Abstract
Sepsis — severe life-threatening infection with organ dysfunction — initiates a complex interplay of host pro- and anti-inflammatory processes. In a real sense, sepsis can be considered a race to the death between the pathogens and the host immune system. It is the proper balance between the often competing pro- and anti-inflammatory pathways that determines the fate of the individual. Although the field of sepsis research has witnessed the failure of many highly-touted clinical trials, a better understanding of the pathophysiological basis of the disorder and the mechanisms responsible for the associated pro- and anti-inflammatory responses is leading to a novel approach to treat this highly lethal condition. Biomarker-guided immunotherapy administered to patients at the proper immune phase of sepsis represents a potential major advance in the treatment of sepsis and more broadly in the field of infectious disease.
Introduction
Sepsis is defined as the host inflammatory response that occurs due to severe life-threatening infection with the presence of organ dysfunction1. Sepsis is the most frequent cause of mortality in most intensive care units (ICUs) and is responsible for over 250,000 deaths in the United States annually2. The incidence of sepsis is increasing due to the aging population, who has impaired immunity due to immunosenescence2. Despite the litany of failed clinical trials in sepsis, a better understanding of different immunological phases of the disorder and encouraging results from several phase II clinical trials of immunotherapies in sepsis is bringing cautious optimism to the field3–7.
Until recently, most research on sepsis was focused on blocking the initial hyper-inflammatory cytokine-mediated phase of the disorder. Improved treatment protocols have resulted in most patients surviving this initial hyper-inflammatory phase and entering a protracted immunosuppressive phase8–13. Deaths in this immunosuppressive phase are typically due to failure to control the primary infection or due to the acquisition of secondary hospital-acquired infections, often with opportunistic pathogens14,15. The recent remarkable success of cytotoxic T lymphocyte antigen 4 (CTLA4)- and programmed cell death 1 (PD1)-specific antibodies as immunotherapies to improve host immunity and increase survival in cancer patients16,17 is highly encouraging to the field of sepsis because of the many similarities in the immune defects observed in cancer and sepsis and because both agents have improved survival in animal models of sepsis7,10 In this Review, we discuss the panoply of sepsis-induced defects in innate and adaptive immune cells and discuss several highly promising immunotherapies for the treatment of sepsis.
Controversies on host immunity in sepsis
The current paradigm regarding the host immune response to sepsis is debated 2–7,18,19. Traditionally, the host immune response to sepsis was considered to be characterized by an initial hyper-inflammatory phase that evolved over several days into a more protracted immunosuppressive phase7–9. However, recent studies have shown that both pro-inflammatory and anti-inflammatory responses occur early and simultaneously in sepsis (Figure 1 theory 1) 18–20, although the net initial effect of these competing processes is typically manifested by an early dominant hyper-inflammatory phase characterized by shock, fever and hyper-metabolism. The robustness of the hyper-inflammatory phase depends on numerous factors, including patients’ pre-existing co-morbidities, nutritional status, microorganism load and virulence factors8,9.
Figure 1. Competing theories of the host immune response in sepsis.
(Theory #1): Recent studies show that activation of both pro- and anti-inflammatory immune responses occurs promptly after sepsis onset. Cells of the innate immune system including monocytes and neutrophils release large amounts of pro-inflammatory cytokines that drive inflammation (blue line – days 1–3). The intensity of the initial inflammatory response varies in individual patients, depending on multiple factors, including pathogen load and virulence, patient co-morbidities and host genetic factors. Early deaths in sepsis (top red line – day 3) are typically due to a hyper-inflammatory “cytokine storm” response with fever, refractory shock, acidosis and hyper-catabolism. An example of this scenario would be a young patient dying of toxic shock syndrome or meningococcemia. Most patients have restoration of innate and adaptive immunity and survive the infection (recovery – day 6). If sepsis persists, failure of critical elements of both innate and adaptive immune system occurs such that patients enter a profound immunosuppressive state (blue and red lines –after day 6). Deaths are due to an inability of the patient to clear infections and development of secondary infections. (Theory #2): A competing theory of sepsis agrees that there is an early activation of innate immunity and suppression of adaptive immunity. This theory holds that deaths in sepsis are due to persistent activation of the innate immunity with resultant intractable inflammation and organ injury. According to this theory, late deaths in sepsis are due to persistent underlying innate immune-driven inflammation.
Investigators recently presented a new paradigm (Figure 1- theory 2) to describe the host immune response in trauma and sepsis. Circulating leukocyte gene expression data in trauma and burn patients showed rapid and sustained upregulation of genes that regulate innate immune response and simultaneous down-regulation of genes regulating adaptive immunity19. These investigators hypothesized that the best model to describe the host immune response in trauma and sepsis is one of protracted, unabated inflammation driven by the innate immune system with resultant organ dysfunction and failure. Although these investigators agree that the adaptive immune system is impaired, they theorize that patients who die of sepsis have a longer duration of and a more profound degree of organ injury caused by unabated innate immune-driven inflammation. They postulate that this inflammation exists despite the down regulation of the expression of genes that regulate the adaptive immune response and is ultimately responsible for patient morbidity and mortality19.
Although we agree with the provocative findings of this group, we believe that this new model proposing that morbidity and mortality in sepsis is due to unremitting innate immune-driven inflammation is unlikely to reflect the actual clinical scenario in the majority of patients. We have demonstrated that patients dying of sepsis have marked immunosuppression12. Immune cells from spleens or lungs of septic patients that were harvested in the ICU within 30–180 minutes of death had profoundly decreased production of both pro- and anti-inflammatory cytokines, upregulated expression of inhibitory receptors including PD1, expansion of T regulatory (Treg) cell and myeloid- derived suppressor cell (MDSC) populations, and downregulation of CD28 and HLA-DR-mediated activation pathways12. Collectively, these results show that sepsis induces numerous overlapping mechanisms of immunosuppression involving both innate and adaptive immunity.
There are several potential explanations for the different findings in the two studies. First, results of the gene expression study involved patients with trauma and burns and although many of these patients became septic, this patient population differs from patients who developed sepsis as a primary phenomenon. The mean age of the trauma patients in the transcriptome study was 33 years, whereas the mean age of septic patients in most developed countries is at least twice this age (65–68 years). This difference in age in the two studies is important because the older patients have baseline underlying defects in immunity due to immunosenescence 21. Second, these investigators based their conclusions on gene expression data which, because of post-transcriptional regulation, may not reflect protein levels. Conversely, the postmortem study of septic patients quantitated actual cytokine levels. Other possible reasons for differences between these two studies are the duration and severity of illnessof the patients that were analyzed. Many septic patients had protracted disease with high severity of illness and increased mortality. We believe that immunosuppression rather than low grade inflammation is the predominant driving force for morbidity and mortality in sepsis. First, our postmortem findings are consistent with numerous studies that have examined cytokine production by peripheral blood mononuclear cells and whole blood from patients with sepsis and have shown severely decreased pro-inflammatory cytokine production22–25. Second, recent postmortem studies have reported that a large number of patients dying of sepsis have unresolved opportunistic infections14,15, which is consistent with defective host immunity as a dominant cause of death. Third, the failure of over 30 clinical trials of different anti-inflammatory agents is inconsistent with the hypothesis that inflammation is a key driving mechanism. Undoubtedly, focal regions of inflammation occur in infected tissue in patients with sepsis. Areas of ischemic and necrotic tissue also likely contribute to local inflammation. It is possible that this focal inflammation contributes to organ dysfunction, morbidity and mortality in a subset of septic patients. Nevertheless, we hypothesize that protracted sepsis is predominantly characterized by systemic immunosuppression leading to a failure to eradicate primary infections and acquisition of lethal secondary infections. Intriguingly, gene expression data in children who develop sepsis demonstrate findings similar to those reported for adult trauma patients: for example, the expression of genes that modulate innate immunity are upregulated whereas genes modulating adaptive immunity are downregulated 26. Although these gene expression data would seem to favor theory 2, the postmortem studies of pediatric patients dying of sepsis have shown immunosuppressive features characteristic of those observed in adult septic patients with a remarkable depletion of immune effector cells (see below). Hopefully, future studies will determine which of these two conflicting hypotheses is correct as resolution of this controversy has important therapeutic implications.
Apoptosis and immunosuppression
A fundamental discovery in the field of sepsis occurred when investigators demonstrated that apoptosis causes profound depletion of immune cells, including CD4+ and CD8+ T cells, B cells, follicular dendritic cells (DCs) and interdigitating DCs in various organs of patients dying of sepsis leading to immunosuppression 27–31 (Figure 2). Sepsis-induced immune cell apoptosis has now been confirmed in several postmortem studies and occurs in all age groups (neonatal, pediatric, and adult populations) 27,30,31, and in response to different classes of microorganisms. Apoptosis of immune cells occurs in lymphoid (spleen, thymus, and lymph nodes) and in gut-associated lymphoid tissues (GALTs)32. The loss in intestinal intraepithelial and lamina propria lymphocytes may facilitate bacterial translocation into the systemic circulation, thereby perpetuating the systemic inflammatory response and predisposing to secondary infections. Sepsis-induced apoptosis occurs through both death receptor- and mitochondrial-mediated pathways suggesting that multiple cell death stimuli are activated during sepsis33. Therefore, it is unlikely that blockade of a single apoptotic trigger will prevent loss of lymphocyte cell death during the disorder.
Figure 2. Immunohistochemical staining for CD4+ T cells, CD8+ T cells, and HLA-DR in spleens from septic or trauma patients.
Spleens from patients with sepsis or patients requiring splenectomy for traumatic injury were obtained and underwent immunostaining for CD4+ or CD8+ T cells or HLA-DR. Sepsis induces apoptotic death of splenocytes including CD4+ and CD8+ T cells leading to profound depletion of these critical immune effector cells. Note the loss in periarteriolar CD4+ and CD8+ T cells (stained a brown color) in spleens from septic versus trauma patients (200X). The loss in CD4+ T cells in patients with sepsis is frequently as severe as occurs in patients with AIDS. The number of HLA-DR+ cells (predominantly B cells) and the levels of HLA-DR expression (as determined by the intensity of the staining) are both decreased in septic versus trauma patients. In contrast to the spleen from trauma patients, the spleen from septic patients also demonstrated an increase in the expression of HLA-DR by capillary endothelial cells.
A key question is whether the extensive sepsis-induced apoptosis of immune cells is an epiphenomenon or a major pathophysiological mechanism? Multiple independent laboratories have demonstrated, through the use of various strategies — including transgenic and knockout mice, anti-apoptotic cytokines, caspase inhibitors and death receptor blockers — that preventing lymphocyte apoptosis improves survival in sepsis34–37. The detrimental effects of apoptosis are not only related to the severe loss of immune cells but also the impact that apoptotic cell uptake has on the surviving immune cells38. Uptake of apoptotic cells by monocytes, macrophages, and DCs results in immune tolerance by inducing anergy or a TH2 cell-associated immune phenotype with increased interleukin-10 (IL-10) production39. The net result of this effect is that the surviving phagocytic cells cannot combat the remaining pathogenic organisms. Thus, sepsis-induced apoptosis has multiple profound effects that impair host defenses.
Impact of sepsis on immune cells
Sepsis directly or indirectly impairs the function of virtually all types of immune cells. The following section provides an overview of these various immunosuppressive effects on the different cells of the innate and adaptive immune systems.
Neutrophils and MDSCs
Neutrophils are essential for early control of invading pathogens40. Investigations on neutrophils obtained during the first hours of sepsis uncovered numerous abnormalities (Figure 3). Normally, most neutrophils undergo apoptosis within 24 hours after release from bone marrow40. Surprisingly, and in contrast to lymphocytes which undergo accelerated apoptosis, neutrophil apoptosis is delayed during sepsis40. Because of an increase in release in immature neutrophils and delayed apoptosis of circulating neutrophils, septic patients typically have markedly increased numbers of circulating neutrophils of various degrees of maturation 41. Animal models of sepsis and experimental studies in patients revealed disrupted neutrophil functions including impaired bacterial clearance, reduced reactive oxygen species (ROS) production, and decreased recruitment to infected tissues42,43. Loss of chemotactic activity is likely the most frequently documented dysfunction of circulating neutrophils during sepsis43,44. Possible explanations for this include reduced expression of CXC-chemokine receptor 2 (CXCR2) and nitric-oxide-mediated suppression44. A current theory is that these alterations in neutrophil function are due to abnormalities in Toll-like receptor (TLR) signalling, analogous to the phenomenon that occurs in monocytes during endotoxin tolerance43 (see below).
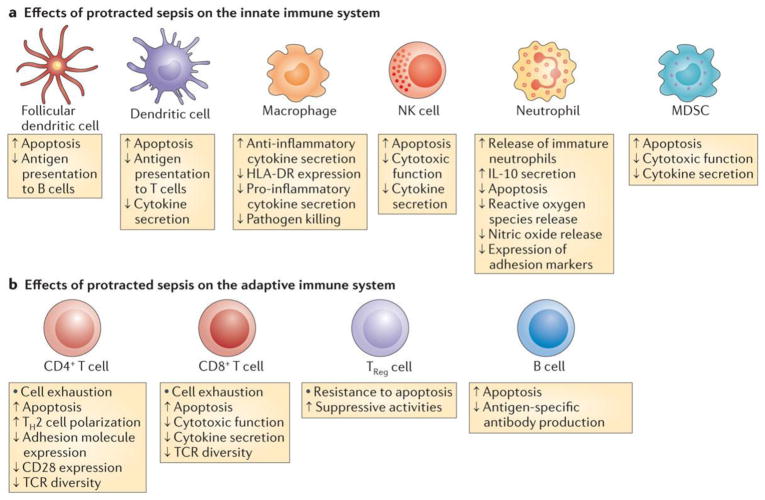
Figure 3. Impact of sepsis on innate and adaptive immunity.
a)Sepsis has diverse and profound effects on all cellular elements comprising the innate immune system. Sepsis rapidly triggers extensive apoptosis in dendritic cells, monocytes and immature macrophages, natural killer (NK) cells, and myeloid derived suppressor cells (MDSCs). Conversely, sepsis delays neutrophil apoptosis, a result thought to be secondary to the mechanisms of neutrophil activation. After initial mobilization and activation of neutrophils, subsequent neutrophils that are released from bone marrow have lower bactericidal functions and decreased cytokine production. Recent data show that a subset of neutrophils release large amounts of the immunosuppressive cytokine interleukin-10 (IL-10). Decreased HLA-DR expression on antigen presenting cells including monocyte/macrophages and dendritic cells is a hallmark of sepsis, which may impair the optimal presentation of microbial antigens to T cells. b) Sepsis causes massive loss of CD4+ and CD8+ T cells as well as B cells. T regulatory (Treg) cells are more resistant to sepsis-induced apoptosis and consequently, there is an increased percentage of TReg cells in the circulation relative to the other lymphocyte subsets. This contributes on a more immunosuppressive phenotype. Surviving CD4+ and CD8+ T cells have either a shift from a pro-inflammatory Th1 cell to an anti-inflammatory Th2 cell phenotype or develop an “exhaustive” phenotype characterized by increased programmed cell death-1 expression and reduced cytokine secretion. CD4+ T cells have decreased expression of CD28 and reduced T cell receptor (TCR) diversity, which both likely contributing to the impaired anti-microbial response to invading pathogens.
Several studies have reported that impaired neutrophil function precedes development of nosocomial infections. Patients with the most severely reduced neutrophil functions are most at risk for acquiring nosocomial infections45. Reduced neutrophil function has also been reported in a mouse model of polymicrobial sepsis leading to increased susceptibility to secondary Pseudomonas aeruginosa infection resulting in pneumonia46. Furthermore, extracorporeal cell therapy of 10 septic shock patients with donor granulocytes lead to improvement in various biomarkers of sepsis and decreased sepsis severity47.
Neutrophils exhibit a great degree of plasticity in response to a wide range of physiological or pathological conditions43. Sepsis may induce a subset of neutrophils with suppressive properties. Although neutrophils are often not considered to produce significant amount of cytokines, it has been shown that neutrophils produce large amounts of the immunosuppressive cytokine IL-10 during sepsis48. Recently, a subset of mature CD16hiCD62Llow neutrophils that suppressed T cell proliferation were identified in a human model of endotoxemia in which healthy volunteers were administered low dose endotoxin49. Immunosuppressive neutrophils were also detected in five severely injured trauma patients49.
The recently explored heterogeneity in neutrophils, including immunosuppressive subsets of myeloid cells, offers parallels with MDSCs. In experimental models of sepsis, these cells have been shown to block specific T cell functions including proliferation and production of interferon-γ (IFN-γ) and IL-2 50,51. However, the actual effect of MDSCs may evolve during sepsis, as studies show that MDSCs can either enhance or attenuate the sepsis-associated inflammatory response, depending on the stage of the disorder50–53. Recently, our group showed that there was an increase in the number of cells consistent with a MDSC phenotype in the lungs of patients dying of sepsis12. Currently, few clinical studies have investigated MDSCs in patients with sepsis, possibly due to the complexity of immunophenotyping these cells and to the absence of a universally accepted phenotypic definition for these cells in humans54.
DCs
DCs are particularly vulnerable to sepsis-induced apoptosis29. In a postmortem study of septic and trauma patients, a dramatic reduction in the number of splenic DCs and in percentage area of spleen occupied by DCs was observed29. Similarly, reduced numbers of circulating DCs occurs in septic and burn patients and after major surgery55–57. Both plasmacytoid and myeloid DCs are effected by sepsis-induced apoptosis55,58 (Figure 3). Interestingly, DC loss was more profound in septic patients who died than in survivors55, and was more profound in patients who subsequently developed nosocomial infections than in those patients who did not59.
Not only are DC numbers decreased in septic patients but, the surviving DCs have lower expression of HLA-DR and produce increased amounts of IL-10 57,60. In addition, monocyte-derived DCs from patients with sepsis were unable to induce a robust effector T cell response but instead induced either T cell anergy or Treg cell proliferation61. In mouse models of burn injury, DCs possess immunosuppressive properties that impair defences against a subsequent bacterial challenge. Several investigators have shown that prevention of sepsis-induced DC apoptosis or improving DC function during the disorder results in enhanced survival62–64. Indeed, mice that had selective overexpression of the anti-apoptotic factor B cell lymphoma 2 (BCL-2) in DCs were reported to have improved survival in a lethal model of endotoxic shock 62. Importantly, these BCL-2-overexpressing DCs were resistant to sepsis-induced apoptosis, which suggests that DC death is an important determinant of sepsis-induced immunosuppression and mortality.
FMS-like tyrosine kinase 3 ligand (FLT3L) is a DC growth factor that induces a rapid increase in DC numbers. Treatment of burn-injured animals with FLT3L restored DC function and enhanced survival when mice were challenged with Pseudomonas aeruginosa, a pathogen that commonly infects burn patients 63–65. The protective effect of FLT3L was also observed following the adoptive transfer of FLT3L-treated DCs. Additional studies documented that treatment with FLT3L increased secretion of IL-12, IL-15 and IFNγ and had broad effects on function of CD4+ T cells, natural killer (NK) cells, and neutrophils in models of burn infection 63–65. Intrapulmonary transfer of bone marrow-derived DCs restored DC function and prevented fatal Aspergillus fumigatus infection in mice that had recovered from primary peritonitis 66. TLR agonists also improve survival from pneumonia occurring in mice after haemorrhagic shock 67,68. A possible mechanism for the beneficial effect of TLR agonists includes increased MHC class II molecules, CD80 and CD86 expression on DCs67,68. Given these encouraging results, some authors have argued that preserving and/or restoring DC function should be a primary target of investigations in sepsis 63–69.
Monocyte and macrophages
A diminished capacity of monocytes from septic patients to release pro-inflammatory cytokines in response to endotoxin (lipopolysaccharide (LPS)), other TLR agonists and various other bacterial compounds is a hallmark of the disorder 70,71 (Figure 3). This finding is consistent with the phenomenon of endotoxin tolerance. Monocytes from septic patients typically exhibit diminished capacity to release pro-inflammatory cytokines, such as tumour necrosis factor (TNF), IL-1α, IL-6, and IL-12, whereas release of anti-inflammatory mediators, such as IL-1 receptor antagonist and IL-10, is either not impaired or enhanced 70,71. These findings demonstrate that LPS can still activate monocytes but that intracellular signalling has shifted toward production of anti-inflammatory molecules, thereby supporting the concept of monocyte reprogramming 72. In clinical studies, the magnitude and persistent nature of this refractory state is associated with increased mortality and nosocomial infections73.
The mechanisms responsible for endotoxin tolerance are not fully understood70,72. Analysis of mRNA expression levels demonstrated increased expression of genes encoding inhibitory signalling molecules and inhibitory cytokines and reduced expression of pro-inflammatory and chemokine receptor genes71–74. Although mainly reported to be useful as biomarkers to predict sepsis onset or prognosis, microRNAs are also thought to participate in endotoxin tolerance 71. Similarly, recent work highlights a prominent role for epigenetic regulation at different levels such as histone modification and chromatin remodelling75,76. In vitro, these epigenetic modifications are restored following IFNγ stimulation and associated with recovery of monocyte cytokine release76,77. Thus, IFNγ may abrogate endotoxin tolerance by facilitating TLR-induced chromatin remodelling78.
Two major consequences of endotoxin tolerance on monocytes and macrophages are increased release of immunosuppressive mediators (mainly IL-10) and decreased antigen presentation through decreased HLA-DR expression; both are associated with worse outcome in sepsis 79–82 (Figure 2). Continued release of IL-10 may contribute to or amplify sepsis-induced immunosuppression and thus augment susceptibility to secondary microbial infections 83–85. Indeed, blocking IL-10 reverses endotoxin tolerance ex vivo 84,86 and can reverse sepsis-induced immunosuppression and improve survival in a clinically relevant animal model of sepsis 87.
Low monocyte HLA-DR expression serves as a surrogate marker of monocyte anergy79,88. Several studies demonstrated an association of low monocyte HLA-DR expression with impaired monocyte function, for example, lower TNF and IL-1β release in response to bacterial challenges89, and decreased lymphocyte proliferation in response to tetanus toxin, presumably due to impaired antigen presentation90. Most importantly, decreased monocyte HLA-DR expression is associated with increased risk of nosocomial infections and death91,92. It is noteworthy that after adjustment for usual clinical confounders through multivariate analysis, decreased monocyte HLA-DR expression remains an independent predictor of nosocomial infection occurrence and mortality after sepsis73,93. This finding demonstrates that monocyte anergy and immunosuppression independently contribute to increased risk of adverse events in sepsis.
NK cells
The preferential location of NK cells in tissues along with their low numbers in peripheral blood render the study of this cell subset difficult, especially in the context of human sepsis. These facts explain why this cell subset has not been heavily investigated in sepsis 94,95. Studies indicate that both CD56hi and CD56low NK cell subsets are affected during sepsis; the number of circulating NK cells is markedly decreased in septic patients 96,97, which frequently persists for weeks98 and is associated with increased mortality 99. Furthermore, the absolute number of both NK cell subsets is decreased in sepsis 100. Reduced NK cell cytotoxic function and cytokine secretion occur during sepsis and following burn and traumatic injuries in animal models and in patients 97,101–104.
Cytokine production by NK cells in response to TLR agonists was impaired after polymicrobial sepsis105 suggesting that NK cells become tolerant to TLR agonists thereby recapitulating some features of endotoxin tolerance that occurs in monocytes 106. Impaired IFNγ production in response to TLR agonists such as LPS and CpG has also been reported in NK cells from septic patients 100. Due to their central role in antiviral defence, one may speculate that impaired NK cell function favours reactivation of latent viruses as has been frequently described in ICU patients 107–110. Decreased NK cell IFNγ production occurs in septic patients and frequently precedes cytomegalovirus reactivation 106 in those patients with protracted sepsis 97. In addition, defective NK cell production of IFNγ could be a factor in the increased incidence of secondary infections and the decrease in monocyte HLA-DR expression that occur in septic patients 111.
γδ T cells
γδ T cells are a distinct subset of lymphocytes that possess qualities common to both innate and adaptive immune cells and which reside in large numbers in the intestinal mucosa. Although the breadth of antigens to which γδ T cells respond is not completely known, it is clear that they recognize lipid antigens that are present on a variety of invading pathogens 112. γδ T cells present within the intestine and at other mucosal surfaces recognize invading pathogens and mount a prompt innate-like immune response with the release of IFNγ, IL-17 and various chemokines. As such, γδ T cells can be considered to represent the first line of defence against particular pathogens 113. The number of circulating γδ T cells is significantly decreased in septic patients with more severe depletion occurring in patients with the greatest severity of illness and mortality 113. The loss of γδ T cells in the intestinal mucosa may be particular detrimental to the host by enabling intestinal pathogens to become invasive and enter the circulation or peritoneal cavity, thereby causing secondary infections.
CD4+ TH cell subsets
Numerous studies have reported profound effects of sepsis on circulating and tissue T cells with some of the most significant effects occurring on CD4+ T cells 114–119. Mature CD4+ TH cells have been characterized into Th1, Th2 and Th17 cell subsets depending on the type of cytokines they produce upon stimulation. Early studies suggested that both Th1 and Th2 cell-associated cytokine production was decreased during the initial immune response to sepsis and trauma (Figure 3) 120–123. Additional work showing marked reductions in T-bet and GATA3 expression, which are transcription factors that modulate the Th1 and Th2 cell response respectively, reinforced the idea that both Th1 and Th2 cell lineages were suppressed after trauma and sepsis 124. These studies also showed that unlike T-bet and GATA3, expression of the Treg cell-associated transcription factor FOXP3, was not altered during sepsis, supporting the idea that regulatory functions are maintained or increased during sepsis whereas effector T cells responses are down-regulated (see below) 96,123,125. Histone methylation and chromatin remodeling are thought to contribute to suppression of Th1 and Th2 cell functions by acting at promoter regions of IFNG and GATA3 genes 126.
Although less explored than Th1 and Th2 cell subsets, there is now general agreement that Th17 cells play an important role in protection against extracellular bacterial and fungal infections by production of IL-17 and IL-22 127–130. The Th17 cell response is reduced in sepsis, possibly secondary to decreased expression of retinoic acid receptor-related orphan receptor-γt (RORγt), the transcription factor specific for Th17 cells 96,124. This defect in the Th17 cell phenotype in sepsis is likely one factor contributing to the increased susceptibility of these patients to secondary fungal infections 130. Indeed, mice that survived an initial polymicrobial bacterial infection (but not control mice) become highly susceptible to and rapidly succumb to pulmonary Aspergillosis fumigatus infection 66. Recently, IL-7 therapy has been shown to increase the Th17 cell response and decrease deaths due to secondary fungal infection with Candida albicans 131,154.
T cell exhaustion
T cell exhaustion was first described in mice suffering from chronic viral infections and is typified by T cells that have severely impaired effector functions 132. Subsequently, T cell exhaustion has been demonstrated in patients with bacterial and parasitic infections, HIV, and cancer 133. The prolonged duration of sepsis is characterized by high antigen load and elevated pro- and anti-inflammatory cytokines, an ideal setting for development of T cell exhaustion. A recent study12, in which spleens were obtained rapidly after death of septic patients showed evidence highly consistent with T cell exhaustion including: one, profound suppression of the production of IFN-γ and TNF-α by stimulated T cells; two, increased expression of PD1 on CD4+ T cells and of programmed cell death ligand 1 (PDL1) on macrophages; and three, decreased T cell expression of CD127 (the IL-7 receptor α-chain), which is another phenotypic feature of exhausted T cells. Furthermore, this study showed that capillary endothelial and bronchial epithelial cells in patients with sepsis had increased PDL1 expression, which in this setting can impair the function of T cells that have migrated to the local area of infection, thereby seriously compromising the ability of the host to eradicate the organisms. A potential causal link between T cell exhaustion and morbidity and mortality in sepsis was provided by studies showing that elevated expression of PD1 on circulating T cells from patients with sepsis correlated with decreased T cell proliferative capacity, increased nosocomial infections, and mortality134. Finally, animal studies demonstrate that inhibition of PD1–PDL1 interaction improves survival in several clinically-relevant models of sepsis, consistent with a key role for T cell exhaustion in the pathogenesis of sepsis (see below) 135–137.
Treg cells
An increased percentage of circulating Treg cells has been described in septic shock patients and this increase was observed immediately after onset of sepsis but persisted only in those septic patients who subsequently died138. These results were further extended by showing that this relative increase was due to a decrease in effector T cell numbers rather than changes in absolute numbers of Treg cells120. This finding suggests that Treg cells are more resistant to sepsis-induced apoptosis possibly because of increased expression of anti-apoptotic protein BCL-2. Alarmins, including heat shock proteins and histones, are increased in sepsis and they, as strong inducers of Treg cells, are also likely to contribute to increased Treg cell numbers in sepsis139. Since this initial work, many groups have confirmed an increase in Treg cell numbers in blood or spleens in various animal models of and patients suffering from trauma and sepsis 115,140,141.
Initial studies in mice investigating the role of Treg cells in sepsis-induced immune dysfunction were inconclusive possibly due to the use of CD25-specific antibodies which lack specificity in inhibiting Treg cells142. Recent studies indicate that increased Treg cells are deleterious in sepsis and associated with decreased effector T cell proliferation and function141. Importantly, these suppressive effects were totally abrogated by using small interfering RNA (siRNA) specific for Foxp3 to block their differentiation of Treg cells141. Other investigators used glucocorticoid-induced TNF-receptor-related protein (GITR)-specific antibodies to block Treg cells and showed that immune function was improved in sepsis leading to enhanced microbial killing 143. Additionally, sepsis-induced immune suppression was shown to facilitate rapid growth of solid tumors via a Treg cell-mediated effect144.
Treg cells can also suppress innate immune cells. In a setting of LPS stimulation, Treg cells inhibit both monocyte and neutrophil function145. Furthermore, Treg cells inhibit IFN-γ production by γδ T cells in response to Mycobacterium tuberculosis and precipitate an NK cell-dependent endotoxin tolerance-like phenomenon that is characterized by decreased production of IFNγ and GM-CSF114,146,147. In summary, there is extensive evidence that septic and trauma patients have increased Treg cell numbers which, by acting both on innate and adaptive immune cells, impair immunity and contribute to nosocomial infections and mortality.
Immunotherapies in sepsis
Recombinant human IL-7
Considering the severe quantitative and qualitative alterations in T cells induced by sepsis, IL-7 has recently emerged as a promising therapeutic agent for septic patients. IL-7 is essential for T cell development and function148. Its effects are mediated via the heterodimeric IL-7 receptor (IL-7R) composed of the IL-7R α-chain (CD127) and the common cytokine receptor γ-chain (CD132). This receptor is expressed by most resting human T cells with highest levels on naïve and central memory T cells and lowest levels on Treg cells. IL-7 up-regulates expression of the anti-apoptotic molecule BCL-2, induces proliferation of peripheral T cells, and sustains increased circulating blood CD4+ and CD8+ T cell numbers. In addition, IL-7 expands T cell receptor (TCR) repertoire diversity, is associated with a reduction in the proportion of Treg cells in the circulation, rejuvenates exhausted T cells by decreasing PD1 expression, and increases expression of cell adhesion molecules thereby facilitating trafficking of T cells to sites of infection149,150 (Figure 4). Recently, a massive loss in diversity of TCR repertoire was observed in septic patients151. Importantly, this low TCR diversity was also associated with increased risk of death and nosocomial infections. An optimal diversity of TCR repertoire greatly facilitates an effective immune response against invading pathogens and this may be one of the most significant benefits of IL-7. Thus, beyond supporting replenishment of lymphocytes, which are seriously depleted during sepsis, IL-7 improves multiple functional aspects of T cells that are profoundly altered in sepsis.
Figure 4. Immunotherapy of sepsis.

There are several immunotherapeutic agents that have shown promise in reversing the immunosuppressive phase of sepsis including recombinant interleukin-7 (IL-7), programmed cell death 1 (PD1)- or PDL1-specific antibodies, recombinant interferon-γ (IFNγ) and recombinant granulocyte/macrophage colony-stimulating factor (GM-CSF). GM-CSF and IFNγ act primarily on monocytes and macrophages to increase HLA-DR expression and induce activation. IL-7 and PD1-specific antibodies have the advantage of targeting CD4+ and CD8+ T cells to restore the function of adaptive immune system. By reengaging CD4+ T cells, both recombinant IL-7 and PD1-specific antibodies will have effects not only on adaptive immune cells but indirectly on monocytes and macrophages. PD1—and PDL1-specific antibodies will prevent and/or reverse T cell exhaustion. Thus, the net effects of these antibodies will be to prevent decreased interferon-γ (IFNγ) production, T cell apoptosis and decreased CD8+ T cell cytotoxicity. IL-7 will block T cell apoptosis, induce T cell proliferation, increase IFNγ production by T cells, increase T cell receptor (TCR) diversity, and increase T cell trafficking by increasing the expression of cell integrins such as lymphocyte function-associated antigen 1 (LFA1). The application of immunotherapeutic agents will depend on the use of various biomarkers or tests of immune function to document that patients have entered the immunosuppressive phase of sepsis.
In clinical trials, recombinant IL-7 has been used to treat patients with idiopathic lymphopenia and with lymphopenia-driven diseases including patients who are infected with HIV and have persistently low lymphocyte numbers despite effective anti-retroviral therapy 152. Clinical studies in over 250 patients have shown that IL-7 is safe and well-tolerated 148. The use of IL-7 in clinical trials of sepsis is supported by data from animal models of sepsis150,153,154. In mice with polymicrobial sepsis due to peritonitis, treatment with IL-7 improved T cell viability, trafficking and IFN-γ production, and restored the delayed-type hypersensitivity response to recall antigens 150. Similar beneficial effects of IL-7 were observed in a murine fungal sepsis model that reproduces the delayed secondary infections that frequently affect ICU patients131. Importantly, in these mouse experiments, there was also an over 2-fold improvement in survival. The ability of IL-7 to reverse the defects in lymphocyte function that occur in septic patients has been tested in an in vitro model155. Ex vivo treatment of T cells from septic patients with IL-7 corrected sepsis-induced defects including CD4+ and CD8+ T cell proliferation abnormalities, decreased IFN-γ production, impaired phosphorylation of signal transduction and activator of transcription-5 (STAT5), and decreased BCL-2 levels155. These data indicate that the IL-7 signalling pathway remains fully operative during sepsis and that IL-7 reverses critical sepsis-induced defects in T cell function.
PD1- and PDL1-specific antibodies
Another approach that holds great potential in reversing immunosuppression in sepsis involves blockade of the co-inhibitory molecules PD1 and PDL1 (Figure 4). As discussed previously, PD1 and PDL1 are widely expressed on immune effector cells, endothelial cells, and bronchial epithelial cells in patients with sepsis12. Blockade of PD1–PDL1 signaling improves survival in clinically-relevant animal models of bacterial sepsis135–137 and markedly decrease mortality in primary and secondary fungal sepsis due to Candida albicans156. Thus, PD-1 expressed by lymphocytes or PDL1 expressed by monocytes could serve as potential biomarkers for selection of septic patients who would be ideal candidates for therapy. A recent important study showed that in vitro blockade of PD-1 improved IFN-γ production and decreased apoptosis of T cells from patients with active infections due to M. tuberculosis157. A second major finding in this study was that when patients with tuberculosis effectively treated, the number of PD-1-expressing T cells decreased and inversely correlated with IFNγ T cell response against M. tuberculosis. We believe that this work has major implications for the broader field of sepsis because of the similarities of active tuberculosis with protracted sepsis.
IFNγ
A key immunological defect in sepsis is decreased production of IFN-γ, a cytokine which is essential for activation of innate immunity (Figure 4). Small clinical trials with recombinant IFNγ have been conducted in sepsis. Treatment with recombinant IFNγ reversed monocyte dysfunction in patients with sepsis whose monocytes had decreased HLA-DR expression and produced reduced amounts of TNF in response to LPS. This IFNγ-induced monocyte improvement was associated with 8 of 9 septic patients surviving the severe septic insult158. Most recently, IFNγ treatment in a patient with protracted Staphylococcus aureus sepsis caused an increase in monocyte HLA-DR expression, increased IL-17-expressing CD4+ T cells, and led to eradication of the bacteria 159. Furthermore, IFNγ is effective in treating fungal sepsis in patients with chronic granulomatous disease160. Finally, a recent trial of HIV patients who had cryptococcal meningitis and who were treated with IFNγ demonstrated enhanced clearing of fungi from the cerebrospinal fluid compared to controls161. Thus, IFNγ may be effective in sepsis if targeted to patients with sepsis who have entered the immunosuppressive phase, as identified by decreased monocyte HLA-DR expression and/or development of fungal sepsis. A clinical trial of IFNγ is currently underway in the Netherlands in patients with sepsis who are determined to have entered the immunosuppressive phase of the disorder (clinicaltrials.gov).
While IFNγ offers real promise as a potential immunotherapy in sepsis because of its ability to restore monocyte function, it will not correct the fundamental defect in T cells that is a major pathological abnormality in sepsis. In the authors’ opinion, recombinant IL-7 and PD1-specific antibodies are more likely to be of benefit to septic patients because of their effects to improve CD4+ and CD8+ T cell function: they both act to restore IFN-γ production in sepsis and also have many other beneficial effects on a variety of other immune effector cells.
G-CSF and GM-CSF
Given the many defects in neutrophil function occurring in sepsis, investigators conducted two randomized clinical trials with recombinant granulocyte colony-stimulating factor (G-CSF), a drug that increases neutrophil numbers and function, in patients with sepsis due to hospital-acquired and community-acquired pneumonia81,82. G-CSF had already been shown to be highly beneficial in decreasing the incidence of sepsis in patients who have abnormally low absolute neutrophil counts due to chemotherapy or radiation therapy. Although neutrophil counts are typically elevated in sepsis, investigators postulated that administration of G-CSF to further increase neutrophil numbers might improve pathogen killing in sepsis. Although white cell numbers increased in these patients, there was no benefit on overall survival162,163. These two clinical studies indicate that it is unlikely that drugs that enhance neutrophil numbers or function are likely to be of benefit in non-neutropenia patients with sepsis.
Another immunotherapy that is being actively investigated in sepsis is granulocyte macrophage colony stimulating factor (GM-CSF), a cytokine that causes accelerated production of neutrophils, monocytes and macrophages11 (Figure 4). Treatment of ventilator-dependent patients with sepsis who had entered the immunosuppressive phase of the disorder, as identified by persistent decreases in monocyte HLA-DR expression, were treated with recombinant GM-CSF, resulting in the restoration of HLA-DR expression, fewer days on the ventilator and decreased days in the ICU164. In addition, treatment of immunosuppressed pediatric patients with sepsis treatment with recombinant GM-CSF restored TNF production and reduced acquired nosocomial infections11.
Biomarker-guided therapy
A prerequisite for application of immunotherapy in sepsis is proper selection of patients. A variety of biomarkers should help in deciphering whether the patient is in the hyper- versus hypo-inflammatory phase of the disorder (Figure. 1). Indeed, immunotherapy could worsen outcome by causing an over-exuberant inflammatory response if applied during the wrong phase of the disorder. Readers are referred to numerous recent reviews which discuss utility of various biomarkers in sepsis92,165,166. Box 2 provides a list of clinical and laboratory findings that could be used to identify immunosuppressed patients who might benefit from immunotherapies. Potential markers include some currently available parameters such as decreased monocyte HLA-DR expression, increased circulating IL-10 concentration (both assessing innate immune function and useable for stratification of GM-CSF or IFN-γ treatments) and decreased absolute CD4+ T cell count and increased percentage of Treg cells (both assessing adaptive immunity and usable for stratification for IL-7 therapy). Ideally, ex vivo functional testing of circulating immune cells remains the gold standard for evaluation of immunity because it directly measures the capacity of cells to respond to pathogens.
BOX #1. Do mouse sepsis models reflect the human condition?
While key differences in the mouse and human immune systems have been recognized for years, for example differences in Toll-like receptor (TLR) expression and nitric oxide regulation, much discussion arose with the recent publication of a study indicating that mouse models of inflammation and sepsis do not reflect the complex scenario that occurs in patients with these disorders 168,169. Investigators performed gene analysis on total blood leukocytes from trauma and burn patients (many of whom were septic) as well as human volunteers who received endotoxin. The results in these three human disorders were compared with those generated in three mouse models of trauma, sepsis and burn injury169. Gene expression data in the three human conditions demonstrated very similar genomic responses, implying a commonality of the human immune response during inflammation induced by distinct mechanisms. In contrast, gene expression data from the three mouse models did not reflect any of the three human states. This lack of similarity in the human and mouse genomic response to injury led the investigators to suggest that mouse models are poor predictors of the more complex human conditions and that results from animal models may be misleading. The investigators argued that this lack of correlation of mouse and human immune response during sepsis likely explains why so many drugs that were efficacious in animal models of sepsis failed in clinical trials of patients with sepsis.
Although major differences exist in the human and mouse immune response to sepsis, there are several important factors that should be discussed. First, the pathological anatomical changes that occur in the mouse and human immune system during sepsis share many common features; specifically, the profound apoptotic depletion of immune cells and gastrointestinal epithelial cells that occurs in mouse sepsis is closely replicated in patients dying of sepsis27,28,33,34. Second, the lack of correlation of the genomic response between mouse and human blood leukocytes may be partly due to differences in the relative proportions of the various white blood cell components, such as neutrophils, lymphocytes and monocytes. Perhaps, if the comparisons were restricted to a particular cell class, such as circulating lymphocytes or monocytes, a much better correlation of the genomic response between mice and humans might have existed. In this regard, the dramatic upregulation in mouse lymphocytes of the genes encoding pro-apoptotic B cell lymphoma 2 (BCL-2) family members and downregulation of genes encoding anti-apoptotic BCL-2 family members has also been documented in lymphocytes from patients with sepsis170,171. The significance of this correlation for a particular class of genes in mice and humans is highlighted by the fact that apoptosis is a key pathogenic mechanism in sepsis. A few of the other immunological abnormalities that have been reported in both mouse and human sepsis include: one, a shift from a T helper 1 (Th1) to a Th2 cell phenotype; two, an increase in Treg cells numbers; three, increased myeloid-derived suppressor cell (MDSCs) numbers; and four, upregulation of co-inhibitory molecule expression 12,50,58,156. Thus, in our opinion, mouse sepsis models do provide important insights into particular mechanisms of sepsis that appear to play a comparable role in patients.
Box 2. Potential biomarker and clinical laboratory findings for applied immunotherapy in sepsis.
Innate immunity
↓ HLA-DR expression on monocytes
↓ TNF production by LPS-stimulated whole blood cells
↑ PDL1 expression on monocytes
Adaptive immunity
Persistent severe lymphopaenia
Reactivation of cytomegalovirus or herpes simplex virus infections
↑ PD1 expression on CD4+ or CD8+ cells
↑ Number of circulating regulatory T cells
↓ IFNγ production by T cells
↓ T cell proliferation
Innate and adaptive immunity
Infections with relatively avirulent or opportunistic pathogens, such as Enteroroccus spp., Acinetobacter spp. or Candida spp.
↑ IL-10:TNF ratio
↓ Delayed-type hypersensitivity response
Two hallmarks of sepsis-induced immunosuppression are reported: one, decreased monocyte capacity to produce pro-inflammatory cytokines (mainly TNF) in response to endotoxin challenge and, two, decreased lymphocyte proliferation. Currently, these two assays are thought to provide a reasonable assessment of immune effector cell status but remain barely usable on a routine clinical monitoring basis due to methodological challenges, such as long incubation time, lengthy cell purification procedures and low standardization. A major challenge is to abolish the gap between automated/standardized biomarkers and functional testing that is usable in routine daily practice. More precise cellular monitoring may be possible based on innovative targeted therapies including PD1 and CTLA4 cell expression to enable specific monoclonal-based therapies. Quantitation of mRNA for a panel of candidate genes in whole blood may enable clinicians to identify immunosuppressed patients and follow response to treatment. In the authors’ opinion, the ideal method to identify immunosuppressed patients would be a combination of cell phenotypic assay (for example measuring HLA-DR or PD-1 expression), functional assays (for example measuring whole blood TNF production) and genomic assays167.
Conclusions
The current understanding of the immune response to sepsis is controversial with investigators proposing competing theories of sepsis-induced morbidity and mortality due to persistent immune activation with accompanying inflammation versus sepsis-induced immunosuppression. We support the concept of sepsis-induced immunosuppression with T cell exhaustion as a likely major abnormality. As discussed in this review, sepsis induces numerous defects in host innate and adaptive immunity such that if the invading organisms are not promptly eliminated, the host becomes more susceptible to intractable infection or new secondary infections. Methods to identify when patients have entered an immunosuppressive phase of sepsis and to detect particular defects in immunity will enable the application of potent new immunotherapies that have shown great promise in animal models of sepsis and in early clinical studies of various infectious disorders. In particular, recombinant IL-7 and PD1-specific antibodies reverse fundamental immune defects in sepsis, have improved survival in multiple clinically relevant animal models of sepsis, are clinically well tolerated and have a demonstrable ability to improve immunity in patients with cancer and chronic viral infections. Immunotherapy may therefore represent the next major advance in the treatment of infectious disease and sepsis.
Acknowledgments
The authors would like to thank Dr. Paul Swanson (Professor of Pathology, University of Washington School of Medicine) for providing the immunohistochemical images of patient spleens (Figures 2 and 3). This work (R.S.H.) was supported by US National Institutes of Health (NIH) grant GM44118 and GM55194. G.M. was supported by funding from Hospices Civils de Lyon.
Glossary terms
- immunosenescence
The decreased function of the immune system with age. In particular, the number of naive T cells decreases as thymic function decreases
- myeloid-derived suppressor cells (MDSCs)
A population of cells composed of mature and immature myeloid cells. They are generated and/or activated during an inflammatory immune response. Through direct interactions and secreted components, they negatively affect T cells, which leads to impaired T cell function
- follicular DC (FDC)
Cell with a dendritic morphology that is present in lymph nodes. These cells display on their surface intact antigens that are held in immune complexes, and B cells present in the lymph node can interact with these antigens. FDCs are of non-haematopoietic origin and are not related to dendritic cells
- Interdigitating dendritic cells
A potent antigen-presenting cell that is rich in MHC class II molecules. Interdigitating dendritic cells take up antigen in the periphery and migrate to the paracortical region of lymph nodes and spleen where they interact with T cells
- Gut-associated lymphoid tissues (GALTs)
Lymphoid structures and aggregates associated with the intestinal mucosa, specifically the tonsils, Peyer’s patches, lymphoid follicles, appendix or coecal patch and mesenteric lymph nodes. They are enriched in conventional and unconventional lymphocytes and specialized dendritic-cell and macrophage subsets
- Anergy
A state of immune unresponsiveness. Anergic B cells or T cells do not respond to their cognate antigens
- Endotoxin tolerance
A transient state of hyporesponsiveness of the host or of cultured macrophages and/or monocytes to lipopolysaccharide (endotoxin) following previous exposure to LPS
- extracorporeal cell therapy
A therapy whereby the patient’s blood is circulated through a hemodialysis-like device through which particular types of cells, for example donor granulocytes, are added to the blood and subsequently infused back into the patient. Extracorporeal cell therapy of autologous granulocytes has been shown success in small clinical trials in sepsis
- FMS-like tyrosine kinase 3 ligand (FLT3L)
An endogenous cytokine that stimulates the proliferation of stem and progenitor cells through binding to the FLT3 receptor (a type III receptor tyrosine kinase member of the PDGF family). FLT3L administration substantially increases the number of DCs in lymphoid and non-lymphoid tissues
- MicroRNAs
Single-stranded RNA molecules of approximately 21–23 nucleotides in length that are thought to regulate the expression of other genes
- T cell exhaustion
The condition of functionally impaired antigen-specific T cells, typified by elevated surface expression of PD1, which occurs in the setting of persistent high antigen load. The defects in effector T cell function include a progressive decrease in their ability to produce cytokines, loss in proliferative capacity and decreased cytotoxicity and can result in apoptotic cell death
- Alarmins
Endogenous molecules that are released follwing tissue injury and which promote activation of the innate immune system. Excessive release of alarmins may promote uncontrolled inflammation and exacerbate tissue injury. Conversely, alarmins may have benefical effects by activating the immune system to mobilize and combat potential pathogens
- Small interfering RNA (siRNA)
Synthetic RNA molecules of 19–23 nucleotides that are used to ‘knockdown’ (that is, silence the expression of) a specific gene. This is known as RNA interference (RNAi) and is mediated by the sequence-specific degradation of mRNA
- Central memory T cells
Antigen-experienced T cells that express cell-surface receptors required for homing to secondary lymphoid organs. These cells are generally thought to be long-lived and can serve as the precursors for effector T cells for recall responses
- Delayed-type hypersensitivity (DTH)
A cellular immune response to antigen that develops over 24–72 hours with the infiltration of T cells and monocytes, and is dependent on the production of T helper 1 cell-associated cytokines
- chronic granulomatous disease
An inherited disorder caused by defective oxidase activity in the respiratory burst of phagocytes. It results from mutations in any of four genes that are necessary to generate the superoxide radicals required for normal neutrophil function. Affected patients suffer from increased susceptibility to recurrent infections
Contributor Information
Richard S. Hotchkiss, Department of Anesthesiology, Medicine, and Surgery; Washington University School of Medicine, St Louis, MO; United States
Guillaume Monneret, Hospices Civils de Lyon, Immunology laboratory, Hôpital E, Herriot, 5, place d’Arsonval - Lyon cedex 03 - France.
Didier Payen, Department of Anesthesiology & Critical Care & SAMU, Hôpital Lariboisière, Assistance Publique Hôpitaux de Paris, Paris, France, UFR de Médecine; UMR 940; University Paris 7 Denis Diderot.
References
- 1.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381:774–5. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J, Opal S, Calandra T. Sepsis studies need new direction. Lancet Infect Dis. 2012;12:503–5. doi: 10.1016/S1473-3099(12)70136-6. [DOI] [PubMed] [Google Scholar]
- 4.Wenzel RP, Edmond MB. Septic shock--evaluating another failed treatment. N Engl J Med. 2012;366:2122–4. doi: 10.1056/NEJMe1203412. [DOI] [PubMed] [Google Scholar]
- 5.Ward PA. New approaches to the study of sepsis. EMBO Mol Med. 2012;4:1234–43. doi: 10.1002/emmm.201201375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306:2614–5. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–8. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. This review article presents one of the first conceptual theories for a new understanding of the immunological basis of sepsis and points to potential new therapeutic approaches based upon the immunological phase of sepsis. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Opal S. Immunotherapy for sepsis--a new approach against an ancient foe. N Engl J Med. 2010;363:87–9. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payen D, Monneret G, Hotchkiss R. Immunotherapy - a potential new way forward in the treatment of sepsis. Crit Care. 2013;17:118. doi: 10.1186/cc12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall MW, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–32. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boomer JS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. This study is the first investigation to document profound defects in immunity in tissues from patients dying of sepsis and determined that T cell exhaustion is an important mechanism of immunosuppression in the disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward PA. Immunosuppression in sepsis. JAMA. 2011;306:2618–9. doi: 10.1001/jama.2011.1831. [DOI] [PubMed] [Google Scholar]
- 14.Torgersen C, et al. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth Analg. 2009;108:1841–7. doi: 10.1213/ane.0b013e318195e11d. [DOI] [PubMed] [Google Scholar]
- 15.Otto GP, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011;15:R183. doi: 10.1186/cc10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. This study confirmed that immunotherapy represents a major breakthrough in the treatment of cancer, a disorder which shares many immune defects with sepsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–21. doi: 10.1164/ajrccm.163.2.2007102. One of the first studies to demonstrate that both pro-inflammatory and anti-inflammatory processes occur rapidly after sepsis. [DOI] [PubMed] [Google Scholar]
- 19.Xiao W, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13:376–89. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 22.van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Frolich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–3. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 23.Ertel W, et al. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341–7. [PubMed] [Google Scholar]
- 24.Munoz C, et al. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–54. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigato O, Salomao R. Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock. 2003;19:113–6. doi: 10.1097/00024382-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Wong HR. Genome-wide expression profiling in pediatric septic shock. Pediatr Res. 2013;73:564–9. doi: 10.1038/pr.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotchkiss RS, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–51. doi: 10.1097/00003246-199907000-00002. This study was the first to demonstrate that apoptosis causes massive death and depletion of immune effector cells in patients with sepsis. [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–63. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, et al. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 30.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174:3765–72. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 31.Toti P, et al. Spleen depletion in neonatal sepsis and chorioamnionitis. Am J Clin Pathol. 2004;122:765–71. doi: 10.1309/RV6E-9BMC-9954-A2WU. [DOI] [PubMed] [Google Scholar]
- 32.Hotchkiss RS, et al. Rapid onset of intestinal epithelial and lymphocyte apoptotic cell death in patients with trauma and shock. Crit Care Med. 2000;28:3207–17. doi: 10.1097/00003246-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Chang KC, et al. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–19. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 34.Hotchkiss RS, et al. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–56. [PubMed] [Google Scholar]
- 35.Chung CS, et al. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J Leukoc Biol. 2003;74:344–51. doi: 10.1189/jlb.0102006. [DOI] [PubMed] [Google Scholar]
- 36.Oberholzer C, et al. Targeted adenovirus-induced expression of IL-10 decreases thymic apoptosis and improves survival in murine sepsis. Proc Natl Acad Sci U S A. 2001;98:11503–8. doi: 10.1073/pnas.181338198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwata A, et al. Over-expression of Bcl-2 provides protection in septic mice by a trans effect. J Immunol. 2003;171:3136–41. doi: 10.4049/jimmunol.171.6.3136. [DOI] [PubMed] [Google Scholar]
- 38.Voll RE, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–1. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 39.Green DR, Beere HM. Apoptosis. Gone but not forgotten. Nature. 2000;405:28–9. doi: 10.1038/35011175. [DOI] [PubMed] [Google Scholar]
- 40.Tamayo E, et al. Evolution of neutrophil apoptosis in septic shock survivors and nonsurvivors. J Crit Care. 2012;27:415 e1–11. doi: 10.1016/j.jcrc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Drifte G, Dunn-Siegrist I, Tissieres P, Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med. 2013;41:820–32. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 42.Alves-Filho JC, Spiller F, Cunha FQ. Neutrophil paralysis in sepsis. Shock. 2010;34 (Suppl 1):15–21. doi: 10.1097/SHK.0b013e3181e7e61b. [DOI] [PubMed] [Google Scholar]
- 43.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis. 2012;25:321–7. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 44.Cummings CJ, et al. Expression and function of the chemokine receptors CXCR1 and CXCR2 in sepsis. J Immunol. 1999;162:2341–6. [PubMed] [Google Scholar]
- 45.Stephan F, et al. Impairment of polymorphonuclear neutrophil functions precedes nosocomial infections in critically ill patients. Crit Care Med. 2002;30:315–22. doi: 10.1097/00003246-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Delano MJ, et al. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186:195–202. doi: 10.4049/jimmunol.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altrichter J, et al. Extracorporeal cell therapy of septic shock patients with donor granulocytes: a pilot study. Crit Care. 2011;15:R82. doi: 10.1186/cc10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasten KR, Muenzer JT, Caldwell CC. Neutrophils are significant producers of IL-10 during sepsis. Biochem Biophys Res Commun. 2010;393:28–31. doi: 10.1016/j.bbrc.2010.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pillay J, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–36. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delano MJ, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–74. doi: 10.1084/jem.20062602. This study identified MDSCs an important mechanism of immunosuppression in sepsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–94. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 52.Cuenca AG, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17:281–92. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brudecki L, Ferguson DA, McCall CE, El Gazzar M. Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infect Immun. 2012;80:2026–34. doi: 10.1128/IAI.00239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61:1155–67. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guisset O, et al. Decrease in circulating dendritic cells predicts fatal outcome in septic shock. Intensive Care Med. 2007;33:148–52. doi: 10.1007/s00134-006-0436-7. [DOI] [PubMed] [Google Scholar]
- 56.Riccardi F, et al. Flow cytometric analysis of peripheral blood dendritic cells in patients with severe sepsis. Cytometry B Clin Cytom. 2011;80:14–21. doi: 10.1002/cyto.b.20540. [DOI] [PubMed] [Google Scholar]
- 57.Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C. Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit Care. 2009;13:R119. doi: 10.1186/cc7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dreschler K, et al. Altered phenotype of blood dendritic cells in patients with acute pneumonia. Respiration. 2012;83:209–17. doi: 10.1159/000328406. [DOI] [PubMed] [Google Scholar]
- 59.Grimaldi D, et al. Profound and persistent decrease of circulating dendritic cells is associated with ICU-acquired infection in patients with septic shock. Intensive Care Med. 2011;37:1438–46. doi: 10.1007/s00134-011-2306-1. [DOI] [PubMed] [Google Scholar]
- 60.Pastille E, et al. Modulation of dendritic cell differentiation in the bone marrow mediates sustained immunosuppression after polymicrobial sepsis. J Immunol. 2011;186:977–86. doi: 10.4049/jimmunol.1001147. [DOI] [PubMed] [Google Scholar]
- 61.Faivre V, et al. Human monocytes differentiate into dendritic cells subsets that induce anergic and regulatory T cells in sepsis. PLoS One. 2012;7:e47209. doi: 10.1371/journal.pone.0047209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gautier EL, et al. Enhanced dendritic cell survival attenuates lipopolysaccharide-induced immunosuppression and increases resistance to lethal endotoxic shock. J Immunol. 2008;180:6941–6. doi: 10.4049/jimmunol.180.10.6941. [DOI] [PubMed] [Google Scholar]
- 63.Bohannon J, Cui W, Sherwood E, Toliver-Kinsky T. Dendritic cell modification of neutrophil responses to infection after burn injury. J Immunol. 2010;185:2847–53. doi: 10.4049/jimmunol.0903619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J Immunol. 2005;174:404–10. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 65.Toliver-Kinsky TE, Lin CY, Herndon DN, Sherwood ER. Stimulation of hematopoiesis by the Fms-like tyrosine kinase 3 ligand restores bacterial induction of Th1 cytokines in thermally injured mice. Infect Immun. 2003;71:3058–67. doi: 10.1128/IAI.71.6.3058-3067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benjamim CF, Hogaboam CM, Lukacs NW, Kunkel SL. Septic mice are susceptible to pulmonary aspergillosis. Am J Pathol. 2003;163:2605–17. doi: 10.1016/S0002-9440(10)63615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benjamim CF, Lundy SK, Lukacs NW, Hogaboam CM, Kunkel SL. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood. 2005;105:3588–95. doi: 10.1182/blood-2004-08-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roquilly A, et al. TLR-4 agonist in post-haemorrhage pneumonia: role of dendritic and natural killer cells. Eur Respir J. 2013 Jan 11; doi: 10.1183/09031936.00152612. [DOI] [PubMed] [Google Scholar]
- 69.Scumpia PO, et al. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol. 2005;175:3282–6. doi: 10.4049/jimmunol.175.5.3282. [DOI] [PubMed] [Google Scholar]
- 70.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–87. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X, Morrison DC. Lipopolysaccharide structure-function relationship in activation versus reprogramming of mouse peritoneal macrophages. J Leukoc Biol. 1993;54:444–50. doi: 10.1002/jlb.54.5.444. [DOI] [PubMed] [Google Scholar]
- 73.Monneret G, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–83. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 74.Rossato M, et al. IL-10-induced microRNA-187 negatively regulates TNF-alpha, IL-6, and IL-12p40 production in TLR4-stimulated monocytes. Proc Natl Acad Sci U S A. 2012;109:E3101–10. doi: 10.1073/pnas.1209100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishii M, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244–54. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carson WF, Cavassani KA, Dou Y, Kunkel SL. Epigenetic regulation of immune cell functions during post-septic immunosuppression. Epigenetics. 2011;6:273–83. doi: 10.4161/epi.6.3.14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turrel-Davin F, et al. mRNA-based approach to monitor recombinant gamma-interferon restoration of LPS-induced endotoxin tolerance. Crit Care. 2011;15:R252. doi: 10.1186/cc10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Ivashkiv LB. IFN-gamma abrogates endotoxin tolerance by facilitating Toll-like receptor-induced chromatin remodeling. Proc Natl Acad Sci U S A. 2010;107:19438–43. doi: 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monneret G, et al. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett. 2004;95:193–8. doi: 10.1016/j.imlet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 80.van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Frolich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–3. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 81.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–80. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 82.Hynninen M, et al. Predictive value of monocyte histocompatibility leukocyte antigen-DR expression and plasma interleukin-4 and -10 levels in critically ill patients with sepsis. Shock. 2003;20:1–4. doi: 10.1097/01.shk.0000068322.08268.b4. [DOI] [PubMed] [Google Scholar]
- 83.Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: A complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30:S58–S63. [PubMed] [Google Scholar]
- 84.Sfeir T, Saha DC, Astiz M, Rackow EC. Role of interleukin-10 in monocyte hyporesponsiveness associated with septic shock. Crit Care Med. 2001;29:129–33. doi: 10.1097/00003246-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 85.Muehlstedt SG, Lyte M, Rodriguez JL. Increased IL-10 production and HLA-DR suppression in the lungs of injured patients precede the development of nosocomial pneumonia. Shock. 2002;17:443–50. doi: 10.1097/00024382-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 86.Randow F, et al. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;181:1887–92. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muenzer JT, et al. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78:1582–92. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lukaszewicz AC, et al. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med. 2009;37:2746–52. doi: 10.1097/CCM.0b013e3181ab858a. [DOI] [PubMed] [Google Scholar]
- 89.Astiz M, Saha D, Lustbader D, Lin R, Rackow E. Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J Lab Clin Med. 1996;128:594–600. doi: 10.1016/s0022-2143(96)90132-8. [DOI] [PubMed] [Google Scholar]
- 90.Rode HN, et al. Lymphocyte function in anergic patients. Clin Exp Immunol. 1982;47:155–61. [PMC free article] [PubMed] [Google Scholar]
- 91.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Venet A, Lukaszewica A, Payen D, Hotchkiss R, Monneret G. Monitoring the immune response in sepsis: a rational approach to administration of immunoadjuvant therapies. Curr Opin Immunol. 2013;25:477–483. doi: 10.1016/j.coi.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Landelle C, et al. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 2010;36:1859–66. doi: 10.1007/s00134-010-1962-x. [DOI] [PubMed] [Google Scholar]
- 94.Chiche L, et al. The role of natural killer cells in sepsis. J Biomed Biotechnol. 2011;2011:986491. doi: 10.1155/2011/986491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Souza-Fonseca-Guimaraes F, Adib-Conquy M, Cavaillon JM. Natural killer (NK) cells in antibacterial innate immunity: angels or devils? Mol Med. 2012;18:270–85. doi: 10.2119/molmed.2011.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Venet F, et al. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock. 2010;34:358–63. doi: 10.1097/SHK.0b013e3181dc0977. [DOI] [PubMed] [Google Scholar]
- 97.Forel JM, et al. Phenotype and functions of natural killer cells in critically-ill septic patients. PLoS One. 2012;7:e50446. doi: 10.1371/journal.pone.0050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holub M, et al. Lymphocyte subset numbers depend on the bacterial origin of sepsis. Clin Microbiol Infect. 2003;9:202–11. doi: 10.1046/j.1469-0691.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- 99.Giamarellos-Bourboulis EJ, et al. Early changes of CD4-positive lymphocytes and NK cells in patients with severe Gram-negative sepsis. Crit Care. 2006;10:R166. doi: 10.1186/cc5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Souza-Fonseca-Guimaraes F, et al. Toll-like receptors expression and interferon-gamma production by NK cells in human sepsis. Crit Care. 2012;16:R206. doi: 10.1186/cc11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puente J, et al. In vitro studies of natural killer cell activity in septic shock patients. Response to a challenge with alpha-interferon and interleukin-2. Int J Clin Pharmacol Ther Toxicol. 1993;31:271–5. [PubMed] [Google Scholar]
- 102.Georgeson GD, et al. Natural killer cell cytotoxicity is deficient in newborns with sepsis and recurrent infections. Eur J Pediatr. 2001;160:478–82. doi: 10.1007/s004310100773. [DOI] [PubMed] [Google Scholar]
- 103.Blazar BA, et al. Suppression of natural killer-cell function in humans following thermal and traumatic injury. J Clin Immunol. 1986;6:26–36. doi: 10.1007/BF00915361. [DOI] [PubMed] [Google Scholar]
- 104.Bender BS, et al. Depressed natural killer cell function in thermally injured adults: successful in vivo and in vitro immunomodulation and the role of endotoxin. Clin Exp Immunol. 1988;71:120–5. [PMC free article] [PubMed] [Google Scholar]
- 105.Chiche L, et al. Interferon-gamma production by natural killer cells and cytomegalovirus in critically ill patients. Crit Care Med. 2012;40:3162–9. doi: 10.1097/CCM.0b013e318260c90e. [DOI] [PubMed] [Google Scholar]
- 106.Souza-Fonseca-Guimaraes F, Parlato M, Fitting C, Cavaillon JM, Adib-Conquy M. NK cell tolerance to TLR agonists mediated by regulatory T cells after polymicrobial sepsis. J Immunol. 2012;188:5850–8. doi: 10.4049/jimmunol.1103616. [DOI] [PubMed] [Google Scholar]
- 107.Luyt CE, et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175:935–42. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 108.Limaye AP, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–22. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cook CH, Trgovcich J. Cytomegalovirus reactivation in critically ill immunocompetent hosts: a decade of progress and remaining challenges. Antiviral Res. 2011;90:151–9. doi: 10.1016/j.antiviral.2011.03.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chiche L, Forel JM, Papazian L. The role of viruses in nosocomial pneumonia. Curr Opin Infect Dis. 2011;24:152–6. doi: 10.1097/QCO.0b013e328343b6e4. [DOI] [PubMed] [Google Scholar]
- 111.Wesselkamper SC, et al. NKG2D is critical for NK cell activation in host defense against Pseudomonas aeruginosa respiratory infection. J Immunol. 2008;181:5481–9. doi: 10.4049/jimmunol.181.8.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vantourout P, Hayday A. Six-of-the-best:unique contributions of γδ T cells to immunology. Nature Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andreu-Ballester JC, et al. Association of γδ T cells with disease severity and mortality in septic patients. Clin Vaccine Immunol. 2012;20:738–46. doi: 10.1128/CVI.00752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hotchkiss RS, et al. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–8. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 115.Gouel-Cheron A, Venet F, Allaouchiche B, Monneret G. CD4+ T-lymphocyte alterations in trauma patients. Crit Care. 2012;16:432. doi: 10.1186/cc11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Le Tulzo Y, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18:487–94. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 117.Inoue S, et al. Reduction of immunocompetent T cells followed by prolonged lymphopenia in severe sepsis in the elderly. Crit Care Med. 2013;41:810–9. doi: 10.1097/CCM.0b013e318274645f. [DOI] [PubMed] [Google Scholar]
- 118.Heffernan DS, et al. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Crit Care. 2012;16:R12. doi: 10.1186/cc11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheadle WG, et al. Lymphocyte subset responses to trauma and sepsis. J Trauma. 1993;35:844–9. doi: 10.1097/00005373-199312000-00007. [DOI] [PubMed] [Google Scholar]
- 120.Venet F, et al. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25− lymphocytes. Crit Care Med. 2004;32:2329–31. doi: 10.1097/01.ccm.0000145999.42971.4b. [DOI] [PubMed] [Google Scholar]
- 121.De AK, et al. Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin Immunol. 2000;96:52–66. doi: 10.1006/clim.2000.4879. [DOI] [PubMed] [Google Scholar]
- 122.Heidecke CD, et al. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 1999;178:288–92. doi: 10.1016/s0002-9610(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 123.Wick M, Kollig E, Muhr G, Koller M. The potential pattern of circulating lymphocytes TH1/TH2 is not altered after multiple injuries. Arch Surg. 2000;135:1309–14. doi: 10.1001/archsurg.135.11.1309. [DOI] [PubMed] [Google Scholar]
- 124.Pachot A, et al. Longitudinal study of cytokine and immune transcription factor mRNA expression in septic shock. Clin Immunol. 2005;114:61–9. doi: 10.1016/j.clim.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 125.Venet F, et al. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol. 2008;83:523–35. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 126.Carson WFt, et al. Impaired CD4+ T-cell proliferation and effector function correlates with repressive histone methylation events in a mouse model of severe sepsis. Eur J Immunol. 2010;40:998–1010. doi: 10.1002/eji.200939739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–88. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 128.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2011;10:112–22. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van de Veerdonk FL, et al. Deficient Candida-specific T-helper 17 response during sepsis. J Infect Dis. 2012;206:1798–802. doi: 10.1093/infdis/jis596. [DOI] [PubMed] [Google Scholar]
- 130.Monneret G, Venet F, Kullberg BJ, Netea MG. ICU-acquired immunosuppression and the risk for secondary fungal infections. Med Mycol. 2011;49 (Suppl 1):S17–23. doi: 10.3109/13693786.2010.509744. [DOI] [PubMed] [Google Scholar]
- 131.Unsinger J, et al. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J Infect Dis. 2012;206:606–16. doi: 10.1093/infdis/jis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. An outstanding review of the importance of T cell exhaustion in chronic infections and cancer. [DOI] [PubMed] [Google Scholar]
- 134.Guignant C, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15:R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang X, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–8. doi: 10.1073/pnas.0809422106. This was the first study to show that blocking the PD1 pathway could improve survival in sepsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brahmamdam P, et al. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–40. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Monneret G, et al. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–71. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 139.Zanin-Zhorov A, et al. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–32. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 140.Huang LF, et al. Association between regulatory T cell activity and sepsis and outcome of severely burned patients: a prospective, observational study. Crit Care. 2010;14:R3. doi: 10.1186/cc8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Venet F, et al. Increased circulating regulatory T cells (CD4(+)CD25 (+)CD127 (−)) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009;35:678–86. doi: 10.1007/s00134-008-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carrigan SO, et al. Depletion of natural CD4+CD25+ T regulatory cells with anti-CD25 antibody does not change the course of Pseudomonas aeruginosa-induced acute lung infection in mice. Immunobiology. 2009;214:211–22. doi: 10.1016/j.imbio.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 143.Scumpia PO, et al. Treatment with GITR agonistic antibody corrects adaptive immune dysfunction in sepsis. Blood. 2007;110:3673–81. doi: 10.1182/blood-2007-04-087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cavassani KA, et al. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010;115:4403–11. doi: 10.1182/blood-2009-09-241083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Venet F, et al. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J Immunol. 2006;177:6540–7. doi: 10.4049/jimmunol.177.9.6540. [DOI] [PubMed] [Google Scholar]
- 146.Li L, Wu CY. CD4+ CD25+ Treg cells inhibit human memory gammadelta T cells to produce IFN-gamma in response to M tuberculosis antigen ESAT-6. Blood. 2008;111:5629–36. doi: 10.1182/blood-2008-02-139899. [DOI] [PubMed] [Google Scholar]
- 147.Tiemessen MM, et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11:330–42. doi: 10.1038/nri2970. An outstanding review article on a remarkable cytokine that has great potential as an immunotherapeutic agent to reverse immunosuppression in a variety of disorders, including sepsis and cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Perales MA, et al. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120:4882–91. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Unsinger J, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184:3768–79. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Venet F, et al. Decreased T-cell repertoire diversity in sepsis: a preliminary study. Crit Care Med. 2013;41:111–9. doi: 10.1097/CCM.0b013e3182657948. [DOI] [PubMed] [Google Scholar]
- 152.Levy Y, et al. Effects of Recombinant Human Interleukin 7 on T-Cell Recovery and Thymic Output in HIV-Infected Patients Receiving Antiretroviral Therapy: Results of a Phase I/IIa Randomized, Placebo-Controlled, Multicenter Study. Clin Infect Dis. 2012;55:291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pellegrini M, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–13. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 154.Kasten KR, et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect Immun. 2010;78:4714–22. doi: 10.1128/IAI.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Venet F, et al. IL-7 restores lymphocyte functions in septic patients. J Immunol. 2012;189:5073–81. doi: 10.4049/jimmunol.1202062. An important study demonstrating that IL-7 restores function in immune effector cells from patients with sepsis. [DOI] [PubMed] [Google Scholar]
- 156.Chang KC, et al. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17:R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh A, Mohan A, Dey AB, Mitra DK. Inhibiting the Programmed Death 1 Pathway Rescues Mycobacterium tuberculosis-Specific Interferon gamma-Producing T Cells From Apoptosis in Patients With Pulmonary Tuberculosis. J Infect Dis. 2013;208:603–15. doi: 10.1093/infdis/jit206. [DOI] [PubMed] [Google Scholar]
- 158.Docke WD, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–81. doi: 10.1038/nm0697-678. One of the first studies to show that therapy with agents that enhance host immunity administered to patients in the immunosuppressive phase of the disorder can improve survival. [DOI] [PubMed] [Google Scholar]
- 159.Nalos M, et al. Immune effects of interferon gamma in persistent staphylococcal sepsis. Am J Respir Crit Care Med. 2012;185:110–2. doi: 10.1164/ajrccm.185.1.110. [DOI] [PubMed] [Google Scholar]
- 160.Marciano BE, et al. Long-term interferon-gamma therapy for patients with chronic granulomatous disease. Clin Infect Dis. 2004;39:692–9. doi: 10.1086/422993. [DOI] [PubMed] [Google Scholar]
- 161.Jarvis JN, et al. Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26:1105–13. doi: 10.1097/QAD.0b013e3283536a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Root RK, et al. Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hospitalized with pneumonia and severe sepsis. Crit Care Med. 2003;31:367–73. doi: 10.1097/01.CCM.0000048629.32625.5D. [DOI] [PubMed] [Google Scholar]
- 163.Nelson S, et al. A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalized patients with community-acquired pneumonia. CAP Study Group. J Infect Dis. 1998;178:1075–80. doi: 10.1086/515694. [DOI] [PubMed] [Google Scholar]
- 164.Meisel C, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–8. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 165.Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25:609–34. doi: 10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leentjens J, Kox M, van der Hoeven JG, Netea MG, Pickkers P. Immunotherapy for the adjunctive treatment of sepsis: from immunosuppression to immunostimulation. Time for a paradigm change? Am J Respir Crit Care Med. 2013;187:1287–93. doi: 10.1164/rccm.201301-0036CP. [DOI] [PubMed] [Google Scholar]
- 167.Wong HR. Genetics and genomics in pediatric septic shock. Crit Care Med. 2012;40:1618–26. doi: 10.1097/CCM.0b013e318246b546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 169.Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brahmamdam P, et al. Targeted delivery of siRNA to cell death proteins in sepsis. Shock. 2009;32:131–9. doi: 10.1097/SHK.0b013e318194bcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Weber SU, et al. Induction of Bim and Bid gene expression during accelerated apoptosis in severe sepsis. Crit Care. 2008;12:R128. doi: 10.1186/cc7088. [DOI] [PMC free article] [PubMed] [Google Scholar]