests these mutations may confer a faster rate of leukemia cell proliferation. This prediction is in opposition to the phenotypes of SF3B1 knockout in mice and of shRNA knockdown in human cells. We, therefore, hypothesize that SF3B1 mutations in CLL are in fact oncogenic gain-of-function mutations, rather than tumor suppressive loss-of-function mutations. Due to technical hurdles in the cloning of full-length SF3B1 cDNA, we are unable to experimentally demonstrate the oncogenic activity of SF3B1 mutations at this moment. To overcome this difficulty, we took an in silico approach using the Catalog of Somatic Mutations in Cancer (COSMIC)13 database. It is well known that cancer develops upon mutational inactivation of tumor suppressor genes or mutational hyperactivation of oncogenes. We profiled all of the mutation data for the top 35 known cancer genes in this database including 18 tumor suppressor genes and 17 proto oncogenes for their frequencies of mutations that lead to ultimate protein truncation, namely frameshifting, and nonsense mutations. As anticipated, a significant portion of the mutations in 18 tumor suppressor genes are truncation mutations (varying from 97.9% in NPM1 to 13.6% in EZH2), while very few truncation mutations (0.03% in JAK2 to 2.5% in ERBB2) were found in all 17 known proto-oncogenes. This analysis suggests that the frequency of truncation mutations can accurately predict if an unknown gene is a tumor suppressor or an oncogene. We next profiled all 637 entries of the SF3B1 gene mutations deposited in the COSMIC database (v63 release). We found that only 2 (0.3%) were protein truncation mutations and that most of the mutations are in the hotspot sites (K700, K666, H662, R625 and E622), strongly suggesting that SF3B1 is a proto-oncogene that disfavors protein inactivating truncation mutations (Figure 2B). The results emerging from gene targeting in mice, gene knockdown in human cells, and in silico mutational analysis collectively suggest that SF3B1 is a proto-oncogene, and is consistent with its dominant role in clonal evolution as we suggested above. Our results are also in agreement with the observation that SF3B1 is often overexpressed in CLL cells,4 and inhibitors to wild-type SF3B1 protein exhibit potent antitumor activity.14 In fact, the splicing factor SRSF1 has long been recognized as a potent oncogene.15 Armed with this information, we suggest that specific targeting of SF3B1 mutations such as K700E may be of therapeutic benefit for patients with CLL and other cancers housing SF3B1 mutations.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Ms. Phoebe Wu for donating her countless hours in compiling the mutation data from the COSMIC database. This work was supported by the National Institutes of Health grant CA136591 (D.F.J.).
Footnotes
AUTHORSHIP Contribution: X.W., D.F.J. designed the study; X.W. performed experiments; X.W., R.C.T, D.F.J analyzed data; X.W. wrote the manuscript; D.F.J. edited the manuscript.
CONFLICTS OF INTEREST DISCLOSURE The authors declare no competing financial interests.
REFERENCES
- 1.Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. The New England journal of medicine. 2011 Dec 29;365(26):2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quesada V, Conde L, Villamor N, Ordonez GR, Jares P, Bassaganyas L, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nature genetics. 2012 Jan;44(1):47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 3.Schwaederle M, Ghia E, Rassenti LZ, Obara M, Dell’Aquila ML, Fecteau JF, et al. Subclonal evolution involving SF3B1 mutations in chronic lymphocytic leukemia. Leukemia. 2013 doi: 10.1038/leu.2013.22. Advance online publication 15 February 2013; doi: 10.1038/leu.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi D, Bruscaggin A, Spina V, Rasi S, Khiabanian H, Messina M, et al. Mutations of the SF3B1 splicing factor in chronic lymphocytic leukemia: association with progression and fludarabine-refractoriness. Blood. 2011 Dec 22;118(26):6904–6908. doi: 10.1182/blood-2011-08-373159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013 Feb 14;152(4):714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oscier DG, Rose-Zerilli MJ, Winkelmann N, Gonzalez de Castro D, Gomez B, Forster J, et al. The clinical significance of NOTCH1 and SF3B1 mutations in the UK LRF CLL4 trial. Blood. 2013 Jan 17;121(3):468–475. doi: 10.1182/blood-2012-05-429282. [DOI] [PubMed] [Google Scholar]
- 7.Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. The New England journal of medicine. 2011 Oct 13;365(15):1384–1395. doi: 10.1056/NEJMoa1103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn CN, Scott HS. Spliceosome mutations in hematopoietic malignancies. Nature genetics. 2012 Jan;44(1):9–10. doi: 10.1038/ng.1045. [DOI] [PubMed] [Google Scholar]
- 9.Malcovati L, Papaemmanuil E, Bowen DT, Boultwood J, Della Porta MG, Pascutto C, et al. Clinical significance of SF3B1 mutations in myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms. Blood. 2011 Dec 8;118(24):6239–6246. doi: 10.1182/blood-2011-09-377275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garvin AM, Holzgreve W, Hahn S. Highly accurate analysis of heterozygous loci bysingle cell PCR. Nucleic acids research. 1998 Aug 1;26(15):3468–3472. doi: 10.1093/nar/26.15.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lespinet V, Terraz F, Recher C, Campo E, Hall J, Delsol G, et al. Single-cell analysis of loss of heterozygosity at the ATM gene locus in Hodgkin and Reed-Sternberg cells of Hodgkin’s lymphoma: ATM loss of heterozygosity is a rare event. International journal of cancer Journal international du cancer. 2005 May 10;114(6):909–916. doi: 10.1002/ijc.20825. [DOI] [PubMed] [Google Scholar]
- 12.Isono K, Mizutani-Koseki Y, Komori T, Schmidt-Zachmann MS, Koseki H. Mammalian polycomb-mediated repression of Hox genes requires the essential spliceosomal protein Sf3b1. Genes & development. 2005 Mar 1;19(5):536–541. doi: 10.1101/gad.1284605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic acids research. 2011 Jan;39(Database issue):D945–950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotake Y, Sagane K, Owa T, Mimori-Kiyosue Y, Shimizu H, Uesugi M, et al. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nature chemical biology. 2007 Sep;3(9):570–575. doi: 10.1038/nchembio.2007.16. [DOI] [PubMed] [Google Scholar]
- 15.Das S, Anczukow O, Akerman M, Krainer AR. Oncogenic splicing factor SRSF1 is a critical transcriptional target of MYC. Cell reports. 2012 Feb 23;1(2):110–117. doi: 10.1016/j.celrep.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.