Abstract
More active high-dose chemotherapy (HDC) regimens are needed for refractory Hodgkin's lymphoma (HL). We report a cohort analysis of 180 consecutive patients with primary refractory or poor-risk relapsed HL treated with busulfan-melphalan (Bu-Mel) (n = 39), gemcitabine-busulfan-melphalan (Gem-Bu-Mel) (n = 84), or BEAM (BCNU, etoposide, ara-C, melphalan; n = 57) between 2005 and 2010. Their pre-HDC positron emission tomography (PET) scans were interpreted prospectively. Despite more prevalent poor-risk features in the Gem-Bu-Mel cohort, such as PET-positive tumors at HDC, tumors growing at HDC, extranodal disease, or bulky tumors at prior relapse, this cohort had improved outcomes compared with the Bu-Mel and BEAM cohorts, with event-free survival (EFS) rates of 57%, 33%, and 39%, respectively (P = .01), at median follow-up of the whole population of 36 months (range, 3 to 72). Their respective overall survival (OS) rates were, respectively, 82%, 52%, and 59% (P = .04). Secondary acute myelogenous leukemia was seen in 5 patients after BEAM but was not seen in Gem-Bu-Mel and Bu-Mel cohorts (P = .004). Multivariate analyses showed independent adverse effects of an HDC regimen different from Gem-Bu-Mel (hazard ratio [HR] for EFS = 2.3, P = .0008; HR for OS = 2.7, P = .0005), positive PET at HDC (HR for EFS 2.2, P = .004, HR for OS = 3.1, P = .0001), and >1 previous salvage line (HR for EFS = 1.9, P = .008, HR for OS = 1.8, P = .07). Gem-Bu-Mel improved outcomes in this cohort analysis of patients with refractory/poor-risk relapsed HL and merits evaluation in randomized phase III trials.
Keywords: Gemcitabine, High-dose chemotherapy, Autologous transplantation, Refractory, Hodgkin
INTRODUCTION
High-dose chemotherapy (HDC) with autologous stem cell transplant (ASCT) is standard treatment of patients with relapsed or primary refractory Hodgkin's lymphoma (HL) [1,2]. Adverse clinical predictors of post-HDC outcome include the length of the prior disease-free interval, presence of B symptoms, extranodal disease or bulky tumors at the time of relapse or progressive disease (PD), relapse within a prior radiation field, and number of prior salvage lines of chemotherapy [3–5]. In addition, there is emerging evidence that persistence of metabolically active tumor in pre-HDC seen with positron emission tomography (PET) is a major adverse prognostic factor [6–9]. These last observations come mostly from retrospective analyses and, therefore, need prospective confirmation.
BEAM (carmustine, etoposide, cytarabine, and melphalan) remains the standard HDC combination for HL despite its suboptimal results in patients with refractory tumors or poor prognostic features, with long-term event-free survival (EFS) rates of 30% to 50% and 15% to 40%, respectively [3–5]. With the goal of improving these results, since 2005 we have conducted sequential studies of busulfan-melphalan (Bu-Mel) and gemcitabine-busulfan-melphalan (Gem-Bu-Mel), enrolling patients with refractory or poor-prognosis relapsed HL [10,11]. Bu-Mel is an all-alkylator regimen that achieves a precise systemic exposure to busulfan through prospective pharmacokinetic monitoring, which increases its safety and tolerability [10]. The Gem-Bu-Mel combination builds on this construct and exploits the synergy between gemcitabine and alkylating agents based on DNA damage repair inhibition [11]. Both regimens cause a manageable toxicity profile of mucositis and skin rash and have shown high activity among patients with non-Hodgkin lymphoma (HL) and HL enrolled in their trials. All other HL patients receiving an ASCT at our institution since 2005 have received BEAM. The pre-HDC PET status of patients with refractory or poor-prognosis relapsed HL has been prospectively evaluated at our institution since 2005. We report here a cohort outcome comparison of patients with refractory or poor-prognosis relapsed HL transplanted with Bu-Mel, Gem-Bu-Mel, or BEAM between 2005 and 2010, as well as the prospective prognostic evaluation of their pre-HDC PET status.
METHODS
Patients
Patients subject to this analysis had refractory or poor-risk relapsed HL defined as (1) primary refractory HL documented histologically, defined as active disease after front-line chemotherapy or a combined modality treatment program, or relapse within 3 months of its completion; (2) poor-risk relapsed HL, defined as that occurring less than 12 months from the end of front-line chemotherapy or combined modality therapy or with extra-nodal involvement at the time of relapse; or (3) refractory relapse, defined as no response to first-line salvage therapy. Second or third lines of salvage chemotherapy were defined as different regimens used to treat persistent or PD afer a prior line. They did not include additional cycles of a different regimen given with peripheral blood progenitor cell mobilizing purposes after a complete response (CR).
Description of Cohorts
Although the Bu-Mel trial (MDA 2004-0190, NCI registration number: NCT00427765) was open to all patients with relapsed non-HL and HL [10], this analysis only includes its refractory or poor-risk relapsed HL subsets, as defined above. Bu-Mel consisted of four daily doses of busulfan targeting an average daily area under the curve of 5000 μM · min−1 or 130 mg/m2/day and melphalan at 70 mg/m2/day × 2.
The succeeding Gem-Bu-Mel study (MDA 2006-0803, NCI registration number: NCT00410982) was only open to patients with refractory or poor-risk relapsed HL and non-HL, defined for the former group by the criteria outlined above [11]. The Gem-Bu-Mel regimen added 2 doses of gemcitabine at 225 to 2775 mg/m2/day (as part of a dose escalation trial) infused over 15 to 40 minutes at a fixed dose rate of 10 mg/m2/min, combined with busulfan (targeting an average daily area under the curve of 4000 μM · min−1 or 105 mg/m2/day) and melphalan (60 mg/m2/day × 2). All HL patients enrolled in this study, 73% of whom were treated at or within 2 dose levels of the maximum tolerated dose (2175 to 2775 mg/m2/day), were included in this analysis.
The sequential Bu-Mel and Gem-Bu-Mel studies shared the same organ function eligibility criteria [10,11]. These trials enrolled patients from 2005 to 2007 and from 2007 to 2010, respectively. During this time period, all other HL patients meeting those trials' criteria of refractory or poor-risk relapsed disease and other eligibility criteria, but who were not enrolled, received BEAM off study and were prospectively registered in our departmental database. The reasons for treating these patients with BEAM included their declination to participate on study, the requirement by their third-party payer to only cover treatment off study, or during periods when enrollment to the Gem-Bu-Mel trial was temporarily held for safety analyses during its dose escalation portion. BEAM consisted of carmustine (300 mg/m2), etoposide (200 mg/m2 every 12 hours for 8 doses), ara-C (200 mg/m2 for 4 doses), and melphalan (140 mg/m2). Patients with relapsed HL receiving BEAM not meeting poor-risk or refractory disease criteria were excluded. This cohort analysis of Bu-Mel, Gem-Bu-Mel, and BEAM was approved by the M.D. Anderson Cancer Center Institutional Review Board (MDA 2010-0859).
Institutional transplantation guidelines for antiemetics; antibacterial, antifungal, and antiviral prophylaxis; and red blood and platelet transfusions were followed in the 3 cohorts. Specific supportive care measures in the Bu-Mel and Gem-Bu-Mel trials were previously described [10,11]. Peripheral blood progenitor cell was infused on day 0. Granulocyte colony-stimulating factor was administered at 5 μg/kg/day subcutaneously beginning on day +5 until neutrophil recovery.
Restaging studies were obtained within 30 days before enrollment and subsequently at 1, 3, and 6 months after SCT and every 6 months thereafter as feasible. Patients in the three cohorts had pre-HDC PET or computed tomography scans that were prospectively interpreted as positive (active tumor) or negative (no active tumor) using mediastinal blood pool activity as the reference background [12]. Posttransplantation involved field radio-therapy (IFRT) at 30.6 to 41.4 Gy was uniformly considered across the 3 cohorts for lesions greater than 5 cm at the time of HDC or persistently PET-positive at the 1-month post-HDC evaluation time point.
Statistical Analyses
Overall response rate and CR rates were calculated among patients with measurable disease at the time of HDC following the usual criteria [13]. EFS was defined as the time from HDC to either relapse, secondary tumor, or death, whichever occurred first, or to last contact. Overall survival (OS) was defined as the time from HDC to death or last contact. Kaplan-Meier survival curves were used to estimate unadjusted time-to-event distribution [14]. The log-rank test was used to compare distributions of time-to-event variables [15]. For the multivariate analyses, which included variables signifi-cant at the .05 level, hazard ratios (HRs) were estimated from Cox regression models. The Fisher exact or chi-square tests were used to assess correlations across prognostic factors and to compare the incidence of secondary leukemias across the 3 groups. The Wald test was used in testing hypothesis on covariates. Results from correlation guided the selection of variables in the multivariate stepwise regression analyses. All calculations used R v2.12.1 and OpenBUGS v3.1.2 rev 668.
RESULTS
Between January 2005 and July 2010, we treated 180 HL patients with primary refractory (n = 86) or poor risk-relapsed (n = 94) HL with HDC using Bu-Mel (n = 39, January 2005 to January 2007), Gem-Bu-Mel (n = 84, January 2007 to July 2010), or BEAM (n = 57, January 2005 to July 2010) (Table 1). Sixty-one patients additionally met criteria for refractory relapse as previously described. Median follow-up of the whole population is 36 months (range, 6 to 84). Previous front-line chemotherapy consisted of ABVD (doxorubicin-vinblastine-dacarbazine ± bleomycin) for almost all patients. Fifty-two patients also received IFRT as part of a combined modality program, 22 of whom subsequently relapsed within the RT field. First-line salvage regimens included ICE (ifosfamide-carboplatin-etoposide) (n = 101), ESHAP (etoposide–methylprednisolone–ara-C–cisplatin) (n = 54), or IGEV (ifosfamide-gemcitabinevinorelbine) (n = 25). Subsequent lines of salvage treatment included GND (gemcitabine-vinorelbine-liposomal doxorubicin) (n = 26 in second line and n = 18 in subsequent lines), IGEV (n = 24 and n = 17, respectively), or ICE (n = 20 and n = 8, respectively). PET at the time of admission for HDC, performed in 97% of the patients, was negative in 106 patients and positive in 69 patients, in 26 of whom it showed tumor that was growing.
Table 1.
Patient Characteristics (N = 180)
| Characteristic | N (%) Value |
|---|---|
| Age, median (range) | 32 (17–65) |
| Male/female | 106/74 |
| HDC regimen | |
| Gem-Bu-Mel | 84 |
| BEAM | 57 |
| Bu-Mel | 39 |
| Front-line chemotherapy | |
| ABVD | 176 (98%) |
| Stanford-V | 3 (1.5%) |
| Other | 1 (0.5%) |
| First-line salvage therapy | 180 |
| ICE | 101 (56%) |
| ESHAP | 54 (30%) |
| 1GEV | 25 (14%) |
| Second-line salvage therapy | 78 |
| GND | 26 (33%) |
| IGEV | 24 (31%) |
| ICE | 20 (26%) |
| Other | 8 (10%) |
| Third-line salvage therapy or beyond | 45 |
| GND | 18 (34%) |
| IGEV | 17 (32%) |
| ICE | 8 (14%) |
| Other | 10 (19%) |
| Radiation with initial treatment | 52 (29%) |
| First remission | |
| Primary induction failure | 86 (48%) |
| 3–6 mo | 50 (28%) |
| 6–12 mo | 17 (9%) |
| >12 mo | 27 (15%) |
| Relapse within prior radiation field | 22 (12%) |
| Number of salvage regimens, median (range) | 1 (1–5) |
| 1 | 119 (66%) |
| >1 | 61 (34%) |
| Extranodal disease at relapse/PD | 72 (40%) |
| B symptoms at relapse/PD | 21 (12%) |
| Bulky disease at relapse/PD | 55 (30%) |
| PET at HDC | 175 (97%) |
| Negative | 106 (62%) |
| After first salvage line | 72 (41%) |
| After second salvage line | 25 (15%) |
| After third salvage line or beyond | 9 (5%) |
| Positive | 69 (38%) |
| After response to first salvage | 22 (12%) |
| After response to second salvage or beyond | 22 (12%) |
| No response to prior line | 26 (14%) |
Eleven patients with low-risk features enrolled in the Bu-Mel trial were excluded from this analysis. Their outcome was significantly better than the 38 high-risk Bu-Mel patients (73% versus 33% EFS, P < .05). Likewise, 59 patients with low-risk HL, who received BEAM during this period, excluded from this analysis, had superior EFS rates compared to the 57 high-risk BEAM patients (89% versus 39%, P < .001).
There were no treatment-related deaths in any of the 3 cohorts. Five patients developed secondary acute myelogenous leukemia at a median 15 months (range, 4 to 46) after BEAM, versus none in the Gem-Bu-Mel and Bu-Mel cohorts (P = .004). These 5 patients presented the following cytogenetic abnormalities: del(7) (n = 2), inv(16) (n = 1), t(8;21) (n = 1), and t(3;21)/t(8) (n = 1). All 5 patients died: 4 with progressive HL and 1 with HL in remission. One patient in the Bu-Mel cohort developed metastatic colorectal cancer at 28 months posttransplantation and is currently receiving second-line treatment. No secondary tumors have been observed to date in the Gem-Bu-Mel cohort.
Comparison of Cohorts Receiving Different HDC Regimens
The Gem-Bu-Mel group had significantly more patients with PET-positive tumors at HDC, tumors growing at HDC, primary refractory disease, extranodal disease, or bulky tumors at prior relapse or PD (Table 2). The three cohorts received a similar number of prior salvage lines. There was bone marrow involvement in 1 Gem-Bu-Mel patient, 2 Bu-Mel patients, and no BEAM patients. Median follow-up of the Gem-Bu-Mel, Bu-Mel, and BEAM groups was 32 months (range, 12 to 68), 36 months (range, 3 to 72), and 49 months (range, 8 to 66), respectively. The response rates among patients with measurable tumors after Gem-Bu-Mel, Bu-Mel, and BEAM were 91%, 67%, and 88%, respectively (P = .2), with CR rates of 74%, 58%, and 56%, respectively (P = .16).
Table 2.
Distribution of Prognostic Features among the Cohorts of Refractory HL Patients Treated with Gem-Bu-Mel, Bu-Mel, or BEAM
| Gem-Bu-Mel (n = 84) | Bu-Mel (n = 39) | BEAM (n = 57) | P (Gem-Bu-Mel versus Bu-Mel or BEAM) | |
|---|---|---|---|---|
| PET-positive at HDC | 51% | 28% | 27% | .001 |
| Tumor growth at HDC | 26% | 6% | 3% | <.0001 |
| Extranodal disease at relapse or PD | 51% | 30% | 32% | .01 |
| Primary refractory tumor | 61% | 31% | 40% | .004 |
| Bulky (>5 cm) tumor at relapse/PD | 38% | 20% | 24% | .03 |
| Age, median (range) | 32 (19–61) | 31 (17–69) | 36 (20–63) | .7 |
| Prior CR1 | ||||
| <6 mo | 83% | 75% | 82% | .2 |
| 6–12 mo | 5% | 10% | 13% | |
| >12 mo | 12% | 15% | 5% | |
| No. prior salvage regimens, mean (range) | 1.8 (1–5) | 1.55 (1.5) | 1.5 (1–5) | .15 |
| >1 salvage regimens | 44% | 49% | 39% | .6 |
| Prior RT | 27% | 30% | 30% | .9 |
| Relapse within prior RT field | 27% | 30% | 30% | .9 |
| B symptoms at relapse/PD | 15% | 14% | 8% | .6 |
| Post-HDC IFRT | 26% | 21% | 9% | .02 |
CR1 indicates first complete remission; RT, radiation therapy.
Although criteria for consideration of posttransplantation IFRT were uniform, more Gem-Bu-Mel patients received it (n = 22, 26% of patients in this cohort) than in the Bu-Mel (21%) or BEAM groups (9%) (P = .04). Reasons for IFRT in Gem-Bu-Mel patients included bulky tumors at HDC (n = 14), persistently post-HDC PET-positive tumors (n = 2), or both (n = 6). One, 3, and 1 BEAM patients, respectively, received IFRT for those same reasons. Three, five, and one Bu-Mel patients, respectively, received IFRT for those reasons. Therefore, the higher prevalence of posttransplantation IFRT for Gem-Bu-Mel patients was explained by more bulky tumors at HDC in this cohort.
The EFS rates of the Gem-Bu-Mel, Bu-Mel, and BEAM cohorts were 57%, 33%, and 39%, respectively, with median EFS times not reached, 13 and 12 months, respectively (Figure 1A). The Bu-Mel and BEAM groups had similar EFS rates (P = .6). Therefore, we combined these two cohorts in a group we named “Bu-Mel + BEAM” in all other comparative and prognostic analyses. The Gem-Bu-Mel cohort had significantly improved EFS rates compared with the combined “Bu-Mel + BEAM” group (57% versus 35%, P = .01, Figure 1B).
Figure 1.
Outcomes of cohorts of patients with refractory HL treated with Gem-Bu-Mel, Bu-Mel, or BEAM (2005–2010). (A) EFS of the 3 groups. (B) EFS of Gem-Bu-Mel versus the combined Bu-Mel + BEAM groups (log-rank: P = .01, Cox proportional hazards regression: P = .0008). (C) OS of the 3 groups. (D) OS of Gem-Bu-Mel versus the combined Bu-Mel + BEAM groups (log-rank P = .04, Cox proportional hazards regression: P = .0005).
The OS rates of the Gem-Bu-Mel, Bu-Mel, and BEAM cohorts were 82%, 52%, and 59%, respectively, with median OS times not reached, 63 and 53 months, respectively (Figure 1C). The Bu-Mel and BEAM cohorts had similar OS rates (P = .8). The OS rate of the Gem-Bu-Mel cohort was superior to that of the combined “Bu-Mel + BEAM” group (82% versus 54%, P = .04, Figure 1D).
Prognostic Factor Analyses
In addition to an HDC regimen different from Gem-Bu-Mel, univariate analyses identified the following unfavorable factors for EFS: PET-positive tumor at HDC (P = .0001), tumor growth at HDC (P < .0005), disease status at HDC (PET negative, responsive PET positive, or unresponsive PET positive) (P < .00002), number of prior salvage regimens (P = .0006), number of previous episodes of relapse or PD (P = .002), and B symptoms at relapse (P < .05) (Table 3).
Table 3.
Univariate Analyses
| Variable | % EFS | P | % OS | P |
|---|---|---|---|---|
| HDC regimen | ||||
| Gem-Bu-Mel | 57 | 82 | ||
| Bu-Mel | 33 | 52 | ||
| BEAM | 39 | 59 | ||
| Bu-Mel versus BEAM | .6 | .7 | ||
| Gem-Bu-Mel versus other | .01 | .04 | ||
| PET at HDC | ||||
| Negative | 55 | .0001 | 78 | .00004 |
| Positive | 32 | 49 | ||
| Tumor growth at HDC | ||||
| No | 50 | <.0005 | 71 | .01 |
| Yes | 18 | 52 | ||
| Disease status at HDC | ||||
| PET negative | 55 | .00002 | 77 | .0002 |
| Responsive PET positive | 39 | 53 | ||
| Unresponsive PET positive | 18 | 48 | ||
| No. prior salvage regimens | ||||
| 1 | 59 | .0006 | 77 | .04 |
| >1 | 33 | 62 | ||
| No. prior relapses or PD | ||||
| 1 | 52 | .001 | 71 | .04 |
| >1 | 31 | 61 | ||
| B symptoms at relapse/PD | ||||
| No | 48 | 68 | ||
| Yes | 27 | <.05 | 58 | .1 |
| Prior radiotherapy | ||||
| No | 44 | .1 | 66 | .1 |
| Yes | 52 | 75 | ||
| Relapse within a prior radiation field | ||||
| No | 45 | 68 | ||
| Yes | 52 | .4 | 74 | .6 |
| Primary induction failure | ||||
| No | 47 | 69 | ||
| Yes | 45 | .8 | 67 | .6 |
| Bulky tumor at relapse/PD | ||||
| No | 40 | 65 | ||
| Yes | 49 | .3 | 76 | .2 |
| Extranodal disease at relapse/PD | ||||
| No | 48 | 67 | ||
| Yes | 43 | .4 | 71 | .9 |
| Disease-free interval | ||||
| ≤6 mo | 52 | .2 | 71 | .4 |
| >6 mo | 45 | 71 | ||
| ≤12 mo | 49 | .7 | 68 | .3 |
| >12 mo | 45 | 79 | ||
| Post-HDC radiotherapy | ||||
| No | 48 | 68 | ||
| Yes | 37 | .3 | 69 | .9 |
| ALC | ||||
| Pre-HDC | ||||
| <600/mm3 | 48 | .4 | 69 | .2 |
| ≥600/mm3 | 43 | 64 | ||
| Day 15 | ||||
| <500/mm3 | 45 | .9 | 65 | .9 |
| ≥500/mm3 | 47 | 68 | ||
| Day 30 | ||||
| <1000/mm3 | 65 | .3 | 65 | .9 |
| ≥1000/mm3 | 68 | 68 |
ALC indicates absolute lymphocyte count.
Patients with primary refractory disease had similar EFS rates to those with relapsed tumors (47% and 45% EFS, respectively, P = .8). Neither extranodal disease (P = .4) nor bulky tumors (P = .3) had a significant effect on EFS. None of these other variables correlated with EFS: duration of prior CR1, age, prior radiotherapy, or relapse within prior radiation field. Administration of post-HDC IFRT did not have an effect on EFS (P = .3). The absolute lymphocyte count, determined pre-HDC, day +15, and day +30 post-HDC did not correlate with any outcome parameter at any of those time points. Likewise, the absolute monocyte count was not associated with any outcome parameter on any of those time points (data not shown).
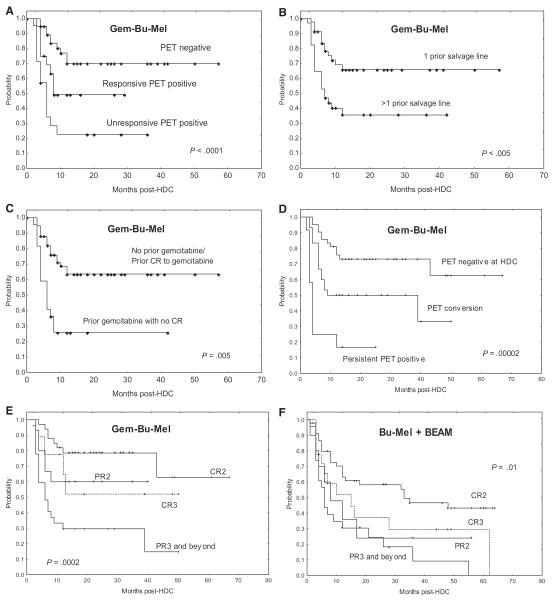
In specific analyses of the Gem-Bu-Mel cohort, patients with PET-negative tumors at HDC, responsive PET-positive tumors, and unresponsive PET-positive tumors (stable disease or PD to latest salvage therapy) had EFS rates of 55%, 39%, and 18%, respectively (P < .00005, Figure 2A). Patients with 1 and >1 prior salvage lines had EFS rates of 71% and 33%, respectively (P < .005, Figure 2B). Prior treatment with gemcitabine without achieving a CR (n = 22) was associated with worse EFS than a prior CR (n = 13) or no exposure to this drug (n = 49) (P = .0003 and P = .0002, respectively, Figure 2C). The 3 subsets of patients with negative PET at HDC, PET conversion from positive to negative after HDC, and a persistently positive PET scan after HDC had significantly different EFS rates (71% versus 47% versus 17%, P = .00002, Figure 2D), with significant differences between the PET-negative and PET-conversion subsets (P = .01). Disease status at HDC (CR2, PR2, CR3, and PR3 or beyond) showed a major impact on EFS (76% versus 60% versus 50% versus 23%, P = .0002, Figure 2E), with the difference between the CR2 and CR3 subsets approaching significance (P = .06), but not between those in PR2 and CR3 (P = .9). The effect of disease status on EFS was also noticed in analysis of the “Bu-Mel + BEAM” group (51% in patients in CR2, 30% in PR2, 33% in CR3, and 13% in PR3 or beyond, P = .01, Figure 2F). In univariate OS analyses of the entire population, the following variables were shown to have a significant effect: PET at HDC (P = .00004), tumor growth at HDC (P = .01), disease status at HDC (P = .0002), number of prior salvage therapy lines (P = .04), and number of previous episodes of relapse or tumor growth (P = .02) (Table 3).
Figure 2.
Prognostic analysis of EFS. (A) PET and disease status at the time of HDC (Gem-Bu-Mel cohort). (B) Number of prior lines of salvage therapy (Gem-Bu-Mel). (C) Prior exposure/response to gemcitabine (Gem-Bu-Mel). (D) Pre- and post-HDC PET (Gem-Bu-Mel). (E) Disease status at HDC (Gem-Bu-Mel). (F) Disease status at HDC (combined Bu-Mel + BEAM group).
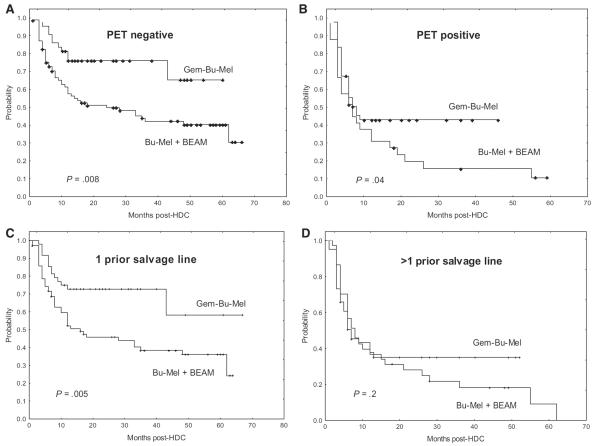
Multivariate Cox models for EFS showed that a treatment different from Gem-Bu-Mel was independently associated with worse EFS (HR = 2.3, P = .0008), along with PET-positive tumors at HDC (HR = 2.2, P = .004) and number of prior salvage lines (HR = 1.9, P = .008) (Table 4). A different treatment from Gem-Bu-Mel (HR = 2.7, P = .0005) and a PET-positive status (HR = 3.1, P = .0001) were independent adverse factors for OS (Table 4). The EFS benefit from Gem-Bu-Mel over “Bu-Mel + BEAM” was observed within the subgroups with PET-negative tumors (74% versus 46%, P = .008, Figure 3A), PET-positive tumors at HDC (42% versus 18%, P = .04, Figure 3B), and 1 prior relapse or PD (76% versus 44%, P = .005, Figure 3C), whereas the differences within the subgroups with >1 prior relapse did not reach significance (33% versus 28%, P = .2, Figure 3D).
Table 4.
Cox Proportional Hazard Regression Analyses of the Groups of Refractory HL Patients Treated with Gem-Bu-Mel, Bu-Mel, or BEAM
| EFS |
OS |
||||
|---|---|---|---|---|---|
| Hazard ratio (95% CI | P | Hazard ratio (95% CI) | P | ||
| High-dose regimen | Gem-Bu-Mel | 1 | .0008 | 1 | .0005 |
| Other | 2.3 (1.4–3.7) | 2.7 (1.3–5.4) | |||
| PET | Negative | 1 | .004 | 1 | .0001 |
| Positive | 2.2 (1.2–3.1) | 3.1 (1.7–5.5) | |||
| No. prior lines of salvage therapy | 1 | 1 | .008 | 1 | .07 |
| >1 | 1.1 (.6–2) | 1.8 (.9–3.2) | |||
| B symptoms | No | 1 | .7 | 1 | .6 |
| Yes | 1.1 (.6–2) | 1.5 (.5–2.5) | |||
CI indicates confidence interval.
Figure 3.
EFS comparisons across main patient subgroups. (A) Patients with PET-negative tumors at HDC. (B) Patients with PET-positive tumors. (C) Patients with 1 prior salvage treatment line. (D) Patients with >1 prior salvage treatment line.
DISCUSSION
Our cohort analysis showed that patients with primary-refractory or poor-prognosis relapsed HL receiving high-dose Gem-Bu-Mel showed improved outcome compared with the cohorts treated with Bu-Mel or BEAM. Despite its higher prevalence of poor prognosis features, the Gem-Bu-Mel cohort showed superior EFS and OS.
Gem-Bu-Mel differs from Bu-Mel in the addition of infusional gemcitabine. At standard doses, this agent has clinical antitumor activity against HL [16,17]. Gem-Bu-Mel exploits the antitumor profile of gemcitabine, which is highly dependent on both dose and duration of exposure [18–20], by administering high doses of this drug over prolonged periods of time at its optimal infusion rate. The marked myelotoxicity of this administration schedule is circumvented by hematopoietic cell support. Gem-Bu-Mel was designed based on the overriding principles of using a pharmacologically guided prolonged infusion schedule of gemcitabine in a therapeutic sequence that would facilitate a synergistic interaction with busulfan and melphalan based on DNA damage repair inhibition [21]. In addition to this well-established mechanism of synergy, we recently reported that gemcitabine induces chromatin relaxation, increasing access of busulfan to DNA and its cytotoxicity in lymphoma cell lines [22].
We observed a major and independent prognostic effect of PET status at HDC determined prospectively in our patients. Our study constitutes the largest analysis to date of pretransplantation PET scans and confirms in a prospective fashion prior reports. Jabbour et al. reviewed retrospectively 211 patients treated at our institution from 1993 to 2004, 68 of whom had a PET performed before transplantation [6]. This previous study showed that a negative PET and the use of BEAM, compared with cyclophosphamide-carmustine-etoposide (CBV), were associated with favorable post-transplantation outcomes. Moskowitz and colleagues reviewed retrospectively 153 patients treated with CBV or cyclophosphamide-carmustine and total lymphoid irradiation between 1994 and 2003 and observed a prognostic effect of pretransplantation functional imaging, which consisted of PET in 42 patients [7]. Smeltzer et al. described similar results in their retrospective analysis of 106 patients treated with BEAM, 46 of whom had pretransplantation PET scans [8]. Similar observations were made by Arai et al. in their study of 96 patients treated with gemcitabine-vinorelbine combined with high-dose CBV, 77 of whom had pretransplantation PET scans [9]. All these prior studies and our present report indicate the prognostic significance of pretransplantation PET for patients with HL receiving HDC.
A major challenge remains the approach to patients with PET-positive tumors after their salvage standard-dose chemotherapy. Moskowitz et al. evaluated prospectively this question and observed similar outcomes between a group of 59 patients in CR2 after salvage ICE and a second group of 17 patients with persistently positive PET tumors after second-line therapy who subsequently achieved a CR3 after third-line GND [23]. These patients received CBV ± IFRT or cyclophosphamide-carmustine + total lymphoid irradiation as transplant-conditioning regimens. In contrast, we observed a trend for better outcomes in our Gem-Bu-Mel patients in CR2 than in those in CR3 (P = .06), with no significant differences between the Gem-Bu-Mel PR2 and CR3 subsets (P = .9). Similar observations were made in the combined “Bu-Mel + BEAM” group. Different patient populations or transplant regimens may account for the differing results of that study and ours. Although the sequence of salvage regimens in most of our patients (ESHAP or ICE in second line and IGEV or GND in third line) appears to be similar to those used by Moskowitz and colleagues, our transplant trials were designed to test new HDC combinations and not to address the relative values of different standard salvage regimens. No patients in either report received brentuximab vedotin, which is non–cross-resistant with chemotherapy [24], and may induce metabolic CRs before HDC in patients with a persistently positive PET scans after salvage therapy, thus potentially serving as a bridge to more successful HDC. In addition, brentuximab vedotin may show to be an effective posttransplantation maintenance therapy, as it is currently being evaluated.
This analysis was based on intent to treat. The clinical data of all patients, both those enrolled in the 2 trials and those treated off protocol, were collected prospectively. Because the 3 cohorts included in this report were treated at our institution over a relatively short time period, we consider the supportive care to be the same for all patients. In fact, there were no treatment-related deaths in any of the 3 cohorts. However, given the nonrandomized nature of this comparison, our observations require confirmation in a randomized trial.
In conclusion, Gem-Bu-Mel improved outcomes compared with Bu-Mel and BEAM in this analysis of contemporary cohorts of patients with refractory or poor-risk relapsed HL. These observations warrant a randomized phase III trial of Gem-Bu-Mel in this poor-prognosis population.
ACKNOWLEDGMENTS
We are grateful to our colleague, Anas Younes, MD, for his comments on the manuscript.
Financial disclosure: Y.N. received research funding from Otsuka Pharmaceuticals. B.A. reports a consultancy with Otsuka Pharmaceuticals and research funding from Otsuka Pharmaceuticals. M.Q. received research funding from Otsuka Pharmaceuticals.
Authorship Statement: Y.N. designed and performed research, participated in patient management, analyzed data, and wrote the article. B.V. designed the research. B.A. designed and performed research and participated in patient management. P.L. analyzed data. C.H., E.J.S., A.A., P.K., U.P., P.A., M.Q., S.P., Q.B., N.S., and I.K. participated in patient management. G.R. performed data collection. R.C. performed research and participated in patient management. R.B.J. designed and performed research and participated in patient management.
Footnotes
Presented in part at the 2010 annual meeting of the American Society of Hematology and the 2012 annual meeting of the American Society for Blood and Marrow Transplantation, February 2012, San Diego, CA.
REFERENCES
- 1.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemo-sensitive Hodgkin's disease: a randomised trial. Lancet. 2002;15:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus HM, Loberiza FR, Jr, Zhang MJ, et al. Autotransplants for Hodgkin's disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR) Bone Marrow Transplant. 2001;27:387–396. doi: 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]
- 4.Popat U, Hosing C, Saliba RM, et al. Prognostic factors for disease progression after high-dose chemotherapy and autologous hematopoietic stem cell transplantation for recurrent or refractory Hodgkin's lymphoma. Bone Marrow Transplant. 2004;33:1015–1023. doi: 10.1038/sj.bmt.1704483. [DOI] [PubMed] [Google Scholar]
- 5.Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16:625–633. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 6.Jabbour E, Hosing C, Ayers G, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109:2481–2489. doi: 10.1002/cncr.22714. [DOI] [PubMed] [Google Scholar]
- 7.Moskowitz A, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116:4934–4937. doi: 10.1182/blood-2010-05-282756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smeltzer JP, Cashen AF, Zhang Q, et al. Prognostic significance of FDG-PET in relapsed or refractory classical Hodgkin lymphoma treated with standard salvage chemotherapy and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1646–1652. doi: 10.1016/j.bbmt.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai S, Letsinger R, Wong RM, et al. Phase I/II trial of GN-BVC, a gemcitabine and vinorelbine-containing regimen for autologous hematopoietic cell transplantation in recurrent and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2010;16:1145–1154. doi: 10.1016/j.bbmt.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kebriaei P, Madden T, Kazerooni R, et al. Intravenous busulfan plus melphalan is a highly effective, well-tolerated preparative regimen for autologous stem cell transplantation in patients with advanced lymphoid malignancies. Biol Blood Marrow Transplant. 2011;17:412–420. doi: 10.1016/j.bbmt.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieto Y, Thall P, Valdez B, et al. High-dose infusional gemcitabine combined with busulfan and melphalan with autologous stem-cell transplant in patients with refractory lymphoid malignancies. Biol Blood Marrow Transplant. 2012;18:1677–1686. doi: 10.1016/j.bbmt.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 16.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 17.Santoro A, Bredenfeld H, Devizzi L, et al. Gemcitabine in the treatment of refractory Hodgkin's disease: results of a multicenter phase II study. J Clin Oncol. 2000;18:2615–2619. doi: 10.1200/JCO.2000.18.13.2615. [DOI] [PubMed] [Google Scholar]
- 18.Hanauske AR, Degen D, Marshall MH, et al. Activity of 2',2'-difluorodeoxycytidine (gemcitabine) against human tumor colony forming units. Anticancer Drugs. 1992;3:143–146. doi: 10.1097/00001813-199204000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Csoka K, Liliemark J, Larsson R, et al. Evaluation of the cytotoxic activity of gemcitabine in primary cultures of tumor cells from patients with hematologic or solid tumors. Semin Oncol. 1995;22(4 Suppl 11):47–53. [PubMed] [Google Scholar]
- 20.Braakhuis BJ, van Haperen VW, Boven E, et al. Schedule-dependent antitumor effect of gemcitabine in in vivo model system. Semin Oncol. 1995;22(4 Suppl 11):42–46. [PubMed] [Google Scholar]
- 21.Plunkett W, Huang P, Searcy CE, et al. Gemcitabine: preclinical pharmacology and mechanisms of action. Semin Oncol. 1996;23(5 Suppl 10):3–15. [PubMed] [Google Scholar]
- 22.Valdez BC, Nieto Y, Murray D, et al. Epigenetic modifiers enhance the synergistic cytotoxicity of combined nucleoside analog-DNA alkylating agents in lymphoma cell lines. Exp Hematol. 2012;40:800–810. doi: 10.1016/j.exphem.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119:1665–1670. doi: 10.1182/blood-2011-10-388058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]