Abstract
In a subset of cases, metastatic renal cell carcinoma can demonstrate significant morphologic overlap with germ cell neoplasms, making accurate diagnosis challenging. In such cases, immunohistochemistry is often used as an adjunct diagnostic tool. Expression of the putative renal cell carcinoma markers PAX-2, PAX-8, and hKIM-1 has been reported in a small series of certain germ cell tumors, raising doubt about their specificity for renal cell carcinoma. To further characterize these markers, we evaluated PAX-2, PAX-8, and hKIM-1 staining in 100 germ cell tumors using tissue microarrays. PAX-2 and PAX-8 staining was identified in 50% and 25% of yolk sac tumors (respectively), with hKIM-1 staining identified in 48% of embryonal carcinomas and 50% of yolk sac tumors. All other germ tumor cells (notably including 62 seminomas) were negative for all 3 markers, in contrast to prior reports of PAX-8 reactivity in seminoma. This study indicates that PAX-2, PAX-8, and hKIM-1 should be used cautiously in distinguishing renal cell carcinoma from nonseminomatous germ cell neoplasia and also adds to the growing list of nonrenal tumors that express these 3 markers.
Keywords: metastatic renal cell carcinoma, immunohistochemistry, hKIM-1, PAX-2, PAX-8, germ cell tumor
Germ cell tumors and renal cell carcinoma may show morphologic overlap, particularly on a small biopsy sample, with immunohistochemistry often used for accurate classification. Expression of putative renal cell carcinoma markers PAX-2, PAX-8, and hKIM-1 has been reported in a few germ cell tumors, including seminomas1 (PAX-8) and yolk sac tumors2,3 (PAX-2, PAX-8, hKIM-1); however, a thorough characterization in a large series of germ cell tumors has not been previously reported.
MATERIALS AND METHODS
A tissue microarray composed of 100 randomly distributed germ cell tumors [including choriocarcinoma (1), embryonal carcinoma (21), intratubular germ cell neoplasia unclassified (2), seminoma (61), spermatocytic seminoma (1), teratoma (5), and yolk sac tumor (8)] using 1.2-mm diameter cores was prepared in triplicate (Stanford tissue microarray 136) and evaluated as described elsewhere.4 Immunohistochemical expression of PAX-2 (Z-RX2, 1:100, Zymed, San Francisco, CA), PAX-8 (polyclonal, 1:20, Proteintech, Chicago, IL), and anti-hKIM-1 (AKG7, prediluted, Joseph Bonventre Lab, Boston, MA) was evaluated using the standard avidin-biotin technique with a Dako (Carpinteria, CA) autostainer with citrate retrieval on 4-mm thick formalin-fixed, paraffin-embedded freshly cut sections mounted on charged slides and baked at 60°C for 1 hour. Clear cell renal cell carcinoma was used as an external positive control tissue, with non-neoplastic and nongerm cell testicular tissues present on the germ cell tumor tissue microarray used as negative controls. Positive reactivity was scored as nuclear (PAX-2, PAX-8) or membranous/cytoplasmic (hKIM-1) with staining intensity scored as none, weak, or strong by 1 author (A.R.S.) manually and intraobserver variability assessed by 3 separate scoring sessions with an interval of 3 weeks between each session.
RESULTS
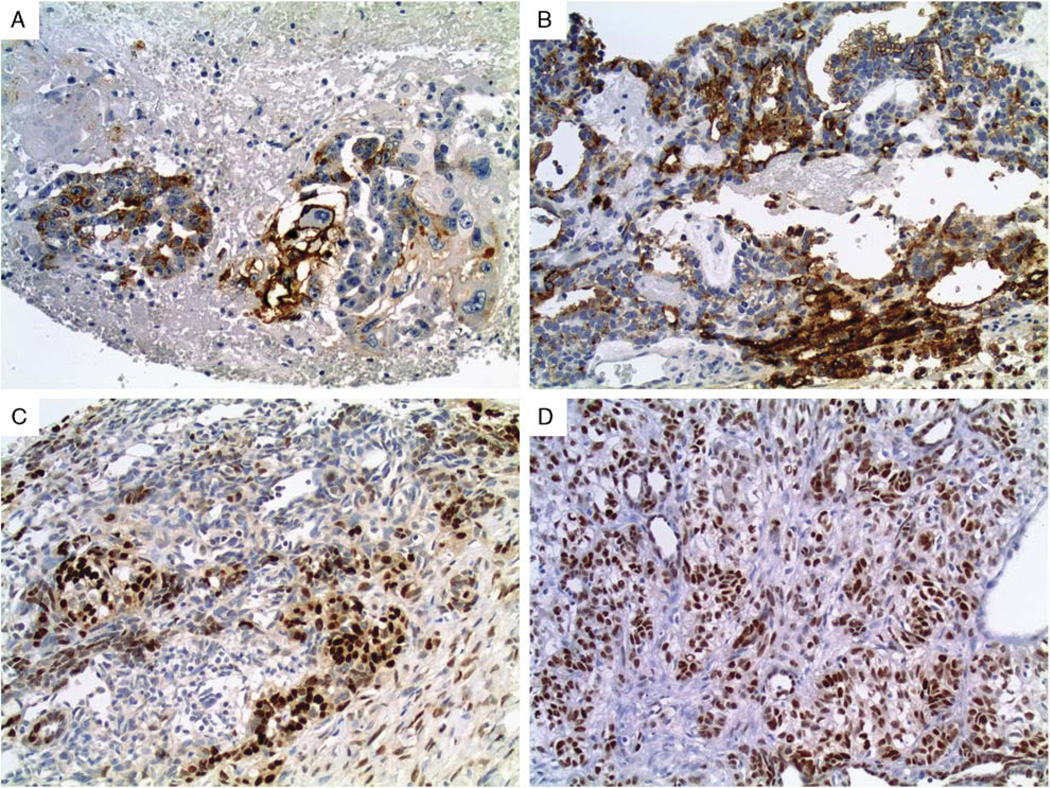
Of the 100 germ cell tumors evaluated, expression for hKIM-1 was identified in 10 of 21 (48%) embryonal carcinomas (Fig. 1A) and 4 of 8 (50%) yolk sac tumors (Fig. 1B) (weak to strong in both tumors). PAX-2 and PAX-8 reactivity was identified in 4 of 8 (50%) and 2 of 8 (25%) of yolk sac tumors (Figs. 1C, D) (weak to strong and strong, respectively). All other germ cell tumors were negative for all 3 markers. Table 1 summarizes the immunohistochemical staining results. There was no evidence of any intraobserver variability.
FIGURE 1.
Membranous/cytoplasmic immunostaining for hKIM-1 was seen in approximately half of (A) embryonal carcinomas and (B) yolk sac tumors. Yolk sac tumors were the only germ cell tumor with immunoreactivity for (C) PAX-2 and (D) PAX-8, seen in half/one-quarter of cases, respectively.
TABLE 1.
Positive Cases With PAX-2, PAX-8, and hKIM-1 Antibodies in Germ Cell Tumors
| Germ Cell Tumor | hKIM-1 | PAX-2 | PAX-8 |
|---|---|---|---|
| Choriocarcinoma | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) |
| Embryonal carcinoma | 10/21 (48%) | 0/21 (0%) | 0/21 (0%) |
| Intratubular germ cell neoplasia | 0/2 (0%) | 0/2 (0%) | 0/2 (0%) |
| Seminoma | 0/62 (0%) | 0/62 (0%) | 0/62 (0%) |
| Spermatocytic seminoma | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) |
| Teratoma | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) |
| Yolk sac tumor | 4/8 (50%) | 4/8 (50%) | 2/8 (25%) |
| Total | 14/100 (14%) | 4/100 (4%) | 2/100 (2%) |
DISCUSSION
The frequency of renal cell carcinoma metastasis to the testis is approximately 1%.5 Despite this low prevalence, reports of unusual clinical presentations of metastatic renal cell carcinoma (testicular metastasis as initial diagnostic presentation of primary renal tumor, ipsilateral primary renal tumor with contralateral testicular metastasis, long interval to testicular metastasis, and paratesticular location of metastasis)6–22 coupled with certain cases showing significant morphologic overlap can make accurate diagnosis challenging. Emerging data on the utility of diagnostic testicular mass biopsies23 yielding small tissue sample can further complicate this problem.
The main goal of this study was to expand on our initial report of PAX-2, PAX-8, and hKIM-1 immunoreactivity in yolk sac tumors (4 of 5, 1 of 5, and 1 of 5 tumors, respectively)3 by using additional and more numerous germ cell tumor subtypes. The current study’s addition of 8 yolk sac tumors corroborates our initial findings, with PAX-2 and PAX-8 staining found in 50% (4/8) cases and hKIM-1 staining found in 25% (2/8) cases. Although our initial report showed no PAX-2/ PAX-8/hKIM-1 staining in 4 embryonal carcinomas, in this study we identified hKIM-1reactivity in 48% (10/21) of embryonal carcinomas. These detailed results, in conjunction with only 2 previously published findings [reported PAX-8 positivity in 3 of 7 “mixed germ cell tumor”24 and reported PAX-8 positive in 1 of 49 germ cell tumors (single positive case was yolk sac tumor2)], further demonstrate the lack of unequivocal specificity of these 3 putative renal cell carcinoma markers and add to the growing list of reported immunoreactive tumor types. Although PAX-2/PAX-8/hKIM-1 immunoreactivity was not identified in the single choriocarcinoma and 5 teratomas, additional studies using more cases are needed for more definitive assessment.
Another goal of this study was to investigate the immunoreactivity of PAX-2, PAX-8, and hKIM-1 in seminomas given a prior report of weak (1 to 2+/4+) PAX-8 staining in 2 of 3 cases.1 Corroborating our initial report of no staining with these 3 markers in 20 seminomas,3 we found no staining in 62 additional seminomas. The recent study by Tong et al2 similarly found no PAX-8 staining in 32 seminomas. These findings may prove useful in separating a subset of morphologically challenging cases (in particular, metastatic clear cell renal cell carcinoma vs. seminoma).
It should be noted that although the hKIM-1 antibody as used in this study is not currently commercially available, development is underway and should be accessible in the near future (personal communication, J.V.B., Boston, MA). Although some antibodies reportedly sharing homology with hKIM-1 are commercially available (TIM-1; R&D Systems, Minneapolis, MN and KIM-1; Immunology Consultants Laboratory, Newberg, OR), the diagnostic utility of these markers has not been fully evaluated in this study, limiting appropriate comparative analysis.
In summary, we confirm PAX-2/PAX-8/hKIM-1 reactivity in yolk sac tumor as we previously noted in a smaller series3 and demonstrate hKIM-1 reactivity in embryonal carcinoma. Our findings indicate that these putative renal cell carcinoma markers should be used cautiously, particularly in cases with morphologic overlap, unusual clinical presentation, and small sample size. A panel approach inclusive of germ cell tumor-specific markers (eg, SALL4) may be useful in these cases. In contrast to prior reports, PAX-8 showed no staining in seminoma. Although the results of this study could conceivably be applicable to extragonadal germ cell tumors, additional studies are needed for verification.
Footnotes
Presented in part at the 100th meeting of the United States and Canadian Academy of Pathology, San Antonio, TX, March 2011.
The authors declare no conflict of interest.
REFERENCES
- 1.Nonaka D, Tang Y, Chiriboga L, et al. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol. 2008;21:192–200. doi: 10.1038/modpathol.3801002. [DOI] [PubMed] [Google Scholar]
- 2.Tong GX, Memeo L, Colarossi C, et al. PAX8 and PAX2 immunostaining facilitates the diagnosis of primary epithelial neoplasms of the male genital tract. Am J Surg Pathol. 2011;35:1473–1483. doi: 10.1097/PAS.0b013e318227e2ee. [DOI] [PubMed] [Google Scholar]
- 3.Sangoi AR, West RB, Bonventre JV, et al. Exploring the specificity of putative renal cell carcinoma markers in non-renal tissues and neoplasms from various organ systems: a tissue microarray study of 501 cases. Mod Pathol. 2010;23(suppl 1):216A. [Google Scholar]
- 4.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 5.Sangoi AR, Fujiwara M, West RB, et al. Immunohistochemical distinction of primary adrenal cortical lesions from metastatic clear cell renal cell carcinoma: a study of 248 cases. Am J Surg Pathol. 2011;35:678–686. doi: 10.1097/PAS.0b013e3182152629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandler CG, Roen PR. Solitary testicular metastasis simulating primary tumor and antedating clinical hypernephroma of the kidney; report of a case. J Urol. 1946;55:663–669. doi: 10.1016/S0022-5347(17)69962-2. [DOI] [PubMed] [Google Scholar]
- 7.Camerini A, Tartarelli G, Martini L, et al. Ipsilateral right testicular metastasis from renal cell carcinoma in a responder patient to interleukine-2 treatment. Int J Urol. 2007;14:259–260. doi: 10.1111/j.1442-2042.2007.01674.x. [DOI] [PubMed] [Google Scholar]
- 8.Correa JJ, Fishman M, Chuang ST, et al. Surgery plus targeted therapy for renal cell carcinoma with isolated spermatic cord metastasis. Clin Genitourin Cancer. 2009;7:E101–E103. doi: 10.3816/CGC.2009.n.034. [DOI] [PubMed] [Google Scholar]
- 9.Daniels GF, Jr, Schaeffer AJ. Renal cell carcinoma involving penis and testis: unusual initial presentations of metastatic disease. Urology. 1991;37:369–373. doi: 10.1016/0090-4295(91)80269-d. [DOI] [PubMed] [Google Scholar]
- 10.Datta MW, Ulbright TM, Young RH. Renal cell carcinoma metastatic to the testis and its adnexa: a report of five cases including three that accounted for the initial clinical presentation. Int J Surg Pathol. 2001;9:49–56. doi: 10.1177/106689690100900108. [DOI] [PubMed] [Google Scholar]
- 11.Fallick ML, Long JP, Ucci A. Metachronous renal cell carcinoma metastases to spermatic cord and penis. Scand J Urol Nephrol. 1997;31:299–300. doi: 10.3109/00365599709070353. [DOI] [PubMed] [Google Scholar]
- 12.Gohji K, Nakanishi T, Yoshimura K, et al. Metastatic tumor of the spermatic cord from renal cell carcinoma. Hinyokika Kiyo. 1990;36:827–829. [PubMed] [Google Scholar]
- 13.Hicks JA, Britton JP, Carter PG, et al. A right-sided renal carcinoma uniquely presenting as a mass in the spermatic cord. Urol Int. 2003;70:247–248. doi: 10.1159/000068763. [DOI] [PubMed] [Google Scholar]
- 14.Lauro S, Lanzetta G, Bria E, et al. Contralateral solitary testis metastasis antedating renal cell carcinoma: a case-report and review. Anticancer Res. 1998;18:4683–4684. [PubMed] [Google Scholar]
- 15.Lioe TF, Biggart JD. Tumours of the spermatic cord and paratesticular tissue. A clinicopathological study. Br J Urol. 1993;71:600–606. doi: 10.1111/j.1464-410x.1993.tb16033.x. [DOI] [PubMed] [Google Scholar]
- 16.Markovic B, Opric M, Prica V, et al. Metastasis to the funiculus spermaticus as the first sign of renal cell carcinoma. World J Surg. 1983;7:669–671. doi: 10.1007/BF01655352. [DOI] [PubMed] [Google Scholar]
- 17.Nabi G, Gania MA, Sharma MC. Solitary delayed contralateral testicular metastasis from renal cell carcinoma. Indian J Pathol Microbiol. 2001;44:487–488. [PubMed] [Google Scholar]
- 18.Sharma S, Nath P, Srivastava AN, et al. Tumours of the male urogenital tract: a clinicopathologic study. J Indian Med Assoc. 1994;92:357–360. 372. [PubMed] [Google Scholar]
- 19.Steiner G, Heimbach D, Pakos E, et al. Simultaneous contralateral testicular metastasis from a renal clear cell carcinoma. Scand J Urol Nephrol. 1999;33:136–137. doi: 10.1080/003655999750016168. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M, Kurokawa Y, Nakanishi R, et al. Laparoscopic findings of transverse testicular ectopia. Urology. 2008;71:e3–e5. doi: 10.1016/j.urology.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 21.Talerman A, Kniestedt WF. Testicular tumor as the first manifestation of renal carcinoma. J Urol. 1974;111:584–586. doi: 10.1016/s0022-5347(17)60021-1. [DOI] [PubMed] [Google Scholar]
- 22.Ulbright TM, Young RH. Metastatic carcinoma to the testis: a clinicopathologic analysis of 26 nonincidental cases with emphasis on deceptive features. Am J Surg Pathol. 2008;32:1683–1693. doi: 10.1097/PAS.0b013e3181788516. [DOI] [PubMed] [Google Scholar]
- 23.Dieckmann KP, Kulejewski M, Heinemann V, et al. Testicular biopsy for early cancer detection—objectives, technique and controversies. Int J Androl. 2011;34:e7–e13. doi: 10.1111/j.1365-2605.2011.01152.x. [DOI] [PubMed] [Google Scholar]
- 24.Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816–826. doi: 10.1097/PAS.0b013e318216c112. [DOI] [PubMed] [Google Scholar]