Abstract
Aims
Alterations in organic acid biomarkers from fatty acid and carbohydrate metabolism have been documented in type 2 diabetes patients. However, their association with gestational diabetes mellitus (GDM) is largely unknown.
Methods
Participants were 25 GDM cases and 25 non-GDM controls. Biomarkers of fatty acid (adipate, suberate and ethylmalonate) and carbohydrate (pyruvate, l-lactate and β-hydroxybutyrate) metabolism were measured in maternal urine samples collected in early pregnancy (17 weeks) using liquid chromatography–mass spectrometry methods. Logistic regression were used to calculate odds ratios (OR) and 95% confidence intervals (CI).
Results
GDM cases and controls differed in median urinary concentrations of ethylmalonate (3.0 vs. 2.3 µg/mg creatinine), pyruvate (7.4 vs. 2.1 µg/mg creatinine), and adipate (4.6 vs. 7.3 µg/mg creatinine) (all p-values <0.05). Women in the highest tertile for ethylmalonate or pyruvate concentrations had 11.4-fold (95%CI 1.10–117.48) and 3.27-fold (95%CI 0.72–14.79) increased risk of GDM compared with women in the lowest tertile for ethylmalonate and pyruvate concentrations, respectively. Women in the highest tertile for adipate concentrations, compared with women in the lowest tertile, had an 86% reduction in GDM risk (95%CI 0.02–0.97).
Conclusions
These preliminary findings underscore the importance of altered fatty acid and carbohydrate metabolism in the pathogenesis of GDM.
Keywords: Organic acid profile, Adipate, Ethylmalonate, Fatty acid metabolism, Carbohydrate metabolism, Gestational diabetes mellitus
1. Introduction
Gestational diabetes (GDM), a common complication of pregnancy occurring in 5–10% of all pregnancies [1,2], is characterized by glucose intolerance that first appears during pregnancy. GDM is associated with adverse maternal (e.g., C-sections and increased risk of type 2 diabetes [T2DM]) and offspring (e.g., fetal hyperinsulinism, macrosomia, and birth injuries) outcomes [3–5]. Underlying pathophysiological disturbances commonly identified in GDM include reduced insulin secretion and abnormal insulin resistance [1] which have been linked to abnormal fatty acid and carbohydrate metabolism.
Higher maternal dietary intake of saturated fat, cholesterol, red and processed meats prior to pregnancy or during early pregnancy were significantly associated with increased GDM risk [6–8]. During pregnancy, women with GDM do have higher serum triglyceride concentrations as compared with women with pregnancies not complicated by GDM [9,10]. Endogenous hepatic glucose production has been shown to be less sensitive to increased insulin concentration among women with GDM affected pregnancies [11], accompanied by an increased contribution of carbohydrates to oxidative metabolism [11]. Although limited, available evidence also suggests that diets with low-dietary glycemic index are associated with reduced risk of GDM [12].
Organic acids, organic compounds with acidic properties measured in human blood and urine, are degradation products of amino acids, neurotransmitters, and intestinal bacterial action on food components that provide information on energy production, neurotransmitter metabolism, intestinal dysbiosis, dietary fat, carbohydrate and protein metabolism [13]. Measurement of organic acids such as ethylmalonate, suberate, and adipate reflect metabolic processes involved in long-chain fatty acid metabolism (such as carnitine-dependent pathways) and related mitochondrial function [13]. Additionally, altered concentrations of organic acids such as pyruvate, l-lactate and β-hydroxybutyrate are biological markers of disturbed carbohydrate metabolism [13].
Alterations in organic acid biomarkers, including those that are intermediate products of fatty acid or carbohydrate metabolism have been documented in experimental models of insulin resistance [14–17]. Furthermore, some [18–23], but not all, studies [14] of organic acid profiles among humans have documented alterations of circulating or urinary organic profiles associated with insulin resistance, hyperglycemia or type 2 diabetes (T2DM). Some investigators have suggested that quantitative profiling of organic acids in urine may be a simple, sensitive test that can reveal evidence of functional inadequacy of specific nutrients for laboratory evaluations in insulin resistance and T2DM [21].
In addition to inconsistencies in previous reports, most prior investigations were case-control studies that did not help to clarify the temporal relationship between altered organic acids concentrations and risk of T2DM. Importantly, we are aware of no prior published studies that have evaluated urinary organic acid profiles in GDM, which is biochemically and epidemiologically similar to T2DM. We therefore, used urine samples (which were collected 16 weeks gestation, on average) from a prospective cohort study and examined the extent to which selected organic acids known to be biomarkers of fatty acid (adipate, suberate and ethylmalonate) and carbohydrate (pyruvate, l-lactate and β-hydroxybutyrate) metabolism are associated with incident GDM risk.
2. Materials and methods
Study subjects were selected from participants of the Omega study, a prospective cohort study designed to investigate risk factors of pregnancy complications such as preeclampsia and GDM. Study population and data collection procedures, described before [6], are briefly summarized here. Participants were women who attended prenatal care clinics affiliated with the Swedish Medical Center, Seattle, WA, USA. Eligible women were those who initiated prenatal care before 20 weeks gestation, spoke and read English, were ≥18 years of age, and planned to carry the pregnancy to term and deliver at the study hospital. Participants completed a questionnaire administered in English by a trained interviewer at or near enrollment. These questionnaires were used to gather information on socio-demographic, anthropometric, and behavioral characteristics and reproductive and medical histories. After delivery, maternal and infant medical records were abstracted for information on the course and outcomes of pregnancy. The Institutional Review Board of the Swedish Medical Center approved study protocols. All participants provided written informed consent. Between 1996 and 2006, 5063 eligible women were approached and 4000 (approximately 79%) agreed to participate. A total of 3886 pregnant women provided biological samples (i.e., blood and urine samples) and completed interviews.
Participants provided a clean-catch spot urine sample around 16–17 weeks of gestation. Immediately after collection, samples were separated into 2 ml aliquots and stored at −80 8C until analysis. Pregnancy outcome information was abstracted from hospital and clinic medical records. We used the food frequency questionnaire (FFQ) from the Women’s Health Initiative Clinical Trial [24] to assess maternal dietary intake during the three-month period (before conception and during the first trimester). Participants completed FFQs at an average of 15.9 weeks gestation (standard deviation: 4.6 weeks). Dietary intake values of nutrients were estimated using food composition tables from the University of Minnesota Nutrition Coding Center nutrient database (Nutrition Coordinating Center, Minneapolis, MN).
In our study settings, according to the recommendations from the American Diabetes Association (ADA) [1], pregnant women were screened at 24–28 weeks gestation using a 50 g 1-h oral glucose challenge test. Those patients who failed this screening test (glucose ≥7.8 mmol/l or 140 mg/dl) were then followed-up within 1–2 weeks with a 100 g 3-h oral glucose tolerance test (OGTT). We also abstracted laboratory results from participants’ 50 g 1-h glucose challenge test and from the diagnostic 100 g 3-h OGTT. Women were diagnosed with GDM if two or more of the 100 g OGTT glucose concentrations exceeded the ADA criteria: fasting ≥5.3 mmol/l (≥95 mg/dl); 1-h ≥10.0 mmol/l (≥180 mg/dl); 2-h ≥8.6 mmol/l (≥155 mg/dl); 3-h ≥7.8 mmol/l (≥140 mg/dl) [1]. From the sample of women who developed GDM and delivered a live born infant, we sampled 25 women to serve as cases. For controls, we sampled 25 women who did not develop GDM and who delivered a singleton live born infant. The 25 controls were frequency-matched to GDM cases for gestational age a urine collection.
Urinary biomarkers were measured using liquid chromatography with tandem mass spectrometric detection (LC/MS–MS) method in Metametrix Clinical Laboratory (now named Genova Diagnostics) (Duluth, Georgia). Intra-assay coefficients of variation for this method are 11.8%. Urinary biomarkers were normalized using participants’ urine creatinine concentrations, and were expressed as µg/mg creatinine. Urinary creatinine concentration was assessed using a commercially available kit (Genzyme Diagnostics, Catalogues 221-30/221-50) with improved Jaffe Reaction.
We examined differences in median concentrations between cases and controls using the Mann–Whitney test. Spearman correlation coefficients (CC) were also examined between the urine biomarkers and selected maternal, newborn characteristics as well as maternal dietary macronutrients intake. We categorized GDM cases and controls according to the tertiles of each urine organic acid biomarker concentrations, determined by the distribution among controls. We used logistic regression models to estimate odds ratios (OR) and 95% confidence interval (95% CI). Adjusted models included a variable for pre-pregnancy body mass index. In multivariate analyses, we evaluated linear trends in risk by treating the three tertiles as a continuous variable after assigning a score (1, 2, and 3) as its value [25]. All analyses were performed using Stata 9.0 (Stata, College Station, TX). All reported confidence intervals were calculated at the 95% level and all reported p-values are two-tailed.
3. Results
Characteristics of GDM cases (16 diet-controlled and 9 insulin-dependent) and controls are presented in Table 1. GDM cases and controls were largelysimilar with regards to maternal age, parity, gestational age at delivery, and total energy intake. As expected, GDM cases had higher pre-pregnancy BMI (27.0 kg/m2) than controls (25.3 kg/m2). GDM cases were less likely than controls to be non-Hispanic White and more likely to have a family history of diabetes. In addition, GDM cases consumed higher percentage of total calories from protein as compared with controls (18.8% vs. 16.8%, p = 0.03). There were no case-control differences observed for maternal first or second blood pressure values. Further, maternal blood pressure values were associated with post-50-g glucose load the plasma glucose concentrations.
Table 1.
Characteristics of study participants.
| Characteristics | GDM (N = 25) | Controls (N = 25) | p-Value |
|---|---|---|---|
| Maternal age (years) | 34.2 ± 4.2 | 34.2 ± 3.4 | 0.97 |
| Maternal age (years) | |||
| <35 | 10 (40.0) | 14 (56.0) | 0.26 |
| ≥35 | 15 (60.0) | 11 (44.0) | |
| Non-Hispanic White | 18 (72.0) | 24 (96.0) | 0.05 |
| Single marital status | 3 (12.0) | 0 (0.0) | – |
| Nulliparous | 12 (48.0) | 12 (48.0) | 1.00 |
| Smoked during pregnancy | 1 (4.0) | 0 (0.0) | – |
| Family history of hypertension | 19 (76.0) | 13 (52.0) | 0.14 |
| Family history of diabetes | 9 (36.0) | 2 (8.0) | 0.04 |
| Maternal pre-pregnancy BMI (kg/m2) | 27.0 ± 5.7 | 22.9 ± 3.8 | 0.005 |
| Maternal pre-pregnancy BMI (kg/m2) | |||
| <18.5 | 0 (0.0) | 1 (4.0) | 0.002 |
| 18.5–24.9 | 9 (36.0) | 20 (80.0) | |
| 25.0–29.9 | 13 (52.0) | 3 (12.0) | |
| ≥30.0 | 3 (12.0) | 1 (4.0) | |
| Gestational age at delivery (weeks) | 38.8 ± 1.2 | 38.9 ± 1.1 | 0.63 |
| Infant birthweight (grams) | 4191 ± 217 | 3321 ± 277 | <0.001 |
| Gestational age @ urine collection (weeks) | 16.6 ± 1.2 | 17.2 ± 2.0 | 0.17 |
| Maternal mean plasma glucose concentrations after a 50-g glucose challenge (mg/dl) | 158.9 ± 24.2 | 95.3 ± 12.7 | <0.001 |
| 1st Trimester systolic BP (mm Hg) | 113.4 ± 8.5 | 112.4 ± 11.4 | 0.73 |
| 1st Trimester diastolic BP (mm Hg) | 69.9 ± 6.4 | 68.0 ± 6.3 | 0.28 |
| 1st Trimester mean arterial BP (mm Hg) | 84.4 ± 5.8 | 82.8 ± 7.6 | 0.40 |
| 2nd Trimester systolic BP (mm Hg) | 114.9 ± 7.2 | 112.8 ± 8.1 | 0.34 |
| 2nd Trimester diastolic BP (mm Hg) | 68.4 ± 4.8 | 67.2 ± 4.4 | 0.35 |
| 2nd Trimester mean arterial BP (mm Hg) | 83.9 ± 4.8 | 82.3 ± 5.1 | 0.25 |
| Total energy intake (kcal) | 1934 ± 686 | 1960 ± 638 | 0.89 |
| Calories from carbohydrate (%) | 49.5 ± 9.2 | 53.5 ± 7.8 | 0.12 |
| Calories from protein (%) | 18.8 ± 3.7 | 16.8 ± 2.2 | 0.03 |
| Calories from total fat (%) | 33.8 ± 6.6 | 32.1 ± 6.8 | 0.37 |
Data in mean ± standard deviation (SD) or number (%).
p-Value from Student t test for continuous variable and Chi-square test or Fisher’s exact test for categorical variables.
24 GDM cases and 23 controls had dietary macronutrients intake information in early pregnancy.
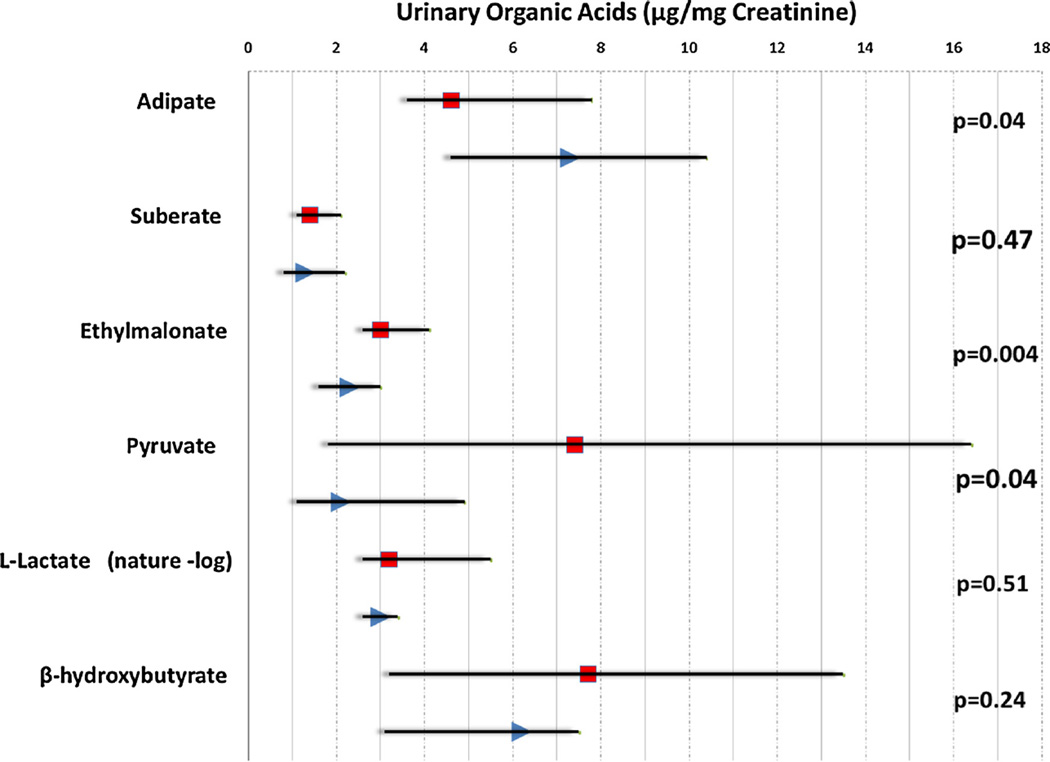
GDM cases and controls differed in median urinary concentrations of ethylmalonate (3.0 vs. 2.3 µg/mg creatinine), pyruvate (7.4 vs. 2.1 µg/mg creatinine), and adipate (4.6 vs. 7.3 µg/mg creatinine) (all p-values <0.05) (Fig. 1). Differences in urinary median concentrations of suberate, l-lactate and β-hydroxybutyrate were not different between GDM cases and controls.
Fig. 1.
Maternal early pregnancy urinary organic acid biomarkers of fatty acid or carbohydrate metabolism for GDM and control subjects (the scatterplot and dropline show the median and interquartile ranges in GDM (square) and control (triangle)).
Early pregnancy urinary organic acids from carbohydrate metabolism were positively correlated among themselves and with maternal 1 h plasma glucose concentrations after a 50-g oral glucose challenge test in GDM cases (Spearman correlation coefficients = 0.32 to 0.80) (Supplemental Fig. 1), but not in controls. Similar correlations were not observed for urinary organic acids from fatty acid metabolism.
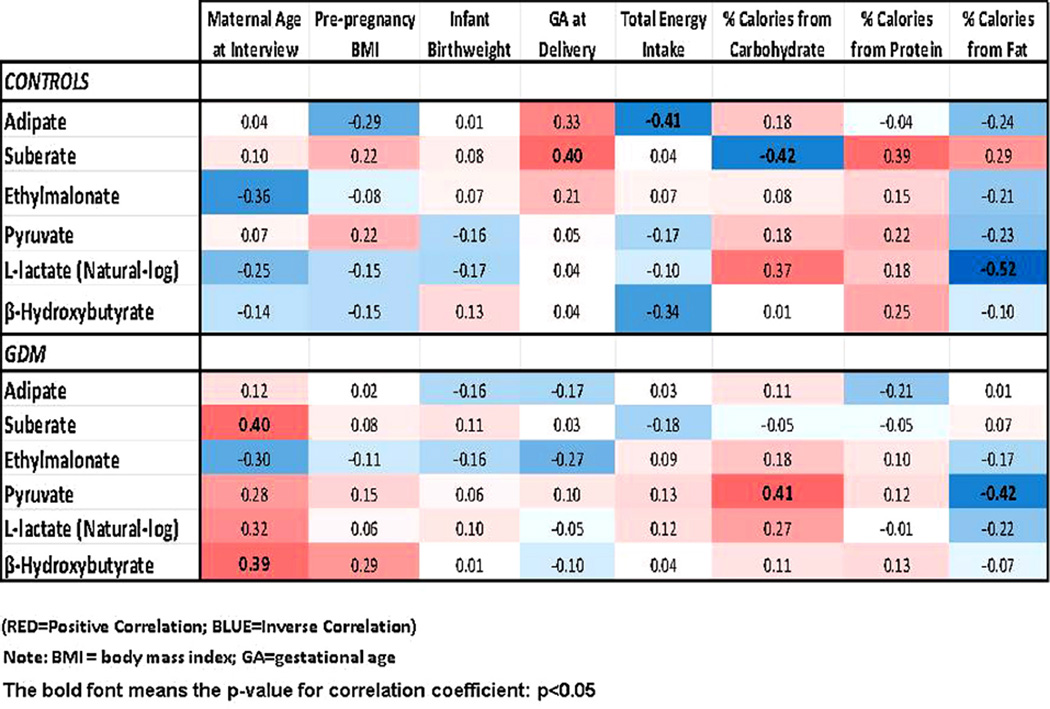
Results of analyses evaluating correlation of urinary biomarkers with selected maternal characteristics are shown in Fig. 2. Overall, correlations between maternal age with urine organic acid biomarkers of fatty acid and carbohydrate metabolism were stronger among GDM cases than controls. Results of analyses evaluating correlation of urinary biomarkers with total energy and macronutrients intake are also shown in Fig. 2. Among GDM cases, urinary pyruvate concentrations was positively related to the percentage of total calories from carbohydrate and inversely related to the percentage of calories from fat (ρ = 0.41, p-value = 0.04; and ρ = −0.42, p-value = 0.04, respectively). Similarly, among controls, l-lactate was also positively correlated with percent calories from carbohydrate (ρ = 0.37, p-value = 0.08) and inversely correlated with percentage of dietary calories from fat (ρ = −0.52, p-value = 0.01).
Fig. 2.
Spearman correlation coefficients (CC) matrix between urine biomarkers and selected maternal, newborn characteristics as well as selected maternal dietary macronutrients intake. (bold indicating the p-value < 0.05).
After adjusting for maternal pre-pregnancy BMI, increased concentrations of early pregnancy urinary adipate were associated with reduced GDM risk (trend test p-values < 0.05). 0.05). Women who had adipate concentrations in the highest tertile (≥10.1 µg/mg creatinine) had a 86% reduced risk of GDM as compared with those women whose adipate concentration fell in the lowest tertile (<5.2 µg/mg creatinine) (OR = 0.14; 95% CI 0.02–0.97). The corresponding adjusted ORs for women with the successive tertiles of suberate was 1.00 (referent), 1.37 (95% CI 0.28–6.59) and 1.27 (95% CI 0.24–6.66). GDM risk was 11.36-fold higher among women with ethylmalonate concentrations in highest tertile (≥2.90 µg/mg creatinine), as compared with those women whose concentrations were in lowest tertile (<1.90 µg/mg creatinine) (OR = 11.36, 95% CI 1.10–117.48) (Table 2).
Table 2.
OR and 95% CI of GDM according to categories of early pregnancy urinary organic acid biomarkers of fatty acid or carbohydrate metabolism.
| Urine organic acids (µg/mg creatinine) | GDM (N = 25) N (%) |
Controls (N = 25) N (%) |
Unadjusted OR (95% CI) | * Adjusted OR (95% CI) |
|---|---|---|---|---|
| Fatty acid metabolism | ||||
| Adipate | ||||
| Tertile 1 (<5.2) | 14 (56.0) | 8 (32.0) | 1.00 (referent) | 1.00 (referent) |
| Tertile 2 (5.2–10.0) | 8 (32.0) | 8 (32.0) | 0.57 (0.15–2.12) | 0.69 (0.17–2.81) |
| Tertile 3 (≥10.1) | 3 (12.0) | 9 (36.0) | 0.19 (0.04–0.91) | 0.14 (0.02–0.97) |
| p-Value for trend | 0.04 | 0.05 | ||
| Suberate | ||||
| Tertile 1 (<1.00) | 5 (20.0) | 7 (28.0) | 1.00 (referent) | 1.00 (referent) |
| Tertile 2 (1.00–1.59) | 10 (40.0) | 10 (40.0) | 1.40 (0.33–5.93) | 1.37 (0.28–6.59) |
| Tertile 3 (≥1.60) | 10 (40.0) | 8 (32.0) | 1.75 (0.40–7.66) | 1.27 (0.24–6.66) |
| p-Value for trend | 0.46 | 0.80 | ||
| Ethylmalonate | ||||
| Tertile 1 (<1.90) | 1 (4.0) | 7 (28.0) | 1.00 (referent) | 1.00 (referent) |
| Tertile 2 (1.90–2.89) | 9 (36.0) | 10 (40.0) | 6.30 (0.64–61.6) | 4.20 (0.39–44.97) |
| Tertile 3 (≥2.90) | 15 (60.0) | 8 (32.0) | 13.13 (1.36–126.31) | 11.36 (1.10–117.48) |
| p-Value for trend | 0.02 | 0.02 | ||
| Carbohydrate metabolism | ||||
| Pyruvate | ||||
| Tertile 1 (<1.2) | 4 (16.0) | 12 (48.0) | 1.00 (referent) | 1.00 (referent) |
| Tertile 2 (1.2–4.7) | 7 (28.0) | 5 (20.0) | 4.20 (0.84–21.05) | 2.87 (0.53–15.69) |
| Tertile 3 (≥4.8) | 14 (56.0) | 8 (32.0) | 5.25 (1.26–21.86) | 3.27 (0.72–14.79) |
| p-Value for trend | 0.03 | 0.14 | ||
| l-lactate | ||||
| Tertile 1 (<15.0) | 8 (32.0) | 8 (32.0) | 1.00 (referent) | 1.00 (referent) |
| Tertile 2 (15.0–27.9) | 8 (32.0) | 9 (36.0) | 0.89 (0.23–3.49) | 1.18 (0.27–5.11) |
| Tertile 3 (≥28.0) | 9 (36.0) | 8 (32.0) | 1.13 (0.29–4.41) | 1.05 (0.23–4.83) |
| p-Value for trend | 0.86 | 0.95 | ||
| β-Hydroxybutyrate | ||||
| Tertile 1 (<4.0) | 7 (28.0) | 8 (32.0) | 1.00 (referent) | 1.00 (referent) |
| Tertile 2 (4.0–7.0) | 5 (20.0) | 8 (32.0) | 0.71 (0.16–3.23) | 1.07 (0.21–5.34) |
| Tertile 3 (≥7.1) | 13 (52.0) | 9 (36.0) | 1.65 (0.44–6.20) | 1.50 (0.35–6.37) |
| p-Value for trend | 0.41 | 0.57 |
Adjusted for maternal pre-pregnancy body mass index.
As can be seen from the bottom panel of Table 2, unadjusted risk of GDM increased across successively higher tertile of pyruvate concentrations (p-value for trend = 0.03). However, after adjustment for pre-pregnancy BMI, the statistical significance for trend disappeared (p-value for trend = 0.14). Relative to women in the lowest tertile of maternal urinary pyruvate concentrations (<1.2 µg/mg creatinine), women with concentrations in the highest tertile (≥4.8 µg/mg creatinine) experienced a 3.27-fold increased risk of GDM (95% CI 0.72–14.79). There is no apparent association between maternal urinary l-lactate or β-hydroxybutyrate concentrations and subsequent GDM risk.
4. Discussion
In this pilot study, we observed that maternal early pregnancy urinary ethylmalonate concentrations were positively associated with subsequent GDM risk while adipate concentrations were inversely associated with GDM risk (both p value for trend < 0.05). Unadjusted risk of GDM increased across successively higher tertiles of pyruvate concentrations (p-value for trend = 0.03). However, after adjustment for pre-pregnancy BMI, the statistical significance for trend disappeared (p-value for trend = 0.14). Our findings suggest that alterations in maternal fatty acid and carbohydrate metabolism might be detectable at 16 weeks. Our findings are consistent with results from other studies conducted in men and non-pregnant women with hyperglycemia or T2DM [21,22]. We are aware of very limited publication that has investigated circulating organic acids among pregnant women with GDM [18,19] and to our best knowledge; there is no publication that examined pre-diagnostic urinary samples.
In the dominant fatty acid metabolism pathway, carnitine is required as a carrier for the transport of fatty acids from the cytosol into the mitochondria for β-oxidation [13]. When carnitine is inadequate, degradation of fatty acids takes place through an alternate, less efficient pathway known as “ω-oxidation” [26]. Adipate and suberate are products of this incomplete oxidation in the ω-oxidation pathway. Notably, urinary excretion of these dicarboxylic acids have been shown to be elevated in malnourished and diabetic rodent models [27] and patients with congenital defects in fatty acid metabolism [28]. Limited previous literature suggests that urinary organic acids are altered in diabetic animal models [14] and in insulin resistant men [21]. Yoshioka [14] reported elevated urinary adipate concentrations in streptozotocin (STZ)-induced diabetic rats as compared to control animals. In a human clinical study of 18 T2DM patients and 15 non-diabetic controls, the same investigative team reported that urinary adipate (6.6 ± 7.9 vs. 2.8 ± 1.6 µg/mg creatinine) and suberate (4.2 ± 4.8 vs. 1.8 ± 1.4 µg/mg creatinine) concentrations were higher among cases than controls. However, these alterations did not reach statistical significance [14], possibly due to the relatively small sample size and low statistical power of the study. In a recent study, Tai reported that urinary adipate concentrations were increased in urine in individuals with insulin resistance compared to controls (1.09 vs. 0.82 mmol/mol creatine, p-value = 0.03) [21].
Obesity and diabetes are tightly related [29]. Before becoming grossly obese, genetically obese (ob/ob) mice develop hyperinsulinemia and abnormalities of glucose metabolism [30] as well as hyperplasia of the pancreatic islets [31]. McDevitt et al. [16] compared the 45 urinary organic acids in fatty (fa/fa) versus lean Zucker rats. The authors observed that obese Zucker rats excreted more urinary ethylmalonate and adipate than their lean littermates. In a study using ob/ob mice, Lai [15] reported that daily excretion of urinary ethylmalonate were significantly greater in obese mice as compared to lean mice. When allowed to eat an all-fat (Crisco) diet for four days, the excretion of adipate rose 10-fold in lean mice, meanwhile only 3-fold in obese mice. This observation suggested the obese mice may have a defect in the pathway by which adipate is synthesized. In this pilot study, we did not observe evidence of correlations between all six urinary biomarkers and maternal pre-pregnancy BMI in both GDM and control groups. Our observation that maternal early pregnancy urinary ethylmalonate concentrations were positively associated with subsequent GDM risk is consistent with the results from animal studies [15,16] which indicate a possible elevation of its precursor, butyryl-CoA. However, we noted that adipate concentrations were inversely associated with GDM risk, and this observation is inconsistent with previous reports [14,16,21,27]. The discrepancy suggests a possible defect in the pathway by which adipate is synthesized in GDM patients [15] Supplemental Fig. 2). This step is a cytochrome P-450 dependent microsomal hydroxylation reaction [32].
Abnormalities of urinary excretion of pyruvate (anaerobic breakdown product of glucose) and lactate (product of glucose oxidation in the muscle) due to their position in the energy production process, provide useful insights into metabolic abnormalities that may precede insulin resistance, T2DM and possibly GDM [13]. After glucose loading, circulating pyruvate and lactate concentrations are significantly increased, and precede hyperglycemia in the general population [22]. In another study of 263 non-obese (BMI approximately 24 kg/m2) Asian–Indian and Chinese men, Tai et al. [21] reported that pyruvate and lactate concentrations were increased in urine in the high-HOMA individuals of both ethnic groups; these changes were accompanied by significant increases in blood lactate concentrations as a function of HOMA, again in both ethnic groups. This is potentially exaggerated in T2DM patients [23]. β-Hydroxybutyrate is a classic ketone body found alongside acetone and acetoacetate. A failure of glucose utilization as with diabetes will result in the formation and urinary elevation of these ketone bodies and sometimes results in diabetic ketoacidosis, a complication predominantly seen in type 1 diabetes mellitus [33]. Ketone body production increases in diabetes because the oxidation of free fatty acids is stimulated, and excess acetyl-CoA is converted to the four-carbon acid, β-hydroxybutyrate. This acetyl-CoA spillover phenomenon occurs because the control of ATP production from fatty acids cannot be regulated as well as from carbohydrate oxidation. An early [18] and a recent [19] study, both case control study designs, showed that GDM patients had higher circulating β-hydroxybutyrate concentrations than controls. Animal study showed that elevations of β-hydroxybutyrate, in an overnight urine collection, may be related to inefficient utilization or mobilization of glucose [34]. Our results concerning pyruvate are consistent with other reports [20–22]. However, we did not observe association between maternal urinary l-lactate or β-hydroxybutyrate concentrations and subsequent GDM risk.
Several methodological caveats should be considered when interpreting the results of our pilot study. Because of our prospective study design, we are able to establish the temporal relationship between maternal early pregnancy organic acid excretion profile and GDM risk later in pregnancy. However, the small sample size hindered our capability to formally access statistical interactions of pre-pregnancy obesity and alteration in urinary organic acids on GDM risk. As discussed above, our review of the available animal literature suggest that systematic evaluations of organic acids in relation to individual obesity status may be of etiologic importance. Future studies, with substantially large sample sizes which allow for serial longitudinal measures of urinary organic acids are needed to further clarify the temporal relationship of urine organic acids, pregnancy weight gain and the incidence of GDM. Although we controlled for confounding, it cannot be concluded with certainty that the reported odds ratios are unaffected by residual confounding. Finally, maternal pre-gestational diabetes status was based on self-report and confirmed in medical records. Chances of undiagnosed diabetes, however, are unlikely because the 5.1% GDM incidence in our study cohort was consistent with US rates. In addition, our cohort is comprised of well-educated, upper income women with over 95% having medical exam within 24 months of pregnancy. Thus it is unlikely that a large proportion of these women were undiagnosed type 2 diabetics.
In summary, in this pilot study, we observed that maternal early pregnancy urinary ethylmalonate and pyruvate concentrations were higher in women who subsequently developed GDM while adipate concentrations were lower in those GDM women. Our results and those of others [15,16,20–22] suggest that evaluation of urinary organic acids may provide some important insights into maternal fatty acid and carbohydrate metabolism during pregnancy and may help elucidate the mechanisms involved in the pathogenesis of GDM.
Our results, if confirmed by future larger sample size studies, highlight the medically relevant potential of determining adipate and ethylmalonate levels in early pregnancy urinary sample, which may promise to serve as a new useful clinical biomarker for predicting GDM risk.
Supplementary Material
Acknowledgment
This research was supported by an award from the National Institutes of Health (R01HD-32562).
Footnotes
Conflict of interest statement
There is no conflict of interest to declare.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.diabres.2014.03.001.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30:S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 3.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009;94:2464–2470. doi: 10.1210/jc.2009-0305. [DOI] [PubMed] [Google Scholar]
- 4.Verier-Mine O. Outcomes in women with a history of gestational diabetes. Screening and prevention of type 2 diabetes. Literature review. Diabetes Metab. 2010;36:595–616. doi: 10.1016/j.diabet.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Black MH, Sacks DA, Xiang AH, Lawrence JM. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care. 2013;36:56–62. doi: 10.2337/dc12-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu C, Frederick IO, Zhang C, Sorensen TK, Enquobahrie DA, et al. Risk of gestational diabetes mellitus in relation to maternal egg and cholesterol intake. Am J Epidemiol. 2011;173:649–658. doi: 10.1093/aje/kwq425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowers K, Tobias DK, Yeung E, Hu FB, Zhang C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am J Clin Nutr. 2012;95:446–453. doi: 10.3945/ajcn.111.026294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao W, Bowers K, Tobias DK, Hu FB, Zhang C. Prepregnancy dietary protein intake, major dietary protein sources, and the risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care. 2013;36:2001–2008. doi: 10.2337/dc12-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enquobahrie DA, Williams MA, Qiu C, Luthy DA. Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2005;70:134–142. doi: 10.1016/j.diabres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Rebuffé-Scrive M, Enk L, Crona N, Lönnroth P, Abrahamsson L, et al. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J Clin Invest. 1985;75:1973–1976. doi: 10.1172/JCI111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71:1256s–1261s. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- 12.Louie JC, Brand-Miller JC, Moses RG. Carbohydrates glycemic index, and pregnancy outcomes in gestational diabetes. Curr Diab Rep. 2013;13:6–11. doi: 10.1007/s11892-012-0332-1. [DOI] [PubMed] [Google Scholar]
- 13.Lord R, Bralley JA. Laboratory evaluations for integrative and functional medicine. 2nd ed. Duluth, GA: Metametrix Institution; 2008. [Google Scholar]
- 14.Yoshioka K, Shimojo N, Nakanishi T, Naka K, Okuda K. Measurements of urinary adipic acid and suberic acid using high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1994;655:189–193. doi: 10.1016/0378-4347(94)80022-7. [DOI] [PubMed] [Google Scholar]
- 15.Lai RK, Goldman P. Urinary organic acid profiles in obese (ob/ob) mice: indications for the impaired omega-oxidation of fatty acids. Metabolism. 1992;41:97–105. doi: 10.1016/0026-0495(92)90197-i. [DOI] [PubMed] [Google Scholar]
- 16.McDevitt J, Wilson S, Her GR, Stobiecki M, Goldman P. Urinary organic acid profiles in fatty Zucker rats: indications for impaired oxidation of butyrate and hexanoate. Metabolism. 1990;39:1012–1020. doi: 10.1016/0026-0495(90)90159-a. [DOI] [PubMed] [Google Scholar]
- 17.Shearer J, Duggan G, Weljie A, Hittel DS, Wasserman DH, Vogel HJ. Metabolomic profiling of dietary-induced insulin resistance in the high fat-fed C57BL/6J mouse. Diabetes Obes Metab. 2008;10:950–958. doi: 10.1111/j.1463-1326.2007.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maresh M, Gillmer MD, Beard RW, Alderson CS, Bloxham BA, et al. The effect of diet and insulin on metabolic profiles of women with gestational diabetes mellitus. Diabetes. 1985;34:88s–93s. doi: 10.2337/diab.34.2.s88. [DOI] [PubMed] [Google Scholar]
- 19.Pappa KI, Anagnou NP, Salamalekis E, Bikouvarakis S, Maropoulos G, et al. Gestational diabetes exhibits lack of carnitine deficiency despite relatively low carnitine levels and alterations in ketogenesis. J Matern Fetal Neonatal Med. 2005;17:63–68. doi: 10.1080/14767050400028733. [DOI] [PubMed] [Google Scholar]
- 20.Konrad T, Vicini P, Kusterer K, Höflich A, Assadkhani A, et al. alpha-Lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes Care. 1999;22:280–287. doi: 10.2337/diacare.22.2.280. [DOI] [PubMed] [Google Scholar]
- 21.Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian–Indian men. Diabetologia. 2010;53:757–767. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Würtz P, Tiainen M, Mäkinen VP, Kangas AJ, Soininen P, et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care. 2012;35:1749–1756. doi: 10.2337/dc11-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheijen JL, Hanssen NM, van de Waarenburg MP, Jonkers DM, et al. l(+) and d(−) lactate are increased in plasma and urine samples of type 2 diabetes as measured by a simultaneous quantification of l(+) and d(−) lactate by reversed-phase liquid chromatography tandem mass spectrometry. Exp Diabetes Res. 2012 Mar 8;2012(2012):234812. doi: 10.1155/2012/234812. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 25.Rothman KJ, Greenland S. Modern epidemiology. 2nd ed. Philadelphia, PA: Lippincott-Raven Publishers; 1998. pp. 311–316. [Google Scholar]
- 26.Feller AG, Rudman D. Role of carnitine in human nutrition. J Nutr. 1988;118:541–547. doi: 10.1093/jn/118.5.541. Review. [DOI] [PubMed] [Google Scholar]
- 27.Mortensen PB. C6–C10-dicarboxylic aciduria in starved, fatfed and diabetic rats receiving decanoic acid or mediumchain triacylglycerol. An in vivo measure of the rate of beta-oxidation of fatty acids. Biochim Biophys Acta. 1981;664:349–355. doi: 10.1016/0005-2760(81)90057-6. [DOI] [PubMed] [Google Scholar]
- 28.Björkhem I, Blomstrand S, Hågå PBF, Kase, Palonek E, et al. Urinary excretion of dicarboxylic acids from patients with the Zellweger syndrome. Importance of peroxisomes in beta-oxidation of dicarboxylic acids. Biochim Biophys Acta. 1984;795:15–19. doi: 10.1016/0005-2760(84)90099-7. [DOI] [PubMed] [Google Scholar]
- 29.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 30.Dubuc PU. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism. 1976;25:1567–1574. doi: 10.1016/0026-0495(76)90109-8. [DOI] [PubMed] [Google Scholar]
- 31.Herberg L, Coleman DL. Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism. 1977;26:59–99. doi: 10.1016/0026-0495(77)90128-7. [DOI] [PubMed] [Google Scholar]
- 32.Wada F, Shibata H, Goto M, Sakamoto Y. Participation of the microsomal electron transport system involving cytochrome P-450 in omega-oxidation of fatty acids. Biochim Biophys Acta. 1968;162:518–524. doi: 10.1016/0005-2728(68)90058-3. [DOI] [PubMed] [Google Scholar]
- 33.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oshida Y, Iwao N, Ohsawa I, Sato J, Nakao T, Sato Y. Effect of insulin on intramuscular 3-hydroxybutyrate levels in diabetic rats. Horm Metab Res. 1998;30:70–71. doi: 10.1055/s-2007-978837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.