Abstract
Stress and associated alterations in hypothalamic-pituitary-adrenal (HPA) function have deleterious impacts on the development of multiple mental and physical health problems. Prior research has aimed to identify individuals most at risk for the development of these stress-related maladies by examining factors that may contribute to inter-individual differences in HPA responses to acute stress. The objectives of this study were to investigate, in adolescents, 1) whether differences in neurocognitive abilities influenced cortisol reactivity to an acute stressor, 2) whether internalizing psychiatric disorders influenced this relationship, and 3) whether acute cognitive stress-appraisal mechanisms mediated an association between neurocognitive function and cortisol reactivity. Subjects were 70 adolescents from a community sample who underwent standardized neurocognitive assessments of IQ, achievement, and declarative memory measures at mean age 14 and whose physiological and behavioral responses to a standardized psychosocial stress paradigm (Trier Social Stress Test, TSST) were assessed at mean age 18. Results showed that, among all adolescents, lower nonverbal memory performance predicted lower cortisol reactivity. In addition, internalizing disorders interacted with verbal memory such that the association with cortisol reactivity was strongest for adolescents with internalizing disorders. Finally, lower secondary cognitive appraisal of coping in anticipation of the TSST independently predicted lower cortisol reactivity but did not mediate the neurocognitive–cortisol relationship. Findings suggest that declarative memory may contribute to inter-individual differences in acute cortisol reactivity in adolescents, internalizing disorders may influence this relationship, and cognitive stress appraisal also predicts cortisol reactivity. Developmental, research, and clinical implications are discussed.
Keywords: Neurocognitive Function, Memory, Stress Appraisal, Cortisol, TSST, Adolescents, Anxiety, Depression
1. Introduction
Extensive research has focused on the deleterious impact of stress on the development of multiple mental and physical health problems including depression, anxiety, cardiovascular disease, and cancer, and the association of these disorders with functional alterations in the hypothalamic-pituitary-adrenal (HPA) axis, the primary physiological mediator of the stress response (Tsigos and Chrousos, 1994; Heim et al., 2000). Much less is known about which individuals are at increased risk for the development of HPA dysregulation and associated stress-related disorders. Thus, while many studies differentiate patterns of HPA dysregulation between groups (e.g., depressed vs. healthy adults), a growing number of investigations have begun to focus on inter-individual differences to identify mechanisms of increased risk for adverse outcomes. Studies to date have largely concentrated on demographic and health-behavior variables (e.g., age, gender, contraceptive use, caffeine, and alcohol), as well as personality features, using laboratory psychosocial stress challenges such as the Trier Social Stress Test (TSST Kirschbaum et al., 1993) within healthy subjects (for reviews see Foley and Kirschbaum, 2010; Kudielka and Wust, 2010).
Neurocognitive factors may contribute to differences in the physiological transduction of psychosocial stress given their significant influence on skills essential for adaptation to the stress and demands of daily life (Fiocco et al., 2007; Stawski et al., 2011). Accordingly, neurocognitive weaknesses may increase subjective and physiological experiences of stress, including activation of the HPA axis (Power et al, 2008; Franz et al., 2011). Existing studies have varied in assessing the temporal relationship of neurocognitive and HPA function, and whether neurocognitive and HPA function is assessed at baseline or under stress. Most investigations have examined the impact of reactive cortisol levels on measures of neurocognitive function, with most, but not all (Domes et al., 2002; Nater et al., 2007), suggesting that increased cortisol levels predict impaired cognition, particularly in both verbal and visual memory (Kirschbaum et al., 1996; Lee et al., 2007; Quesada et al., 2012). Several longitudinal community studies, however, suggest a bidirectional effect of neurocognitive and HPA function; that is, lower baseline neurocognitive performance predicts changes in HPA axis diurnal function characterized by lower morning and higher afternoon/evening cortisol levels and a flatter diurnal cortisol slope presumably due to chronic stress (Lupien et al., 2005; Power et al, 2008; Franz et al., 2011;Stawski et al., 2011). Some, but not all of these investigations, also controlled for anxiety and depressive symptoms (Lupien et al., 2005; Franz et al., 2011).
Little is known about whether and how baseline neurocognitive abilities predict adolescent HPA reactivity to acute stress. Ginty et al. (Ginty and Conklin, 2012) assessed the relationship of baseline general intelligence (IQ) and verbal memory with acute cortisol and cardiovascular (CV) responses following a series of laboratory stress tasks in a large community sample of adults and found that lower cortisol and CV responses to the stressors were associated with lower verbal memory ability, but not IQ. A study of children 5 to 18 years of age similarly found that higher HPA reactivity to a speech task was associated with higher global academic achievement scores post-task (Mathewson et al., 2012). Using education level as a proxy for academic abilities, Fiocco and colleagues (Fiocco et al., 2007) found that adults with lower educational levels had greater cortisol responses to the TSST. Secondary analyses found that those with lower levels of education also performed more poorly on tests of verbal fluency, but groups did not differ on measures of digit span, self-esteem, or appraisal of the TSST as stressful. Although limited in scope and number, these investigations suggest that specific domains of neurocognitive function and acute cortisol reactivity to stress may be related and influenced by developmental factors.
Very little is also understood about whether the bidirectional associations of neurocognitive and HPA function are influenced by the presence of psychiatric conditions such as anxiety and depression despite evidence of the 1) high prevalence of these disorders among adults and children (Kessler et al., 2005; Merikangas et al., 2010), 2) frequent co-occurrence of neurocognitive deficits and learning disorders with depression and anxiety (Wenz-Gross and Siperstein, 1998; Feurer and Andrews, 2009), and 3) association of depressive and anxiety disorders with stress and HPA dysfunction (Tafet and Bernardini, 2003; Von Werne Baes et al., 2012). Existing studies of post-traumatic stress disorder, depression, and anxiety suggest a differential effect among clinical subjects compared to controls, with higher levels of cortisol generally associated with greater deficits in neurocognitive function in those with internalizing disorders (Gomez et al., 2009; Hinkelmann et al., 2009; Lagarde et al., 2010; Lenze et al., 2012; Wingenfeld et al., 2012).
A separate growing body of cognition-focused studies have examined the proximal impact of cognitive stress appraisal on cortisol reactivity. A transactional model of stress posits that perceptions of increased threat and decreased ability to cope are associated with greater experience of subjective stress to specific stressors (Folkman et al., 1986). Support for this model is derived from a recent meta-analysis of laboratory acute stress challenges that found social evaluative threat and lack of control as central to inducing cortisol reactivity (Dickerson and Kemeny, 2004). Contemporary measures of acute cognitive stress processes distinguish between primary appraisal (threat and personal relevance of a situation) and secondary appraisal (self-assessment of ability to cope with the stressor) (Gaab et al., 2005). Although limited, findings support an association of subjective reports of increased acute cognitive stress appraisal with increased cortisol reactivity (Wirtz et al., 2007; Juster et al., 2012), with one investigation suggesting an effect of increased primary (threat) but not secondary (coping ability) stress appraisal (Gaab et al., 2005).
The current study aims to investigate the relationship of neurocognitive function and cortisol reactivity in a longitudinal community sample of adolescents using verbal and nonverbal subtests of commonly used measures of IQ, academic achievement, and declarative memory; and whether these relationships are influenced by the presence of psychiatric internalizing disorders. The study further examines the influence of acute cognitive appraisal of stress in this relationship. We hypothesize that 1) youth with lower cognitive abilities in both verbal and nonverbal domains will have decreased cortisol reactivity, 2) that this finding will be more robust in those with internalizing disorders due to the compounding, or interactive, effect of having anxiety and/or depression, and 3) that higher levels of acute cognitive appraisal of stress to the TSST will mediate these relationships (See Figure 1).
Figure 1.
Conceptual model of trait neurocognitive abilities and cognitive appraisal predicting acute cortisol reactivity
Note: The letters [a, b, c, c’] correspond to analyses in Results.
2. Methods
2.1. Sample
Subjects included 70 (40 female) adolescents participating in a longitudinal study (Wisconsin Study of Families and Work; WSFW) who had data on neurocognitive function (at mean age 14.8 years) as part of a larger study of emotion regulation, and who later participated in a laboratory study of adolescent acute stress reactivity (mean age 18.4 years). Recruitment for both assessments was limited to subjects who passed MRI exclusionary criteria, participated in past salivary cortisol sampling, and lived in geographic proximity (2-3 hr drive) of project offices; sub-sample size was also limited by funding constraints. Thirteen adolescents who did the neurocognitive assessments are not included here because they did not complete the age 18 follow-up due to MRI exclusionary criteria (metal dental work acquired since age 15, n = 2), medical screen-out for major medical disorders with known cortisol associations(diabetes, n = 1), unavailability (away serving in military, n = 1), or lack of time and/or interest (n = 9). Most participants (n = 68) had recently graduated from high school at the time of the acute stress reactivity component of the study; the remaining two were between 11th and 12th grades in school.
The original WSFW included 570 women recruited from prenatal clinics, with eligibility criteria of age greater than 18, in the second trimester of pregnancy, living with the baby's father, and either employed or homemakers (for details see Hyde et al., 1995). At the time of recruitment (1990–1991), the socioeconomic status (SES) of the families was predominantly working- or middle-class, and they were largely Caucasian: family income ranged from $10,000 to $120,000, (Mdn = $46,000); approximately half of parents were high school graduates, and half were college graduates; and 11% self-identified as a racial/ethnic minority. There were no significant differences on these family demographic characteristics between the 70 participating adolescents and the remaining 500 families in the original WSFW. All study procedures were approved by the Institutional Review Board of the University of Wisconsin-Madison. Informed consent was obtained from adults at each assessment; children provided assent at the neurocognitive assessment and informed consent at the TSST visit.
Subjects in the current study were grouped according to lifetime presence of a DSM-IV internalizing disorder (i.e., depressive or anxiety disorder) established with the Structured Clinical Interview for DSM Disorders (SCID; First et al., 2002), administered within one month prior to the TSST lab session by a doctoral-level clinical psychologist extensively trained in the administration of the SCID. All SCID interviews were reviewed by a board-certified child and adolescent psychiatrist (MJS) with the interviewer, and disagreements were resolved by consensus.
The SCID yielded 24 adolescents with a lifetime diagnosis of an internalizing disorder (“Cases”; 16 female) including Generalized Anxiety Disorder (GAD; n = 2), Social Phobia (7), Specific Phobia (3), Panic Disorder (1), Posttraumatic Stress Disorder (PTSD; 1), Major Depressive Disorder (MDD; 9), and Co-morbid MDD with Social Phobia (5); current diagnoses (mutually exclusive) included Social Phobia (n = 2), Specific Phobia (3), GAD (2) and comorbid MDD and Social Phobia (3). In addition to these internalizing disorders, 3 Cases had a substance use disorder (cannabis abuse, n = 1current; alcohol abuse, n = 2 current). Similarly, among the 46 adolescents with no internalizing diagnosis (“Controls”; 24 female), 7 had a substance use disorder (cannabis abuse, n = 1 current; alcohol abuse, n = 4 lifetime, 2 current). With one minor exception, Cases and Controls did not differ by family SES (parental education and family income), gender, or racial/ethnic status: fathers of Control participants had slightly more years of education than fathers of Case participants, M = 15.3 versus 14.1, respectively, t(67) = 2.15, p = .035.
Health status and medication use were assessed at the time of the TSST visit. Use of oral contraception was reported by seven subjects (2 Cases, 5 Controls) and therefore considered in all analyses.
2.2. Procedure
2.2.1. Neurocognitive Assessment
Subjects completed a battery of neuropsychological measures assessing verbal and nonverbal domains of general cognitive abilities (IQ), academic achievement, and memory using three instruments frequently used in clinical and academic centers. Tests were administered according to each assessment's manual by an experienced member of the study team trained in standardized testing procedures.
IQ. The Wechsler Abbreviated Scale of Intelligence (WASI) is a brief measure of intelligence for individuals between the ages of 6-89 years (Wechsler, 1999). Vocabulary and Matrix Reasoning subtests were administered to assess general verbal and nonverbal cognitive abilities, requiring subjects to provide spoken definitions of increasingly complex words and complete missing components to visual patterns, respectively. Raw scores of the WASI subtests convert to T-scores (M = 50, SD = 10) with higher scores representing better performance. In the development and standardization of the measure, internal consistency for composite scores ranged from .92 to .98; test-retest coefficients were adequate, ranging from .87 to .92. Construct validity was supported by strong correlations with the Wechsler Intelligence Scale for Children (WISC-III) and Wechsler Adult Intelligence Scale (WAIS-III).
Academic Achievement. The Wide Range Achievement Test—Third Edition (WRAT-3; Wilkinson, 1993) is a standardized measure of academic achievement for ages 3-89 years. Raw scores on this measure translate to standard scores (M = 100, SD = 15), with higher scores indicating better performance. Reading and Arithmetic subtests were administered, which required subjects to perform increasingly complex single-word decoding and written mathematical calculations, respectively. Both subtests have demonstrated high content validity and internal consistency across adolescent age groups (alpha coefficients: Arithmetic .88-.89, Reading .90-.92).
Memory. The Wide Range Assessment of Memory and Learning—Second Edition (WRAML-2; Sheslow and Adams, 2003) is a standardized assessment battery for memory, appropriate for ages 5-90 years. Story Memory and Design Memory subtests were administered to assess verbal and visual declarative short-term memory. The tasks required subjects to immediately repeat two stories read out loud by an examiner and reproduce sets of geometric forms following a five-second exposure and a ten-second delay, respectively. Raw scores on these tests translate to scaled scores (M = 10, SD = 3), with higher scores indicating better performance. Both subtests have established strong internal consistency (alpha coefficients: Story Memory .91 - .92, Design Memory .86 - .91) and moderate to strong test-retest reliability (Story Memory r = .75, Design Memory r = .53).
2.2.2. State Cognitive Stress Appraisal
Cognitive stress appraisal was measured with the Primary Appraisal / Secondary Appraisal scale (PASA; Gaab et al., 2005), immediately after arrival at the TSST testing area and receiving instructions about the acute stress paradigm. Constructs of the PASA are based on a transactional stress model, and designed to specifically assess anticipatory anxiety to the TSST (Kirschbaum et al., 1993). Two eight-item scales measure situation-specific threat and challenge (Primary Appraisal) and self-concept of abilities and control expectancies (Secondary Appraisal). Participants evaluate the extent to which statements apply to themselves on a six-point scale ranging from “Strongly disagree” to “Strongly agree.” PASA scales have demonstrated reasonable to good internal consistency (alpha coefficients: Primary Appraisal .80, Secondary Appraisal .74), and negative inter-correlations between primary and secondary appraisal scales support the complementary relationship between the concepts (Gaab et al., 2005).
2.2.3. Laboratory Acute Stress Paradigm
The TSST was used to elicit HPA reactivity to acute stress (Kirschbaum et al., 1993). The TSST is a highly standardized laboratory psychosocial stress test consisting of five minutes of free speech and five minutes of mental arithmetic performed in front of an audience. All visits were conducted in the late afternoon. Subjects were thoroughly instructed on standardized cortisol sampling procedures including refraining from eating, brushing their teeth, and exercise one hour before the study visit. Serial samples of saliva were obtained at baseline, which followed an acclimation period (approximately 60 minutes of questionnaires and neutral-content DVDs) and immediately preceded the TSST; and 0, 10, 20, 30, 45, and 60 minutes following the TSST. Following the acclimation period, subjects were escorted by the research assistant (RA) to a separate room and standardized TSST testing “booth” where two TSST “judges” (one male, one female) were seated. At the conclusion of the TSST, the judges exited and the RA rejoined the adolescent in the booth where both remained during a 60-min recovery period for completion of questionnaires and saliva collection. At the conclusion of the visit, adolescents completed a writing exercise (Cohen et al., 2000) previously shown to boost self-esteem (Schmeichel and Vohs, 2009) and were thoroughly debriefed.
Saliva samples were stored in a −80° C freezer until assayed with the Salimetrics (State College, PA) extended-range cortisol enzyme immunoassay (EIA) kit using a monoclonal antibody. All samples were assayed in duplicate; results were considered acceptable only if the coefficient of variation (CV%) for the duplicate measurement was <20% for samples with values >0.02 ug/dL and <30% for samples with values ≤0.02 ug/dL. The mean inter-assay CV% was 5.1% and the mean intra-assay CV% was 3.5%.
2.3. Statistical Analysis
Cortisol reactivity was assessed as area under the curve with respect to increase (AUCi; Pruessner et al., 2003) and was the primary dependent variable of interest. A logarithmic transformation was applied to raw cortisol values to yield a normal distribution; all cortisol analyses are based on logarithmic transformed values.
Descriptive analysis of neurocognitive variables was accomplished by examining full sample means and standard deviations in relation to the average range values per each measure. Group differences by diagnostic (internalizing Case versus Control) and gender groups were examined via independent samples t-tests on all study variables. A value of p < .05 was considered statistically significant for all analyses.
Primary study aims were addressed via hierarchical multiple regression analyses, with each predictor variable centered at its mean (Kraemer and Blasey, 2004). To examine the relationship of baseline neurocognitive function with acute cortisol reactivity to the TSST, neurocognitive variables were entered stepwise with p ≤ .05 as the criterion for inclusion, predicting cortisol AUCi. Although neurocognitive predictors were not highly inter-correlated (Table 1), stepwise entry was chosen to limit the number of variables in the model given the sample size. The impact of an internalizing disorder on this relationship was examined by entering diagnostic group membership (Case vs. Control) into the model, followed by interaction terms of neurocognitive variables × diagnostic group entered stepwise with p ≤ .05 as the criterion for inclusion in the final model. Dummy variables for oral contraceptive use and gender were entered as control variables in the first step of each regression model, given the possibility of steroid medications and hormonal gender differences modulating cortisol responses to acute stress (Kumsta et al., 2007).
Table 1.
Descriptive statistics and inter-correlations of Cortisol, neurocognitive and stress appraisal variables
| Measure | M | SD | 1a | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Cortisol AUCi | 36.46 | 41.67 | - | .25* | .09 | .26* | .13 | .10 | .33** | −.05 | .41** |
| 2. Vocabulary | 56.64 | 8.13 | - | .18 | .62** | .46** | .23 | .04 | −.02 | .24* | |
| 3. Matrix Reasoning | 52.19 | 5.50 | - | .33** | .20 | .13 | .18 | −.09 | .25* | ||
| 4. Reading | 102.63 | 9.70 | - | .48** | .15 | .13 | .07 | .19 | |||
| 5. Arithmetic | 103.39 | 12.69 | - | .13 | .08 | .02 | .10 | ||||
| 6. Story Memory | 11.94 | 1.99 | - | .25* | .04 | −.10 | |||||
| 7. Design Memory | 8.44 | 2.30 | - | .05 | .14 | ||||||
| 8. Primary Appraisal | 3.63 | 0.80 | - | −.41** | |||||||
| 9. Secondary Appraisal | 3.67 | 0.92 | - |
Note. N = 70; WASI (Vocabulary and Matrix Reasoning) Standardized M = 50, SD = 10; WRAT-3 (Reading and Arithmetic) M = 100, SD = 15; WRAML-2 (Story Memory and Design Memory) M = 10, SD = 3. PASA Primary Appraisal and Secondary Appraisal are mean response values. Independent samples t-tests revealed no significant differences between Case and Control participants on any of the study variables (p range .416 - .868).
Inter-correlations are presented as Pearson's r values.
p < .05
p < .01
To assess the possibility of cognitive appraisal mediating the model of neurocognitive variables predicting cortisol reactivity, two sets of analyses evaluated PASA-Primary (threat, challenge) and PASA-Secondary (control expectancy, self-concept of abilities) as mediator variables using the criteria outlined by Kraemer et al. (2001), which specifies temporal ordering as in the present study. First, variables in the initial model were entered simultaneously, with PASA-Primary evaluated as the dependent variable. Next, the initial model variables were entered simultaneously, followed by the entry of PASA-Primary, as independent variables predicting cortisol AUCi. An identical procedure was performed to evaluate PASA-Secondary as a mediating variable.
3. Results
3.1. Preliminary Analyses
Full sample means and standard deviations suggest scores in the average range on all neurocognitive measures (Table 1). There were no differences on any study variable (neurocognitive functioning, cognitive appraisal, or cortisol AUCi) between Cases and Controls, t(68) range = −1.83 – 1.62; p range = .071 - .868, or between males and females, t(68) range = −0.40 – 1.85; p range = .069 - .852. Baseline cortisol level (obtained after an acclimation period of approximately 60 minutes after arriving for the TSST visit) also did not differ between Cases and Controls t(68) = .14, p = .893.
3.2. Influence of Neurocognitive Functioning and Internalizing Disorders on Cortisol Reactivity
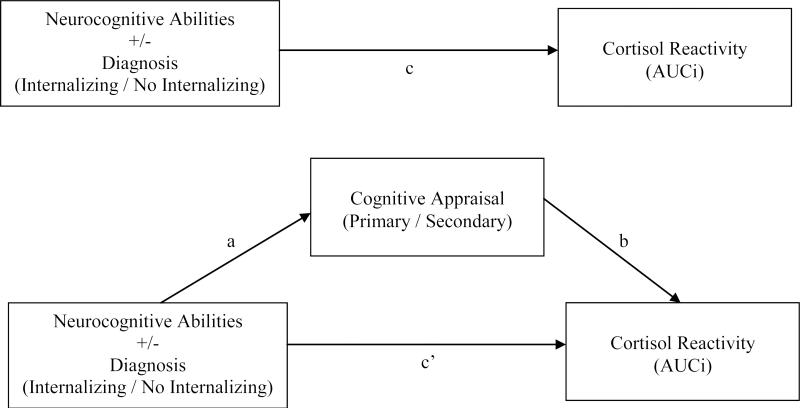
Initial analyses investigated whether neurocognitive function as assessed by IQ, academic achievement, and memory predicted cortisol reactivity to the TSST in the full sample and when considering those with/without psychiatric internalizing disorders. A significant model emerged with WRAML-2 Design Memory predicting cortisol AUCi (R2 = .150, p = .013) in the full sample, such that those who exhibited better immediate nonverbal memory had a higher cortisol response to the TSST (β = .285, p = .018). No other neurocognitive variables met criterion for inclusion in the model.
The presence or absence of an internalizing disorder did not significantly improve the predictive value of the model (β = .089, p = .427), although its addition still yielded a significant predictive model for cortisol AUCi (R2 = .158, p = .023). Stepwise entry of interaction terms added WRAML-2 Story Memory × diagnostic group to the analysis (β = −.265, p = .019), yielding a significant initial model of neurocognitive abilities and internalizing diagnostic status (Figure 1, path c) that accounted for an unadjusted 22.7% of variance in cortisol responses (adjusted R2 = .167, p = .005). To examine whether a main effect of Story Memory was unaccounted for in the stepwise entry procedure, Story Memory was subsequently entered as a final step. Story Memory was not a significant predictor in this step (β = .018, p = .881), thus confirming the significance of the Story Memory × diagnostic group interaction. Graphical representation of this interaction (Figure 2) revealed a significant positive association of Story Memory and cortisol AUCi for Cases (r = .45, p = .028) and a non-significant negative association for Controls (r = −.17, p = .256).
Figure 2.
Association of the main effect of Design Memory, and interaction effect of Story Memory with internalizing diagnosis in predicting cortisol AUCi
3.3. Role of Acute Cognitive Stress Appraisal in Predicting Cortisol Reactivity
Subsequent analyses examined whether acute cognitive stress appraisal mediated the relationship of neurocognitive function and cortisol AUCi. PASA-Primary, a measure of threat and challenge perceived by subjects immediately after receiving instructions for the TSST, was first assessed as a potential mediator. The initial model of neurocognitive function and diagnostic group did not significantly predict PASA-Primary (Figure 1, path a; R2 = .071, p = .441), and PASA-Primary was not a significant predictor of cortisol AUCi (Figure 1, path b; β = .020, p = .865) when controlling for the initial model variables. Thus, PASA-Primary did not mediate the relationship of neurocognitive function and diagnostic group with cortisol reactivity.
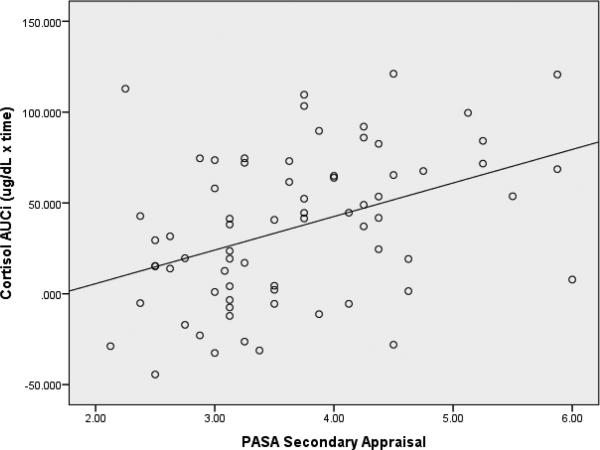
Analyses next assessed whether PASA-Secondary, a measure of self-perception of control and coping abilities, mediated the relationship of neurocognitive function and cortisol AUCi. Similar to findings for PASA-Primary, the initial model of neurocognitive function and diagnostic group did not predict PASA-Secondary (Figure 1, path a; R2 = .065, p = .494). However, in contrast to PASA-Primary, PASA-Secondary was a significant predictor of cortisol AUCi (Figure 1, path b; β = .337, p = .002) when controlling for the initial model variables, with Design Memory (β = .238, p = .031) and Story Memory × diagnostic group interaction term (β = −.246, p = .020) each contributing unique variance to the overall model (Figure 1, path c’; R2 = .333, p < .001). Higher levels of self-perceived coping abilities in anticipation of the TSST were associated with higher cortisol responsivity (Figure 3), but the relationship of neurocognitive function and diagnostic group with cortisol reactivity was not mediated via secondary appraisal, i.e., PASA-Secondary was found to have a unique main effect in predicting cortisol AUCi (Table 2).
Figure 3.
Association between Secondary Appraisal and cortisol AUCi
Table 2.
Regression models predicting Cortisol (AUCi) response to TSST
| Predictor Variables | β | p | Model Summary |
|||
|---|---|---|---|---|---|---|
| R2 | p | Δ R2 | p | |||
| Design Memory | .285 | .018 | .150 | .013 | .076 | .018 |
| Design Memory | .276 | .023 | .158 | .023 | .008 | .441 |
| Internalizing Diagnosis | .090 | .441 | ||||
| Design Memory | .270 | .021 | .227 | .005 | .070 | .019 |
| Internalizing Diagnosis | .089 | .427 | ||||
| Story Memory × Internalizing Diagnosis | −.265 | .019 | ||||
| Design Memory | .267 | .027 | .228 | .010 | <.001 | .881 |
| Internalizing Diagnosis | .089 | .435 | ||||
| Story Memory × Internalizing Diagnosis | −.267 | .020 | ||||
| Story Memorya | .018 | .881 | ||||
| Design Memory | .238 | .031 | .333 | <.001 | .106 | .002 |
| Internalizing Diagnosis | .025 | .818 | ||||
| Story Memory × Internalizing Diagnosis | −.246 | .020 | ||||
| Secondary Appraisalb | .337 | .002 | ||||
Note. All steps included entry of oral contraceptive use and gender as a control variable.
Post hoc entry to confirm significance of interaction term.
Entered following mediation analyses
4. Discussion
Of the three standardized verbal and nonverbal neurocognitive assessments of IQ, academic achievement, and memory, only memory variables were predictive of cortisol reactivity to acute stress. Findings partially supported our hypothesis that both verbal and nonverbal weakness would predict lower cortisol reactivity in that only nonverbal memory had a main effect among all subjects. Results also partially supported our hypothesis of a compounding effect of having an internalizing disorder, i.e., internalizing disorders interacted with verbal memory performance in differentially predicting cortisol responses, with a significant positive association of moderate magnitude within Cases and a negative non-significant association within Controls. While findings did not support a hypothesized mediational role of cognitive stress appraisal in linking memory and cortisol reactivity, results did reveal a significant relationship between acute cognitive stress appraisal and cortisol reactivity among all adolescents. More specifically, subjects’ self-perception of their abilities and level of control (Secondary Appraisal), but not threat (Primary Appraisal), in anticipation of the TSST was a main predictive factor of cortisol reactivity and strengthened the overall model.
Findings add to extant studies supporting a relationship between stress and declarative memory (Lupien and Lepage, 2001; Lupien et al., 2005), and uniquely extend these findings to adolescents. Our results, combined with findings from longitudinal studies (Lupien et al., 2005; Power et al, 2008; Franz et al., 2011; Stawski et al., 2011), suggest that neurocognitive weaknesses, including memory, may portend risk of downregulation of HPA function due to a putative model of chronic psychological stress arising from repeated difficulties navigating day-to-day demands that require memory and related neurocognitive abilities. The experience of this stress during childhood and adolescence confers additional risk for adverse stress-related outcomes given the immense psychological changes during this developmental epoch including development of self-identity, self-esteem, and locus of control (Stortelder and Ploegmakers-Burg, 2010; Thomaes et al., 2010). School is the major source of developmental experiences away from home for most youth, and is traditionally characterized by an overarching emphasis on academic performance compared to one's peers as a primary measure of competence and self-worth. Youth with neurocognitive weaknesses may have repeated experiences of feeling less capable and embarrassed in academic and social demands when comparing themselves to peers, with subsequent development of self-doubt and low self-esteem. Low self-esteem and feeling unable to control one's experiences have, in turn, been shown to heighten psychological and physiological reactivity to stress (Pruessner et al., 2005). Finally, repeated stress-activated release of glucocorticoids might dynamically worsen baseline neurocognitive function, including memory, due to the detrimental effects of corticosteroids on the hippocampus (Lupien and Lepage, 2001). In contrast to other investigations (Fiocco et al., 2007; Stawski et al., 2011; Mathewson et al., 2012), this study did not find a main effect of IQ or academic achievement in predicting cortisol reactivity. This may be related to several factors. One possibility is that, given the strength of the influence of memory on cortisol reactivity, weaker effects of the other neurocognitive domains were overshadowed in this relatively small and homogeneous sample. Alternatively, relative weaknesses in memory may be more influential in triggering a stress response compared to relative weaknesses in IQ and achievement when considering a normative range of performance as was found in this sample. Finally, given that this is the first study to systematically investigate the relationship of neurocognitive function with cortisol reactivity in adolescents, results may reflect neurocognitive domains of risk unique to this developmental epoch.
This is also the first study to examine the influence of lifetime history of internalizing disorders on the association of neurocognitive function and acute cortisol reactivity, and does so in a longitudinal study of adolescents. Several clinical studies of depression and anxiety report a negative association of increased diurnal cortisol levels with weaknesses in both verbal and nonverbal memory (Gomez et al., 2009; Hinkelmann et al., 2009). Findings may reflect an additive effect of negative affect on alterations in HPA function in addition to the variance due to memory deficits (Buchanan et al., 1999; Abercrombie et al., 2012). The interaction of internalizing disorders with verbal memory in this study may also be partially related to the verbal demands of the TSST as suggested by similar findings in adults with lower educational levels and relative weaknesses in verbal abilities (Fiocco et al., 2007).
Finally, findings support existing evidence linking increased cognitive appraisal of stress with alterations in cortisol reactivity (Gaab et al., 2005; Wirtz et al., 2007; Juster et al., 2012). This investigation also adds to the limited but growing number of studies that have begun to employ a more fine-grained approach to assessing this relationship by specifically investigating primary and secondary subcomponents of acute cognitive appraisal. Results of this study found that secondary appraisal, i.e., self-perception of coping abilities, predicted acute cortisol reactivity to the stress of the TSST, whereas perception of threat (primary appraisal) was not predictive of cortisol response. This is in contrast to an existing report of a positive relationship with primary appraisal and cortisol reactivity (Gaab et al., 2005). Findings may reflect a model of “chronic” cognitive appraisal of stress akin to downregulation of the HPA axis with repeated wear-and-tear activation, i.e., initial repeated cognitive experience of increased (primary appraisal) threat and associated increased cortisol reactivity, followed by a “downregulation” of response to threat and shift due to “chronic” expectation of inadequate abilities and coping resources (secondary appraisal), and low self-esteem, in approaching day-to-day demands requiring neurocognitive abilities. Differences in study outcomes may also be related to age of study subjects given that this is the first investigation to examine these relationships among adolescents in contrast to adults. Adolescence is a developmental period focused on self-concept and self-control (Stortelder and Ploegmakers-Burg, 2010) akin to the cognitive constructs of secondary appraisal. Components of secondary cognitive stress appraisal may therefore be of higher salience to adolescents in contrast to adults. Additional studies of cognitive stress appraisal mechanisms are also needed to further delineate the specificity of these cognitive factors with different physiological responses to stress as exemplified in a recent study that found that primary and secondary appraisal to the TSST were differentially associated with unique cytokine profiles (Wirtz et al., 2007).
There are several clinical and research implications to this study. First, findings support the consideration of memory function as a potential variable in research studies investigating inter-individual differences in cortisol acute stress reactivity. Second, future investigation of trait neurocognitive function and cortisol reactivity in children and adolescents of different ages will be important to assess potential developmental distinctions in this relationship, given known differences in HPA activity (Gunnar and Quevedo, 2007) and cognitive abilities (Casey et al., 2000) in younger children compared to adolescents. Finally, if findings are replicated in larger studies, identification of youth at risk for decreased HPA function related to chronic neurocognitive-related stress experiences could open new doors to the development of early stress-focused treatments and prevention interventions to decrease repeated experiences of perceived stress. More specifically, interventions might expand neurocognitive adapational abilities commonly needed for successful navigation of day-to-day demands, while also emphasizing cognitive behavioral components to enhance positive self-esteem, self-efficacy, locus of control, and cognitive appraisal of stress in youth identified as having lower neurocognitive, including memory, abilities. Such interventions may importantly prevent chronic alterations in HPA function, thereby also potentially preventing the development of stress-related mental and physical health problems among youth.
Limitations of this study include the use of single measures of verbal and nonverbal tasks of each of the three neurocognitive domains assessed. Moreover, the limited unique number of anxiety and depressive disorders required combined categorization into internalizing disorders. This approach prevented investigation of the relationship of neurocognition and acute stress reactivity separately in youth with anxiety and depressive disorders, and is an important consideration in light of existing reports of differences in cortisol reactivity in studies of anxiety and depressive disorders (Burke et al., 2005; Duncko et al., 2006). The use of lifetime history of internalizing disorders was chosen as a trait-like diagnostic variable given the use of trait neurocognitive function measures administered earlier in the longitudinal study. Larger studies that include subgroups of current and past history of specific anxiety and depressive disorders would further clarify the temporal influence of each of these disorders on neurocognition. Finally, the neurocognitive domains included in this study were only assessed at mean age 14.8 years. While existing studies strongly suggest stability in all measures during the approximate 3 year period during mid to late adolescence between our study points (Wilkinson, 1993; Wechsler, 1999; Sheslow and Adams, 2003), longitudinal concurrent assessment of neurocognitive abilities and HPA function over time would permit more precise assessment of this relationship.
There are several strengths of the current investigation including the novel design and research questions linking neurocognitive function, acute cortisol reactivity to stress, and influence of internalizing psychiatric disorders and acute cognitive appraisal of stress in adolescents. Methodological strengths include the longitudinal community design, rigorous adminstration of the TSST, and use of standardized measures of internalizing disorders and cognition. All subjects were assessed with three standardized neurocognitive measures commonly used in clinic and school settings, thus providing an initial gauge of the use of these instruments as potential probes for identified areas of risk associated with stress and alterations in HPA function. All subjects were near the same age, level of education, and SES thereby minimizing these potential confounds in assessing the underlying relationship between neurocognition and cortisol reactivity (Fiocco et al., 2007; Hackman et al., 2010). Lastly, results of this study were found in the context of normative neurocognitive performance scores in a community sample, thus suggesting the strength of these associations amidst a large range of youth. Future studies should include assessment of youth with established neurocognitive deficits to determine whether findings are similar or unique for these groups.
Acknowledgements
The authors wish to express their appreciation to the participating families, the staff of the Wisconsin Study of Families and Work, and Dr. Elizabeth Shirtcliff for providing consultation on the cortisol measures.
Funding Bodies
This work was supported by NIH Grants P50-MH069315, P50-MH084051, R21-MH082705, R01-MH044340, and 1UL1RR025011 from the Clinical and Translational Science Award program of the National Center for Research Resources. Additional support was provided by NIH grant P20-DA017589; the John D. and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development; and the Graduate School, the School of Medicine and Public Health, the Department of Psychiatry, and the HealthEmotions Research Institute at the University of Wisconsin-Madison.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Marcia Slattery, Jeffrey Armstrong, and Marilyn Essex designed the study, performed data analyses, and wrote the manuscript. Michelle Ames and Adam Grieve performed data analyses and wrote the manuscript.
Conflicts of Interest
The authors report no conflicts of interest.
References
- Abercrombie HC, Wirth MM, Hoks RM. Inter-individual differences in trait negative affect moderate cortisol's effects on memory formation: preliminary findings from two studies. Psychoneuroendocrinology. 2012;37:693–701. doi: 10.1016/j.psyneuen.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, al'Absi M, Lovallo WR. Cortisol fluctuates with increases and decreases in negative affect. Psychoneuroendocrinology. 1999;24:227–241. doi: 10.1016/s0306-4530(98)00078-x. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cohen GL, Aronson J, Steele CM. When Beliefs Yield to Evidence: Reducing Biased Evaluation by Affirming the Self. Pers Soc Psychol Bull. 2000;26:1151–1164. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Reichwald U, Hautzinger M. Hypothalamic-pituitary-adrenal axis reactivity to psychological stress and memory in middle-aged women: high responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology. 2002;27:843–853. doi: 10.1016/s0306-4530(01)00085-3. [DOI] [PubMed] [Google Scholar]
- Duncko R, Makatsori A, Fickova E, Selko D, Jezova D. Altered coordination of the neuroendocrine response during psychosocial stress in subjects with high trait anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1058–1066. doi: 10.1016/j.pnpbp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Feurer DP, Andrews JJW. School-Related Stress and Depression in Adolescents With and Without Learning Disabilities: An Exploratory Study. Alberta J Educ Res. 2009;55:92–108. [Google Scholar]
- Fiocco AJ, Joober R, Lupien SJ. Education modulates cortisol reactivity to the Trier Social Stress Test in middle-aged adults. Psychoneuroendocrinology. 2007;32:1158–1163. doi: 10.1016/j.psyneuen.2007.08.008. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam Gibbon, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Foley P, Kirschbaum C. Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci Biobehav Rev. 2010;35:91–96. doi: 10.1016/j.neubiorev.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Folkman S, Lazarus RS, Dunkel-Schetter C, DeLongis A, Gruen RJ. Dynamics of a stressful encounter: cognitive appraisal, coping, and encounter outcomes. J Pers Soc Psychol. 1986;50:992–1003. doi: 10.1037//0022-3514.50.5.992. [DOI] [PubMed] [Google Scholar]
- Franz CE, O'Brien RC, Hauger RL, Mendoza SP, Panizzon MS, Prom-Wormley E, Eaves LJ, Jacobson K, Lyons MJ, Lupien S, Hellhammer D, Xian H, Kremen WS. Cross-sectional and 35-year longitudinal assessment of salivary cortisol and cognitive functioning: the Vietnam Era twin study of aging. Psychoneuroendocrinology. 2011;36:1040–1052. doi: 10.1016/j.psyneuen.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater UM, Ehlert U. Psychological determinants of the cortisol stress response: The role of anticipatory cognitive appraisal. Psychoneuroendocrinology. 2005;30:599–610. doi: 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Ginty AT, Conklin SM. High perceived stress in relation to life events is associated with blunted cardiac reactivity. Biol Psychol. 2012;86:383–385. doi: 10.1016/j.biopsycho.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Gomez RG, Posener JA, Keller J, DeBattista C, Solvason B, Schatzberg AF. Effects of major depression diagnosis and cortisol levels on indices of neurocognitive function. Psychoneuroendocrinology. 2009;34:1012–1018. doi: 10.1016/j.psyneuen.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Moritz S, Botzenhardt J, Riedesel K, Wiedemann K, Kellner M, Otte C. Cognitive impairment in major depression: association with salivary cortisol. Biol Psychiatry. 2009;66:879–885. doi: 10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Klein MH, Essex MJ, Clark R. Maternity leave and women's mental health. Psychol Women Q. 1995;19:257–285. [Google Scholar]
- Juster RP, Perna A, Marin MF, Sindi S, Lupien SJ. Timing is everything: Anticipatory stress dynamics among cortisol and blood pressure reactivity and recovery in healthy adults. Stress. 2012;15:569–577. doi: 10.3109/10253890.2012.661494. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Blasey CM. Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Wust S. Human models in acute and chronic stress: Assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Entringer S, Hellhammer DH, Wust S. Cortisol and ACTH responses to psychosocial stress are modulated by corticosteroid binding globulin levels. Psychoneuroendocrinology. 2007;32:1153–1157. doi: 10.1016/j.psyneuen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Lagarde G, Doyon J, Brunet A. Memory and executive dysfunctions associated with acute posttraumatic stress disorder. Psychiatry Res. 2010;177:144–149. doi: 10.1016/j.psychres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry. 2007;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Dixon D, Mantella RC, Dore PM, Andreescu C, Reynolds CF, 3rd, Newcomer JW, Butters MA. Treatment-related alteration of cortisol predicts change in neuropsychological function during acute treatment of late-life anxiety disorder. Int J Geriatr Psychiatry. 2012;27:454–462. doi: 10.1002/gps.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can't live with it, can't live without it. Behav Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Miskovic V, Cunningham CE, McHolm AE, Boyle MH, Schmidt LA. Salivary Cortisol, Socioemotional Functioning, and Academic Performance in Anxious and Non-Anxious Children of Elementary and Middle School Age. Early Education & Development. 2012;23:74–95. [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, Moor C, Okere U, Stallkamp R, Martin M, Ehlert U, Kliegel M. Performance on a declarative memory task is better in high than low cortisol responders to psychosocial stress. Psychoneuroendocrinology. 2007;32:758–763. doi: 10.1016/j.psyneuen.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Power C, Li L, Hertzman C. Cognitive development and cortisol patterns in mid-life: findings from a British birth cohort. Psychoneuroendocrinology. 2008;33:530–539. doi: 10.1016/j.psyneuen.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Baldwin MW, Dedovic K, Renwick R, Mahani NK, Lord C, Meaney M, Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage. 2005;28:815–826. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Quesada AA, Wiemers US, Schoofs D, Wolf OT. Psychosocial stress exposure impairs memory retrieval in children. Psychoneuroendocrinology. 2012;37:125–136. doi: 10.1016/j.psyneuen.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Vohs K. Self-affirmation and self-control: affirming core values counteracts ego depletion. J Pers Soc Psychol. 2009;96:770–782. doi: 10.1037/a0014635. [DOI] [PubMed] [Google Scholar]
- Sheslow D, Adams W. Wide Range Assessment of Memory and Learning Second Edition administration and technical manual. Psychological Assessment Resources Inc.; Lutz, FL.: 2003. [Google Scholar]
- Stawski RS, Almeida DM, Lachman ME, Tun PA, Rosnick CB, Seeman T. Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: timing is everything. J Gerontol 66 Suppl. 2011;1:i71–81. doi: 10.1093/geronb/gbq094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stortelder F, Ploegmakers-Burg M. Adolescence and the reorganization of infant development: a neuro-psychoanalytic model. J Am Acad Psychoanal Dyn Psychiatry. 2010;38:503–531. doi: 10.1521/jaap.2010.38.3.503. [DOI] [PubMed] [Google Scholar]
- Tafet GE, Bernardini R. Psychoneuroendocrinological links between chronic stress and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:893–903. doi: 10.1016/S0278-5846(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Thomaes S, Reijntjes A, Orobio de Castro B, Bushman BJ, Poorthuis A, Telch MJ. I like me if you like me: on the interpersonal modulation and regulation of preadolescents’ state self-esteem. Child Dev. 2010;81:811–825. doi: 10.1111/j.1467-8624.2010.01435.x. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metab Clin North Am. 1994;23:451–466. [PubMed] [Google Scholar]
- Von Werne Baes C, de Carvalho Tofoli SM, Martins CMS, Juruena MF. Assessment of the hypothalamic-pituitary-adrenal axis activity: glucocorticoid receptor and mineralocorticoid receptor function in depression with early life stress - a systematic review. Acta Neuropsychiatrica. 2012;24:4–15. doi: 10.1111/j.1601-5215.2011.00610.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- Wenz-Gross M, Siperstein GN. Students with learning problems at risk in middle school: stress, social support, and adjustment. Except Child. 1998;65:91–100. [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test – Revision 3. Psychological Assessment Resources, Inc.; Lutz, FL.: 1993. [Google Scholar]
- Wingenfeld K, Driessen M, Terfehr K, Schlosser N, Fernando SC, Otte C, Beblo T, Spitzer C, Lowe B, Wolf OT. Cortisol has enhancing, rather than impairing effects on memory retrieval in PTSD. Psychoneuroendocrinology. 2012;37:1048–1056. doi: 10.1016/j.psyneuen.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, von Kanel R, Emini L, Suter T, Fontana A, Ehlert U. Variations in anticipatory cognitive stress appraisal and differential proinflammatory cytokine expression in response to acute stress. Brain Behav Immun. 2007;21:851–859. doi: 10.1016/j.bbi.2007.02.003. [DOI] [PubMed] [Google Scholar]