Abstract
Background
Incidences of gastrointestinal (GI) motility disorders increase with age. However, there is a paucity of knowledge about the aging mechanisms leading to GI dysmotility. Motility in the GI tract is a function of smooth muscle contractility, which is modulated in part by the enteric nervous system (ENS). Evidence suggests that aging impairs the ENS, thus we tested the hypothesis that senescence in the GI tract precipitates abnormalities in smooth muscle and neurally mediated contractility in a region-specific manner.
Methods
Jejunal and colonic circular muscle strips were isolated from young (4–10 years) and old (18+ years) baboons. Myogenic responses were investigated using potassium chloride (KCl) and carbachol (CCh). Neurally mediated contractile responses were evoked by electrical field stimulation (EFS) and were recorded in the absence and presence of atropine (1 μM) or NG-Nitro-l-arginine methyl ester (l-NAME; 100 μM).
Key Results
The myogenic responses to KCl in the jejunum and colon were unaffected by age. In the colon, but not the jejunum, CCh-induced contractile responses were reduced in aged animals. Compared to young baboons, there was enhanced EFS-induced contractility of old baboon jejunal smooth muscle in contrast to the reduced contractility in the colon. The effect of atropine on the EFS response was lower in aged colonic tissue, suggesting reduced participation of acetylcholine. In aged jejunal tissue, higher contractile responses to EFS were found to be due to reduced nitregic inhibition.
Conclusions & Inferences
These findings provide key evidence for the importance of intestinal smooth muscle and ENS senescence in age-associated GI motility disorders.
Keywords: enteric neurodegeneration, myenteric plexus, non-human primate, normal aging
Key Messages
The presented experiments in a non-human primate model demonstrate age-induced changes to a phenotype that cannot be investigated in humans, and provide significant functional evidence supporting histological data previously reported for the aging human gut.
The goal of this study was to investigate the effect of aging on smooth muscle contractility in a non-human primate model with comparable rates and patterns of aging to humans.
Jejunal and colonic tissue were isolated from young and old baboons. Myogenic responses were investigated using potassium chloride (Kcl) and carbachol (Cch), and neurally mediated contractile responses were evoked by electric field stimulation (EFS) in the presence and absence of neuronal inhibitors.
Jejunal contractility increased with age and was associated with an impairment of nitrergic mechanisms. In contrast, colonic contractile responses were significantly reduced in aged baboons and involved blunted cholinergic mechanisms in the enteric nervous system and in the smooth muscle tissue.
INTRODUCTION
Current knowledge of biological aging in the human gastrointestinal (GI) tract is lacking because many aspects of biological aging in the GI tract cannot be investigated directly in man. Moreover, aging studies in animals often report inconsistent results with human observations likely because humans age differently than other species such as rodents and swine.1 Although some studies have shown that intestinal transit time appears to be preserved with age,1–3 it is our belief that further investigation is necessary to delineate the precise mechanisms by which aging may lead to increased susceptibility to age-associated GI disorders.
Aging induces profound effects on the GI tract, causing progressive deterioration of physiological function and greater incidences of GI disorders.4,5 Specifically, the prevalence of motility disorders, such as fecal incontinence and constipation, increases substantially as a function of age. Although there is a paucity of information regarding the precise mechanisms of aging that leads to abnormalities in GI motility, degenerative neural mechanisms in the enteric nervous system (ENS) have been implicated.5 Observations of the ENS in the human GI tract reveal significant progressive neuronal attrition as a function of age,6,7 and similar findings are reported in aging mice,8 rats,9, and guinea pigs.10 Moreover, neuronal cell loss within the ENS significantly correlates with many of the negative traits of aging in the GI tract, including GI motility dysfunction.5,11
Although the precise mechanism by which enteric senescence alters GI motility is unclear, dysmotility may be the result of degenerative neural control of GI smooth muscle contractility. Contractility of the smooth muscles that line the gut is a fundamental constituent of GI transit and is significantly modulated by neural innervations from the myenteric plexus of the ENS. The myenteric plexus is composed of various neuronal cell types, including nitrergic and cholinergic neurons. Thus far, attempts in animal studies to delineate the precise neuronal populations afflicted by aging have been inconsistent and conflicting with human studies.11 Therefore, the primary objective of the present investigation was to develop a more relevant animal model to investigate aging in the GI tract, specifically focusing on smooth muscle function. Toward that end, we hypothesized that the mechanisms of senescence in a non-human primate GI tract more closely resemble humans than reports in other animal models. To test our hypothesis, we specifically examined myogenic and neurally mediated intestinal smooth muscle contractility using a baboon model with comparable rates and patterns of aging to humans.12 The results of our experiments in the baboons not only demonstrate a phenotype that cannot be investigated in humans but also provide significant functional evidence supporting histological data previously reported for the aging human gut.
METHODS
Animals
Tissue samples were obtained from baboons (Papio anubis) housed at the University of Oklahoma Health Science Center (OUHSC), Department of Comparative Medicine Annex, Oklahoma City, OK, an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility. The animal studies were approved by the OUHSC Institutional Animal Care and Use Committees (#06-147) and are in accordance with the Guide for the Care and Use of Laboratory Animals and National Research Council guidelines. All baboons were free from major GI pathologies, and a full pathology report of the baboon has been previously reviewed.13 All baboons used in this study had a non-GI-related rationale for euthanization independent of these experiments. Based on bone density, the lifespan (up to 40 years) and rate of aging of baboons in captivity is directly proportional to humans.14 An estimate of baboon age relative to human is limited to observations of the developmental milestones of baboons in captivity, and the age of baboons 18–20 years of age is equivalent to humans ∼55–60 years of age.15 Therefore, baboons 4–10 years of age were considered ‘young’ and baboons 18+ years of age were considered ‘old’ for this study.
Isolation of intestinal smooth muscle
In the morning of the procedure, the baboons were transported to the surgical suite. The animals were then sedated with an intramuscular dose of ketamine (10 mg/kg) and euthanized with an overdose of pentobarbital. Death was determined by monitoring vitals using a non-invasive blood pressure cuff, pulse oximeter, and visual observation of respiratory rate. Postmortem jejunal and colonic tissue samples were collected from the young (n = 4) and old (n = 9) baboons. A midline vertical incision was made, and segments of the jejunum and transverse colon (3 cm in length) were extracted and immediately placed in ice-cold Krebs-bicarbonate solution that was constantly aerated with 95% O2 and 5% CO2. The muscle/myenteric plexus and mucosa/submucosal plexus were separated from each other by sharp dissection, keeping the myenteric plexus intact with the muscle layer. The smooth muscle orientated into the direction of the circular muscle was isolated and cut into strips (2–3 × 9–11 mm). Muscle segments remained in the oxygenated Krebs solution until mounting in the organ bath.
Assessment of smooth muscle contractility
Individual muscle strip preparations were mounted into organ baths and allowed to equilibrate for up to 1 h before initializing experiments. Resting tension (To) was set at 7.5 mN, and produced adequate contractile responses to potassium chloride (KCl), carbachol (CCh), or EFS frequencies with minimal baseline drift. Individual muscle strips were attached to isometric force transducers (Radnoti Instruments, Monrovia, CA, USA), which were connected to a PowerLab data acquisition system (AD Instruments Ltd, Bell Vista, NSW, Australia). The maximum contractile response to KCl (80 mM) was used to quantify non-neuronally mediated smooth muscle contractility. Receptor-mediated cholinergic mechanisms were investigated using gradient doses of CCh from 10−9 M to 10−5 M. Neurally mediated responses were elicited by EFS applied via pairs of platinum wire electrodes with vertical spiral leads placed parallel to the muscle strips. An electrical stimulus was generated by a Grass-88 stimulator (Grass Technologies, West Warwick, RI, USA), and contractile responses of isolated circular muscles were evoked by 5 s trains of rectangular pulses (0.5 ms pulse duration, 30 V) applied for a total of 10 s. Pulse frequencies within the trains were increased from 1 to 32 Hz to induce frequency-dependent responses. Tetrodotoxin (TTX; 1 μM), which inhibits synaptic neurotransmission, was added to the bathing solution to verify that the responses to EFS were neurally mediated. All EFS-induced contractile responses were completely blocked by the addition of TTX. To assess the role of nitric oxide (NO) and acetylcholine (ACh) on smooth muscle contractility, standard concentrations of NG-Nitro-l-arginine methyl ester (l-NAME; 100 μM) and atropine (1 μM) were added to the organ bath, replacing the Krebs solution between each pharmacological treatment. At the end of the experiment, traces were processed using the LabChart software (v6; AD Instruments Ltd).
Drugs and solutions
The modified Krebs solution contained: (pH 7.2–7.4) 120 mM NaCl, 6 mM KCl, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 2.5 mM CaCl2, 14.4 mM NaHCO3, and 11.5 mM of glucose. Atropine sulfate, l-NAME, KCl, and CCh were obtained from Sigma-Aldrich (St. Louis, MO, USA) and were dissolved in Krebs solution. All drugs were added to the baths in volumes less than 1% of the total bath volume.
Data analysis and statistics
Contractions induced by KCl, CCh, or EFS were measured as changes from basal current (mN) and normalized per cm2 of cross-sectional area. Cross-sectional area = tissue wet weight (mg)/tissue length (cm) × tissue density (mg/cm3). The tissue length was measured at resting tension at the beginning of each experiment, whereas tissue wet weight was measured upon completion of the experiment. Specific muscle strip density was assumed to be 1.05 mg/mm3.16,17 Percent inhibition or excitation was calculated for each preparation at each frequency as the difference between maximum contractility pre- and posttreatment, divided by pretreatment, and multiplied by 100. All results are expressed as mean ± SEM, and statistical significance was determined using two-way repeated anova with Bonferroni post hoc analysis. Significance for basal tension and maximum contractility was calculated using a Student's unpaired t-test. Statistical significance was p < 0.05 and analyzed with GraphPad Prism 5.2 (La Jolla, CA, USA). All data are represented in the figures, and the F-value statistic describing the main effects is reported in the text.
RESULTS
The effects of aging on smooth muscle contractility induced by KCl
In the first series of studies, we investigated the magnitude of the maximum contractile response to KCl to assess the effect of age on non-neurally mediated smooth muscle contractility. There was no significant difference in smooth muscle contractility of the jejunum or colon isolated from old or young animals in response to KCl (Table1; p > 0.05; F3,82 = 0.51).
Table 1.
Intestinal smooth muscle contractile response to KCl
| Young |
Old |
|||||
|---|---|---|---|---|---|---|
| Mean (ΔmN/cm2) | SEM | N | Mean (ΔmN/cm2) | SEM | N | |
| Jejunum | 19.28 | 1.35 | 14 [4] | 17.69 | 1.58 | 30 [9] |
| Colon | 15.52 | 2.30 | 14 [4] | 19.69 | 2.94 | 28 [9] |
Data shown represent the maximal contractile response to KCl (80 mM). The number inside the parentheses indicates the total number of animals used, and the number outside the parentheses denotes the total number of preparations.
The effects of aging on smooth muscle contractility induced by CCh
Intestinal smooth muscle contractility in response to receptor-mediated cholinergic stimulation was further examined via the administration of CCh into the bath and measuring the contractile response. No significant difference in jejunal contractility was seen following increasing doses of CCh (p > 0.05; F1,407 = 0.00; Fig.1A) or in maximum contractility (p > 0.05) in the old vs young tissue (Fig.1B). However, age had a significant inhibitory effect on the magnitude of the contractile response of colonic smooth muscle to increasing concentrations of CCh (p < 0.001; F1,439 = 11.71; Fig.1D) and in maximum contractility (p < 0.05).
Figure 1.
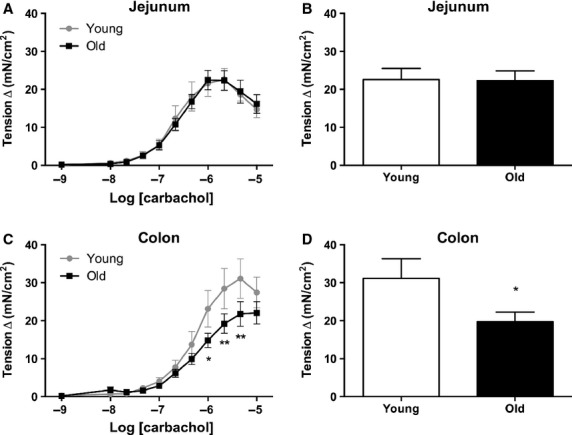
Intestinal smooth muscle contractile response to CCh. Addition of CCh dose-dependently enhanced contractility in both the (A and B) jejunum (n = 13[4] young; n = 24[7] old) and the (C and D) colon (n = 14[4] young; n = 26[8] old). A dose–response curve is shown on the left and maximum contractility is shown on the right. In the jejunum, there was no significant difference between old vs young baboons. However, there was a significant decrease in overall response to CCh in the old colonic smooth muscle when compared to young. Activation of cholinergic mechanisms by CCh revealed no significant differences in maximal jejunal contractile responses observed in the presence of 6 × 10−6 M CCh. However, there was a significant decline in contractile responses in the old colonic smooth muscle tissue in comparison to young. *p < 0.05, **p < 0.01 significance was determined using two-way repeated measures anova followed by Bonferonni posttest or Student's unpaired t-test for analysis of maximum contractility.
The effect of aging on EFS-induced contractile response
Neurally mediated smooth muscle contractility was assessed following graded EFS frequencies. Under basal conditions, there were no differences in resting tone between old and young colon (p > 0.05), but there was a significant difference in tension (p < 0.05) between old jejunal smooth muscle (21 ± 3.0 mN) compared to young (11 ± 2.0 mN). Electrical field stimulation induced a frequency-dependent increase in contractile responses in the jejunum and colon (Fig.2). Representative traces are shown at 32 Hz for the jejunum (Fig.2A; lower) and for the colon (Fig.2B; lower). We found a significant effect of age on the EFS-induced contractility of smooth muscles collected from the jejunum (p < 0.01; F1,174 = 10.45; Fig.2A). Specifically, in the jejunum from old baboons compared to young, there was a significant increase in smooth muscle contractility at 16 and 32 Hz (p < 0.05). There was a significant effect of age on EFS-induced contractility in colonic smooth muscles of old baboons compared to young (p < 0.001; F1,228 = 24.91). Frequency-dependent attenuation of colonic contractility in tissue taken from aged baboons reached statistical significance at 32 Hz (Fig.2B; p < 0.001).
Figure 2.
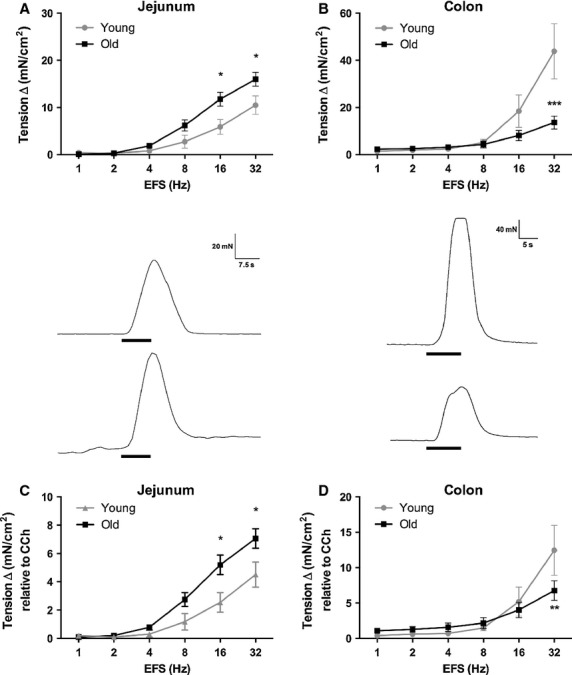
Intestinal smooth muscle response to electrical field stimulation (EFS). EFS (1–32 Hz, 0.5 ms, 10 s trains) of enteric nerve terminals cause a stimulus-dependent increase in contractility in the (A) jejunum (n = 8[3] young; n = 24[7] old) and (B) colon (n = 10[3] young; n = 30[8] old). Representative traces are shown (bottom). The vertical scale bar represents 20 and 40 mN, and the horizontal scale bar represents 7.5 and 5.0 s, respectively. The onset and duration of EFS is denoted by the dark black bar and represents 10 s. In response to increasing stimulation frequencies, there was a significant increase in contractility of aged jejunual smooth muscle tissue compared to young, whereas the opposite effect was seen in the colon. EFS-induced contractility was normalized to the maximal CCh response (C and D). There was no change in the differences between young and old baboon jejunum. However, age differences were minimized in the colon following normalization. *p < 0.05, **p < 0.01, ***p < 0.001, significance was determined using two-way repeated measures anova followed by Bonferonni posttest.
The EFS responses were then normalized to the maximum contractility induced by CCh to remove the myogenic influence and to specifically determine the extent of the neurogenic contribution to age-induced changes in contractility. Normalization to maximum CCh response did not affect the differences in EFS-induced contractility in the jejunum (p < 0.01; F1,174 = 11.05; Fig.2C). Normalization of the EFS-induced responses to the maximum CCh response eliminated most of the age-related effects in the colon (p > 0.05; F1,228 = 0.8396; Fig.2D), but post hoc analysis indicated a statistical difference between young and old contractility at 32 Hz (p < 0.01).
The effects of atropine on EFS-induced smooth muscle contractility
Cholinergic antagonism with the addition of atropine significantly reduced EFS-induced jejunal smooth muscle contractility in tissue taken from young baboons (p < 0.001; F1,84 = 21.71), with significant differences seen at 16 and 32 Hz (p < 0.01, p < 0.001). Atropine also had a marked inhibitory effect on colonic smooth muscle strips (p < 0.001; F1,108 = 28.20) isolated from young animals with significance seen at 16 and 32 Hz (p < 0.01, p < 0.001). In old baboon jejunal smooth muscle, atropine significantly inhibited EFS-induced contractility (p < 0.001; F1,276 = 17.59), with significant differences seen at 8, 16, and 32 Hz (p < 0.01, p < 0.001), and also significantly decreased contractility in colonic smooth muscles (p < 0.001; F1,228 = 17.81) with differences seen at 32 Hz (p < 0.001). The magnitude of inhibition induced by cholinergic antagonism was compared between young and old (Table2), revealing a significant effect of age on the percent change in contractile response to atropine between old and young baboon colon (p < 0.001; F1,98 = 24.09), but not jejunum (p > 0.05; F1,90 = 0.00).
Table 2.
Percent Inhibition of contractile response following EFS + atropine
| Jejunum |
Colon |
|||
|---|---|---|---|---|
| EFS (Hz) | Young (n = 8 [3]) | Old (n = 14 [4]) | Young (n = 9 [3]) | Old (n = 10 [3]) |
| 1 | 105 ± 21.1 | 100 ± 12.1 | 97.2 ± 7.30 | 75.4 ± 11.4 |
| 2 | 114 ± 21.6 | 86.6 ± 10.3 | 104 ± 10.7 | 71.0 ± 12.8 |
| 4 | 82.9 ± 20.2 | 95.3 ± 6.70 | 109 ± 11.6 | 67.4 ± 12.2* |
| 8 | 91.8 ± 7.60 | 107 ± 27.2 | 97.8 ± 6.69 | 66.6 ± 10.7 |
| 16 | 71.8 ± 11.4 | 88.3 ± 14.3 | 94.6 ± 4.76 | 77.2 ± 9.31 |
| 32 | 60.1 ± 10.8 | 48.5 ± 12.1 | 94.2 ± 3.58 | 71.5 ± 9.23 |
Analysis of the percent inhibition revealed no difference in percent inhibition in old jejunal smooth muscle tissue compared to young, whereas there was less percent inhibition in the old colon smooth muscles compared to young.
p < 0.05, significance was determined using two-way repeated measures anova followed by Bonferonni posttest.
The effects of l-NAME on EFS-induced smooth muscle contractility
The addition of the NO synthase inhibitor, l-NAME enhanced smooth muscle contractility with increasing frequency of EFS. l-NAME significantly increased EFS-induced smooth muscle contractility in young baboon jejunum (p < 0.001; F1,90 = 12.96) and colonic smooth muscle tissue (p < 0.05; F1,108 = 4.47). Post hoc analysis revealed significant differences at 16 and 32 Hz in the jejunum (p < 0.05), but no significant differences were seen at any specific frequency in the colon. The addition of l-NAME had significant effects on old jejunum (p < 0.001; F1,276 = 17.59) and colonic smooth muscles (p < 0.05; F1,348 = 6.20). Post hoc analysis showed significant differences at 8 and 32 Hz in jejunum smooth muscles (p < 0.05), but there were no significant differences at any specific frequency in the colon. When percent inhibition was analyzed between young and old baboons, there was a significant difference in the percent change in contractile responses to EFS in the presence of l-NAME (Table3) in the jejunum (p < 0.05; F1,220 = 5.678), but not in the colon (p > 0.05; F1,217 = 0.02).
Table 3.
Percent increase in contractile response following EFS + L-NAME
| Jejunum |
Colon |
|||
|---|---|---|---|---|
| EFS (Hz) | Young (n = 8 [3]) | Old (n = 20 [6]) | Young (n = 10 [3]) | Old (n = 30 [8]) |
| 1 | 254 ± 150 | 206 ± 69.3 | 104 ± 51.1 | 81.0 ± 42.6 |
| 2 | 252 ± 167 | 242 ± 62.0 | 121 ± 63.4 | 109 ± 41.1 |
| 4 | 420 ± 101 | 369 ± 97.5 | 136 ± 47.2 | 102 ± 24.2 |
| 8 | 492 ± 137 | 167 ± 51.3* | 149 ± 55.2 | 154 ± 42.0 |
| 16 | 218 ± 75.7 | 65.1 ± 15.1 | 111 ± 31.8 | 123 ± 43.6 |
| 32 | 104 ± 23.4 | 33.4 ± 04.60 | 25.5 ± 7.01 | 55.8 ± 10.2 |
In comparison of the percent changes in contractility in response to EFS in the presence of L-NAME, there was no difference seen in the colon of old and young baboons. However, there was significantly less percent excitation observed in the jejunal smooth muscle tissue from old baboons compared to young.
p < 0.01, significance was determined using two-way repeated measures anova followed by Bonferonni posttest.
DISCUSSION
The overall objective of this study was to investigate the consequences of enteric senescence on intestinal and colonic neuromuscular function in a non-human primate model. In summary, we found that age-associated changes in neurally mediated contractile responses induced by EFS were region-specific. In the jejunum, EFS-induced contractions were enhanced by age, whereas they were attenuated in colonic smooth muscle from aged animals. Blocking cholinergic neurotransmission inhibited EFS-induced contractions to a greater extent in the jejunum of the old baboon smooth muscle tissue compared to young, but significantly less in the old baboon colon. When neuronal nitric oxide (nNOS) was inhibited with l-NAME, there was no difference in the degree of change in contractile responses of old and young smooth muscle tissue in the jejunum, whereas the contractility response to l-NAME in the old baboon colons was significantly less than young. There was no difference between groups in contractile response of the jejunum smooth muscles to CCh; however, smooth muscle contractile responses of the old baboon colon to CCh were significantly less than young. Finally, there was no effect of aging on K+-induced contractions of the intestinal smooth muscle tissue. Overall, our findings illustrate that impairment of neurally mediated smooth muscle contractility, specifically NO-dependent mechanisms in the jejunum and ACh-dependent mechanisms in the colon, are evident in the aging gut.
Aging does not affect KCl-mediated smooth muscle contractility
The aging process could potentially affect muscle functionality, for example changes in the coupling of neurotransmitter binding sites on potassium channels, or altered kinetics of the potassium channels. To identify the potential changes in contractility of the smooth muscles due to aging muscle tissue, muscle contractions were evoked using a high concentration of KCl. When KCl was added to the bath, there was no difference in maximal contractility between the muscles extracted from the jejunum or colon from old or young baboons. Therefore, age-associated changes in intestinal smooth muscle contractility in response to EFS are not attributed to muscular atrophy in our preparations. Although we did not observe many changes in non-neuronal myogenic traits in the intestine with age, it is important to note that recent studies in mice have reported enhanced gastric smooth muscle contractility, including KCl responses, concomitant with an upregulation of contractile proteins.18 These findings, in addition to the present results, highlight the importance of region specificity when investigating age-associated changes in smooth muscle contractility.
Aging impairs cholinergic receptor-mediated mechanisms in the colon
To further investigate whether the changes in contractility with age may be due to a degeneration of receptor-mediated functions on the smooth muscle, contractility was induced by exposing the smooth muscle to increasing concentrations of CCh. There was no difference between old and young baboon jejunal smooth muscle responses to CCh. However, we found a significant decrease in maximal contractile responses to CCh in aged colonic smooth muscles in comparison to young. Furthermore, normalization of the EFS response in the colon eliminated some of the age-related effects on smooth muscle contractility. Therefore, age-induced changes in EFS-induced contractility of colonic smooth muscles may be due, at least in part, to a decline in cholinergic receptor-mediated mechanisms in the smooth muscle tissue. In addition, the observed changes in CCh responses support previous studies indicating blunted secondary signaling pathways in the colonic smooth muscle tissue.19
The effect of aging on neurally mediated intestinal smooth muscle contractility
One of the hallmark characteristics of aging in the GI tract is delayed transit, which correlates with the decline in neurons of the myenteric and submucosal plexus.20 The myenteric plexus innervates the smooth muscles of the GI tract, and thus neurodegeneration in the ENS may lead to aberrant smooth muscle contractility.11 To investigate the age-associated changes in smooth muscle contractility, we stimulated the intestinal strips with EFS and observed the contractility of the intestinal smooth muscles. We found that EFS induced frequency-dependent contractions in both jejunum and colonic tissue from old and young baboons. However, there was a significant enhancement of contractility in the old jejunum smooth muscle tissue compared to young, suggesting an impairment of inhibitory mechanisms with age. As aging is primarily associated with degeneration of function, our results direct attention to the possibility of a deficit in inhibitory tone in the jejunum, potentially via degradation of nitrergic mechanisms. In contrast, there was a decline in contractility of old colonic smooth muscle tissue compared to young, suggesting an impairment of the excitatory mechanisms in the colonic myenteric plexus, such as ACh-mediated neurotransmission.
Cholinergic inhibition implicates divergent mechanisms of aging
Studies to date on enteric neurodegeneration have suggested that the effects of aging are exclusive to specific populations of neurons—either cholinergic or nitrergic. However, the reports on the neuronal population affected by the aging process have been conflicting. In rodent models, there are marked losses of nNOS-immunoreactive cells associated with aging, but not cholinergic cells that express choline acetyltransferase (ChAT),21,22 which supports observations in patients showing a decline in inhibitory junction potentials with age.23 In this study, the influence of cholinergic innervation was eliminated with atropine to isolate the effects of aging on inhibitory neural control of smooth muscle contractility. Our key finding was that the effect of atropine was lower in aged tissues for the colon, and the lower impact of atropine treatment is indicative of reduced participation of ACh that may explain the reduction in the neurogenic response in aged colon. Our results show that blocking cholinergic mechanisms with atropine did not eliminate the discrepancy in EFS-induced contractility between old and young jejunum smooth muscle tissue, but the percent inhibition was significantly greater in the old jejunal tissue, which can either be due to a facilitation of cholinergic excitatory mechanisms or a deterioration of inhibitory mechanisms. An inverse effect was observed in the colon of aged baboon compared to young. The percent inhibition significantly decreased with age, indicating less cholinergic innervation of colonic smooth muscles and representing a mechanism potentially responsible for the diminished neurally mediated contractility induced by EFS. In the presence of atropine, contractility of colonic smooth muscles in aged baboons was greater than young, which may indicate a modest impairment of inhibitory mechanisms masked by a substantial decline in excitatory mechanisms.
Inhibiting nNOS reveals impaired nitrergic mechanisms in aged jejunum
Studies in rodents have also identified age-associated neurodegeneration to be limited to loss of neurons that express ChAT and not those expressing nNOS.24 The specific degeneration of cholinergic neurons has also been reported in the human myenteric plexus.20 Overall, our results indicate that in aged jejunal tissue there is less participation of NO and consequently a higher contractile response to EFS in the jejunum of old animals. Our study demonstrated that, as a result of the presence of l-NAME, there was an increase in contractility of both young and old jejunal smooth muscles, but more importantly, the level of contractility between the two groups in the presence of l-NAME were equivalent. Therefore, our data indicate that the increase in neurally mediated contractility induced by EFS in the old jejunal smooth muscle tissue is most likely due to a loss of nitrergic mechanisms, and the possibility of an enhancement of excitatory mechanisms or the decline in non-nitrergic inhibition in the aging jejunum can be eliminated. Our results support the observation in humans that a significant decline in the amplitude of inhibitory junction potentials, without changes in inhibitory neuropeptides occurs23 and reports of selective neurodegeneration of NO-producing neurons such as previously reported in rodent models.21,22 It is important to note that the results do not preclude the possibilities of a decrease in enzymes that produce inhibitory neurotransmitters such as nNOS, decreased inhibitory NO quanta/quantal content, decline in release probability, or loss of receptor-mediated mechanisms such as those found on interstitial cells of Cajal (ICC). For example, previous studies have demonstrated that NO-mediated relaxation of smooth muscles involve ICC, which express receptors for NO.25 More importantly, there is a significant decline in numbers of ICC with age,26 identifying another potential mechanism by which age-associated decline in nitrergic inhibition of intestinal smooth muscle may occur.
Conclusions
Aging has profound effects on the GI tract, and in this study we demonstrated marked changes in smooth muscle contractility in aged baboon jejunum and colon biopsies compared to young. We also demonstrated that the cause of the decline in muscle contractility was in part due to abnormal neuronal stimulation from the myenteric plexus. Subsequently, we identified deteriorative nitrergic mechanisms in the jejunum and cholinergic mechanisms in the colon as the most probable candidates responsible for the changes in contractility. As smooth muscle contractility is a pivotal factor involved in intestinal transit, these data highlight important mechanisms that may contribute to age-related GI motility dysfunction such as constipation and diarrhea.
Acknowledgments
The authors would like to acknowledge Gary L White, DVM, MMS for providing funding and Roman F Wolf, DVM for assistance in collecting the colonic tissue.
FUNDING
This project was supported in part by grant P40RR012317 from the NCRR/NIH.
DISCLOSURE
The authors declare no conflict of interests.
AUTHOR CONTRIBUTION
LT analyzed, interpreted the data, and wrote the manuscript; BG-VM designed the research study, interpreted the data, and revised the manuscript.
References
- 1.Hall KE, Wiley JW. Age-associated changes in gastrointestinal function. In: Hazzard WR, Blass JP, Ettinger WH, Halter JB, Ouslader JG, editors. Principles of Geriatric Medicine and Gerontology. 4th edn. New York: McGraw-Hill; 1999. pp. 835–42. [Google Scholar]
- 2.Melkersson M, Andersson H, Bosaeus I, Falkheden T. Intestinal transit time in constipated and non-constipated geriatric patients. Scand J Gastroenterol. 1983;18:593–7. doi: 10.3109/00365528309181643. [DOI] [PubMed] [Google Scholar]
- 3.Evans MA, Triggs EJ, Cheung M, Broe GA, Creasey H. Gastric emptying rate in the elderly: implications for drug therapy. J Am Geriatr Soc. 1981;29:201–5. doi: 10.1111/j.1532-5415.1981.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 4.Hall KE, Proctor DD, Fisher L, Rose S. American gastroenterological association future trends committee report: effects of aging of the population on gastroenterology practice, education, and research. Gastroenterology. 2005;129:1305–38. doi: 10.1053/j.gastro.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Bitar K, Greenwood-Van Meerveld B, Saad R, Wiley JW. Aging and gastrointestinal neuromuscular function: insights from within and outside the gut. Neurogastroenterol Motil. 2011;23:490–501. doi: 10.1111/j.1365-2982.2011.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes OA, de Souza RR, Liberti EA. A preliminary investigation of the effects of aging on the nerve cell number in the myenteric ganglia of the human colon. Gerontology. 1997;43:210–7. doi: 10.1159/000213852. [DOI] [PubMed] [Google Scholar]
- 7.de Souza Dutra A. Conditionally exactly soluble class of quantum potentials. Phys Rev A. 1993;47:R2435–7. doi: 10.1103/physreva.47.r2435. [DOI] [PubMed] [Google Scholar]
- 8.El-Salhy M, Sandstrom O, Holmlund F. Age-induced changes in the enteric nervous system in the mouse. Mech Ageing Dev. 1999;107:93–103. doi: 10.1016/s0047-6374(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 9.Santer RM, Baker DM. Enteric neuron numbers and sizes in Auerbach's plexus in the small and large intestine of adult and aged rats. J Auton Nerv Syst. 1988;25:59–67. doi: 10.1016/0165-1838(88)90008-2. [DOI] [PubMed] [Google Scholar]
- 10.Gabella G. Fall in the number of myenteric neurons in aging guinea pigs. Gastroenterology. 1989;96:1487–93. doi: 10.1016/0016-5085(89)90516-7. [DOI] [PubMed] [Google Scholar]
- 11.Wiskur B, Greenwood-Van Meerveld B. The aging colon: the role of enteric neurodegeneration in constipation. Curr Gastroenterol Rep. 2010;12:507–12. doi: 10.1007/s11894-010-0139-7. [DOI] [PubMed] [Google Scholar]
- 12.Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. The aging baboon: comparative demography in a non-human primate. Proc Natl Acad Sci. 2002;99:9591–5. doi: 10.1073/pnas.142675599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosanke SD. 56th Annual Meeting of the American College of Veterinary Pathologists (ACVP) and 40th Annual Meeting of the American Society for Veterinary Clinical Pathology (ASVCP) Boston, MA, Ithaca, NY: International Veterinary Information Service; 2005. Pathology of baboons Acvp, Asvcp. www.ivis.org Document No. P2225.1205. [Google Scholar]
- 14.Havill L. Bone mineral density reference standards in adult baboons (Papio hamadryas) by sex and age. Bone. 2003;33:877–88. doi: 10.1016/s8756-3282(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 15.Shi Q, Aida K, Vandeberg JL, Wang XL. Passage-dependent changes in baboon endothelial cells–relevance to in vitro aging. DNA Cell Biol. 2004;23:502–9. doi: 10.1089/1044549041562294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon AR, Siegman MJ. Mechanical properties of smooth muscle. I. Length-tension and force-velocity relations. Am J Physiol. 1971;221:1243–9. doi: 10.1152/ajplegacy.1971.221.5.1243. [DOI] [PubMed] [Google Scholar]
- 17.Gordon AR, Siegman MJ. Mechanical properties of smooth muscle. II. Active state. Am J Physiol. 1971;221:1250–4. doi: 10.1152/ajplegacy.1971.221.5.1250. [DOI] [PubMed] [Google Scholar]
- 18.Bhetwal BP, An C, Baker SA, Lyon KL, Perrino BA. Impaired contractile responses and altered expression and phosphorylation of Ca(2+) sensitization proteins in gastric antrum smooth muscles from ob/ob mice. J Muscle Res Cell Motil. 2013;34:137–49. doi: 10.1007/s10974-013-9341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bitar KN, Patil SB. Aging and gastrointestinal smooth muscle. Mech Ageing Dev. 2004;125:907–10. doi: 10.1016/j.mad.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL, Linden D, Cima RR, et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009;21:746–e46. doi: 10.1111/j.1365-2982.2008.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprenger N, Julita M, Donnicola D, Jann A. Sialic acid feeding aged rats rejuvenates stimulated salivation and colon enteric neuron chemotypes. Glycobiology. 2009;19:1492–502. doi: 10.1093/glycob/cwp124. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y, Kuro OM, Ishikawa F. Aging mechanisms. Proc Natl Acad Sci. 2000;97:12407–8. doi: 10.1073/pnas.210382097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch TR, Carney JA, Go VL, Szurszewski JH. Inhibitory neuropeptides and intrinsic inhibitory innervation of descending human colon. Dig Dis Sci. 1991;36:712–8. doi: 10.1007/BF01311226. [DOI] [PubMed] [Google Scholar]
- 24.Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003;106:69–83. doi: 10.1016/S1566-0702(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi T, Fujinami K, Fujita A, Okishio Y, Takewaki T, Hata F. Essential role of the interstitial cells of Cajal in nitric oxide-mediated relaxation of longitudinal muscle of the mouse ileum. J Pharmacol Sci. 2004;95:71–80. doi: 10.1254/jphs.95.71. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Pinilla PJ, Gibbons SJ, Sarr MG, Kendrick ML, Shen KR, Cima RR, Dozois EJ, Larson DW, et al. Changes in interstitial cells of Cajal with age in the human stomach and colon. Neurogastroenterol Motil. 2011;23:36–44. doi: 10.1111/j.1365-2982.2010.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


